Abstract
We previously used Gene Expression Signature technology to identify methazolamide (MTZ) and related compounds with insulin sensitizing activity in vitro. The effects of these compounds were investigated in diabetic db/db mice, insulin-resistant diet-induced obese (DIO) mice, and rats with streptozotocin (STZ)-induced diabetes. MTZ reduced fasting blood glucose and HbA1c levels in db/db mice, improved glucose tolerance in DIO mice, and enhanced the glucose-lowering effects of exogenous insulin administration in rats with STZ-induced diabetes. Hyperinsulinemic-euglycemic clamps in DIO mice revealed that MTZ increased glucose infusion rate and suppressed endogenous glucose production. Whole-body or cellular oxygen consumption rate was not altered, suggesting MTZ may inhibit glucose production by different mechanism(s) to metformin. In support of this, MTZ enhanced the glucose-lowering effects of metformin in db/db mice. MTZ is known to be a carbonic anhydrase inhibitor (CAI); however, CAIs acetazolamide, ethoxyzolamide, dichlorphenamide, chlorthalidone, and furosemide were not effective in vivo. Our results demonstrate that MTZ acts as an insulin sensitizer that suppresses hepatic glucose production in vivo. The antidiabetic effect of MTZ does not appear to be a function of its known activity as a CAI. The additive glucose-lowering effect of MTZ together with metformin highlights the potential utility for the management of type 2 diabetes.
Despite significant advances in knowledge, the prevalence of type 2 diabetes and comorbidities continues to increase (1) and alternative ways of tackling this disease are needed. The molecular complexity of insulin resistance is reflected by the requirement in most cases for combination therapy to improve metabolic control; yet even this approach is often not adequate for long-term glycemic control (2). A recent consensus statement from a 29-member international working group of leading experts called for a focus on identifying the polygenic factors related to type 2 diabetes to individualize treatment, and thus increase individual benefit while minimizing risk (3). Therefore, we generated a small subset of genes (Gene Expression Signature [GES]) in which expression pattern differentiated insulin resistance from insulin sensitivity in cells and used this GES as a tool to screen for new antidiabetic compounds (4). The GES is a group of genes in which mRNA expression pattern represents the integrated response of a cell or tissue to its environment, and hence, screening assays can be developed that are not limited by prior knowledge of specific gene candidates (5,6). Using this target- and mechanism-independent screening approach, we identified that the glaucoma drug methazolamide (MTZ) and related carbonic anhydrase inhibitors (CAIs) had potential insulin sensitizing activity in vitro (4).
CAIs are structurally varied, and their physicochemical properties have affected their use as therapeutics (7–9). CAIs have been prescribed as diuretics and for the treatment of glaucoma for several decades; however, potential applications as anticonvulsant, antiobesity, and anticancer drugs have emerged (10). The objectives of our study were to assess the hypoglycemic and insulin sensitizing properties of MTZ and related molecules dichlorphenamide (DCP), chlorthalidone (CTD), furosemide (FUR), acetazolamide, and ethoxyzolamide in animal models of insulin resistance and/or diabetes and to delineate modes of action in vivo.
RESEARCH DESIGN AND METHODS
Animal experiments.
Male C57BL/6J and db/db mice and Sprague-Dawley rats (Animal Resource Centre, Perth, Western Australia, Australia) were housed with free access to water and food, with the exception of the pair-feeding study. Room temperature was maintained at 21 ± 2°C, humidity 40–70%, with a 12-h light/dark cycle. Ten-week-old C57BL/6J mice were fed a high-fat diet for 10 weeks (42% calories from fat; diet SF04-001; Specialty Feeds, Perth, Western Australia, Australia) to generate glucose-intolerant diet-induced obese (DIO) mice. DIO mice were dosed once daily with the CAIs for 2 weeks at 20–200 mg/kg/day via oral gavage. Db/db mice and Sprague-Dawley rats were fed a standard rodent diet (Barastoc Rat & Mouse; Ridley Agriproducts, Melbourne, Victoria, Australia). Db/db mice were treated by oral gavage with 20–100 mg/kg/day MTZ and/or metformin (300 mg/kg/day) for 2–4 weeks. Sprague-Dawley rats were given with 65 mg/kg/day streptozotocin (STZ) for 7 days through an intravenous injection via the lateral tail vein, and blood glucose levels of ≥12 mmol/L were achieved. After STZ administration, animals were treated by oral gavage with 50 mg/kg/day MTZ for 2 weeks. All reagents were purchased from Sigma-Aldrich (Sydney, New South Wales, Australia) except for DCP (US Pharmacopeia, Rockville, MD), saline (PharmaLab, Lane Cove, New South Wales, Australia), and Humulin-insulin (Clifford Hallam Healthcare, Melbourne, Victoria, Australia). Dosing solutions of compounds were prepared fresh in sterile saline:PEG400 at 65:35 (vol/vol), protected from light and stored at room temperature. All experiments were approved by Deakin University’s Animal Welfare Committee and followed National Health and Medical Research Council guidelines.
Metabolic assays.
Glucose tolerance tests (2 g/kg i.p.) were performed in overnight-fasted DIO mice. Insulin tolerance tests (0.5 units/kg i.p.) were performed in 2-h fasted rats with STZ-induced diabetes, and blood glucose levels were measured at 0, 30, 60, 45, and 60 min after the initial administration. Blood glucose levels in db/db mice were determined in 4-h fasted animals. Blood samples were obtained from the tail tip and measured using a glucometer (AccuCheck II; Roche, Castle Hill, New South Wales, Australia). Insulin concentrations were measured using an ELISA (Crystal Chem, Downers Grove, IL). Urine was collected from animals housed in metabolic cages for 24 h with free access to food and water. Glucose in urine was determined by glucose oxidase (Sigma-Aldrich). Glycosylated hemoglobin (HbA1c) levels were measured using DCA2000+ analyzer (Bayer Diagnostics, Tarrytown, NY).
Hyperinsulinemic-euglycemic clamps.
DIO mice were treated with 50 mg/kg/day MTZ for 14 days and then overnight-fasted. Mice were anesthetized, and catheters were inserted in the jugular vein and carotid artery. An insulin bolus and [6-3H]-glucose (3.4 µCi) was given over 2 min followed by infusion of 8 mU/kg/min insulin and 0.14 µCi/min [6-3H]-glucose for 2 h. Blood glucose concentration was maintained by infusion of 5% glucose. Specific activity of [6-3H]-glucose was steady-state after 90 min of infusion (Supplementary Fig. 1). Blood samples were collected during steady-state conditions at 90, 100, and 110 min, where glucose appearance rate equals Rd. Rd was calculated by dividing the infusion rate of [6-3H]-glucose (disintegrations per min) by the plasma [6-3H]-glucose specific activity. Endogenous glucose production (EGP) rate was measured as the difference between the calculated Rd and the glucose infusion rate.
Tracer studies.
A radioactive tracer glucose tolerance test was performed in overnight-fasted DIO mice. A 50% glucose solution containing 100 μCi/mL 2-deoxy-[U-3H]-glucose (PerkinElmer Life Sciences, Melbourne, Victoria, Australia) and 100 μCi/mL [U-14C]-glucose was administered via intraperitoneal injection (2 g/kg body weight, 10 μCi/animal). Blood samples were obtained from the tail tip at the times indicated. Blood glucose levels were measured using a glucometer. Animals were killed, and tissues were removed rapidly and frozen in liquid nitrogen at 90 min.
Cell culture.
FAO rat hepatoma cells were grown in RPMI-1640 containing 10% FBS and 1% penicillin/streptomycin (normal growth media; GIBCO-Invitrogen, Melbourne, Victoria, Australia). Cells were treated when they reached ∼70% confluence.
Cell treatments and glucose production assay.
FAO hepatocytes seeded at 1 × 105 cells per well in 48-well plates were treated with activators of glucose production (100 nM dexamethasone and 100 μmol/L CPT-cAMP) for 24 h in the presence or absence of 0.1 nM insulin (Humulin R; Novo Nordisk, Baulkham Hills, New South Wales, Australia) and 100 μmol/L MTZ or 200 μmol/L metformin. All treatments were performed in serum- and glucose-free RPMI supplemented with 0.1% BSA, 2 mmol/L sodium pyruvate, and 20 mmol/L sodium lactate. After 24 h treatment, 40 μL of conditioned media were incubated with 250 μL Assay Media (0.12M NaH2PO4.2H2O, pH 7.0, with 1 mg/mL phenol, 0.5 mg/mL 4-aminoantipyrine, 1.6 units/mL peroxidize, 10 units/mL glucose oxidase) at 37°C for 25 min in a 96-well plate. Glucose content was determined by measuring absorbance at 490 nm, and glucose production was corrected for protein concentration per sample well. All reagents were from Sigma-Aldrich unless otherwise stated.
RNA isolation and real-time PCR.
Total RNA was extracted from FAO cells and db/db livers using TRIzol (Invitrogen, Melbourne, Victoria, Australia) and RNeasy columns (Qiagen, Hilden, Germany). RNA quality and concentration was determined using RNA-6000 Nano Assay and Agilent-2100 Bioanalyser (Agilent Technologies, Santa Clara, CA). First-strand cDNA was generated using SuperScript First-Strand Synthesis System for real-time PCR (RT-PCR; Invitrogen). Phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) gene expression levels were quantified using SYBR-Green PCR-Master Mix (Applied Biosystems, Worthington, Leicestershire, U.K.) with an ABI PRISM-7700 Sequencing Detector (Perkin Elmer, Waltham, MA). RT-PCR primers were as follows: mouse PEPCK forward 5′-ACTGTTGGCTGGCTCTCACTG-3′, reverse 5′-GGGAACCTGGCGTTGAATGC-3′; rat PEPCK forward 5′-CTCGGTGCCACCTGAAACA-3′, reverse 5′-AGCCATGTGCAACTCATGCA-3′; mouse and rat G6Pase forward 5′-ACGCCTTCTATGTCCTCTTTCCC-3′, reverse 5′-TGTTGCTGTAGTAGTCGGTGTCC-3′.
Oxygen consumption and extracellular acidification rates.
The cellular flux of oxygen (oxygen consumption rate [OCR]) and protons (extracellular acidification rate [ECAR]), which reflect the rate of mitochondrial respiration and rate of lactate production via glycolysis, respectively, were simultaneously measured using the Seahorse-XF24 Extracellular Flux analyzer (Seahorse Bioscience, North Billerica, MA) (11). FAO cells were seeded into Seahorse V7 culture plates at 4 × 104 cells per well in 100 μL normal growth media. After 4 h, a further 400 μL normal growth media were added to each well. Vehicle (0.33% DMSO), MTZ (100 μmol/L), metformin (200 μmol/L), or MTZ/metformin (combination of 100 μmol/L MTZ and 200 μmol/L metformin) were added to culture medium. After 16 h, cells were washed twice with Assay-Running media (Krebs-Ringer Bicarbonate buffer, pH 7.4, supplemented with 2.5 mmol/L glucose and DMSO, MTZ, MET, or MTZ/MET) before incubation in Assay-Running media for 60 min in a non-CO2 incubator at 37°C. Basal ECAR was determined for four measurement cycles (32 min). Basal OCR was determined for four measurement cycles (32 min) before injection of sodium pyruvate (2 mmol/L), sodium lactate (20 mmol/L), and insulin (100 nM).
Statistical analysis.
Data distribution for normality was tested using Kolmogorov-Smirnov. Group mean differences were assessed using Student’s unpaired t test or one-way ANOVA using least significant differences or Games-Howell post hoc assessment for homogenous or nonhomogeneous samples, respectively. Analyses performed using Statistical Package for the Social Sciences software (SPSS version 17). Data considered statistically different at P < 0.05, and each data point was presented as the mean of ≥ 3 independent experiments ± SE unless otherwise stated.
RESULTS
Methazolamide reduced circulating glucose levels in diabetic rodents.
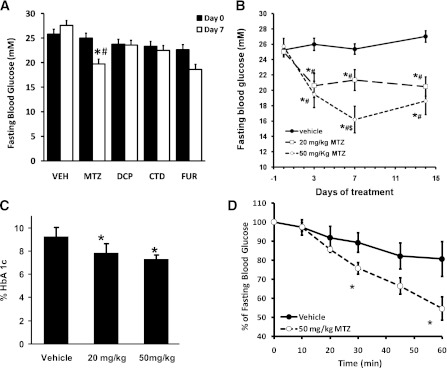
We investigated whether a range of CAIs exhibited insulin sensitizing effects in vivo. Obese and diabetic db/db mice were treated with CAIs administered orally at 50 mg/kg/day for 7 days. DCP, CTD, and FUR had no effect on fasting blood glucose levels (Fig. 1A). In contrast, MTZ reduced fasting blood glucose by 21 ± 7% (P = 0.009 compared with vehicle; Fig. 1A). The glucose-lowering effect of MTZ was also observed in animals treated with 20 mg/kg MTZ for 7 days (−16 ± 5%, P = 0.005 compared with vehicle; Fig. 1B). The reduction in blood glucose as a result of MTZ was significant after 3 days (P = 0.002 and 0.0003 for 20 and 50 mg/kg/day, respectively, compared with vehicle) and was stable for at least 14 days (Fig. 1B). Consistent with sustained reduction in fasting blood glucose levels, treatment of db/db mice with MTZ for 28 days resulted in a dose-dependent decrease in HbA1c levels of 16 ± 5% at 20 mg/kg/day (P = 0.035) and 22 ± 4% at 50 mg/kg/day (P = 0.003) compared with vehicle-treated mice (Fig. 1C). Because CAIs have been prescribed previously as diuretics, we examined whether glucose-lowering properties of MTZ were the result of increased loss of glucose in urine. On the contrary, 20 mg/kg/day MTZ treatment for 14 days reduced glucose urine concentration in db/db mice by 18 ± 4% compared with vehicle-treated (P = 0.014), whereas there was no difference in total urine glucose excretion between the treatment groups (Supplementary Table 1).
FIG. 1.
Methazolamide reduced blood glucose levels in db/db mice and rats with STZ-induced diabetes. A: Fasting blood glucose levels in db/db mice treated with vehicle (VEH, 35% PEG400) or 50 mg/kg/day MTZ, DCP, CTD, or FUR for 7 days. Day 0 (black bars) and day 7 of treatment (white bars) are shown. Data represent the means ± SE of 4–7 mice per group. B: Fasting blood glucose levels in db/db mice treated with 0 (black circles, vehicle, n = 24–36), 20 (white squares, n = 26), or 50 (white diamonds, n = 23) mg/kg/day MTZ for the indicated times. *P < 0.05 compared with day 0; #P < 0.05 compared with the corresponding vehicle; $P < 0.05 compared with 20 mg/kg MTZ. C: The effect of MTZ on HbA1c levels in db/db mice treated at the indicated doses for 28 days. *P < 0.05 compared with the vehicle treatment group (n = 11 to 12). D: Insulin tolerance test (0.5 units/kg i.p.) in insulin-deficient rats with STZ-induced diabetes and diabetic rats treated with vehicle (black circles) or 50 mg/kg/day MTZ (white circles) for 14 days. *P < 0.005 compared with the corresponding vehicle treatment group at the relevant time point (n = 6).
No change in body weight or food intake was observed in the 20 mg/kg MTZ-treated db/db mice at 7 and 14 days compared with day 0 (data not shown). After 7 days, MTZ (50 mg/kg) reduced body weight by 4 ± 3% (P = 0.001) and decreased food intake by 24 ± 8% (P = 0.005) compared with day 0. To investigate whether the decrease in glycemia following MTZ treatment was because of reduced food intake and loss of body weight, changes in blood glucose in pair-fed vehicle- and 50 mg/kg/day MTZ-treated db/db mice were monitored for 8 days. Pair-feeding resulted in a 9 ± 2% reduction in body weight in vehicle-treated mice (P = 0.001). However, only MTZ-treated animals had reduced fasting blood glucose after treatment (+0.6 ± 1.9 vs. −6.2 ± 1.5 mmol/L, pair-fed vehicle- vs. MTZ-treated, P = 0.016). The results indicate that glucose-lowering effects of MTZ were not a result of reduction in food intake or body weight loss.
Methazolamide improved insulin sensitivity in insulin-deficient diabetic rats.
We assessed whether MTZ had hypoglycemic properties in rats with STZ-induced diabetes, a model of insulin-deficient diabetes. There was no reduction in fasting blood glucose levels in rats with STZ-induced diabetes treated with MTZ (50 mg/kg/day) for 2 weeks (21.3 ± 1.0 vs. 19.6 ± 0.3 mmol/L at days 0 and 14, respectively). The vehicle-treated group also remained unaffected (21.4 ± 1.4 vs. 20.4 ± 0.7 mmol/L at days 0 and 14, respectively). In contrast, MTZ enhanced the glucose-lowering effects of exogenous insulin in the STZ-induced diabetic rats by 2.4-fold (P = 0.038 compared with insulin alone; Fig. 1D). The results suggest that MTZ acts as an insulin sensitizing agent in vivo.
Methazolamide improved glucose homeostasis in diet-induced obese mice.
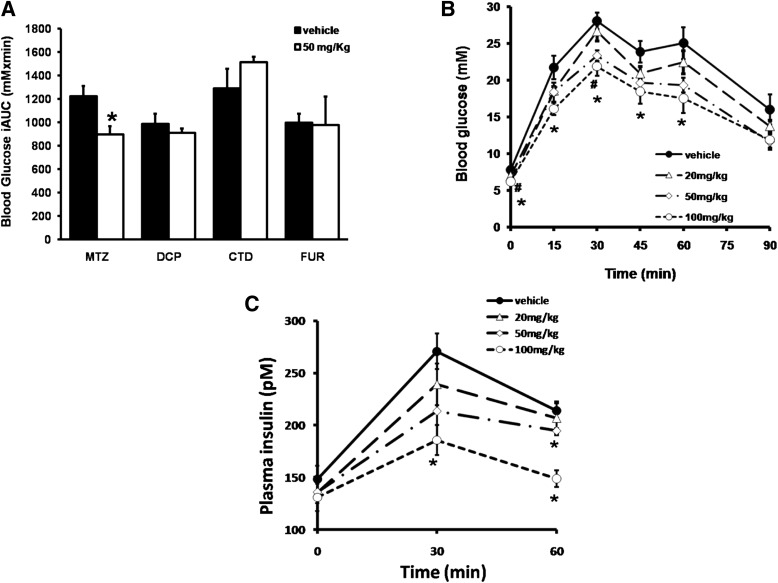
We examined whether MTZ affected glucose homeostasis in normoglycemic, insulin-resistant DIO mice. Animals were treated with vehicle or MTZ at 50 mg/kg/day for 14 days and then intraperitoneal glucose tolerance tests were performed. MTZ elicited a 33 ± 7% reduction (P = 0.016 compared with day 0) in the incremental area under the glucose curve compared with vehicle-treated animals (−7 ± 8%, NS compared with day 0; Fig. 2A). Fasting blood glucose was also significantly reduced in DIO mice treated with MTZ compared with vehicle-treated (P = 0.013). The effect of MTZ on circulating glucose levels was achieved without significant changes in body weight, food and water intake, or epididymal fat mass compared with vehicle-treated mice (Supplementary Table 2). Furthermore, 24 h urine output was not altered between MTZ- and vehicle-treated mice, indicating that the difference observed in glucose metabolism was not because of increased glucose excretion (Supplementary Table 2). Consistent with the studies in db/db mice, 50 mg/kg/day DCP, CTD, or FUR for 14 days did not affect glucose tolerance in DIO mice (Fig. 2A). Dose-response studies with DCP, CTD, or FUR found no effect on glucose tolerance in DIO mice treated up to 100 mg/kg/day (data not shown). However, MTZ caused a dose-dependent improvement in glucose tolerance (P ≤ 0.030 compared with vehicle-treated, Fig. 2B). The glucose-lowering effect of MTZ was accompanied by a dose-dependent decrease in plasma insulin levels (P ≤ 0.040 compared with vehicle-treated, Fig. 2C), suggesting that the effects of MTZ were a result of improved peripheral insulin sensitivity rather than increased insulin secretion by the pancreas.
FIG. 2.
Methazolamide improved glucose tolerance in DIO mice. A: Glucose tolerance test (2 g/kg glucose i.p.) in DIO mice treated with vehicle (35% PEG400) (black bars) or 50 mg/kg/day MTZ, DCP, CTD, or FUR (white bars) for 14 days. Data show the mean incremental area under the glucose curve (iAUC) ± SE. *P < 0.05 vs. corresponding vehicle treatment group, n = 4–10. Circulating blood glucose (B) and insulin levels (C) during an intraperitoneal glucose tolerance test in animals dosed with vehicle (black circles), 20 (white triangles), 50 (white diamonds), or 100 (white circles) mg/kg/day MTZ for 14 days are shown. Data represent the mean ± SE of 5 to 6 mice per treatment group. *P < 0.05 vs. corresponding vehicle; #P < 0.05, 50 mg/kg MTZ vs. corresponding vehicle.
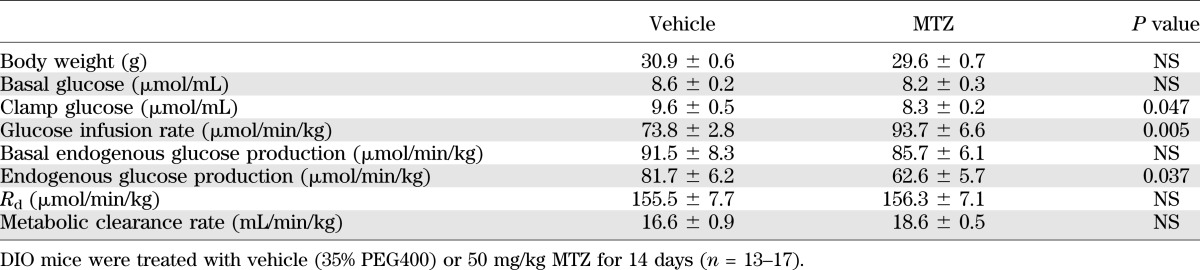
To determine whether MTZ enhanced insulin sensitivity, hyperinsulinemic-euglycemic clamps were performed. DIO mice treated with 50 mg/kg MTZ for 14 days exhibited a 27 ± 9% increase in the glucose infusion rate compared with vehicle-treated mice (P = 0.005, Table 1). This effect was accompanied by a 23 ± 7% reduction in EGP (P = 0.037), whereas no difference was observed in Rd compared with vehicle-treated mice (Table 1). The results indicate that the glucose-lowering effects of MTZ are a result of the inhibition of hepatic glucose production rather than increased glucose disposal into peripheral tissues. In support of this, glucose clearance into skeletal muscle and white adipose tissue was not different between vehicle- and MTZ-treated mice (Supplementary Fig. 2).
TABLE 1.
Hyperinsulinemic-euglycemic clamps in DIO mice
Methazolamide inhibited glucose production from pyruvate.
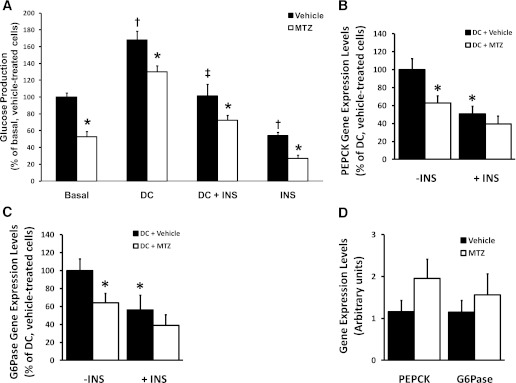
To investigate the effects of MTZ on hepatic glucose production, we examined whether MTZ altered glucose production from pyruvate/lactate in FAO hepatoma cells (Fig. 3A). Insulin (0.1 nM, 24 h) reduced basal and activated (dexamethasone and CPT-cAMP, 24 h termed as DC) glucose production by 46 and 40%, respectively (P = 0.001). In the absence of insulin, MTZ (100 μmol/L, 24 h) reduced basal and activated glucose production by 47% (P = 0.001) and 23% (P = 0.020), respectively. With insulin, MTZ further reduced basal and DC-activated glucose production by 50% (P = 0.001) and 28% (P = 0.050), respectively. The data support our in vivo results that MTZ is an inhibitor of hepatic glucose production.
FIG. 3.
Methazolamide reduced glucose production from pyruvate in FAO hepatoma cells, and the effect on PEPCK and G6Pase gene expression levels in db/db mice livers and FAO cells. A: Glucose production by FAO hepatoma cells was determined 24 h after treatment with vehicle (0.33% DMSO, black bars) or 100 μmol/L MTZ (white bars). Treatments were conducted in basal conditions (Basal) or after glucose production was activated with 100 nmol/L dexamethasone and 100 μmol/L CPT-cAMP (DC) in the presence or absence of submaximal 0.1 nmol/L insulin (DC + INS or INS). Data are the mean percent of basal, vehicle-treated cells ± SE of ≥ 6 independent experiments, where each treatment within an experiment was performed in triplicate. The average amount of glucose produced by the basal, vehicle-treated FAO liver cells was 45.6 ± 17.9 μg glucose per mg protein (mean ± SD). *P ≤ 0.05 vs. corresponding vehicle-treated cells; †vs. basal, vehicle-treated cells; ‡vs. DC, vehicle-treated cells. PEPCK (B) and glucose-6-phosphatase (G6Pase; C) mRNA levels in FAO hepatoma cells treated with 100 nmol/L dexamethasone and 100 μmol/L CPT-cAMP (DC) for 24 h to activate gluconeogenesis in absence (−INS) or the presence of 0.5 nmol/L insulin (+INS), 0.3% DMSO (+Vehicle), or 100 μmol/L methazolamide (+MTZ) are shown. Data are expressed as percent of the activated (DC-treated), non-MTZ treated cells from 3 to 5 independent experiments, where each treatment within an experiment was performed in triplicate. *P < 0.05 vs. DC, vehicle (noninsulin or MTZ) treatment group. D: PEPCK and G6Pase mRNA levels in livers of db/db mice treated with vehicle (35% PEG400) or 50 mg/kg MTZ for 14 days. Data represent the means ± SE of 6 mice per group.
Effect of methazolamide on the gluconeogenic enzymes PEPCK and G6Pase.
To ascertain a mechanism for the ability of MTZ to inhibit glucose production, PEPCK and G6Pase mRNA levels were measured. In activated (DC-treated) FAO cells, insulin (0.1 nM, 24 h) decreased PEPCK mRNA levels by 49 ± 8% (P = 0.015, Fig. 3B) and G6Pase by 77 ± 16% (P = 0.020, Fig. 3C). MTZ (100 μmol/L, 24 h) also reduced PEPCK mRNA levels by 37 ± 8% and G6Pase by 36 ± 10% (P = 0.037 and 0.052, respectively, Figs. 3B and C). There was a trend for MTZ together with insulin to further reduce PEPCK and G6Pase mRNA levels by 22 ± 17% and 34 ± 20%, respectively (Fig. 3B and C). In contrast, PEPCK and G6Pase mRNA levels (Fig. 3D) and G6Pase activity (data not shown) were not different in the livers of vehicle- versus MTZ-treated db/db or DIO mice (data not shown). The results suggest that PEPCK and G6Pase may not be direct targets of the antidiabetic actions of MTZ in the liver.
Methazolamide did not affect the activation of insulin signaling pathways in vivo.
We examined whether the insulin sensitizing effects of MTZ in DIO mice were the result of increased activation of signaling pathways initiated by insulin in the liver. No effect on insulin receptor, insulin receptor substrate proteins, Akt, GSK3β, or CREB phosphorylation levels was observed in MTZ-treated mice (Supplementary Fig. 3). The results suggest that MTZ may not induce its glucose-lowering effects by enhancing these insulin signaling proteins in the liver. Consistent with this data, MTZ did not affect the glucose clearance rate into glycogen or triacylglycerides (Supplementary Fig. 4A and B) or total glycogen and triacylglycerides levels (Supplementary Fig. 4C and D) in the liver.
Methazolamide effects were related to changes in glycolysis.
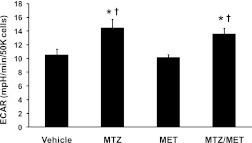
To investigate the effect of MTZ on glycolysis, ECAR, which reflects lactate formation during glycolytic energy metabolism (11), was measured in FAO cells. Metformin was included as a comparison. MTZ (100 μmol/L) for 16 h, but not metformin (200 μmol/L), increased ECAR under basal conditions (2.5 mmol/L glucose) by 37 ± 12% (P = 0.019) compared with vehicle-treated cells (Fig. 4). Cells cotreated with MTZ and metformin had similar effects on glycolytic rate as MTZ alone (Fig. 4). The ECAR data suggest that MTZ may be increasing glycolysis in the liver and favoring carbon substrate break-down rather than synthesis.
FIG. 4.
Methazolamide affected the glycolytic pathway in FAO hepatoma cells. ECAR as an indicator of glycolytic rate was measured in FAO cells treated with vehicle (0.33% DMSO), MTZ (100 μmol/L), metformin (200 μmol/L metformin [MET]), or MTZ/metformin (cotreatment of 100 μmol/L MTZ and 200 μmol/L metformin) for 16 h. ECAR was determined under basal conditions (2.5 mmol/L glucose alone). Data are presented as the means ± SE of 2 independent experiments whereby each treatment was performed in quadruplicate wells. *P ≤ 0.03 vs. vehicle-treated cells; †P ≤ 0.01 vs. metformin-treated cells.
Methazolamide effects were not related to changes in oxidative metabolism.
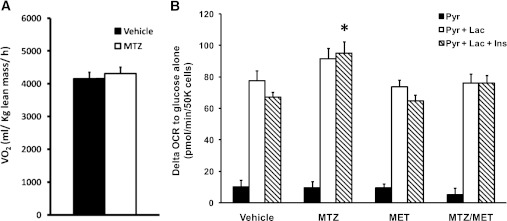
The hypoglycemic properties of metformin are due in part to suppression of gluconeogenesis in the liver (12). Metformin, troglitazone, rosiglitazone, and berberine can interfere with mitochondrial respiration via inhibition of respiratory complex I, and this mechanism has been proposed to contribute to their insulin sensitizing effects (13–16). As MTZ affected glucose production from pyruvate (Fig. 3A), a process that requires mitochondrial enzymes, the effect of MTZ on the whole-body respiratory capacity of DIO mice was examined. MTZ (50 mg/kg/day) for 14 days elicited no effect on OCR (Fig. 5A). Likewise, FAO cells treated with 100 μmol/L MTZ for 16 h did not affect OCR in the presence of glucose (data not shown). However, after the addition of pyruvate and lactate, there was a trend by MTZ for increased OCR above basal levels (glucose as the only substrate) by 18% (NS), which was further enhanced by 42% in the presence of insulin (P = 0.015, Fig. 5B). Metformin alone or together with MTZ had no effect (Fig. 5B). The data suggest that although MTZ does not affect global oxidative metabolism, it does influence the oxidation of specific gluconeogenic precursors such as pyruvate and lactate.
FIG. 5.
Methazolamide did not affect oxygen consumption rate in DIO mice or FAO hepatoma cells. A: DIO mice treated with vehicle (35% PEG400 = black bars) or 50 mg/kg methazolamide (MTZ = white bars) for 14 days were held in sealed cages connected to an indirect calorimeter (Oxymax; Columbus Instruments, Columbus, OH) for 24 h. Oxygen consumption (VO2) and carbon dioxide production were measured and used to calculate fat oxidation, carbohydrate oxidation, respiratory quotient, and energy expenditure (39,40). Physical activity within the calorimeter was measured using an Opto-Varimax Mini-Infrared Animal Activity Monitor System (Columbus Instruments). Data represent the means ± SE of 7 mice in each group. B: OCR was measured in FAO cells treated with vehicle (0.33% DMSO), MTZ (100 μmol/L), metformin (200 μmol/L metformin [MET]), or MTZ/metformin (combination of 100 μmol/L MTZ and 200 μmol/L metformin [MTZ/MET]) for 16 h. OCR was determined in the presence of 2.5 mmol/L glucose alone (basal conditions) or with the addition of 2 mmol/L sodium pyruvate (Pyr = black bars), 2 mmol/L sodium pyruvate and 20 mmol/L sodium lactate (Pyr + Lac = white bars) or 2 mmol/L sodium pyruvate, 20 mmol/L sodium lactate and 100 nmol/L insulin (Pyr + Lac + Ins = striped bars). Data are presented as the difference in OCR from glucose alone (basal) and the means ± SE of 2 independent experiments whereby each treatment was performed in quadruplicate wells. *P = 0.015 vs. vehicle-treated cells with Pyr + Lac.
Methazolamide enhanced the glucose-lowering effects of metformin.
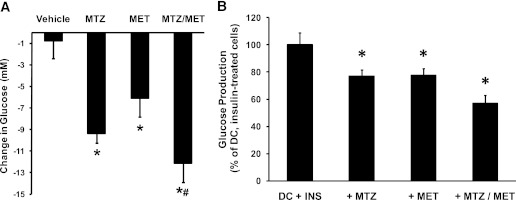
Given the apparent differences on both ECAR and OCR and, hence, potential mechanisms of action, we investigated whether metformin and MTZ have additive hypoglycemic effects. In db/db mice, metformin (300 mg/kg/day, 8 days) induced a similar reduction in fasting blood glucose levels (−18 ± 9%, P = 0.034) to 20 mg/kg/day MTZ (−30 ± 8%, P = 0.001 and Fig. 1B) compared with vehicle (Fig. 6A). Coadministration of metformin and MTZ decreased blood glucose levels by 47 ± 8% compared with vehicle (P = 0.001), 24 ± 12% compared with MTZ alone (NS, P = 0.071), and 35 ± 10% compared with metformin alone (P = 0.002), suggesting an additive effect of the two insulin sensitizers (Fig. 6A). This enhanced hypoglycemic effect was accompanied by decreased urine glucose excretion (63 ± 5%, P = 0.050), water intake (52 ± 7%, P = 0.00001), and urine volume (64 ± 7%, P = 0.022) compared with vehicle-treated animals (Supplementary Table 1). In FAO cells treated simultaneously with 200 μmol/L metformin and 100 μmol/L MTZ for 24 h, DC-activated glucose production in the presence of insulin was further reduced by 43% (P = 0.001) compared with the reductions observed with MTZ by 23% (P = 0.007 and Fig. 3A) or metformin by 22% (P = 0.005) versus DC, insulin-treated cells (Fig. 6B). We have shown that MTZ has insulin sensitizing properties and the ability to improve the efficacy of metformin. The use of these drugs in combination could improve the management of type 2 diabetes.
FIG. 6.
Methazolamide enhanced the antidiabetic effects of metformin in db/db mice and glucose production in FAO hepatoma cells. A: Change in glucose from day 0 in db/db mice treated with vehicle (35% PEG400), 20 mg/kg MTZ (MTZ), 300 mg/kg metformin (MET), or a combination of 20 mg/kg MTZ and 300 mg/kg metformin (MTZ/MET) for 8 days. Data represent the means ± SE of 7 mice per group. *P < 0.04 vs. vehicle; #P = 0.002 vs. metformin alone. B: Glucose production in FAO cells incubated with 100 nmol/L dexamethasone and 100 μmol/L CPT-cAMP for 24 h to activate glucose production (DC) in presence of submaximal (0.1 nmol/L) insulin (INS). At the same time, cells were treated with 0.3% DMSO (DC + INS), 100 μmol/L methazolamide (+ MTZ), 200 μmol/L metformin (+MET), or 100 μmol/L methazolamide together with 200 μmol/L metformin (+MTZ/MET). Data are expressed as the mean percent of the DC and insulin-treated cells ± SE from 8–10 independent experiments, where each treatment was performed in triplicate. Average amount of glucose produced by the basal, vehicle-treated cells was 54.5 ± 7.9 μg glucose per mg cellular protein (n = 10). DC increased glucose production by 185 ± 21% (P = 0.022 vs. basal, vehicle-treated cells), and the addition of insulin effectively reduced DC-activated glucose production by 41 ± 10% (P = 0.014 vs. DC-treated cells) (data not shown). *P < 0.01 vs. DC + INS-treated cells; P = 0.069 between DC + INS + MTZ- and P = 0.060 between DC + INS + MET- vs. DC + INS + MTZ/MET-treated cells.
DISCUSSION
We have shown that MTZ exhibited hypoglycemic properties in three different animal models of insulin resistance and diabetes. Our data indicate that MTZ is an insulin sensitizer that reduces hepatic glucose production without a significant effect on peripheral glucose disposal. We reached this conclusion based on the following key findings: 1) MTZ only exhibited blood glucose-lowering capacity when exogenous insulin was present, but had no effect on fasting blood glucose levels in insulin-deficient rats with STZ-induced diabetes and 2) MTZ treatment decreased EGP in DIO mice under hyperinsulinemic-euglycemic clamp conditions. We have demonstrated that MTZ enhances the effects of metformin to lower blood glucose levels.
Our data suggest that the effects of MTZ on insulin action may be primarily the result of inhibition of hepatic glucose production, since we did not observe significant effect on glycogen synthesis and lipogenesis. Consistent with this, there were no differences in early insulin-activated signaling events (insulin receptor substrate/phosphoinositide 3-kinase/Akt axis). It also appears unlikely that changes in the steady-state expression levels of the gluconeogenic genes PEPCK and G6Pase account for the glucose-lowering effects of MTZ in vivo despite measuring reduced PEPCK and G6Pase gene expression levels in FAO cells. Our findings are consistent with recent studies indicating that differences in hepatic glucose production in vivo can occur without changes in PEPCK or G6Pase expression (17–20). With the use of the same conditions that showed that MTZ reduced glucose production in FAO hepatocytes, MTZ increased the glycolytic rate and increased cellular respiration when pyruvate and lactate (not glucose alone) were provided as precursors. Our studies indicate that MTZ lowers glucose production and increases glycolytic flux.
One hypothesis in terms of potential mechanism for the insulin sensitizing properties of MTZ comes from speculation that the antidiabetic properties of metformin reside in its ability to reduce the energy charge status of liver cells via inhibition of the mitochondrial respiratory complex I (13,15); however, there is controversy as to whether AMPK activation mediates the antigluconeogenic effects of metformin (21–24). We found no evidence that MTZ had adverse effects on either whole-body respiration in vivo or liver global cell respiration in vitro, or any effect on AMPK protein or phosphorylation levels in the livers of MTZ-treated DIO mice (data not shown), suggesting that the mechanism of action of MTZ may differ to metformin. This is supported by the additive effects of these two drugs reported here.
We found that MTZ was the only CAI tested with glucose-lowering properties in vivo. Similar divergence in the effect of CAIs on hypoxic pulmonary vasoconstriction has also been documented (25). In our study, chlorthalidone and furosemide had no effect on hyperglycemia in db/db mice or glucose tolerance in insulin-resistant DIO mice. Our in vivo data are in contrast with earlier publications involving CAI effects on insulin sensitivity. Thiazide diuretics have been associated with glucose intolerance in humans (26), whereas furosemide caused hyperglycemia and glucose intolerance in vivo in mice (27). The glucose intolerance found with these CAIs may reflect their diuretic effects at higher doses, which can cause metabolic acidosis and altered urine potassium release (28). Our in vivo data demonstrate that at the doses used, MTZ was not having a diuretic effect nor was the blood glucose level decreased as a result of reduced food consumption as indicated by pair-feeding studies.
Another explanation for the lack of in vivo efficacy for some CAIs may be because of differences in the sensitivity toward specific carbonic anhydrase (CA) isoforms that may result in effects on a metabolic process of varied magnitude in a particular tissue. For example, CAII, CAIII, CAIV, and CAV are expressed at high levels in liver (29). Although it is difficult to determine the contribution of each CA isoform, mitochondrial CAV is considered to be important for hepatic gluconeogenesis since it provides the bicarbonate required by pyruvate carboxylase to produce oxalacetate from pyruvate (30). Consistent with this, CAV activity increased in livers from diabetic rats, which are characterized by enhanced hepatic glucose production (31,32). Notably, there was no reduction in hyperglycemia in db/db mice treated with acetazolamide or in DIO animals treated with ethoxyzolamide (data not shown). However, these CAIs exhibit similar CAV (and other isoforms) inhibitory activity to MTZ (10). This could be because of differences in the pharmacokinetic properties between the different CAIs (33). Our results (Figs. 1 and 2) suggest that CA inhibition is not the primary mechanism for the antidiabetic properties of MTZ, and other (as yet unknown) proteins may be modulated by MTZ. In support of this hypothesis, the inhibition of hypoxic pulmonary vasoconstriction by CAI has been proposed to be independent of CA activity (25,34). Further studies are currently underway to identify new molecular targets of MTZ.
Published studies have provided mixed results regarding the potential of CAIs to modulate blood glucose levels. Acetazolamide and ethoxyzolamide were found to inhibit gluconeogenesis in vitro or after acute administration in rats (30,35,36). In vivo studies found that acetazolamide caused acidosis and insulin resistance in alloxanized diabetic dogs (37), and decreased glucose tolerance while increasing insulin levels in fed (but not fasted) mice for 24 h postacetazolamide injection (38). Our study is the first to demonstrate that MTZ is a hepatic insulin sensitizer able to improve glucose intolerance and reduce glycemia in insulin-resistant and diabetic animals at doses lower than those required to induce diuresis. Furthermore, MTZ enhanced the glucose-lowering effects of metformin. This may be important for the management of diabetes, since lower doses of metformin used in combination with MTZ may help to prolong its therapeutic utility and reduce the incidence of adverse side effects. Clinical trials testing the efficacy of MTZ in combination with metformin in individuals with type 2 diabetes have commenced (Australian New Zealand Clinical Trials Registry number ACTRN12609000634279).
Supplementary Material
ACKNOWLEDGMENTS
Funding for this work was provided by a research grant from Verva Pharmaceuticals. S.L.M. is supported by the Diabetes Australia Research Trust Viertel Award. L.G.-G. was a recipient of Research Training Programme Fellowship from the Ministry of Science and Innovation of Spain.
G.K. was an employee of Verva Pharmaceuticals, and K.R.W. received research grants from Verva Pharmaceuticals. No other potential conflicts of interest relevant to this article were reported.
N.K., J.C.M., B.S., T.C., M.d.V., S.W., R.F., S.M., C.S., S.J., A.C., and L.G.-G. researched data. S.L.M., B.S., T.C., V.C.F., S.A., and K.R.W. reviewed and edited the manuscript. N.K., J.C.M., S.L.M., G.K., S.A., and K.R.W. contributed to discussion. N.K. and J.C.M. wrote the manuscript. K.R.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Prof. David James of Garvan Institute of Medical Research (Sydney, Australia), Prof. Michael Cowley of Monash University (Melbourne, Australia), and Dr. Lance Macaulay of CSIRO (Melbourne, Australia) for intellectual contribution and Prof. Andy Sinclair and Drs. Kathryn Aston-Mourney and Nicole Stupka of Deakin University (Geelong, Australia) for thoughtful comments on the manuscript. The authors thank Ms. Jane Hosking of Deakin University (Geelong, Australia) for technical assistance.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-0578/-/DC1.
REFERENCES
- 1.Prevention. CfDCa: National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA: U.S., 2011 [Google Scholar]
- 2.Robertson RP, Harmon J, Tran POT, Poitout V. β-Cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes 2004;53(Suppl. 1):S119–S124 [DOI] [PubMed] [Google Scholar]
- 3.Smith RJ, Nathan DM, Arslanian SA, Groop L, Rizza RA, Rotter JI. Individualizing therapies in type 2 diabetes mellitus based on patient characteristics: what we know and what we need to know. J Clin Endocrinol Metab 2010;95:1566–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konstantopoulos N, Foletta VC, Segal DH, et al. A gene expression signature for insulin resistance. Physiol Genomics 2011;43:110–120 [DOI] [PubMed] [Google Scholar]
- 5.Stegmaier K, Ross KN, Colavito SA, O’Malley S, Stockwell BR, Golub TR. Gene expression-based high-throughput screening(GE-HTS) and application to leukemia differentiation. Nat Genet 2004;36:257–263 [DOI] [PubMed] [Google Scholar]
- 6.van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 2002;347:1999–2009 [DOI] [PubMed] [Google Scholar]
- 7.Gamba G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev 2005;85:423–493 [DOI] [PubMed] [Google Scholar]
- 8.Kim GH. Long-term adaptation of renal ion transporters to chronic diuretic treatment. Am J Nephrol 2004;24:595–605 [DOI] [PubMed] [Google Scholar]
- 9.Soleimani M. Na+:HCO3- cotransporters (NBC): expression and regulation in the kidney. J Nephrol 2002;15(Suppl. 5):S32–S40 [PubMed] [Google Scholar]
- 10.Supuran CT. Diuretics: from classical carbonic anhydrase inhibitors to novel applications of the sulfonamides. Curr Pharm Des 2008;14:641–648 [DOI] [PubMed] [Google Scholar]
- 11.Wu M, Neilson A, Swift AL, et al. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol Cell Physiol 2007;292:C125–C136 [DOI] [PubMed] [Google Scholar]
- 12.Hundal RS, Krssak M, Dufour S, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 2000;49:2063–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunmair B, Staniek K, Gras F, et al. Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions? Diabetes 2004;53:1052–1059 [DOI] [PubMed] [Google Scholar]
- 14.El-Mir MY, Nogueira V, Fontaine E, Avéret N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem 2000;275:223–228 [DOI] [PubMed] [Google Scholar]
- 15.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 2000;348:607–614 [PMC free article] [PubMed] [Google Scholar]
- 16.Turner N, Li JY, Gosby A, et al. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes 2008;57:1414–1418 [DOI] [PubMed] [Google Scholar]
- 17.Cleasby ME, Dzamko N, Hegarty BD, Cooney GJ, Kraegen EW, Ye JM. Metformin prevents the development of acute lipid-induced insulin resistance in the rat through altered hepatic signaling mechanisms. Diabetes 2004;53:3258–3266 [DOI] [PubMed] [Google Scholar]
- 18.Edgerton DS, Ramnanan CJ, Grueter CA, et al. Effects of insulin on the metabolic control of hepatic gluconeogenesis in vivo. Diabetes 2009;58:2766–2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samuel VT, Beddow SA, Iwasaki T, et al. Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with type 2 diabetes. Proc Natl Acad Sci USA 2009;106:12121–12126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burgess SC, He T, Yan Z, et al. Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell Metab 2007;5:313–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller RA, Birnbaum MJ. An energetic tale of AMPK-independent effects of metformin. J Clin Invest 2010;120:2267–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foretz M, Hébrard S, Leclerc J, et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest 2010;120:2355–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim YD, Park KG, Lee YS, et al. Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP. Diabetes 2008;57:306–314 [DOI] [PubMed] [Google Scholar]
- 24.Shaw RJ, Lamia KA, Vasquez D, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 2005;310:1642–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Höhne C, Pickerodt PA, Francis RC, Boemke W, Swenson ER. Pulmonary vasodilation by acetazolamide during hypoxia is unrelated to carbonic anhydrase inhibition. Am J Physiol Lung Cell Mol Physiol 2007;292:L178–L184 [DOI] [PubMed] [Google Scholar]
- 26.Greenberg A. Diuretic complications. Am J Med Sci 2000;319:10–24 [PubMed] [Google Scholar]
- 27.Sandström PE, Sehlin J. Furosemide causes acute and long-term hyperglycaemia and reduces glucose tolerance in mice. Acta Physiol Scand 1988;132:75–81 [DOI] [PubMed] [Google Scholar]
- 28.Papadopoulos DP, Papademetriou V. Metabolic side effects and cardiovascular events of diuretics: should a diuretic remain the first choice therapy in hypertension treatment? The case of yes. Clin Exp Hypertens 2007;29:503–516 [DOI] [PubMed] [Google Scholar]
- 29.Dodgson SJ, Quistorff B, Ridderstråle Y. Carbonic anhydrases in cytosol, nucleus, and membranes of rat liver. J Appl Physiol 1993;75:1186–1193 [DOI] [PubMed] [Google Scholar]
- 30.Dodgson SJ, Forster RE, 2nd. Inhibition of CA V decreases glucose synthesis from pyruvate. Arch Biochem Biophys 1986;251:198–204 [DOI] [PubMed] [Google Scholar]
- 31.Carter ND, Dodgson SJ, Quant PA. Expression of hepatic mitochondrial carbonic anhydrase V. Biochim Biophys Acta 1990;1036:237–241 [DOI] [PubMed] [Google Scholar]
- 32.Dodgson SJ, Watford M. Differential regulation of hepatic carbonic anhydrase isozymes in the streptozotocin-diabetic rat. Arch Biochem Biophys 1990;277:410–414 [DOI] [PubMed] [Google Scholar]
- 33.Maren TH, Haywood JR, Chapman SK, Zimmerman TJ. The pharmacology of methazolamide in relation to the treatment of glaucoma. Invest Ophthalmol Vis Sci 1977;16:730–742 [PubMed] [Google Scholar]
- 34.Shimoda LA, Luke T, Sylvester JT, Shih HW, Jain A, Swenson ER. Inhibition of hypoxia-induced calcium responses in pulmonary arterial smooth muscle by acetazolamide is independent of carbonic anhydrase inhibition. Am J Physiol Lung Cell Mol Physiol 2007;292:L1002–L1012 [DOI] [PubMed] [Google Scholar]
- 35.Bode AM, Foster JD, Nordlie RC. Glycogenesis from glucose and ureagenesis in isolated perfused rat livers. Influence of ammonium ion, norvaline, and ethoxyzolamide. J Biol Chem 1994;269:7879–7886 [PubMed] [Google Scholar]
- 36.Cao TP, Rous S. Action of acetazolamide on liver pyruvate carboxylase activity, glycogenolysis and gluconeogenesis of mice. Int J Biochem 1978;9:603–605 [DOI] [PubMed] [Google Scholar]
- 37.Das SN, Sadhu DP. Effect of acetazolamide on insulin sensitivity in dogs with alloxan diabetes. Indian J Physiol Pharmacol 1978;22:301–304 [PubMed] [Google Scholar]
- 38.Boquist L, Bäckman AM, Strömberg C. Effects of acetazolamide on insulin release, serum glucose and insulin, glucose tolerance, and alloxan sensitivity of mice. Med Biol 1980;58:169–173 [PubMed] [Google Scholar]
- 39.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 1983;55:628–634 [DOI] [PubMed] [Google Scholar]
- 40.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949;109:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.