Abstract
Obesity is associated with systemic low-grade inflammation and obesity-related metabolic disorders. Considering that obesity decreases the expression of proinflammatory cytokines in the spleen, we assessed the role of interleukin (IL)-10, an anti-inflammatory cytokine produced by the spleen, in the pathogenesis of obesity. Changes in obesity-related pathogenesis, including inflammatory responses in multiple organs, were assessed after systemic administration of exogenous IL-10 to splenectomy (SPX)-treated obese wild-type and IL-10 knockout (IL-10KO) mice. Obesity resulted in the inability of the spleen to synthesize cytokines, including IL-10, and proinflammatory cytokines in obesity are then likely to emerge from tissues other than the spleen because serum levels of IL-10, but not proinflammatory cytokines, decreased despite the expression of these cytokines in the spleen being reduced in high fat–induced obese mice. SPX aggravated the inflammatory response in white adipose tissue (WAT) and the liver and suppressed adiposity in WAT. However, it accentuated adiposity in the liver. These SPX-induced changes were inhibited by systemic administration of IL-10. Moreover, SPX had little effect on the inflammatory responses in WAT and the liver of IL-10KO mice. These data show the role of spleen-derived IL-10 in diet-induced changes as a result of inflammatory responses in WAT and the liver.
An important recent development in the understanding of obesity is that it is characterized by chronic systemic low-grade inflammation (1–4). Many proinflammatory-related cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and monocyte chemoattractant protein (MCP)-1, are released from macrophage-infiltrated white adipose tissue (WAT) in the obese state (1,5). Obesity also leads to the progression of TNF-α– or MCP-1–induced nonalcoholic steatohepatitis and insulin resistance (6–8). Recent studies regarding crosstalk between the immune system and WAT in obese subjects have attracted a great deal of attention (3,4). The spleen is the largest lymphoid organ in the body, and it plays an important role in host immune function. Obese rats show decreased gene expression of proinflammatory cytokines, such as IL-6 and TNF-α, in the spleen (9). IL-10, which is synthesized by several cell types within multiple organs including the spleen, is a potent anti-inflammatory cytokine that inhibits the synthesis of proinflammatory cytokines. Large amounts of IL-10 are produced by activated B cells, which mature in the marginal zone of the spleen. New evidence shows that IL-10–producing B cells play a regulatory role in suppressing harmful immune responses (10). In fact, low IL-10 production capacity has been demonstrated in obesity (11,12).
On the basis of these findings, obesity is hypothesized to suppress the synthesis of IL-10 and result in chronic inflammation in WAT and the liver. Our data show that obesity reduced IL-10 expression in the spleen and that spleen-derived IL-10 protected against inflammatory responses in WAT and the liver in obese mice.
RESEARCH DESIGN AND METHODS
Male C57Bl/6J mice (wild-type mice, 22–25 g; KBT Oriental, Saga, Japan) and IL-10–deficient mice (IL-10KO mice, 002251-B6.129P2-Il10tm1Cgn/J, a gift from Sandy Morse, The Jackson Laboratory, Bar Harbor, ME) were housed in a room at Oita University under a 12-h light/dark cycle with lights on from 0700 h to 1900 h. IL-10KO mice maintained at our university were used for backcrossing. PCR primers of 5′-CCACACGCGTCACCTTAATA-3′ (mutant forward), 5′-GTTATTGTCTTCCCGGCTGT-3′ (wild-type reverse), and 5′-CTTGCACTACCAAAGCCACA-3′ (common) were used for genotyping. All studies were conducted in accordance with Oita University guidelines, based on the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. All mice were handled for 5 min each on 4 successive days before the experiment (13).
Experimental protocol
Experiment 1.
Wild-type mice were assigned to one of two groups (n = 6 in each group) as follows: group 1 mice were fed standard chow (Standard; 20% fat, 56% carbohydrate, 24% protein; Clea, Tokyo, Japan) for 8 weeks and then subjected to sham operation (Sham); group 2 mice were fed Standard for 8 weeks and then underwent splenectomy (SPX). Anesthesia was then induced by intraperitoneal injection of sodium pentobarbital (50 mg/kg), the abdominal cavity was opened, and the spleen was carefully removed. The abdomen was opened, but the spleen was not removed in the Sham group.
Experiment 2.
Wild-type mice were assigned to one of five groups (n = 6 in each group) as follows: group 1 mice were fed Standard for 8 weeks and administered mouse serum albumin (m-albumin), group 2 mice were fed a high-fat diet (HF; 60% fat, 20% carbohydrate, 20% protein; Research Diets, New Brunswick, NJ) for 8 weeks and then given m-albumin, group 3 mice were fed HF for 8 weeks after SPX and then given m-albumin, group 4 mice were fed HF for 8 weeks after SPX and then given recombinant mouse IL-10 (r-IL-10; 0.5 ng/day; Wako Chemicals, Osaka, Japan), and group 5 mice (pair-fed group) were fed the amount of food consumed by the SPX-treated group for 8 weeks and then given m-albumin.
Experiment 3.
Wild-type and IL-10KO mice were assigned to one of three groups (n = 6 in each group) as follows: group 1 mice were fed HF for 8 weeks and administered m-albumin, group 2 mice were fed HF for 8 weeks after SPX and administered m-albumin, and group 3 mice were fed HF for 8 weeks after SPX and administered r-IL-10 (0.5 ng/day; Wako Chemicals). Food intake during 24 h was calculated by weighing the remaining food, and body weight was determined between 1700 h and 1800 h every day. Food intake was normalized by body weight. All mice were housed for an additional 4 weeks after completion of the interventions.
Cytokine levels in the spleen, WAT, liver, and serum.
ELISA kits (Invitrogen, Carlsbad, CA) were used to measure TNF-α, IL-1β, MCP-1, and IL-10 levels in the spleen, epididymal WAT, liver, and serum. Protein concentrations of each organ were analyzed using the Lowry method. The ratio of IL-10 to TNF-α was calculated from the above measurements.
Western blotting.
Frozen tissue preparations were homogenized with sample buffer, centrifuged, and boiled. Total protein concentration of the tissue was quantified using the Bradford method. Equal amounts of total protein were loaded onto 8% SDS-PAGE and then electrophoretically transferred onto polyvinylidene difluoride membranes (Bio-Rad, Richmond, CA). The membranes were blocked with 5% nonfat milk for 1 h, incubated overnight with primary antibodies at 4°C, and then incubated with the secondary antibody for 1 h at room temperature. The primary antibody solution consisted of polyclonal antiserum with specificity for rabbit F4/80 (Santa Cruz Biotechnology, Santa Cruz, CA). F4/80 was detected by enhanced chemiluminescence (Amersham Life Sciences, Piscataway, NJ) and quantified using Quantity One imaging software (Bio-Rad).
Histological and immunohistochemical analyses.
Epididymal WAT and liver samples were examined with Mayer hematoxylin-eosin (H-E) (Wako Chemicals). Liver samples were also examined with Oil-Red-O staining and lightly stained with hematoxylin (Wako Chemicals). For immunohistochemical staining of F4/80, the slides were incubated with primary antibodies overnight at 4°C with rabbit anti-mouse F4/80 antibody (Santa Cruz Biotechnology). Slides were incubated with biotin-conjugated goat anti-rabbit IgG (ABC reagent; Vector Laboratories, Burlingame, CA). The immunoreactivity of each sample was visualized with diaminobenzidine tetrahydrochloride (Nacalai Tesque, Kyoto, Japan). In addition, normal rabbit serum was used instead of the aforementioned antibodies, and a further incubation with secondary antibody was performed as a negative control. These tests yielded negative staining.
Triglyceride contents in liver and WAT.
Triglyceride (TG) contents in epididymal WAT and liver samples were determined using a commercially available kit (Wako Chemicals).
Serum TG, free fatty acid, total cholesterol, alanine transaminase, and adiponectin levels.
Serum TG, free fatty acid (FFA), total cholesterol (TC), and alanine transaminase (ALT) concentrations were determined using commercially available kits (Wako Chemicals), and serum adiponectin levels were measured with an adiponectin ELISA kit (Otsuka Pharmaceutical, Tokyo, Japan).
Glucose tolerance test.
After an overnight fast, mice were injected intraperitoneally with glucose (2.0 g/kg body wt), and blood samples were taken at 0, 15, 30, 60, and 120 min. Blood glucose concentrations were measured using the glucose oxidase method and a glucose analyzer (MS-GR101; Terumo, Tokyo, Japan). Serum insulin concentrations were determined using an insulin ELISA kit (Shibayagi, Gunma, Japan).
Measurement of Vo2.
Vo2 was calculated using an indirect calorimetry system (Oxymax; Columbus Instruments, Columbus, OH). Vo2 and Vco2 were measured during a 24-h period at room temperature and normalized by body weight. The respiratory quotient (RQ) is the ratio of Vco2 to Vo2. Total Vo2 and Vco2 were determined during a period of 24 h by integrating the areas under the Vo2 and Vco2 curves (AUC Vo2 and AUC Vco2) according to the trapezoidal rule. The AUC RQ was also calculated.
Measurement of visceral and subcutaneous fat.
Visceral and subcutaneous fat areas were quantified by abdominal computed tomography (CT) scans (RmCT; Rigaku, Tokyo, Japan). CT slice scans were acquired at the lower margin of the L4 vertebra with mice in the supine position to measure the amounts of visceral and subcutaneous fat at a single level. We defined the level of the L4 vertebra as the line between the two highest points on the iliac crest. The images were converted to files compatible with the commercial software program i-Dixel-R (J. Morita Corporation, Kyoto, Japan).
Statistics.
Results are expressed as the means ± SEM. Statistical tests included the two-tailed Student t test and two-way ANOVA followed by the Scheffé test. Differences in splenic and serum levels of cytokines between Standard- and HF-fed groups and differences in WAT and liver data between Standard Sham– and Standard SPX–treated groups were analyzed using Student t test. For comparison of data among more than three groups, ANOVA was performed. In all analyses, P < 0.05 was taken to indicate statistical significance.
RESULTS
Effects of HF feeding on splenic and serum levels of pro- and anti-inflammatory cytokines.
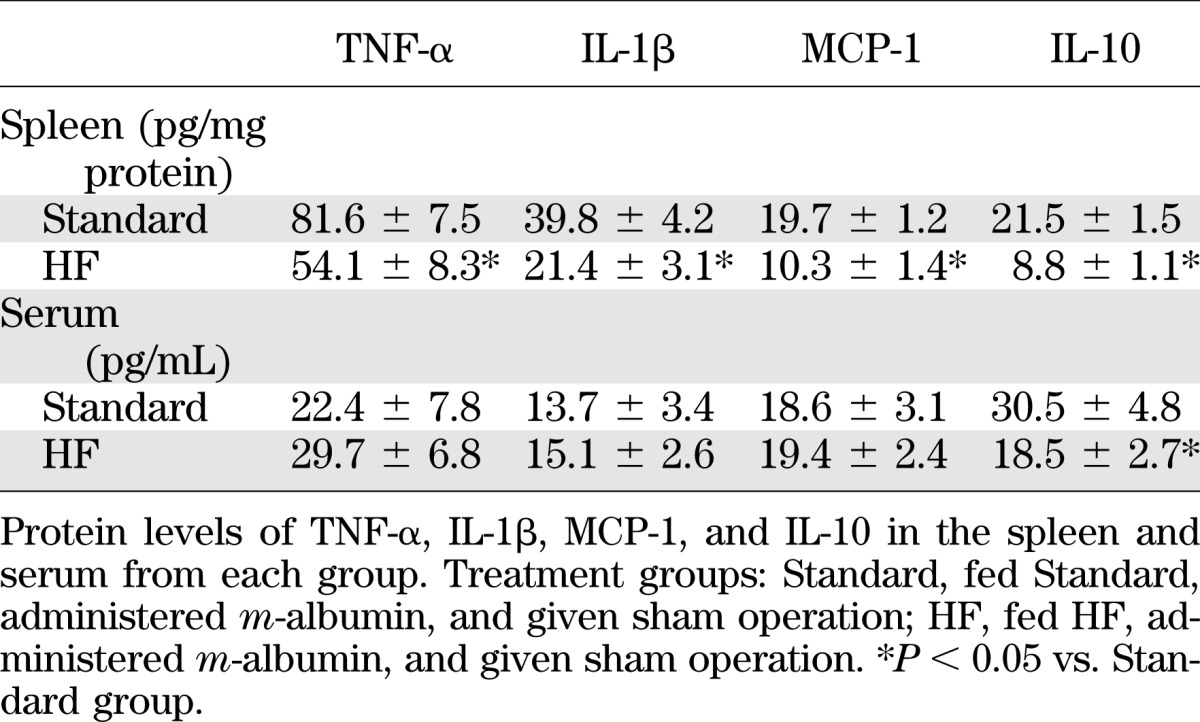
Despite the observation that the levels of TNF-α, IL-1β, MCP-1, and IL-10 expression in the spleen were significantly lower in the HF group than in the Standard group (t test, P < 0.05), serum levels of IL-10, but not TNF-α, IL-1β, or MCP-1, were significantly lower in the HF group than the Standard group (t test, P < 0.05) (Table 1). Serum cytokine levels, except IL-10, are probably maintained by other organs, such as WAT and the liver, when splenic cytokine expression is downregulated by HF feeding. However, serum IL-10 levels remained low, suggesting that large amounts of serum IL-10 are derived from the spleen.
TABLE 1.
HF-induced obesity decreases serum levels of IL-10 but not TNF-α, IL-1β, or MCP-1
SPX causes hypophagia and body weight loss.
We examined the effects of SPX on energy metabolism. SPX decreased energy intake, body weight, and the weight of epididymal WAT compared with the Sham treatment group (t test, P < 0.05) (Supplementary Table 1). SPX also increased AUC Vo2 and Vco2 and decreased AUC RQ compared with the Sham treatment group (t test, P < 0.05) (Supplementary Table 1) and increased serum ALT, TG, and FFA but not TC levels. Serum adiponectin levels were lower compared with the Sham treatment group (t test, P < 0.05) (Supplementary Table 1).
SPX reduces lipid accumulation in WAT but accelerates lipid accumulation in the liver and induces inflammatory responses in WAT and the liver.
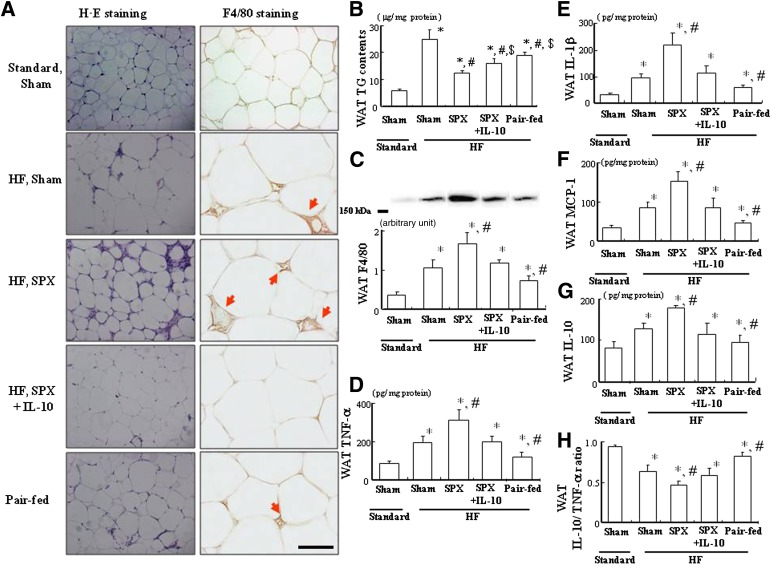
The effects of SPX on fat accumulation and the proinflammatory response in WAT were evaluated. Adipocytes tended to be smaller after SPX treatment, which was probably due to SPX-induced hypophagia and increased energy expenditure (Fig. 1A). SPX treatment also lowered TG content in WAT compared with the Sham treatment group (t test, P < 0.05) (Fig. 1B) and promoted infiltration of macrophages in WAT, as illustrated by immunohistochemical staining for F4/80, a glycoprotein whose levels increase as macrophages mature (Fig. 1A). In addition, SPX treatment increased F4/80 expression (t test, P < 0.05) (Fig. 1C) as well as TNF-α, IL-1β, MCP-1, and IL-10 levels (t test, P < 0.05) (Fig. 1D) in WAT but downregulated the ratio of IL-10 to TNF-α in WAT compared with that in the Sham treatment group (t test, P < 0.05) (Fig. 1E). Abdominal CT scans indicated that visceral fat accumulation, including epididymal WAT but not subcutaneous fat, was significantly lower in SPX-treated mice than in Sham-treated controls (t test, P < 0.05) (Fig. 1F–H).
FIG. 1.
SPX reduces fat deposition in WAT but elevates fat in the liver and accelerates HF feeding–induced inflammation in WAT and the liver. A: Representative H-E staining and immunostaining for F4/80 performed with WAT sections derived from each group. B: TG contents in WAT of each group (n = 6). C: Expression of F4/80 in WAT of each group (n = 6). D: Protein levels of TNF-α, IL-1β, MCP-1, and IL-10 in WAT of each group (n = 6). E: The ratio of IL-10 to TNF-α in WAT of each group (n = 6). F–H: CT images of the abdomen (red arrows; iliac crest) (F), including visceral fat (G) and subcutaneous fat (H) in each group (n = 6). J: Representative Oil-Red-O staining and immunostaining for F4/80 in liver sections from each group. Scale bar = 100 μm. K: TG content in the liver of each group (n = 6). L: Expression of F4/80 in the liver of each group (n = 6). M: Protein levels of TNF-α, IL-1β, MCP-1, and IL-10 in the liver of each group (n = 6). N: The ratio of IL-10 to TNF-α in the liver of each group (n = 6). *P < 0.05 vs. Standard Sham group. Scale bar = 100 μm. Treatment groups: Standard Sham, fed Standard and given sham operation; Standard SPX, fed Standard and given splenectomy. (A high-quality digital representation of this figure is available in the online issue.)
Next, we investigated whether SPX would result in similar inflammation in the liver as in WAT. First, the effects of SPX on fat accumulation in the liver were evaluated because a previous study indicates an important link between chronic inflammation and fat accumulation in the liver (14). In contrast to WAT, however, the morphological findings of Oil-Red-O staining (Fig. 1J) and hepatic TG content analysis (Fig. 1K) showed that SPX treatment accelerated HF feeding–induced hepatic fattiness despite concurrent hypophagia and body weight loss compared with the Sham treatment group (t test, P < 0.05). SPX promoted infiltration of F4/80+ cells (Fig. 1J), including Kupffer cells (KCs), which are thought to participate in hepatic lipid storage, compared with the Sham treatment group (15). Moreover, SPX also increased F4/80 expression and pro- and anti-inflammatory cytokine levels (t test, P < 0.05) (Fig. 1L and M) in the liver but reduced the hepatic ratio of IL-10 to TNF-α compared with the Sham treatment group (t test, P < 0.05) (Fig. 1N), which is consistent with a previous report that the ratio of IL-10 to TNF-α is low in nonalcoholic steatohepatitis (16).
IL-10 treatment suppresses SPX-induced catabolic effects.
SPX significantly attenuated the HF feeding–induced increase in energy intake, whereas IL-10 treatment suppressed this effect (Supplementary Fig. 1). A corresponding pair-feeding experiment was used to assess the net effects of SPX on energy metabolism. SPX diminished the HF-induced increase in body weight, but weight did not decline to the same level as in the Standard group. IL-10 treatment abolished the SPX-induced decreases in body weight, which were greater than those of the pair-fed intact controls (Supplementary Fig. 1). Furthermore, SPX accelerated the HF-induced increase in AUC Vo2 and Vco2 and decrease in AUC RQ, whereas IL-10 inhibited this effect (Supplementary Fig. 1).
IL-10 treatment inhibits the SPX-induced reduction in adiposity and improves the SPX-induced inflammatory condition in WAT.
The effects of IL-10 on SPX-induced changes in fat accumulation in WAT were evaluated. IL-10 treatment tended to inhibit the SPX-induced decrease in adipocyte size (Fig. 2A). The SPX-induced reduction in TG content was diminished by IL-10 treatment, consistent with previous observations indicating that IL-10 treatment reversed the SPX-induced reduction in food intake and body weight (Fig. 2B). The pair-fed intact controls also tended to show decreased lipid accumulation in WAT; however, the reduction in TG content was greater after SPX treatment than in the pair-fed intact control, which was probably the result of SPX-enhanced energy expenditure (Fig. 2A and B).
FIG. 2.
Systemic administration of IL-10 diminishes SPX-induced proinflammation in WAT. A: Representative H-E staining and immunostaining for F4/80 (red arrows) performed with WAT sections derived from each group. Scale bar = 100 μm. B: TG contents in WAT of each group (n = 6). C: Expression of F4/80 in WAT of each group (n = 6). D–G: Protein levels of TNF-α (D), IL-1β (E), MCP-1 (F), and IL-10 (G) in WAT of each group (n = 6). H: The ratio of IL-10 to TNF-α of WAT in each group (n = 6). *P < 0.05 vs. Standard Sham group, #P < 0.05 vs. HF Sham group, $P < 0.05 vs. HF SPX group. Treatment groups: Standard Sham, fed Standard, administered m-albumin, and given sham operation; HF Sham, fed HF, administered m-albumin, and received sham operation; HF SPX, fed HF, administered m-albumin, and given splenectomy; HF SPX+IL-10, fed HF, administered IL-10, and given splenectomy; Pair-fed, administered m-albumin, given sham operation, and fed the same amount of food as consumed by the HF SPX group. (A high-quality digital representation of this figure is available in the online issue.)
We also investigated whether SPX might accelerate the HF-induced inflammatory response in WAT. SPX treatment promoted the HF-induced infiltration of macrophages in WAT, as illustrated by immunohistochemical staining for F4/80 (Fig. 2A). It also elevated the HF-induced increase in F4/80 expression (Fig. 2C) as well as TNF-α, IL-1β, MCP-1, and IL-10 contents (Fig. 2D–G) in WAT but downregulated the HF-induced decrease in the ratio of IL-10 to TNF-α in WAT (Fig. 2H). IL-10 treatment suppressed the SPX-induced increase in F4/80 expression (Fig. 2A and C) and related cytokine levels (Fig. 2D–G) in WAT and inhibited the SPX-induced decrease in the ratio of IL-10 to TNF-α (Fig. 2H). Thus, IL-10 may ameliorate SPX-induced inflammation in WAT. Conversely, the pair-fed intact controls showed reduced expression and an elevated ratio of IL-10 to TNF-α in WAT compared with the HF-fed Sham treatment group (Fig. 2).
IL-10 treatment inhibits SPX-induced lipid accumulation and inflammatory responses in the liver.
The morphological findings (Fig. 3A) and results of hepatic TG content analysis (Fig. 3B) indicate that SPX promoted HF-induced fat accumulation in the liver, despite concurrent hypophagia and body weight loss. These changes were abolished by IL-10 treatment (Fig. 3A and B). In contrast, pair-fed intact controls showed reduced fat accumulation in the liver (Fig. 3A and B), suggesting that this fat accumulation may have been caused by factors other than nutrient excess.
FIG. 3.
Systemic administration of IL-10 improves SPX-induced proinflammatory responses of the liver and liver dysfunction. A: Representative H-E staining and immunostaining for F4/80 in liver sections derived from each group. Scale bar = 100 μm. B: TG contents of the liver in each group (n = 6). C: Serum ALT levels in each group (n = 6). D: Expression of F4/80 in the liver of each group (n = 6). E–H: TNF-α (E), IL-1β (F), MCP-1 (G), and IL-10 (H) contents of the liver in each group (n = 6). J: The ratio of IL-10 to TNF-α in the liver of each group (n = 6). *P < 0.05 vs. Standard Sham group, #P < 0.05 vs. HF Sham group. Treatment groups: Standard Sham, fed Standard, administered m-albumin, and given sham operation; HF Sham, fed HF, administered m-albumin, and given sham operation; HF SPX, fed HF, administered m-albumin, and given splenectomy; HF SPX+IL-10, fed HF, administered IL-10, and given splenectomy; Pair-fed, administered m-albumin, given sham operation, and fed the same amount of food as consumed by the HF SPX group. (A high-quality digital representation of this figure is available in the online issue.)
We found that SPX extended the HF-induced elevation in serum ALT level (Fig. 3C), F4/80 expression (Fig. 3D), and pro- and anti-inflammatory cytokine levels (Fig. 3E–H) in the liver and reduced the hepatic ratio of IL-10 to TNF-α (Fig. 3J). Similar to the findings in WAT, IL-10 treatment improved SPX-induced inflammatory responses in the liver (Fig. 3D–J). Conversely, pair-fed intact controls showed reduced expression and an increase in the ratio of IL-10 to TNF-α in the liver compared with the HF-fed Sham treatment group (Fig. 3).
SPX reduces serum IL-10 levels to a greater extent than other proinflammatory cytokines, and IL-10 treatment improves SPX-induced disorders in glucose and lipid metabolism.
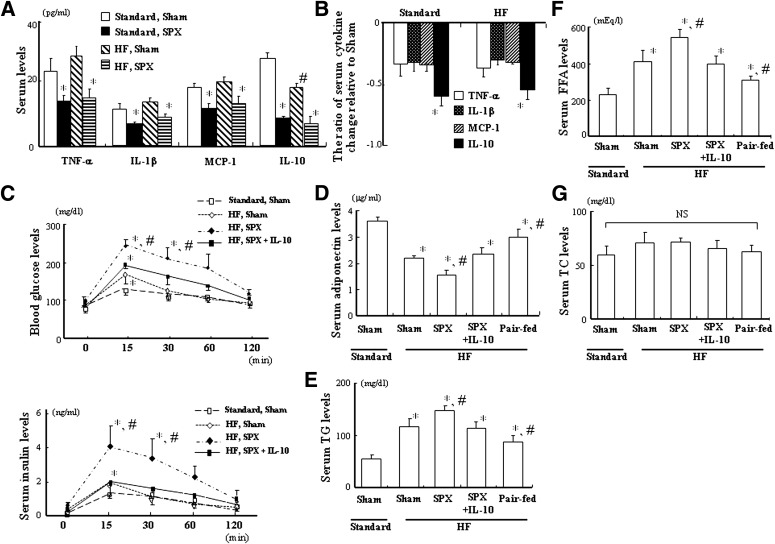
We examined serum cytokine levels between Sham and SPX groups with Standard and HF feeding. SPX decreased both serum pro- and anti-inflammatory cytokine levels irrespective of Standard or HF treatment (Fig. 4A). However, SPX reduced serum IL-10 level by ∼60% on Standard and HF feeding, and the ratio of decrease in IL-10 by SPX was greater than those of proinflammatory cytokines in both the Standard and HF groups (Fig. 4B).
FIG. 4.
SPX reduces serum IL-10 level to a greater extent than other proinflammatory cytokines, and systemic administration of IL-10 improves SPX-induced disorders in glucose and lipid metabolism. A: Serum levels of TNF-α, IL-1β, MCP-1, and IL-10 in Sham and SPX groups on Standard and HF. *P < 0.05 vs. Standard Sham group and HF Sham group, #P < 0.05 vs. Standard Sham group. B: The ratio of serum cytokine change relative to Sham group. *P < 0.05 vs. TNF-α, IL-1β, and MCP-1. C: Blood glucose and plasma insulin levels during glucose tolerance tests in each group (n = 6). D: Fasting serum adiponectin levels in each group (n = 6). E–G: Fasting serum TG (E), FFA (F), and TC (G) levels in each group (n = 6). *P < 0.05 vs. Standard Sham group, #P < 0.05 vs. HF Sham group; NS, not significant. Treatment groups: Standard Sham, fed Standard, administered m-albumin, and given sham operation; HF Sham, fed HF, administered m-albumin, and given sham operation; HF SPX, fed HF, administered m-albumin, and given splenectomy; HF SPX+IL-10, fed HF, administered IL-10, and given splenectomy; Pair-fed, administered m-albumin, given sham operation, and fed the same amount of food as consumed by the HF SPX group.
An intraperitoneal glucose tolerance test was performed to evaluate the effects of IL-10 treatment on SPX and glucose tolerance. SPX significantly extended HF-induced impaired glucose tolerance, and IL-10 treatment inhibited SPX-induced increases in blood glucose and serum insulin concentrations 15 and 30 min after glucose loading (Fig. 4C). Moreover, the HF-induced decrease in fasting serum adiponectin, an adipocyte-derived hormone that may be an insulin sensitizing factor with an antidiabetic effect, was significantly accelerated after SPX, and IL-10 treatment suppressed the SPX-induced change in serum adiponectin level (Fig. 4D). In addition, the HF-induced increases in fasting serum TG (Fig. 4E) and FFA levels (Fig. 4F), but not those of TC (Fig. 4G), were significantly potentiated after SPX treatment, whereas they decreased in the pair-fed intact controls. Furthermore, IL-10 treatment improved the SPX-induced increases in serum TG and FFA levels. These results are consistent with previous findings indicating that serum TG levels were increased in SPX-treated rats (17).
SPX does not influence IL-10 deficiency–induced catabolic effects.
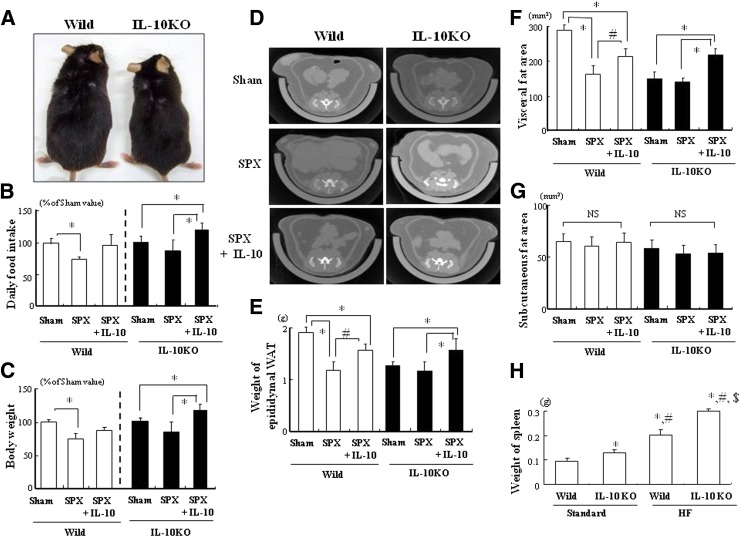
We examined whether IL-10 deficiency would affect SPX-induced inflammation in WAT and the liver using IL-10KO mice to better understand the influence of spleen-derived IL-10 protection. IL-10KO mice fed HF showed growth retardation (Fig. 5A), similar to previously reported results (18). In addition, no SPX-induced reductions in food intake (Fig. 5B) or body weight (Fig. 5C) were seen in IL-10KO mice. However, IL-10 treatment increased food intake and body weight in both SPX-treated wild-type and IL-10KO mice (Fig. 5B and C). SPX decreased the accumulation of visceral fat, including epididymal WAT, but not subcutaneous fat in wild-type mice, but it showed no significant effect in IL-10KO mice (Fig. 5D–G). Furthermore, IL-10 administration facilitated the accumulation of visceral fat but not subcutaneous fat (Fig. 5D–G). In addition, we examined the changes in spleen weight between wild-type and IL-10KO mice on Standard and HF. The spleens of IL-10KO mice were heavier than those of wild-type controls in both Standard and HF groups, and HF increased the weight of the spleen in both wild-type and IL-10KO mice (Fig. 5H). Considering our observation that obesity induces splenomegaly, there may be an association between the size of the spleen and its ability to produce IL-10 (19).
FIG. 5.
IL-10 deficiency induces an anorectic effect and suppresses adiposity in WAT. A: Growth appearance in wild-type (Wild) and IL-10KO mice fed HF. B and C: Comparison of daily food intake (B) and body weight (C) in each group shown as percentages relative to Sham values (n = 6). D: CT images of the abdomen, including subcutaneous and visceral fat, in wild-type and IL-10KO mice fed HF. E–G: Comparison of epididymal WAT weight (E) and accumulation of visceral fat (F) and subcutaneous fat (G) in each group (n = 6). *P < 0.05 vs. Sham (Wild or IL-10KO), #P < 0.05 vs. SPX (Wild); NS, not significant. H: Weight of spleen in each group (n = 6). *P < 0.05 vs. Standard (Wild), #P < 0.05 vs. Standard (IL-10KO), $P < 0.05 vs. HF (Wild). Treatment groups: Sham, fed HF, administered m-albumin, and given sham operation; SPX, fed HF, administered m-albumin, and given splenectomy; SPX+IL-10, fed HF, administered r-IL-10, and given splenectomy. (A high-quality color representation of this figure is available in the online issue.)
SPX has little effect on the inflammatory response in WAT or on glucose metabolism in IL-10KO mice.
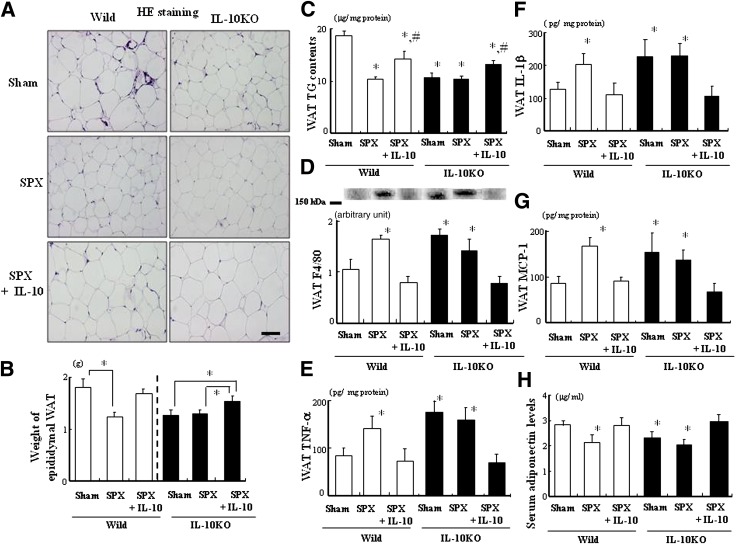
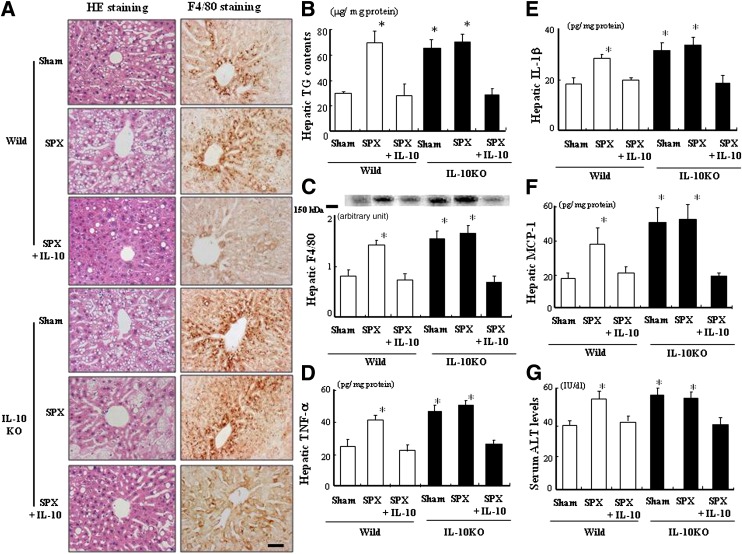
We investigated whether SPX would alter fat accumulation and inflammatory responses in WAT of IL-10KO mice. Fat accumulation in WAT decreased in IL-10KO mice (Fig. 6A). SPX-induced decreases in adipocyte size, weight of epididymal WAT, and TG contents in WAT (Fig. 6A–C) and increases in F4/80 expression and TNF-α, IL-1β, and MCP-1 protein levels (Fig. 6D–G) were not observed in IL-10KO mice. However, IL-10 treatment tended to restore these factors in both SPX-treated mice. In addition, no SPX-induced decrease in fasting serum adiponectin level was observed in IL-10KO mice (Fig. 6H). We also examined the effects of IL-10 treatment on SPX and glucose tolerance in IL-10KO mice. SPX treatment had no effect on glucose tolerance in IL-10KO mice, and IL-10 treatment decreased blood glucose and serum insulin concentrations 15 min after glucose loading (Supplementary Fig. 2).
FIG. 6.
SPX had little effect on adiposity and inflammatory responses in WAT of IL-10KO mice. A: Representative H-E staining performed with WAT sections derived from each group. Scale bar = 100 μm. B: The weight of epididymal WAT in each group (n = 6). *P < 0.05 vs. Sham (Wild or IL-10) group. C: TG contents in WAT of each group (n = 6). D: Expression of F4/80 in WAT of each group (n = 6). E–G: TNF-α (E), IL-1β (F), and MCP-1 (G) contents in WAT of each group (n = 6). H: Serum adiponectin levels in each group (n = 6). *P < 0.05 vs. Sham (Wild) group, #P < 0.05 vs. SPX (Wild and IL-10) group. Treatment groups: Sham, fed HF, administered m-albumin, and given sham operation; SPX, fed HF, administered m-albumin, and given splenectomy; SPX+IL-10, fed HF, administered r-IL-10, and given splenectomy. Wild, wild-type mice. (A high-quality digital representation of this figure is available in the online issue.)
SPX does not affect the proinflammatory state in the liver of IL-10KO mice.
HF-induced hepatic fat accumulation increased in IL-10KO mice but was not influenced by SPX treatment (Fig. 7A and B). Furthermore, IL-10 treatment suppressed the activation of F4/80+ cells in the liver of both SPX-treated mice (Fig. 7A and C). Overall, changes in the above protein levels (Fig. 7D–F) and serum ALT levels (Fig. 7G) in the liver were similar to those seen in WAT in all groups.
FIG. 7.
SPX does not affect fat accumulation or inflammatory responses in the liver of IL-10KO mice. A: Representative H-E staining and immunostaining for F4/80 in liver sections derived from each group. Scale bar = 100 μm. B: Comparison of hepatic TG contents in each group (n = 6). C: F4/80 expression in the liver of each group (n = 6). D−F: Protein levels of TNF-α (D), IL-1β (E), and MCP-1 (F) in the livers of each group (n = 6). G: Comparison of serum ALT levels in each group (n = 6). *P < 0.05 vs. Sham (Wild) group. Treatment groups: Sham, fed HF, administered m-albumin, and given sham operation; SPX, fed HF, administered m-albumin, and given splenectomy; SPX+IL-10, fed HF, administered r-IL-10, and given splenectomy. Wild, wild-type mice. (A high-quality digital representation of this figure is available in the online issue.)
DISCUSSION
Obesity is associated with a low-grade, chronic, proinflammatory condition (1–4). However, the primary cause of obesity-induced inflammation is not well understood. This is the first study to systematically characterize the lack of IL-10 induction from the spleen as a result of HF-induced obesity and SPX in rats with respect to individual organs (e.g., WAT and liver), serum cytokines, and glucose and lipid metabolism. IL-10 is synthesized by several cell types within multiple organs, including the spleen. We paid special attention to spleen-derived IL-10 because the serum levels of IL-10 are specially and significantly decreased despite the significant decreases in expression levels of all cytokines in the spleen in the HF group compared with the Standard group, suggesting that HF feeding reduces IL-10 secretion from the spleen. Our previous study shows that compared with Standard feeding, HF downregulated the expression of CD20, a surface molecule present on B cells that produces IL-10 mainly in the spleen (20). Moreover, splenocyte proliferation stimulated by T-cell and B-cell mitogen was significantly lower in obese subjects; not only T-cell but also B-cell function in the spleen may be impaired in obesity (21). Thus, it was hypothesized that obesity-induced reduction of IL-10 synthesis in the spleen may lead to inflammatory responses in multiple organs and to metabolic disorders.
We demonstrated that IL-10 treatment abolished SPX-induced hypophagia, body weight loss, and increase in energy expenditure, consistent with previous data showing that administration of IL-10 attenuates inflammation-induced anorexia (22). We previously reported that a reduction of spleen-derived IL-10 resulted in activation of microglia and induction of hypothalamic inflammation and that mild reduction of spleen-derived IL-10 by HF causes hyperphagia, whereas severe reduction of splenic IL-10 by SPX causes hypophagia, which may explain why anorexia and body weight loss occur in the setting of sepsis-induced hypothalamic inflammation (20). A recent study shows that intrahypothalamic infusion of r-IL-10 blocked inhibitor of κB kinase/nuclear factor-κB signaling and endoplasmic reticulum stress and restored Akt and signal transducer and activator of transcription 3 phosphorylation, promoting an antiobesity effect (23). These observations suggest that modulation of hypothalamic IL-10 expression may constitute a promising alternative to reduce hypothalamic inflammation and endoplasmic reticulum stress related to obesity.
Inflammation is generally accepted as being closely related to adiposity in WAT; however, our results show that SPX treatment reduced adiposity but increased the inflammatory responses in WAT (24). Furthermore, IL-10 treatment restored the SPX-induced reduction of WAT adiposity, although it suppressed SPX-induced inflammation in WAT, suggesting that SPX-induced inflammation may be independent of adiposity in WAT. In contrast, SPX treatment facilitated both adiposity and inflammatory responses in the liver compared with Sham treatment, and treatment with IL-10 suppressed these SPX-induced responses, suggesting that TNF-α expression in the liver is related to hepatic inflammation and fattiness (15). Our data indicate that spleen-derived IL-10 has a protective effect against pathological fat deposition and inflammation in the liver, supporting previous reports that treatment with the anti–TNF-α antibody infliximab significantly reduces fat deposition in the liver of obese rats and that exogenous IL-10 improves liver fibrosis caused by carbon tetrachloride (16,25). Taken together, these findings suggest that IL-10 originating in the spleen may prevent chronic low-level inflammation induced by obesity in WAT and the liver, although it may increase fat accumulation in WAT while decreasing accumulation in the liver.
We examined whether IL-10 deficiency would affect SPX-induced inflammation in WAT and liver using IL-10KO mice. We observed that food intake and body weight decreased in IL-10KO mice compared with wild-type controls, in agreement with previous findings that spontaneous weight loss occurs in IL-10KO mice (18). However, the SPX-induced hypophagia and body weight loss observed in wild-type mice were not seen in IL-10KO mice, although IL-10 treatment restored these changes in both SPX-treated mice. These results indicate that spleen-derived IL-10 may affect the regulation of energy metabolism. We observed that SPX-induced reduction of adiposity and proinflammatory effects in WAT observed in wild-type mice were not seen in IL-10KO mice. IL-10 treatment restored these alterations in WAT of both SPX-treated mice. In the liver, SPX treatment promoted adiposity as well as inflammatory responses in wild-type mice, but no such effects were observed in IL-10KO mice. In addition, IL-10 treatment improved adiposity and inflammation in the liver of both SPX-treated mice. Therefore, spleen-derived IL-10 may be closely related to the adiposity and inflammatory responses in multiple organs.
We found that IL-10 treatment suppressed the SPX-induced glucose metabolism disorder and the reduction in serum adiponectin levels, suggesting that TNF-α represses the synthesis of adiponectin in adipocytes and that IL-10 interferes with macrophage function and the production of proinflammatory cytokines, including TNF-α (26,27). Moreover, the SPX-induced abnormalities in glucose tolerance observed in wild-type mice were not seen in IL-10KO mice, and administration of IL-10 to both SPX-treated mice improved glucose tolerance, suggesting that spleen-derived IL-10 may be involved in glucose metabolism. The inhibition of TNF-α activity by infliximab improves insulin signaling in the liver of HF-fed rats (18). Moreover, depleting KCs prevents the development of insulin resistance, and KC-derived TNF-α plays a role in mediating the detrimental effects of KCs on insulin action (28).
We examined serum cytokine levels between Sham and SPX groups on Standard and HF. Serum IL-10 level was reduced by more than half by SPX treatment, which induces a strong systemic inflammatory response, whereas SPX treatment elevated the expression of IL-10 as well as proinflammatory cytokines in WAT and the liver, supporting the suggestion that spleen-derived IL-10 is more relevant to systemic inflammation than IL-10 produced locally in WAT or the liver. Indeed, SPX eliminates both pro- and anti-inflammatory cytokines produced by the spleen. However, we identified that SPX had little effect on lipid accumulation or inflammation in WAT and the liver of IL-10KO mice, indicating that SPX-induced changes in WAT and the liver of wild-type mice are not due to the elimination of proinflammatory cytokines. Thus, obesity may be characterized by promotion of proinflammatory pathways accompanied by diminished anti-inflammatory mediators, such as IL-10, adiponectin, M2 macrophages that produce high levels of the anti-inflammatory cytokine IL-10, and others.
In conclusion, we demonstrated a critical role of spleen-derived IL-10 in the inflammation of multiple organs and insulin resistance in the obese state. Although additional studies are required to determine why obesity elicits an inflammatory response, the results of this study indicate that targeting the spleen may be a potential therapeutic strategy for treating metabolic syndrome.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant for the Research on Measures for Intractable Diseases from Japan’s Ministry of Health, Labor, and Welfare.
No potential conflicts of interest relevant to this article were reported.
K.G. researched data and wrote the manuscript. M.I., T.M., S.C., H.A., and K.F. researched data. T.Sh., I.K., T.K., M.S., and T.Sa. contributed to discussion. H.Y. reviewed and edited the manuscript. H.Y. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-1688/-/DC1.
REFERENCES
- 1.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest 2005;115:1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860–867 [DOI] [PubMed] [Google Scholar]
- 3.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006;116:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest 2011;121:2111–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaminski DA, Randall TD. Adaptive immunity and adipose tissue biology. Trends Immunol 2010;31:384–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin M, Wheeler MD, Kono H, et al. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology 1999;117:942–952 [DOI] [PubMed] [Google Scholar]
- 7.Lin HZ, Yang SQ, Chuckaree C, Kuhajda F, Ronnet G, Diehl AM. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med 2000;6:998–1003 [DOI] [PubMed] [Google Scholar]
- 8.Kanda H, Tateya S, Tamori Y, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 2006;116:1494–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamas O, Martínez JA, Marti A. Decreased splenic mRNA expression levels of TNF-alpha and IL-6 in diet-induced obese animals. J Physiol Biochem 2004;60:279–283 [DOI] [PubMed] [Google Scholar]
- 10.Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol 2004;22:929–979 [DOI] [PubMed] [Google Scholar]
- 11.Esposito K, Pontillo A, Giugliano F, et al. Association of low interleukin-10 levels with the metabolic syndrome in obese women. J Clin Endocrinol Metab 2003;88:1055–1058 [DOI] [PubMed] [Google Scholar]
- 12.Waters KA, Mast BT, Vella S, et al. Structural equation modeling of sleep apnea, inflammation, and metabolic dysfunction in children. J Sleep Res 2007;16:388–395 [DOI] [PubMed] [Google Scholar]
- 13.Sakata T. Hunger and satiety related activity induced certain metabolites in rats. In The Neural Basis of Feeding and Reward. Hoebel BG, Ed. Brunswick, ME, Haer Institute for Electrophysiological Research, 1982, p. 339–354 [Google Scholar]
- 14.Cai D, Yuan M, Frantz DF, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med 2005;11:183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Segersvärd R, Tsai JA, Herrington MK, Wang F. Obesity alters cytokine gene expression and promotes liver injury in rats with acute pancreatitis. Obesity (Silver Spring) 2008;16:23–28 [DOI] [PubMed] [Google Scholar]
- 16.Barbuio R, Milanski M, Bertolo MB, Saad MJ, Velloso LA. Infliximab reverses steatosis and improves insulin signal transduction in liver of rats fed a high-fat diet. J Endocrinol 2007;194:539–550 [DOI] [PubMed] [Google Scholar]
- 17.Fatouros M, Bourantas K, Bairaktari E, Elisaf M, Tsolas O, Cassioumis D. Role of the spleen in lipid metabolism. Br J Surg 1995;82:1675–1677 [DOI] [PubMed] [Google Scholar]
- 18.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 1993;75:263–274 [DOI] [PubMed] [Google Scholar]
- 19.Altunkaynak BZ, Ozbek E, Altunkaynak ME. A stereological and histological analysis of spleen on obese female rats, fed with high fat diet. Saudi Med J 2007;28:353–357 [PubMed] [Google Scholar]
- 20.Gotoh K, Inoue M, Masaki T, et al. A novel anti-inflammatory role for spleen-derived interleukin-10 in obesity-induced hypothalamic inflammation. J Neurochem 2012;120:752–764 [DOI] [PubMed] [Google Scholar]
- 21.Sato Mito N, Suzui M, Yoshino H, Kaburagi T, Sato K. Long term effects of high fat and sucrose diets on obesity and lymphocyte proliferation in mice. J Nutr Health Aging 2009;13:602–606 [DOI] [PubMed] [Google Scholar]
- 22.Hollis JH, Lemus M, Evetts MJ, Oldfield BJ. Central interleukin-10 attenuates lipopolysaccharide-induced changes in food intake, energy expenditure and hypothalamic Fos expression. Neuropharmacology 2010;58:730–738 [DOI] [PubMed] [Google Scholar]
- 23.Ropelle ER, Flores MB, Cintra DE, et al. IL-6 and IL-10 anti-inflammatory activity links exercise to hypothalamic insulin and leptin sensitivity through IKKbeta and ER stress inhibition. PLoS Biol 2010;8:e1000465. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993;259:87–91 [DOI] [PubMed] [Google Scholar]
- 25.Zhang LJ, Zheng WD, Chen YX, et al. Antifibrotic effects of interleukin-10 on experimental hepatic fibrosis. Hepatogastroenterology 2007;54:2092–2098 [PubMed] [Google Scholar]
- 26.Cintra DE, Pauli JR, Araújo EP, et al. Interleukin-10 is a protective factor against diet-induced insulin resistance in liver. J Hepatol 2008;48:628–637 [DOI] [PubMed] [Google Scholar]
- 27.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun 2004;323:630–635 [DOI] [PubMed] [Google Scholar]
- 28.Huang W, Metlakunta A, Dedousis N, et al. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes 2010;59:347–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.