Abstract
The overall role of modification of β-cell antigens in type 1 diabetes has not been elucidated and was the focus of a recent workshop on posttranslational modification of proteins in type 1 diabetes. The prevailing opinion of the workshop attendees was that novel insights into the mechanism of loss of immune tolerance might be gained and that novel diagnostic and therapeutic approaches could be developed for type 1 diabetes if protein modifications were shown to play a critical role in the disease.
Tolerance and modified antigens
The mechanisms by which immune tolerance is broken in autoimmune diseases are complex and poorly understood. Data gathered have demonstrated a prominent role for inflammation and cross-talk between the β-cell and the immune system in the pathogenesis of type 1 diabetes (1). The inflammatory response is due to the production of chemokines and cytokines by the β-cell and immune system and leads to β-cell endoplasmic reticulum (ER) and oxidative stress (2,3). Cells undergoing stress are prone to changes in posttranslational modifications (PTMs), alternative splicing, translational infidelity, and misfolding of proteins. The β-cell is highly susceptible to oxidative and ER stress and is potentially a site of alteration in protein expression. Alternative splicing and PTMs are prominent in the face of cytokine-induced β-cell stress (4–6), and there is some evidence for immune recognition of PTMs of β-cell autoantigens.
Tolerance to self-antigens is normally achieved through two mechanisms: central tolerance and peripheral tolerance. In central tolerance, B and T cells that recognize self-antigens are destroyed in the bone marrow and thymus, respectively. Peripheral tolerance exists in cells that have escaped this deletional tolerance. Even with these two mechanisms, tolerance can be broken, causing autoimmunity. One possible explanation is that antigens presented in the periphery undergo protein modifications whereby T cells or antibodies recognize a modified protein as a new antigen. Subsequently, an immune response is generated against this novel epitope.
Observations suggest that modified autoantigens may be critical in type 1 diabetes. Insulin may be the predominant autoantigen in initiating disease. Evidence from both NOD mice and humans with type 1 diabetes suggests that autoreactive T cells are very diverse, and a number of autoantigens have been implicated in disease pathogenesis (7). Loss of central tolerance is a prevalent idea. An attractive hypothesis is that autoreactive T cells escape negative selection because a protein modification prevents the antigenic protein being presented in the thymus. Alternatively, a protein modification may alter presentation in the pancreas or pancreatic lymph nodes (PLNs). PLNs drain the pancreas and are key players in antigen presentation of pancreatic antigens. Using insulin as an experimental model, Unanue and colleagues have focused on differences in antigen presentation in tissue-dwelling antigen-presenting cells (APCs) and the thymus. They have described two types of granules within the β-cell: most are classical insulin-containing granules with C-peptide, and a smaller quantity contain a number of degradation products of insulin (8). Some of these latter granules escape and are captured by a network of APCs, which tightly associate with blood vessels, in the islets. A number of digested peptides are presented that differ from native insulin sequences, possibly resulting in the generation of diabetogenic T cells. This may be common among all endocrine tissues, and using tools previously developed may help us understand the role of protein modifications in type 1 diabetes.
β-Cell antigen presentation
T-cell specificity is dictated by the uniqueness of peptide display by major histocompatibility complex (MHC) molecules on the surface of APCs. In type 1 diabetes, if β-cell antigens are modified, how these peptides might bind to MHC and present to T cells is unknown. It is possible that the altered peptide changes the way it is presented to T cells and in the determination of tolerance. Kappler and colleagues presented data resolving the question of how NOD CD4+ T cells recognize insulin B:9–23 bound to IAg7 (9). In the case of the B:9–23 peptide of insulin that has been implicated in initiating type 1 diabetes, Kappler, Eisenbarth, and colleagues (10) have shown that the immunogenicity of this peptide resides in its ability to bind to MHC in different registers. New research from this group suggests that there is a third register in which the insulin peptide could bind. PTM of insulin peptides may result in additional binding conformations and registers, resulting in diverse T-cell recognition (9). Kappler and colleagues pointed out the dearth of structural information on how peptide modifications alter MHC binding and noted that either altered intracellular antigen processing or PTMs could create novel or cryptic epitopes. To better understand the basis by which T cells escape negative selection in the thymus, Kappler and colleagues (11) have described register three, which they have shown to successfully present multiple insulin epitopes. In the case of chromogranin A, the newly discovered BDC-2.5 antigen, the ligand is a naturally processed peptide, WE14. Similar to the situation with insulin B:9–23, this peptide may occupy an unusual position in the MHC binding groove.
β-Cell cross-talk
The cross-talk between β-cells and the immune system should be considered in the pathogenesis of type 1 diabetes, particularly given that the autoantigens are expressed in the β-cells. The trigger of the initial event may be due to a “dialogue” between the β-cells and the immune system. Activation of the innate immune system by viruses, cytokines, or endogenous ligands may trigger a sequence of events in the β-cell that results in β-cell antigen modifications and local production of inflammatory mediators (1). These events may also contribute to ER stress. When the normal equilibrium of the ER is disrupted, an adaptive program called the unfolded protein response is initiated. This response involves reduced protein synthesis, upregulation of ER chaperones, and increased protein degradation, which help the ER to reestablish equilibrium; if the unfolded protein response fails to restore homeostasis, apoptosis is eventually triggered (12,13). Oxidative stress also results in ER stress in β-cells and can lead to disrupted function and survival. Furthermore, because the assembly of MHC molecules also takes place in the ER, altered assembly and peptide loading in a dysfunctional ER may propagate the autoimmune response in type 1 diabetes. By targeting ER stress responses, alternatives to treat type 1 diabetes may emerge (14,15). The ER has three sensors of misfolded proteins: RNA-dependent protein kinase-like ER kinase, inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6). RNA-dependent protein kinase-like ER kinase and IRE1 are both kinases, and IRE1 also acts as an RNase (3). Engin and Hotamisligil suggest that ATF6 may be a key pathogenic pathway for β-cells exposed to ER stress in the context of insulitis. β-Cells from NOD mice express low levels of ATF6, hampering their response to ER stress. Treating NOD mice with tauroursodeoxycholic acid, a chemical chaperone that alleviates ER stress, impedes diabetes development (16). Insulin production and ATF6 expression are retained and, in spite of islet infiltration by immune cells, there seems to be no ongoing destructive immune response. These data suggest that targeting ER stress and the inflammatory pathway may provide novel therapeutic approaches.
Evidence for PTMs in other autoimmune diseases
In several autoimmune diseases, such as celiac disease and rheumatoid arthritis (RA), many of the antigens targeted by the autoimmune process have been modified (summarized in Table 1). In celiac disease, which is caused by a hypersensitivity to gluten proteins, loss of tolerance is likely due to modification of these proteins and the formation of neoepitopes. Tissue transglutaminase (tTG) deamidates gluten peptides, resulting in strongly enhanced T cell–stimulatory activity due to improved binding of these peptides to disease associated HLA DQ allotypes (17). Sollid and colleagues demonstrated a correlation between the degree of deamidation of gluten and T cell responses in celiac patients (18,19). tTG is ubiquitously expressed and may be critical in other autoimmune diseases, including type 1 diabetes, because it may be expressed within islets (17). Furthermore, as many of the susceptibility genes in the immune system are shared between celiac disease and type 1 diabetes, we may be able to extrapolate knowledge from one disease to the other. Thus, an investigation into deamidation of β-cell antigens and potential modulation of HLA DQ binding is warranted. Type 1 diabetic patients do not have anti-tTG autoantibodies, suggesting distinct pathological processes.
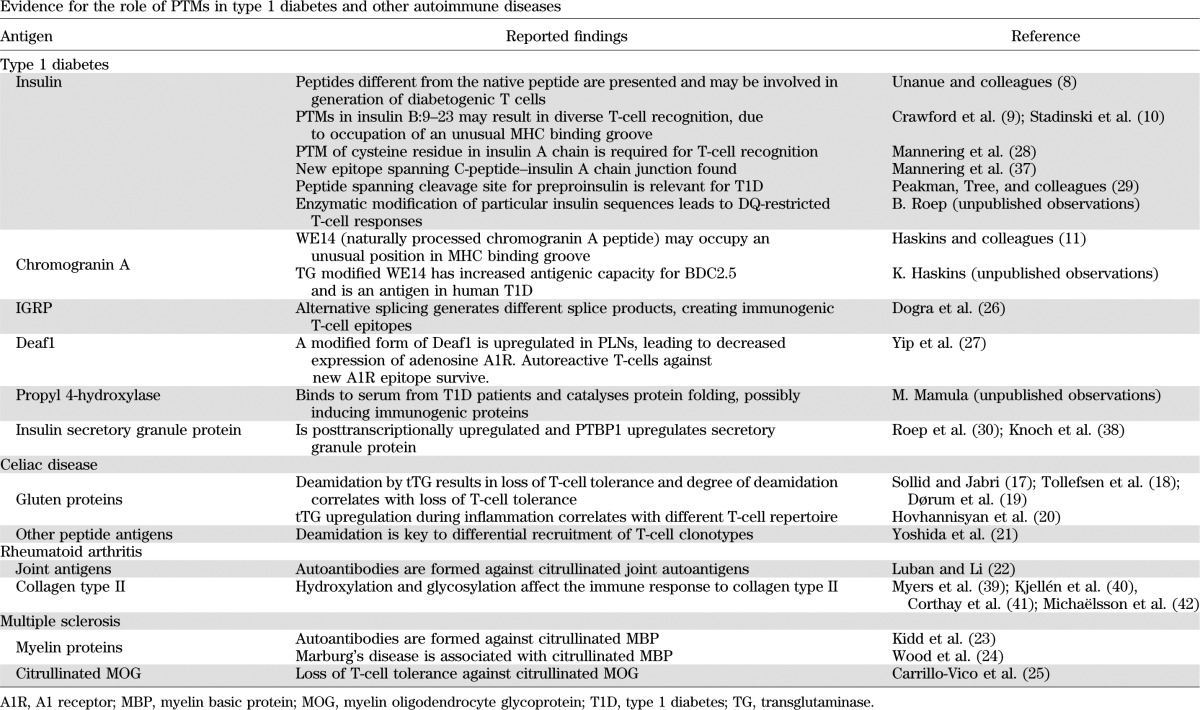
TABLE 1.
Evidence for the role of PTMs in type 1 diabetes and other autoimmune diseases
Since upregulation of tTG often occurs in the context of inflammation, Jabri and colleagues noted that the T-cell repertoire is potentially different than under normal conditions. When tTG is activated as the result of inflammation, the deamidated form of the gluten peptide may be produced, and the T-cell repertoire preferentially recognizing the deamidated peptide can expand, increasing the possibility of disease (20). They showed that the native and deamidated form of the immunodominant gluten peptide induced similar immune responses in a mouse model of celiac disease. The nature of the T-cell repertoire in response to the two peptides was different, suggesting that deamidation was key to the differential recruitment of T-cell clonotypes. In further experiments with L. Teyton’s laboratory, Jabri and colleagues showed that this effect could be reproduced in other peptides, suggesting that this was not particular to the gluten peptide (21). This suggests that immunization with both the native and deamidated peptide would maximize the immune response because different types of T cells would be recruited, and this is what Jabri, Teyton, and colleagues found. Finally, as described below, transglutaminase plays a role in the modification of a murine diabetes antigen, WE14 (K. Haskins, unpublished data). Collectively, these data suggest that transglutaminase may be an important therapeutic target.
Citrullination, the deimidation of arginine to citrulline, has been implicated as a modification to autoantigens in RA (22) and multiple sclerosis (23) (Table 1). In RA, autoantibodies are generated against citrullinated joint autoantigens. The ability of these autoantibodies to recognize cyclic pentapeptide–containing citrulline residues (CCPs) is used diagnostically and has high specificity for RA, with anti-CCP autoantibodies correlating with disease prognosis and severity.
In multiple sclerosis, citrullinated myelin proteins may be a component of autoreactivity. After immunization with proteolipid protein to induce experimental autoimmune encephalomyelitis (EAE), autoantibodies against a citrullinated form of myelin basic protein have been reported (23). Moreover, a severe form, Marburg’s disease, has been associated with extensive citrullination of myelin basic protein (24). Inflammation plays an important role in this modification. The question of whether T cells can specifically recognize citrullinated autoantigens, and whether these T cells are pathogenic, has also been recently addressed (25). Mouse T cells that responded to modified peptides of myelin oligodendrocyte glycoprotein containing citrulline at a T-cell receptor (TCR) contact residue could be generated. These T cells responded efficiently, indicating a lack of tolerance to the modified form of the autoantigen. However, although these T cells could respond at very low concentrations and could exacerbate ongoing EAE upon infusion, they could not initiate clinical signs of EAE. For this, T cells recognizing the native form of myelin oligodendrocyte glycoprotein were required. This led to the conclusion that specific T-cell recognition of citrullinated autoantigen could contribute to pathology, although an initial inflammatory insult in the central nervous system would be required.
Using knowledge about autoreactive T cells and their targets, as well as lessons learned from other autoimmune diseases, it may be possible to work backward to find potential modifications of antigenic epitopes. One of the key findings from other autoimmune diseases points to inflammation being a crucial factor in the initiation of modifications. Although inflammation may cause modifications, the findings that can explain MHC association with type 1 diabetes will most likely be the most relevant.
Evidence in type 1 diabetes
In type 1 diabetes, do modifications of proteins cause the immune system to see certain proteins and ignore others? Many proteins are modified, and a modified protein will likely look different to the immune system. Preliminary evidence shows that β-cell antigens undergo modifications through alternative splicing. Alternative splicing occurs in about 90% of human genes, meaning that a single gene may result in multiple protein isoforms. Importantly, there is a tissue-specific factor, and alternative splicing generates enormous proteome diversity, which may lead to the exposure of novel antigenic epitopes. Islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP), a type 1 diabetes autoantigen, has been shown to have at least eight different splice products, six of which are unique to β-cells (26). These splice variants create very immunogenic T-cell epitopes due to frameshifts and neosequences. However, given the number of type 1 diabetes autoantigens, thousands of splice variants may exist and be specific for β-cells, but proving that these proteins exist and have modified functions is an important step forward to understanding how alternative splicing may impact disease pathogenesis. Eizirik and colleagues are using next generation RNA sequencing to discover new transcripts that result from alternative splicing following exposure to proinflammatory cytokines (4). By comparing these data sets generated from islets to similar data sets made from other human tissues, islet-specific transcripts can be identified. Preliminary results suggest that chemokines found in the islets are alternatively spliced. The functional impact of these changes and their pathogenic role remains to be elucidated, but one hypothesis is that the continuing local production of alternatively spliced cytokines and chemokines will keep the inflammatory process going unless there is an external force to stop it.
In a study from Fathman and colleagues, the role of Deaf1, a transcriptional regulator, in regulating the expression of genes encoding peripheral tissue antigens (PTAs) in the PLNs was investigated (27). PTAs are important in the maintenance of tolerance. A variant form of Deaf1 is upregulated in the PLNs while the canonical form does not appear to lead to autoimmunity. The Deaf1 variant leads to decreased expression of PTAs such as the adenosine A1 receptor, leading to reduced presentation on stromal cells of the PLN. This allows self-reactive T cells against this receptor to escape deletion and self-reactive T cells against the new epitope of the A1 receptor to survive because it is never expressed. In the pancreas, these T cells mediate the destruction of A1 receptor-positive glucagon-positive cells, and with time these cells seem to disappear. The presenters concluded that destruction of the A1-receptor positive cells at the start of insulitis might contribute to bystander destruction of nearby β-cells.
Further evidence that PTMs may be important in type 1 diabetes was provided by Mannering et al., who studied epitopes formed by PTMs of insulin. They showed that spontaneous PTMs of adjacent cysteine residues in the insulin A chain is required for T-cell recognition (28). Peakman, Tree, and colleagues indicated that a peptide spanning the cleavage site for preproinsulin may be of great relevance for type 1 diabetes through presentation to CD8+ T cells, which may be involved in the killing of β-cells (29). B. Roep has studied binding motifs of HLA-DQ binding proteins and found particular sequences in proinsulin that may be subject to enzymatic modification leading to DQ-restricted T-cell responses. Using human islets, he found splice variants of IGRP and islet antigen-2 that were unique to β-cells and were not present in the thymus. K. Haskins reported that the native form of WE14 from chromogranin A is a weak antigen, but is converted to a highly stimulatory peptide by tissue transglutaminase. The enzymatically converted form of WE14 is more antigenic for autoreactive T cells represented by the clonotype BDC-2.5 and appears to be an antigen for human patients with type 1 diabetes (K. Haskins, unpublished data). Haskins concluded that PTMs may be an important mechanism for generation of autoantigenic T-cell epitopes.
M. Mamula showed that an endoplasmic reticulum resident enzyme, prolyl 4 hydroxylase, can bind to sera from type 1 diabetic patients. This protein catalyzes protein folding, suggesting that a modification creates misfolding of proteins, potentially making it immunogenic in some form. Inhibition of this enzyme leads β-cells to have lower glucose-induced released insulin. Early diabetic patients are currently being screened for this enzyme to test its potential use as a disease marker. Mamula expressed how using biochemistry tools to identify these types of proteins is key, but stressed that the real importance remains in realizing how these proteins are in involved in the disease process.
Cell biologists have considered the insulin secretory granule as a potential autoantigen. The upregulation of secretory granule protein is primarily a posttranscriptional event, and understanding its role can lead to a greater understanding of type 1 diabetes, antigen presentation, and immune tolerance (30). Granules are the most abundant component of the β-cell, and the insulin granule is dominant within the β-cell, suggesting a role for the insulin secretory granule in the pathogenesis of type 1 diabetes. M. Solimena has shown that polypyrimidine-tract binding protein 1 (PTBP1) is involved in the posttranscriptional upregulation of insulin biosynthesis following recruitment to β-cell. This data suggests that nuclear retention of PTBP1 is likely to contribute to impaired insulin secretion, noting the importance of understanding the insulin secretory mechanisms in understanding type 1 diabetes. Interestingly, PTBP1 is also required for the virus-mediated translation of baculovirus RNAs. Recent evidence has shown that the RNA from viruses that are implicated in diabetes may exploit this mechanism to express themselves at a high level. Thus, it may be possible in the context of viral-induced type 1 diabetes pathogenesis that the secretory granule containing autoantigens and the virus converge on the same pathway to induce a modification that the immune system detects to initiate an autoimmune response.
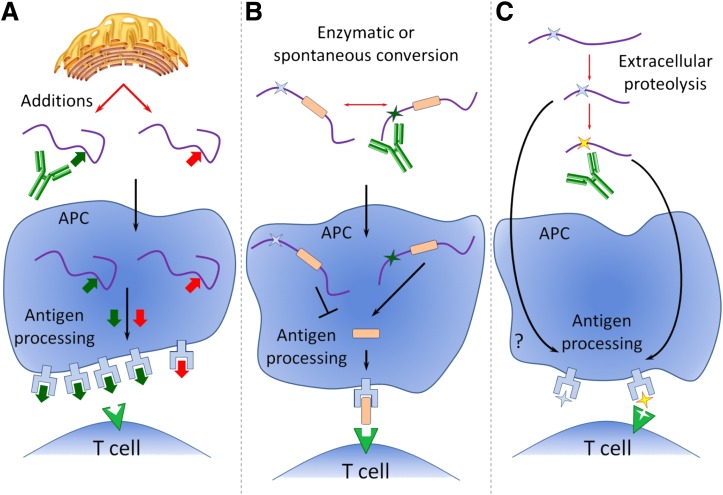
What are the drivers of these events in type 1 diabetes? Evidence suggests that they do exist and can become targets of autoimmune reactions. More needs to be learned to clarify the role for modifications, especially in the pathogenesis of human type 1 diabetes, which is more heterogeneous than the current mouse models. Understanding how these events contribute to disease may lead to better, early therapeutic approaches. At present, we propose a synopsis of how these PTMs could contribute to autoimmunity in type 1 diabetes (Fig. 1).
FIG. 1.
A possible role for PTMs in the pathogenesis of type 1 diabetes. Any form of nonspecific β-cell stress such as inflammation or metabolic stress may lead to alterations in peptide/protein state. These altered peptides/proteins may be processed by the immune system in different immunogenic ways. A: Modifications such as phosphorylation, methylation, or glycosylation may create a new epitope for autoantibody binding. Alternatively, after uptake by APCs, the PTM may create a different nontolerized ligand for T-cell recognition. B: Enzymatic or spontaneous modifications, such as deamidation and citrullination, can also generate neoepitopes or influence antigen processing. C: Extracellular proteolysis to generate peptide fragments may be required to allow subsequent modification. In this case, the posttranslationally modified protein (yellow star) forms part of a neoepitope for T-cell recognition. Alternatively, the PTM itself could enhance or limit extracellular degradation by interference with the cleavage site. Figure adapted from Anderton et al. (43).
Diagnostic potential
Organ-specific diseases such as type 1 diabetes have the difficulty that the target organ is not accessible for direct evaluation, allowing staging of the disease on a pathogenic rather than a clinical basis. We need to rely on biomarkers circulating in blood, such as autoantibodies. Often, these autoantibodies are nonspecific and have limited prognostic value. In type 1 diabetes, combinations of autoantibodies are used in order to fine tune risk in genetically predisposed individuals, but remain of lesser value in the general population. The recent arrival of autoantibodies directed against intracellular proteins has allowed greater refinement, but limitations still exist. Lessons from other autoimmune diseases demonstrate that using PTM peptides may allow the detection of more relevant, sensitive, and new autoantibodies. There was concern among the participants that this avenue may not be fruitful in type 1 diabetes, given that the evidence that autoantibodies are pathogenic is nonexistent.
The use of mass spectrometry to identify peptides isolated from MHC molecules from the surface of β-cells or from professional APCs in associated lymphatic tissue promises to identify not only native but also PTM peptides that may have a role in type 1 diabetes pathogenesis (31). Advances in mass spectrometry and particularly targeted mass spectrometry such as multiple reaction monitoring (32), make in depth analysis of the peptidome a reality. Mass spectrometry is the only technique currently available to robustly identify and characterize PTM antigens. The ability to generate recombinant proteins with preordained PTMs is now also a viable option with work pioneered by Schultz and colleagues (33). Thus the ability to generate chemically defined antigens for immunotherapy may follow rapidly from their discovery.
Development of more sensitive detection systems allowing identification of PTM peptides and proteins opens a new path for biomarkers, namely the detection of circulating modified β-cell–relevant peptides (34). The possibility exists that a β-cell under stress releases misprocessed, misfolded, or modified peptides or proteins. Several groups are performing secretome analyses at the peptide level, trying to identify specific peptides or signatures of peptides that are released by β-cells under stress. Next to these secretome analyses, analyses of the peptidome and proteome of dying β-cells may yield signatures of peptides that can indicate ongoing β-cell destruction. This opens a new aspect of biomarker development that has been exploited in a limited way in type 1 diabetes.
New biomarkers may come from modeling using bioinformatics, allowing for integration of data from different sources and projection of models. Data from microarray and genome-wide association studies can be integrated with the proteomics and peptidomics work, adding the information on PTMs to genetic information (35). Much attention has been given to protein-protein interactions and the protein sites for these interactions, including interactions between putative autoantigens and MHC or TCR molecules. Integrating the data on the strength of the immune response toward peptides with different PTMs, with carefully gathered phenotypic data from the responders, may allow prediction on a whole array of putative autoantigens and their relevance for certain patient phenotypes. On the basis of these data, projection models on preferred PTM types or sites in the peptides that may be relevant for the pathogenesis of type 1 diabetes and the development of biomarkers may be developed. Moreover, provided the system gets input from carefully executed phenotypic databases, bioinformatics could well provide the step toward individualized biomarker development, as certain peptides may be autoantigens for some individuals with a certain immune make-up but not for others (36). Overall, there was support from the participants in using these types of approaches to integrate data from multiple sources, to bridge the gap between what occurs at the molecular level and the phenotypic level.
Applications in immunotherapy
If PTM proteins and peptides are important for the pathogenesis of the disease, can we use modified rather than native proteins to develop vaccination strategies to increase their efficacy? There are still many questions, such as which PTM to select, as studies demonstrate that potentially different PTMs will occur when β-cells are under stress or even more important that different PTMs might be implicated in different individuals in protein-protein interactions taking place in the MHC molecule or TCR context. Some PTMs are also rare or transient and may not be relevant for making a peptide or protein into an autoantigen. Long-lasting PTMs should be more relevant, but at present that is not certain.
A suggested approach to relevant PTM selection for autoantigen development is to start from existing autoantibodies or autoreactive T cells present in newly diagnosed type 1 diabetic patients. PTMs present in the peptides recognized by these antibodies or T cells will be long lasting and relevant. Finding the peptides to which T cells in the inflamed pancreas of type 1 diabetic patients are reactive will most probably point to the most relevant peptides and their eventual PTMs (Fig. 1).
Due to the current lack of understanding of the pathogenesis of type 1 diabetes, it is possible that different PTMs may be relevant in triggering the aggressiveness of the immune system, and that children may react differently to modified proteins or peptides than adults and need different peptides or proteins in vaccination regimens. We need to be more inclusive in the thinking as databases start to be developed around the relevance of PTMs to type 1 diabetes. Recent-onset type 1 diabetes individuals will likely look different than early or late prediabetic individual and intervention in these periods may require different approaches.
Summary
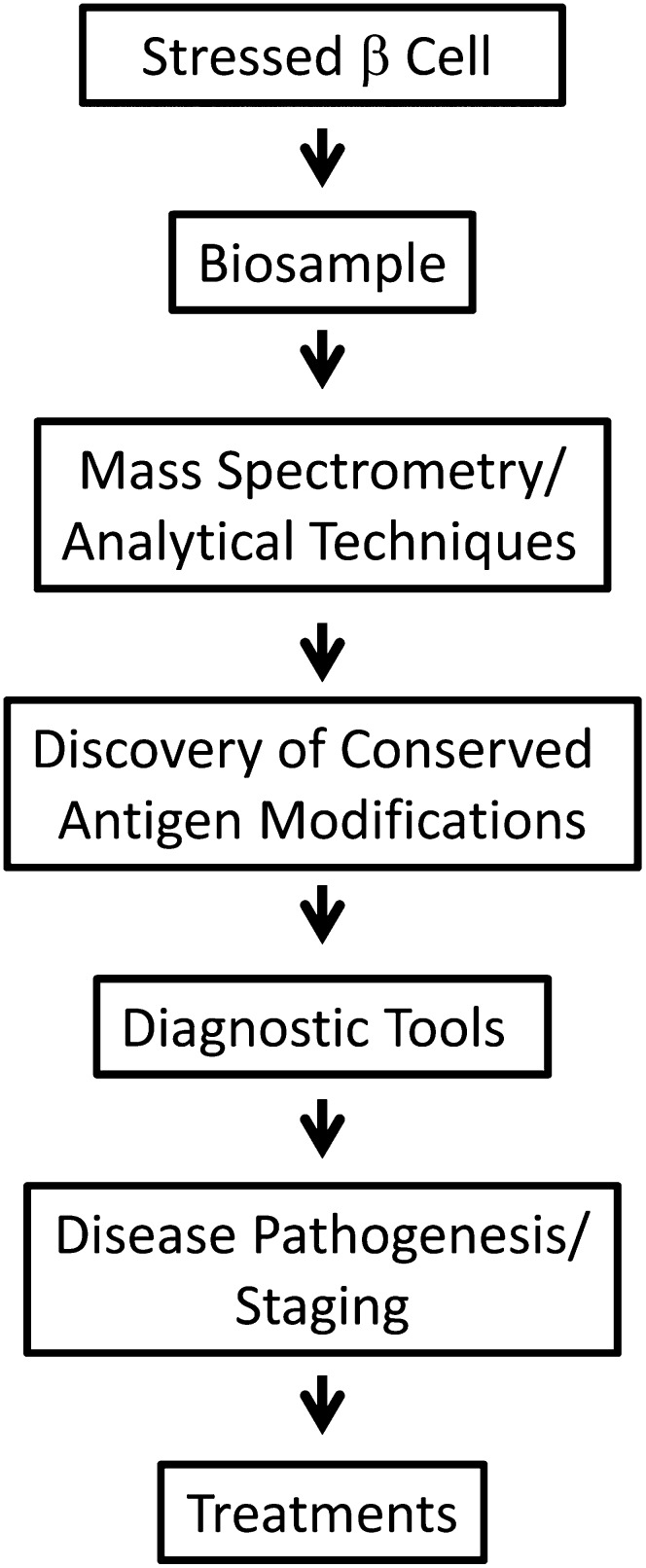
In a workshop held in December of 2010 on the PTM of proteins in type 1 diabetes, there was discussion as to what approaches to take and what the next steps should be to move the field forward in type 1 diabetes. That protein modifications do exist and are relevant to immune recognition is clear. What is less clear is their importance. It will be important to convince ourselves that a particular antigen modification is disease causative or therapeutic in a way that would allow us to move forward. How this will be achieved and assessed in humans is still uncertain. A possible scenario is presented in Fig. 2. If it is an enzymatic change, one could easily measure the level of the enzyme expression in the pancreas. How to measure the level of a modified antigen is more challenging, and whether we have the tools to do that and get to the underlying mechanism is the tougher task. How do we test antigenic modifications and, perhaps, more importantly, do we need to? In terms of therapeutics, the role of modified antigens is even less clear. Once a modified autoantigen has been identified as causative in disease, disrupting the generation of the modified autoantigen may be sufficient. However, the challenge remains to understand the ramifications of inhibiting a particular pathway in an ongoing autoimmune response once the patient has presented in the clinic. A therapeutic alternative could be antigen-specific therapy with modified autoantigens or peptides. Can a person become tolerized to a modified antigen that is disease causative prior to disease onset or after disease onset? Or will therapeutically administered antigens aggravate disease?
FIG. 2.
The value of studying PTMs in type 1 diabetes. Discovering the role of PTMs in altering β-cell proteins may not only lead to better understanding of the pathogenesis of type 1 diabetes but could also open avenues for the development of new diagnostic and therapeutic tools for prevention and intervention in the disease.
In type 1 diabetes, antigenic modifications may play a role. The inherent susceptibility of β-cells will be heterogeneous across individuals and within any given tissue, including the pancreas, not all cells will succumb to stress at the same time. Indeed, pancreatic samples have shown that in patients with long-standing type 1 diabetes, there can remain functioning β-cells. Understanding what causes one β-cell in a pancreas to yield to an immune attack while a β-cell in a neighboring islet withstands the same attack is key to understanding the process of disease, including the role of antigenic modifications.
The keys to understanding PTMs in human type 1 diabetes will likely involve integration of data that involves genomics, proteomics, bioinformatics, and clinical data. While the suspected heterogeneity of type 1 diabetes remains problematic, the availability of biobanked samples from retrospective and longitudinal patient cohorts will help in identifying appropriate biomarkers or therapeutics. The role of PTMs in type 1 diabetes is a nascent field, with many questions unanswered. The Juvenile Diabetes Research Foundation (JDRF) recently solicited applications to assess the role of PTMs in the immunopathogenesis of type 1 diabetes with plans to support research in this area.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
J.L.D., L.O., A.W.P., and C.M. wrote, reviewed, and edited the manuscript. J.L.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors would like to thank JDRF for hosting the workshop on posttranslational modification of proteins in type 1 diabetes in December 2010, and all the participants of this workshop for their contributions to the discussion and reading of the manuscript: Stephen Anderton (University of Edinburgh), Soren Brunak (Technical University of Denmark), Teresa Dilorenzo (Albert Einstein College of Medicine), Decio Eizirik (Universite Libre de Bruxelles), Garry Fathman (Stanford University Medical School), Katie Haskins (University of Colorado School of Medicine), Ann Herman (Genomics Institute of the Novartis Research Foundation), Rikhard Holmdahl (Karolinska Institutet), Gokhan Hotamisligil (Harvard School of Public Health), Bana Jabri (University of Chicago), John Kappler (Howard Hughes Medical Institute), Allan Ertmann Karlsen (Hagedorn Research Institute), Mark Mamula (Yale University School of Medicine), Stuart Mannering (St. Vincent's Institute of Medical Research), Anna Maria Papini (University of Florence and University of Cergy-Pontoise), Mark Peakman (King's College London), Flemming Pociot (Glostrup Research Institute), Bill Robinson (Stanford University Medical School), Bart Roep (Leiden University Medical Center), Peter Schultz (The Scripps Research Institute), Hal Scofield (Oklahoma Medical Research Foundation), Michele Solimena (Dresden University of Technology), Ludvig Sollid (University of Oslo), Emil Unanue (Washington University), Cong-Yi Wang (Medical College of Georgia), and Linda Yip (Stanford University Medical School).
REFERENCES
- 1.Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol 2009;5:219–226 [DOI] [PubMed] [Google Scholar]
- 2.Eizirik DL, Cnop M. ER stress in pancreatic beta cells: the thin red line between adaptation and failure. Sci Signal 2010;3:pe7. [DOI] [PubMed] [Google Scholar]
- 3.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010;140:900–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ortis F, Naamane N, Flamez D, et al. Cytokines interleukin-1beta and tumor necrosis factor-alpha regulate different transcriptional and alternative splicing networks in primary beta-cells. Diabetes 2010;59:358–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Hertog W, Maris M, Ferreira GB, et al. Novel insights into the global proteome responses of insulin-producing INS-1E cells to different degrees of endoplasmic reticulum stress. J Proteome Res 2010;9:5142–5152 [DOI] [PubMed] [Google Scholar]
- 6.D’Hertog W, Overbergh L, Lage K, et al. Proteomics analysis of cytokine-induced dysfunction and death in insulin-producing INS-1E cells: new insights into the pathways involved. Mol Cell Proteomics 2007;6:2180–2199 [DOI] [PubMed] [Google Scholar]
- 7.van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev 2011;91:79–118 [DOI] [PubMed] [Google Scholar]
- 8.Mohan JF, Levisetti MG, Calderon B, Herzog JW, Petzold SJ, Unanue ER. Unique autoreactive T cells recognize insulin peptides generated within the islets of Langerhans in autoimmune diabetes. Nat Immunol 2010;11:350–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crawford F, Stadinski B, Jin N, et al. Specificity and detection of insulin-reactive CD4+ T cells in type 1 diabetes in the nonobese diabetic (NOD) mouse. Proc Natl Acad Sci USA 2011;108:16729–16734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stadinski BD, Zhang L, Crawford F, Marrack P, Eisenbarth GS, Kappler JW. Diabetogenic T cells recognize insulin bound to IAg7 in an unexpected, weakly binding register. Proc Natl Acad Sci USA 2010;107:10978–10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stadinski BD, Delong T, Reisdorph N, et al. Chromogranin A is an autoantigen in type 1 diabetes. Nat Immunol 2010;11:225–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J, Kaufman RJ. From acute ER stress to physiological roles of the Unfolded Protein Response. Cell Death Differ 2006;13:374–384 [DOI] [PubMed] [Google Scholar]
- 13.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev 2008;29:42–61 [DOI] [PubMed] [Google Scholar]
- 14.Kaufman RJ, Back SH, Song B, Han J, Hassler J. The unfolded protein response is required to maintain the integrity of the endoplasmic reticulum, prevent oxidative stress and preserve differentiation in β-cells. Diabetes Obes Metab 2010;12(Suppl. 2):99–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volchuk A, Ron D. The endoplasmic reticulum stress response in the pancreatic β-cell. Diabetes Obes Metab 2010;12(Suppl. 2):48–57 [DOI] [PubMed] [Google Scholar]
- 16.Engin F, Hotamisligil GS. Restoring endoplasmic reticulum function by chemical chaperones: an emerging therapeutic approach for metabolic diseases. Diabetes Obes Metab 2010;12(Suppl. 2):108–115 [DOI] [PubMed] [Google Scholar]
- 17.Sollid LM, Jabri B. Celiac disease and transglutaminase 2: a model for posttranslational modification of antigens and HLA association in the pathogenesis of autoimmune disorders. Curr Opin Immunol 2011;23:732–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tollefsen S, Arentz-Hansen H, Fleckenstein B, et al. HLA-DQ2 and -DQ8 signatures of gluten T cell epitopes in celiac disease. J Clin Invest 2006;116:2226–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dørum S, Arntzen MO, Qiao SW, et al. The preferred substrates for transglutaminase 2 in a complex wheat gluten digest are Peptide fragments harboring celiac disease T-cell epitopes. PLoS ONE 2010;5:e14056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hovhannisyan Z, Weiss A, Martin A, et al. The role of HLA-DQ8 beta57 polymorphism in the anti-gluten T-cell response in coeliac disease. Nature 2008;456:534–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshida K, Corper AL, Herro R, Jabri B, Wilson IA, Teyton L. The diabetogenic mouse MHC class II molecule I-Ag7 is endowed with a switch that modulates TCR affinity. J Clin Invest 2010;120:1578–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luban S, Li ZG. Citrullinated peptide and its relevance to rheumatoid arthritis: an update. Int J Rheum Dis 2010;13:284–287 [DOI] [PubMed] [Google Scholar]
- 23.Kidd BA, Ho PP, Sharpe O, et al. Epitope spreading to citrullinated antigens in mouse models of autoimmune arthritis and demyelination. Arthritis Res Ther 2008;10:R119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood DD, Bilbao JM, O’Connors P, Moscarello MA. Acute multiple sclerosis (Marburg type) is associated with developmentally immature myelin basic protein. Ann Neurol 1996;40:18–24 [DOI] [PubMed] [Google Scholar]
- 25.Carrillo-Vico A, Leech MD, Anderton SM. Contribution of myelin autoantigen citrullination to T cell autoaggression in the central nervous system. J Immunol 2010;184:2839–2846 [DOI] [PubMed] [Google Scholar]
- 26.Dogra RS, Vaidyanathan P, Prabakar KR, Marshall KE, Hutton JC, Pugliese A. Alternative splicing of G6PC2, the gene coding for the islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP), results in differential expression in human thymus and spleen compared with pancreas. Diabetologia 2006;49:953–957 [DOI] [PubMed] [Google Scholar]
- 27.Yip L, Su L, Sheng D, et al. Deaf1 isoforms control the expression of genes encoding peripheral tissue antigens in the pancreatic lymph nodes during type 1 diabetes. Nat Immunol 2009;10:1026–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mannering SI, Harrison LC, Williamson NA, et al. The insulin A-chain epitope recognized by human T cells is posttranslationally modified. J Exp Med 2005;202:1191–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skowera A, Ellis RJ, Varela-Calviño R, et al. CTLs are targeted to kill beta cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope. J Clin Invest 2008;118:3390–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roep BO, Arden SD, de Vries RR, Hutton JC. T-cell clones from a type-1 diabetes patient respond to insulin secretory granule proteins. Nature 1990;345:632–634 [DOI] [PubMed] [Google Scholar]
- 31.Purcell AW, Gorman JJ. Immunoproteomics: Mass spectrometry-based methods to study the targets of the immune response. Mol Cell Proteomics 2004;3:193–208 [DOI] [PubMed] [Google Scholar]
- 32.Tan CT, Croft NP, Dudek NL, Williamson NA, Purcell AW. Direct quantitation of MHC-bound peptide epitopes by selected reaction monitoring. Proteomics 2011;11:2336–2340 [DOI] [PubMed] [Google Scholar]
- 33.Grünewald J, Hunt GS, Dong L, et al. Mechanistic studies of the immunochemical termination of self-tolerance with unnatural amino acids. Proc Natl Acad Sci USA 2009;106:4337–4342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papini AM. The use of post-translationally modified peptides for detection of biomarkers of immune-mediated diseases. J Pept Sci 2009;15:621–628 [DOI] [PubMed] [Google Scholar]
- 35.Pers TH, Hansen NT, Lage K, et al. Meta-analysis of heterogeneous data sources for genome-scale identification of risk genes in complex phenotypes. Genet Epidemiol 2011;35:318–332 [DOI] [PubMed] [Google Scholar]
- 36.Brorsson C, Tue Hansen N, Bergholdt R, Brunak S, Pociot F. The type 1 diabetes - HLA susceptibility interactome—identification of HLA genotype-specific disease genes for type 1 diabetes. PLoS ONE 2010;5:e9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mannering SI, Pang SH, Williamson NA, et al. The A-chain of insulin is a hot-spot for CD4+ T cell epitopes in human type 1 diabetes. Clin Exp Immunol 2009;156:226–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knoch KP, Meisterfeld R, Kersting S, et al. cAMP-dependent phosphorylation of PTB1 promotes the expression of insulin secretory granule proteins in beta cells. Cell Metab 2006;3:123–134 [DOI] [PubMed] [Google Scholar]
- 39.Myers LK, Myllyharju J, Nokelainen M, et al. Relevance of posttranslational modifications for the arthritogenicity of type II collagen. J Immunol 2004;172:2970–2975 [DOI] [PubMed] [Google Scholar]
- 40.Kjellén P, Brunsberg U, Broddefalk J, et al. The structural basis of MHC control of collagen-induced arthritis; binding of the immunodominant type II collagen 256-270 glycopeptide to H-2Aq and H-2Ap molecules. Eur J Immunol 1998;28:755–767 [DOI] [PubMed] [Google Scholar]
- 41.Corthay A, Bäcklund J, Broddefalk J, et al. Epitope glycosylation plays a critical role for T cell recognition of type II collagen in collagen-induced arthritis. Eur J Immunol 1998;28:2580–2590 [DOI] [PubMed] [Google Scholar]
- 42.Michaëlsson E, Malmström V, Reis S, Engström A, Burkhardt H, Holmdahl R. T cell recognition of carbohydrates on type II collagen. J Exp Med 1994;180:745–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderton SM. Post-translational modifications of self antigens: implications for autoimmunity. Curr Opin Immunol 2004;16:753–758 [DOI] [PubMed] [Google Scholar]