Mitochondria are at the crossroads of energy metabolism, and they play a major role in the regulation of cell life and death. Mitochondrial oxidation of fat and glucose substrates generates a transmembrane electrochemical gradient, and this is used to synthesize adenosine triphosphate (ATP), the chemical fuel for cell and tissue functions. In the process, electron transfer through the mitochondrial respiratory chain is inevitably associated with production of reactive oxygen species (ROS). Several physiological roles have been described for ROS (1), but ROS generation in excess of antioxidant defenses is harmful and may cause oxidative stress with damage to DNA, lipids, proteins, and organelles (1,2). Hyperglycemia is a strong inducer of oxidative stress through enhanced ROS generation at mitochondrial as well as nonmitochondrial levels (2–4), and pathways activated by oxidative stress have a major role in the pathogenesis of diabetes complications (2,5). In addition, oxidative stress may contribute to hyperglycemia-induced impairment of β-cell insulin secretion (2), and excess mitochondrial ROS production has been shown to acutely and chronically cause muscle insulin resistance in some (6–8) but not all reports (9). Lowering oxidative stress in diabetes could therefore beneficially modulate pathogenic disease mechanisms, and it could potentially reduce morbidity and mortality by limiting tissue and cardiovascular complications.
Understanding and modulating the pathways that regulate mitochondrial ROS production is particularly relevant, and novel approaches involving mitochondrial dynamics have been hypothesized in recent years. Tissue mitochondrial morphology is continuously reshaped by fusion into tubular-shaped networks of interconnected organelles and by fission into smaller, fragmented mitochondria (10,11). Mutations of fusion or fission proteins cause disease, and the importance of mitochondrial dynamics is underscored by embryonic lethality of knock-out models for its major regulators. The physiological role of fusion and fission is not yet completely understood, but impairment of either process leads to altered mitochondrial function, characterized by the inability to maintain membrane potential and abnormal ROS production in fibroblasts (11). Diabetes is associated with altered mitochondrial morphology, suggesting excess fission in several tissues. Loss of tubular shape has been observed in β-cells and skeletal muscle from people with type 2 diabetes (12,13). Enhanced mitochondrial fission has also been reported in neurons from diabetic animal models (14) and in endothelium from individuals with type 2 diabetes (15), and impaired expression of the fusion regulator mitofusin-2 was reported in diabetic muscle (16). In β-cells, mitochondrial fission has been shown to contribute to apoptosis (17) as well as impaired insulin secretion (18) in the presence of hyperglycemia. Importantly, enhanced mitochondrial fission and fragmentation were demonstrated to be causally involved in ROS overproduction induced by hyperglycemia in liver and muscle cells (19), thereby indicating a causal link between altered mitochondrial dynamics and diabetic oxidative stress.
In this issue of Diabetes, Galloway et al. (20) report the impact of the inhibition of mitochondrial fission through a negative mutant of its regulator dynamin-like protein 1 on mitochondrial bioenergetics and diabetic oxidative stress. In an in vitro model, inhibiting fission in hepatocytes caused mild proton leak across the mitochondrial membrane, i.e., the partial dissipation of mitochondrial membrane potential uncoupled from ATP synthase activity (20). However, these effects were not associated with lower activity of electron transport chain components, or with relevant short-term impairment of ATP production capacity (20). Since membrane hyperpolarization contributes to mitochondrial ROS overproduction in diabetes (5), the authors went on to test the hypothesis that impairment of mitochondrial fission through transgene expression of the same dynamin-like protein 1 mutant would lower ROS generation in a streptozotocin-induced diabetic rodent model. Under these conditions, the authors focused on kidney and liver and observed lower ROS production with low markers of oxidative stress in both tissues (20). Importantly, improvements of the diabetic phenotype were reported in terms of apparent animal wellbeing and indexes of renal function (20).
The relevance of the article by Galloway et al. is twofold. First, the article provides novel insight on the role of fission in regulating both mitochondrial bioenergetics and function through changes in proton leak and membrane potential. Moreover, it demonstrates beneficial effects of inhibiting mitochondrial fission on oxidative stress and diabetic phenotype in vivo. The study therefore introduces fission as a potential target for treatment of diabetes-associated morbidity triggered by oxidative stress. The authors also recognize that the relevant role of mitochondrial dynamics in maintenance of cell and tissue homeostasis warrants extensive additional investigations on the long-term biological impact and safety of this approach before it may be considered for translation into clinical applications. Additional questions relate to the potential long-term consequences of enhanced uncoupling on mitochondrial function, and possibly on tissue and body energy balance. Moreover, mitochondria are not the only source of ROS involved in the pathogenesis of diabetes complications; the roles of NADPH oxidases, xanthine oxidase, and cyclooxygenase have been described in various tissues (2,3). The long-term impact of nonmitochondrial sources of oxidative stress could limit the beneficial impact of lower mitochondrial ROS production, an issue that will also need to be elucidated.
With the above considerations in mind, the findings of the study by Galloway et al. strengthen the rationale for research on mitochondrial dynamics and its alterations in diabetes, and suggest that the sites of common diabetes complications may be logical targets for future investigation. Further studies are also supported in pancreatic β-cells, skeletal muscle, and liver, where altered mitochondrial morphology and function are associated with, and may be causally linked to, impaired insulin secretion, altered lipid metabolism, and insulin resistance (2,17,18,21,22). From a molecular standpoint, the mediators linking changes in mitochondrial dynamics to changes in proton leak and membrane potential also need to be identified and will potentially become key intervention targets. Addressing these issues will likely result in useful information on the pathophysiological role of mitochondrial dynamics in diabetes and diabetes complications that could eventually lead to innovative and effective therapeutic approaches.
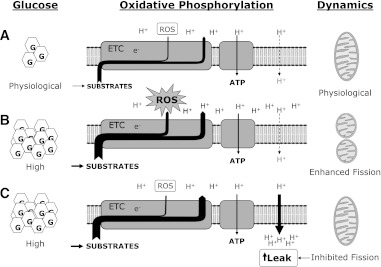
FIG. 1.
Schematic representation of putative interactions at the level of the inner mitochondrial membrane between hyperglycemia, mitochondrial ROS generation by the electron transport chain, proton leak, and mitochondrial fission, based on available knowledge and the contribution by Galloway et al. (20). Compared with euglycemic conditions (A), hyperglycemia (B) ultimately favors both excess respiratory substrates utilization with enhanced electron transfer through the electron transport chain, and enhanced mitochondrial fission. Higher inner membrane potential is not completely utilized for ATP synthesis, may cause proton accumulation in the intermembrane space and hyperpolarization that may contribute to enhanced ROS production. As demonstrated in ref. 20, partial inhibition of mitochondrial fission (C) may result in moderate proton leak with reduced hyperpolarization and lower mitochondrial ROS production. G, glucose; e−, electron(s); ETC, electron transport chain; H+, proton(s).
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 2093.
REFERENCES
- 1.Goldstein BJ, Mahadev K, Wu X. Redox paradox: insulin action is facilitated by insulin-stimulated reactive oxygen species with multiple potential signaling targets. Diabetes 2005;54:311–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sivitz WI, Yorek MA. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid Redox Signal 2010;12:537–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen GX. Oxidative stress and diabetic cardiovascular disorders: roles of mitochondria and NADPH oxidase. Can J Physiol Pharmacol 2010;88:241–248 [DOI] [PubMed] [Google Scholar]
- 4.Bravard A, Bonnard C, Durand A, et al. Inhibition of xanthine oxidase reduces hyperglycemia-induced oxidative stress and improves mitochondrial alterations in skeletal muscle of diabetic mice. Am J Physiol Endocrinol Metab 2011;300:E581–E591 [DOI] [PubMed] [Google Scholar]
- 5.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001;414:813–820 [DOI] [PubMed] [Google Scholar]
- 6.Anderson EJ, Lustig ME, Boyle KE, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 2009;119:573–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barazzoni R, Zanetti M, Cappellari GG, et al. Fatty acids acutely enhance insulin-induced oxidative stress and cause insulin resistance by increasing mitochondrial reactive oxygen species (ROS) generation and nuclear factor-κB inhibitor (IκB)-nuclear factor-κB (NFκB) activation in rat muscle, in the absence of mitochondrial dysfunction. Diabetologia 2012;55:773–782 [DOI] [PubMed] [Google Scholar]
- 8.Lee HY, Choi CS, Birkenfeld AL, et al. Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance. Cell Metab 2010;12:668–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loh K, Deng H, Fukushima A, et al. Reactive oxygen species enhance insulin sensitivity. Cell Metab 2009;10:260–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmer CS, Osellame LD, Stojanovski D, Ryan MT. The regulation of mitochondrial morphology: intricate mechanisms and dynamic machinery. Cell Signal 2011;23:1534–1545 [DOI] [PubMed] [Google Scholar]
- 11.Yoon Y, Galloway CA, Jhun BS, Yu T. Mitochondrial dynamics in diabetes. Antioxid Redox Signal 2011;14:439–457 [DOI] [PMC free article] [PubMed]
- 12.Anello M, Lupi R, Spampinato D, et al. Functional and morphological alterations of mitochondria in pancreatic beta cells from type 2 diabetic patients. Diabetologia 2005;48:282–289 [DOI] [PubMed] [Google Scholar]
- 13.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 2002;51:2944–2950 [DOI] [PubMed] [Google Scholar]
- 14.Edwards JL, Quattrini A, Lentz SI, et al. Diabetes regulates mitochondrial biogenesis and fission in mouse neurons. Diabetologia 2010;53:160–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shenouda SM, Widlansky ME, Chen K, et al. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation 2011;124:444–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bach D, Naon D, Pich S, et al. Expression of Mfn2, the Charcot-Marie-Tooth neuropathy type 2A gene, in human skeletal muscle: effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor alpha and interleukin-6. Diabetes 2005;54:2685–2693 [DOI] [PubMed] [Google Scholar]
- 17.Men X, Wang H, Li M, et al. Dynamin-related protein 1 mediates high glucose induced pancreatic beta cell apoptosis. Int J Biochem Cell Biol 2009;41:879–890 [DOI] [PubMed] [Google Scholar]
- 18.Molina AJ, Wikstrom JD, Stiles L, et al. Mitochondrial networking protects beta-cells from nutrient-induced apoptosis. Diabetes 2009;58:2303–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci USA 2006;103:2653–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galloway CA, Lee H, Nejjar S, et al. Transgenic control of mitochondrial fission induces mitochondrial uncoupling and relieves diabetic oxidative stress. Diabetes 2012;61:2093–2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gambino R, Musso G, Cassader M. Redox balance in the pathogenesis of nonalcoholic fatty liver disease: mechanisms and therapeutic opportunities. Antioxid Redox Signal 2011;15:1325–1365 [DOI] [PubMed] [Google Scholar]
- 22.Lanza IR, Nair KS. Muscle mitochondrial changes with aging and exercise. Am J Clin Nutr 2009;89:467S–471S [DOI] [PMC free article] [PubMed] [Google Scholar]