Abstract
Magnetic resonance spectroscopy (MRS) methods offer a potentially valuable window into cellular metabolism. Measurement of flux between inorganic phosphate (Pi) and ATP using 31P MRS magnetization transfer has been used in resting muscle to assess what is claimed to be mitochondrial ATP synthesis and has been particularly popular in the study of insulin effects and insulin resistance. However, the measured Pi→ATP flux in resting skeletal muscle is far higher than the true rate of oxidative ATP synthesis, being dominated by a glycolytically mediated Pi↔ATP exchange reaction that is unrelated to mitochondrial function. Furthermore, even if measured accurately, the ATP production rate in resting muscle has no simple relationship to mitochondrial capacity as measured either ex vivo or in vivo. We summarize the published measurements of Pi→ATP flux, concentrating on work relevant to diabetes and insulin, relate it to current understanding of the physiology of mitochondrial ATP synthesis and glycolytic Pi↔ATP exchange, and discuss some possible implications of recently reported correlations between Pi→ATP flux and other physiological measures.
Magnetic resonance spectroscopy (MRS) methods offer a window on metabolism in vivo and can yield dynamic information in three main ways: firstly, from the kinetics of changes in metabolite concentrations [examples in muscle include 31P MRS measurements of postexercise phosphocreatine (PCr) resynthesis to probe mitochondrial ATP synthesis (1–3) and of PCr breakdown in ischemia to measure resting ATP turnover (2,4–11)]; secondly, using exogenous tracers [for example, the use of 13C MRS measurements of label transfer from infused [2-13C]acetate to muscle [4-13C]glutamate to estimate tricarboxylic acid cycle (TCAC) rate (12–16)]; and thirdly, measurements of unidirectional reaction rates by magnetization transfer (MT). Like isotope-labeling methods, MT has the advantage of being applicable to resting muscle. However, its interpretation is problematic. In this study, we discuss 31P MRS MT measurements of flux between inorganic phosphate (Pi) and ATP. Since the first report in 1989 by one of us (K.M.B.) of its application in working rat leg muscle (17), this has been applied to resting muscle to assess what is variously described as mitochondrial F1F0 ATPase activity, ATP synthase flux, unidirectional ATP production, or simply mitochondrial function (3,12,14–16,18–30). It has been used to study insulin effects (19,22,25,28,31,32) and insulin resistance (3,12,16,19–21,23–25,28,30,31), training (13,30), mitochondrial biogenesis (22,33), thyroid hormone pathophysiology (14,15,34), acromegaly (29), and burn injury (26,27) and has been combined with 13C MRS measurements of TCAC rate in an effort to quantify changes in mitochondrial coupling (13–15,33–36) [i.e., P:O, the ratio of ATP generated to oxygen consumed (37,38)].
As emphasized by one of us (G.J.K.) (39) and in a recent review (40) and commentary (41), the Pi→ATP flux measured by MT in resting skeletal muscle is far higher than estimates of oxidative ATP synthesis by other means, because [as discussed in the original article (17) and previously (42)], it is dominated by a glycolytically mediated Pi↔ATP exchange that is unrelated to mitochondrial ATP synthesis. Furthermore (39), even if measured accurately, the resting ATP turnover rate has no simple relationship to mitochondrial capacity as measured either in biopsy samples or in vivo from O2 consumption during maximal exercise (30) or indirectly from 31P MRS measurements of PCr recovery after submaximal exercise (43). Although both these points are becoming accepted (2,32), debate continues about the extent to which the measured Pi→ATP flux can be used as a measure of oxidative ATP production.
In this article, we summarize the quantitative evidence, concentrating on work relevant to diabetes and insulin, and relate it to the physiology of mitochondrial ATP synthesis and glycolytic Pi–ATP exchange. We show that the 1989 article (17) provides no warrant for the application of the Pi→ATP flux measurement to resting muscle and discuss some more general aspects of the interpretation of MT measurements and their relationship to metabolic fluxes. We point out how little is actually known about the changes in energy metabolism in insulin-stimulated and insulin-resistant muscle that this technique has been used to probe. We discuss a recent study (2) that addresses directly the relationship between Pi→ATP flux and MR-based measures of mitochondrial function and ATP turnover in normal muscle, and argue that (although interesting and surprising) the findings do not rehabilitate 31P MR MT measurements of Pi→ATP flux as a probe of mitochondrial metabolism in resting muscle.
THE QUANTITATIVE PROBLEM WITH Pi→ATP FLUX MEASUREMENTS
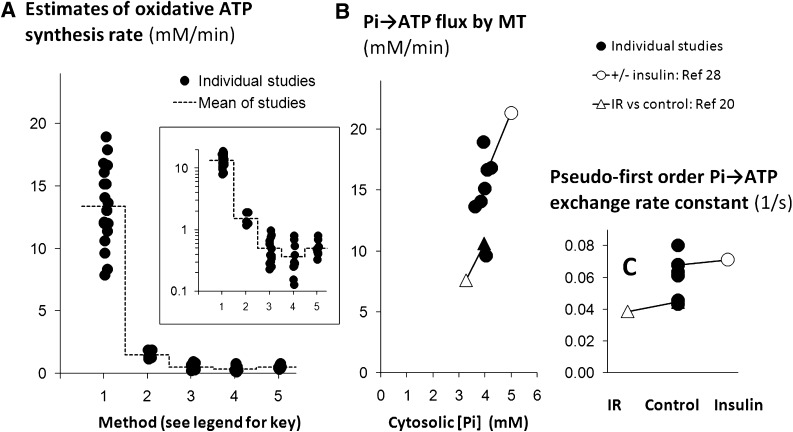
That the Pi→ATP flux far exceeds oxidative ATP turnover in resting skeletal muscle is easily appreciated visually. Figure 1A (which expands on Fig. 1A in Ref. 39) summarizes the published resting Pi→ATP fluxes in healthy adult human muscle (each data point being the mean from one study) in comparison with oxidative ATP synthesis rates derived from published 13C MRS estimates of TCAC rate and with a representative selection of resting ATP turnover measurements by three other methods (see figure legend). Although the 13C MRS values appear somewhat high (39,40), most obvious is the order-of-magnitude difference between the Pi→ATP flux and the rest.
FIG. 1.
Pi→ATP flux and exchange rate constant in resting human muscle: a quantitative summary of the literature. This figure summarizes the results of a number of published studies of human muscle using various experimental methods. Each point (or pair of linked points) shows the mean in a single reported study. A shows estimates of oxidative ATP synthesis by five experimental methods: Method 1, from the Pi→ATP flux measured by 31P MRS MT (1–3,13–16,20,23–25,28–32,45,73); Method 2, from 13C MRS measurements of TCAC rate (12–16); Method 3, from AVD measurements of O2 consumption (47–51,74–79) [and three articles cited in Table 3 of van Beekvelt et al. (11)]; Method 4, from near-infrared spectrophotometry measurements of O2 consumption in ischemic muscle (4,80,81) [and eight articles cited in Table 3 of van Beekvelt et al. (11)]; and Method 5, from 31P MRS measurements of PCr decrease (sometimes with correction for glycolytic ATP synthesis) in ischemic muscle (2,4–10) [and three articles cited in Table 3 of van Beekvelt et al. (11)]. The dashed line shows the overall mean value for each method. The inset shows the same data in logarithmic form to focus on the values obtained by Methods 3–5 (which are similar to values given by 15O positron emission tomography [39], omitted here for brevity). B shows mean Pi→ATP flux measured by 31P MRS MT as a function of cytosolic [Pi] in published studies of normal muscle unstimulated by insulin (closed circles) (1,2,13,28,29,32,73) and also during hyperinsulinemic-euglycemic clamp stimulation in a single study (open circle linked by a line to the corresponding unstimulated point) (28). The figure also shows data from insulin-resistant offspring of patients with type 2 diabetes (open triangle, linked to the closed triangle representing data from the unstimulated muscle) in a single study (20) (as absolute values of [Pi] are not reported, we assume a basal value equal to the mean of the other studies in this figure). C shows the pseudo–first-order rate constant for flux between Pi and ATP in the studies shown in B; lines link the data from insulin stimulation and IR to the matching control points. In this review, concentrations are expressed as mmol/L cytosolic water (which we call mM), recalculated where necessary from published sources assuming leg muscle mass = 10 kg, muscle density = 1.049 kg/L, and muscle cell water = 0.67 L/kg wet weight (17); Vo2/TCAC rate = 3; and 1 mol O2 = 25.5 l (82); for Methods 2, 3, and 4, P:O is taken as 2.16 on the basis of mouse experiments combining near-infrared spectrophotometry and 31P MRS during ischemia (83) (see also legend to Fig. 2).
INTERPRETATIONS OF EXPERIMENTS ON WORKING MUSCLE
Although the 1989 article (17) is often cited in support of the MT measurement in resting muscle (18,21,26–28,34), its message has evidently been misunderstood. In their recent review, From and Ugurbil (40) critically evaluated the analysis in the 1989 article and suggested that although its authors were aware of the problem with Pi↔ATP exchange catalyzed by the glycolytic enzymes glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (EC 1.2.1.12) and phosphoglycerate kinase (PGK) (EC 2.7.2.3), “they concluded that the observed work-associated increase of measured Pi-ATP rate probably did not contain a major GAPDH/PGK component” (40). However, this is not so. Their comparison with published O2 consumption data (40) is essentially the same as that from which Brindle et al. (17) concluded that “… a contribution from the glycolytic enzymes cannot altogether be ruled out. For example, the oxygen consumption at rest in the perfused hind limb [….] corresponds to an ATP turnover rate […] which is at the lower end of the range of fluxes measured with a 10-ms stimulation pulse at a frequency of 0.5 Hz.” The 1989 article went on to point out that working muscle is different. During a 70-ms tetanus at 1 Hz, “the similarity of [the Pi→ATP flux] to the ATP turnover rate calculated from O2 consumption measurements in the perfused hind limb indicates that the contribution of a glycolytic exchange reaction […] is relatively small” (17). From and Ugurbil (40) largely agree with this analysis, although they suggest subtracting the resting rate from the fluxes measured in working muscle to get the true mitochondrial Pi→ATP flux, which Brindle et al. (17) declined to do because the glycolytic Pi↔ATP exchange might well change with work (44). Brindle et al. (17) were therefore correct to conclude that the GAPDH/PGK exchange likely makes only a relatively small contribution to the measured Pi→ATP flux in working muscle. The problem has arisen from the use of this analysis in resting muscle (40), first by Jucker et al. (34), who, citing Brindle et al. (17) as a method reference, combined 31P MRS MT with 13C MRS measures of TCAC rate to try to infer changes in mitochondrial coupling. This article (34) has formed the basis for subsequent MT studies that have claimed to detect mitochondrial dysfunction in skeletal muscle in insulin-resistant and other states, some (12,14,15,22,23,26–28,33,35,36,45) citing it directly as a method reference, and others (16,18–21,32) citing other articles (14,35) that do so.
Pi→ATP FLUX MEASUREMENTS VERSUS OTHER MEASURES
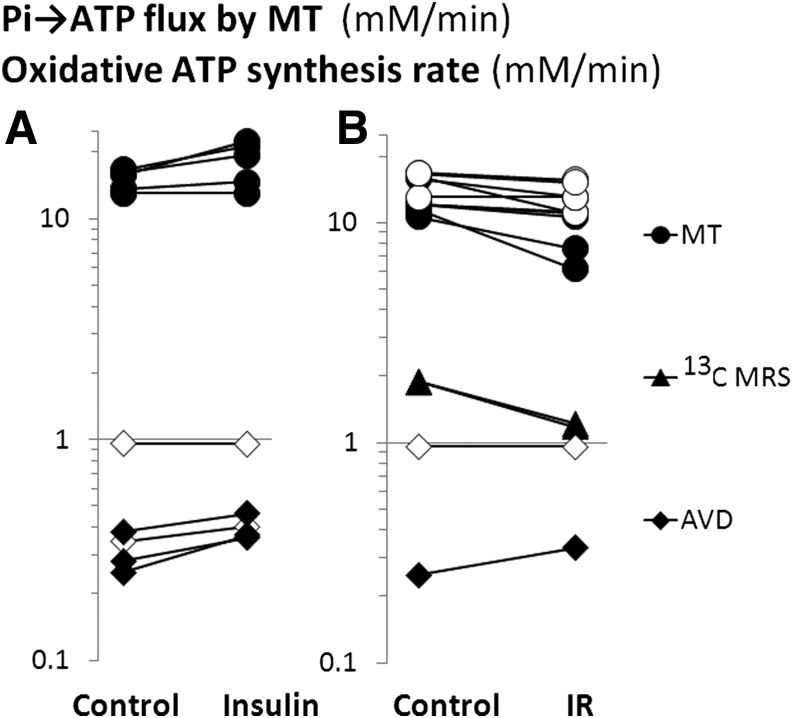
Much of the interest in resting muscle Pi→ATP flux has been in the differences in high-insulin and insulin-resistant states relative to control (3,12,16,19–21,23–25,28,30,31), in the acute response to insulin (19,22,25,28,31,32), and in correlations with other measurements (18,23,28,45,46). Figure 2 summarizes the insulin and insulin-resistance effects in human muscle and compares them with published resting ATP turnover data obtained by two other methods, 13C MRS measurements of TCAC rate and arteriovenous difference (AVD) measurements of O2 consumption.
FIG. 2.
Resting muscle Pi→ATP flux and oxidative ATP synthesis rate in IR and insulin stimulation: a quantitative summary of the literature. The figure shows (in logarithmic form) mean values from published studies of Pi→ATP flux measured by 31P MRS MT (circles) and oxidative ATP synthesis rate calculated from published 13C MRS measurements of TCAC rate (triangles) and from AVD measurements of muscle O2 consumption (diamonds). Each linked pair of points shows the means in single reported studies. A compares control muscle in the fasting state and during hyperinsulinemic-euglycemic clamp, and B compares control and IR states. In both, each linked pair of points represents mean values of basal versus insulin or control versus IR from a single study. Filled symbols show that the difference was statistically significant within the study, and open symbols that it was not. Studies in A are: MT measurements (23,25,28,31,32) and AVD measurements (47–51). [Pi→ATP flux is also stimulated by insulin in Petersen et al. (19), not plotted in the figure because absolute rates are not reported.] Studies in B are: MT measurements in mitochondrial diabetes (myopathy, encephalopathy, lactic acidosis, and stroke-like episodes) (25), type 2 diabetic patients (3,23,28,31), and their first-degree relatives (20,30), women with a history of gestational diabetes (24), patients with inherited insulin receptor signaling defects (1), and elderly (versus young) subjects (16); 13C MRS measurements in elderly (versus young) subjects (16) and first-degree relatives of type 2 diabetic patients (12); and AVD measurements in type 2 diabetic patients (49,50). Note that for the AVD and 13C MRS measurements in this figure, we have assumed that neither insulin nor type 2 diabetes affects the P:O ratio. This is unlikely to be so; if there were no proton leak across the inner mitochondrial membrane, then for stoichiometric reasons, the P:O ratio for glucose oxidation would be ∼10–15% higher than for palmitate oxidation (38); thus, the insulin-induced switch from fat to glucose oxidation (47–51) would increase P:O. However, effective P:O ratios in vivo are influenced (lowered) by significant proton leak (38), and it is unknown how this changes in response to insulin or in type 2 diabetes.
Effects of insulin and insulin resistance.
Figure 2A summarizes how elevation of insulin (typically by euglycemic-hyperinsulinemic clamp) increases Pi→ATP flux (23,25,28,32), the effect being reduced or absent in insulin-resistant states such as lipid infusion (45), high-fat diet (18), type 2 diabetes (23), mitochondrial diabetes (25), and longstanding type 1 diabetes (28). Also shown, for comparison, are reported effects on the (much lower) estimates of oxidative ATP synthesis from AVD O2 consumption measurements: insulin stimulates this significantly in some (47–49) but not all (50,51) reports. As the euglycemic-hyperinsulinemic clamp results in a two- to sixfold increase in glucose oxidation and near-complete suppression of fat oxidation (47–51), the overall effect on oxidative ATP synthesis will depend (in an arithmetical sense) on the balance between these two opposing effects, about which little is known. Figure 2B also summarizes how, compared with controls, Pi→ATP flux in resting muscle is reduced in some (16,20,23–25), but not all (1,3,28,30) studies of insulin-resistant states. Also shown, for comparison, is the decreased oxidative ATP synthesis rate inferred from 13C MRS studies of TCAC rate in insulin-resistant first-degree relatives of type 2 diabetic patients (12) and the elderly (16), and the oxidative ATP synthesis rates estimated from AVD O2 consumption measurements in two studies of type 2 diabetic patients, one showing a significant increase relative to control (49) and one not (50). Again, how this abnormality in oxidative ATP synthesis might relate to the opposing effects of muscle insulin resistance on glucose and fat oxidation (49) is poorly understood.
Some correlations with Pi→ATP flux measurements.
Interest in the MT measurement has been stimulated by reported correlations with measures of insulin sensitivity and insulin effect. Pi→ATP flux correlates with glucose infusion rate in the euglycemic-hyperinsulinemic clamp across test and control groups in studies of fat-fed rats (18) and of lipid infusion in healthy humans (45) and type 2 diabetic patients and control subjects (23,28) [in whom it also correlates with glycogen synthesis rates (31)]. To interpret these findings, we need to consider the biochemistry of the Pi→ATP flux.
COMPONENTS OF Pi→ATP FLUX
The reason why the MT measurement of Pi→ATP flux is so high, as discussed in the 1989 article (17) as well as recently (40), is that the Pi→ATP flux catalyzed by the mitochondrial ATP synthase in resting muscle is dwarfed by the Pi↔ATP exchange catalyzed by the glycolytic enzymes GAPDH and PGK (44). As the net glycolytic flux through GAPDH/PGK is three orders of magnitude smaller than the exchange flux [being ∼10–50 μmol/L/min, increasing three- to eightfold in hyperinsulinemic-euglycemic clamp (47–51)], the GAPDH/PGK reactions are always near to equilibrium.
The Pi→ATP flux has commonly been expressed relative to a control group (16) or a basal state (32) or to TCAC rate measured by 13C MRS (16). However, because we know little about what controls the much larger glycolytic component, it definitely cannot be assumed that a relative decrease in Pi→ATP flux represents, even approximately, the same relative decrease in net oxidative ATP synthesis (which could change by a completely different amount or direction) nor that changes in absolute flux above basal (19) be taken as a guide to the increment in net oxidative ATP synthesis.
Pi→ATP FLUX AND CYTOSOLIC Pi CONCENTRATION
The MT experiment (17,40,44) gives the pseudo–first-order rate constant for flux between Pi and ATP (the absolute rate divided by the Pi concentration), which has no physiological meaning. This rate constant (usually expressed in s−1) must be multiplied by [Pi] (usually obtained from the Pi/ATP peak intensity ratio in a suitable spectrum, and a value of [ATP] assumed or measured by other means) in order to give the physiologically relevant flux (in this study, expressed as mmol/L/min). Cytosolic [Pi] and the exchange rate constant are reported separately (or can be inferred) in only a few MT studies in rat (35,52), mouse (26,27,36), and human muscle (1,2,13,28,32,45) [although relative changes in [Pi] are given in Petersen et al. (19) and can be estimated in multiple studies (20,22,29)]. Figure 1B summarizes how, for studies of healthy human muscle in which [Pi] is reported, relatively little between-study variation in Pi→ATP fluxes is explained by variation in [Pi], there being considerable between-study variation in the rate constant (Fig. 1C). [By contrast, Pi→ATP fluxes in the five published studies of mouse muscle (22,26,27,36,53) span a 10-fold range, and in the four studies in which this is reported, differences in [Pi] account for most of this variation.] Figure 1B shows two studies in which increased Pi→ATP flux in insulin infusion (19) and decreased Pi→ATP flux in insulin-resistant relatives of type 2 diabetic patients (20) is explained by altered cell [Pi], in the sense that the rate constant does not change (Fig. 1C). There are three reports in which Pi→ATP flux is dissociated from changes in [Pi]: slightly in treated acromegaly (29) and strikingly in experimental burn injury (26,27). This distinction is not usually reported.
The glycolytic component of the measured Pi→ATP flux depends on the activities of the two enzymes and the concentrations of their substrates and products, viz. NAD+ and NADH, Pi, ADP, ATP, glyceraldehyde 3-phosphate, 1,3-biphophosphoglycerate, and 3-phosphoglycerate (44), which we can only measure to a limited extent in vivo. Apart from [Pi], 31P MRS can monitor [ADP] (calculated usually from pH and [PCr], assuming literature values for [ATP] and total creatine concentrations), but this is not reported in the cited experiments. PCr concentration (measured as PCr/ATP) is unchanged in insulin infusion (19,54), but insulin increases cytosolic pH (54), which will tend to increase [ADP], and stimulates Na+-linked creatine accumulation (55), neither of which would necessarily change [ADP] (see the discussion of mitochondrial feedback control below). We have seen that there is little evidence of a causal influence of [Pi] on the measured Pi→ATP flux in resting human muscle unstimulated by insulin; however, in the absence of additive or countervailing effects of changes in unmeasured metabolites, the rise in [Pi] in insulin stimulation (Fig. 1B) (19,28) might increase the glycolytic exchange flux (44).
RESTING ATP TURNOVER AND MITOCHONDRIAL FUNCTION
From and Ugurbil (40), among others (56), note that reduction in maximum oxidative capacity should not affect the much lower rates of ATP supply in resting muscle. If a consistent closed-loop feedback relationship controls oxidative ATP synthesis across the whole dynamic range, and if resting ATP demand does not change, then a decrease in the mitochondrial capacity [which in 31P MRS terms is the notional maximum rate of oxidative ATP synthesis at maximum values of the error signal (43)] should mean an increase in the resting error signal (57)—for example, [ADP]. This exemplifies the general principle of metabolic control analysis that inhibition of an enzyme will tend to increase its substrate (upstream) concentrations; whether this results in a decrease in steady-state flux through the whole pathway depends on its regulatory structure (58); this is captured quantitatively by the flux control coefficient, which is related to the elasticity coefficients describing how concentrations of substrates and products affect the activities of individual pathway enzymes (58). In resting skeletal muscle the flux control coefficient of mitochondrial capacity is very low, control instead residing mainly in ATP demand. Even quite large changes in mitochondrial capacity should therefore have a negligible effect on resting ATP turnover.
CORRELATIONS WITH ATP TURNOVER RATE AND MITOCHONDRIAL CAPACITY
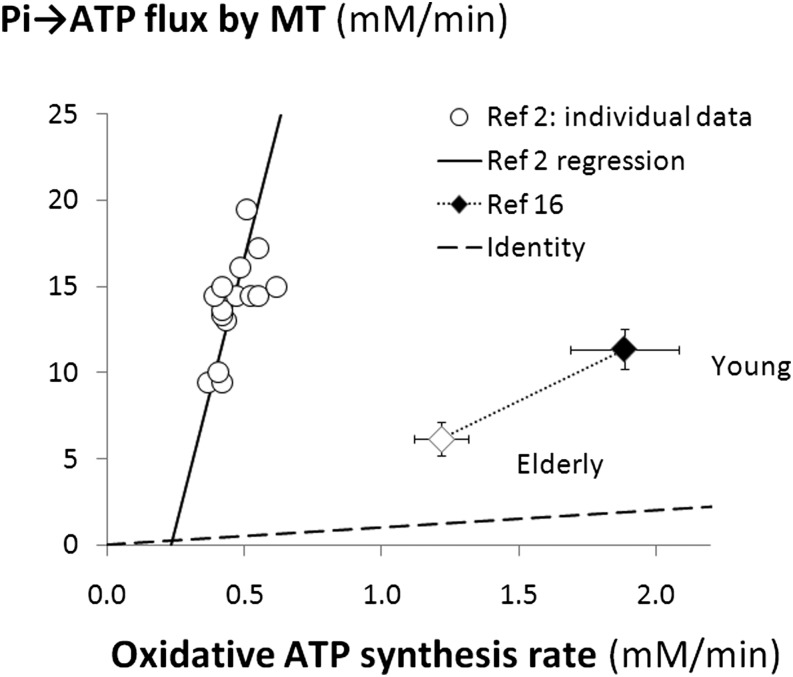
A recent study (2) has directly addressed some of these issues, finding that Pi→ATP flux correlates across healthy subjects with an ischemic measure of ATP turnover and also with PCr recovery-based measures of mitochondrial capacity. This might be thought to be in favor of MT measurements of Pi→ATP flux as a physiologically useful probe of muscle energy metabolism, despite the problems discussed above. However, the scale of the quantitative discrepancy undermines this. Consider the correlation with ATP turnover rate. Figure 3 shows individual data points taken from Schmid et al. (2) and the regression line through them (extrapolated for illustrative purposes); as this intercepts near the origin, the massively dominant nonoxidative component seems to scale with oxidative ATP synthesis rate, presumably because both measurements correlate with an underlying variable, which is so far unknown, For comparison, Fig. 3 shows the only other report (16) in which Pi→ATP flux measurements can be compared with a parallel measurement of oxidative ATP turnover (in this case, from TCAC rate by 13C MRS): mean data points are shown for healthy adults and elderly, insulin-resistant subjects (16), who show a low value by both MT and 31C MRS methods. This has been interpreted as showing reduced basal ATP turnover and normal mitochondrial coupling (16), but it seems safer to regard it as showing a low TCAC rate, with a so far unexplained decrease in the glycolytic component of Pi↔ATP exchange.
FIG. 3.
Pi→ATP flux compared with other measures of oxidative ATP synthesis in some published studies of resting human muscle. The circles represent individual subjects’ data points from Schmid et al. (2), showing the Pi→ATP flux measured by 31P MRS MT plotted against oxidative ATP synthesis rate in normally perfused muscle estimated as the rate of nonoxidative ATP synthesis by 31P MRS during temporary ischemia; also shown is the linear regression line through the individual data points [compared with Fig. 4e in Schmid et al. (2)] extended to the x-axis. The diamonds represent mean ± SEM data from Ref. 16, showing the Pi→ATP flux measured by 31P MRS MT plotted against oxidative ATP synthesis estimated from TCAC rates measured by 13C MRS; results are shown for young subjects (closed symbols) and elderly, relatively IR, subjects (open symbols), linked by a dotted line. The dashed line is the line of identity.
The interesting between-subjects correlation of Pi→ATP flux with measures of mitochondrial capacity was suggested (2) to reflect fiber-type differences. However, there appears to be no independent evidence that more oxidative fibers have greater basal ATP turnover.
MUSCLE MITOCHONDRIAL ABNORMALITY IN TYPE 2 DIABETES?
We will not discuss the wider evidence about mitochondrial dysfunction in type 2 diabetes in detail. From and Ugurbil (40) summarize some evidence against this, and numerous reviews have been published (e.g., Refs. 56,59,60). However, if (as we argue) MT does not tell us anything about mitochondrial function, it is of interest to ask what information other MR-based methods might be able to provide.
Probing mitochondrial function in vivo.
There are three main ways to assess muscle mitochondrial capacity: firstly, ex vivo (biopsy) measurements of mitochondrial number, mitochondrial enzyme and respiratory chain component content (61), and maximal respiration or ATP production rates in isolated mitochondria (61). Results from diabetic muscle are reviewed in Holloszy (56) and Pagel-Langenickel et al. (59). Secondly, in vivo measurements of oxygen consumption during maximal exercise (Vo2max), which test the integrated cardiorespiratory/vascular/muscular response (30), whereas measurements made by arteriovenous sampling can be specific to particular muscles. (Some results in diabetes are reviewed in Refs. 56,59.) Thirdly, MR techniques can provide reasonably well-tolerated noninvasive measurements of muscle in vivo. However, measurements of resting ATP turnover (even if accurate) cannot tell us about the capacity for oxidative ATP synthesis, and practical considerations rule out maximal exercise in the MR scanner. 31P MRS measurements of PCr recovery kinetics after submaximal exercise, however, can yield information about mitochondrial capacity in that this reflects a number of physiological and pathophysiological mechanisms, including mitochondrial number, content of oxidative enzymes or other mitochondrial components, and vascular supply of substrates and oxygen (43).
Evidence of mitochondrial dysfunction from postexercise PCr recovery kinetics.
Evidence of mitochondrial dysfunction, relative to appropriate controls, has been reported in type 2 diabetic patients in some studies (62–65) but not others (3,66), and even when group differences are observed, correlation with insulin resistance may be poor (65). However, a similar defect has also been reported in primary congenital insulin resistance (1) and congenital lipodystrophy (67), suggesting that muscle mitochondrial dysfunction can be a response to insulin resistance, as opposed to a cause. 31P MRS measures of mitochondrial function correlate with ex vivo measurements of muscle mitochondrial function and whole-body measures of aerobic fitness in type 2 diabetic patients (65,68), similarly to healthy controls (65). Exercise training improves 31P MRS measures of mitochondrial function (as well as insulin sensitivity) in some studies (64) but not others (3). A recent report that a short intensive exercise program improves maximal in vitro mitochondrial ATP production in healthy controls and the offspring of mothers with diabetes, but insulin sensitivity only in the former (61), argues against any simple link between mitochondrial function and insulin sensitivity.
Mitochondrial dysfunction and the pathophysiology of insulin resistance.
A popular hypothesis is that impaired lipid metabolism is associated with accumulation of intermediates (notably diacylglycerol) that interfere with postreceptor insulin signaling (e.g., Refs. 69,70). Furthermore, a feature of many insulin-resistant states is increased intramyocellular triglyceride (measurable by 1H MRS) (69), which is commonly attributed to impairment of fat catabolism. Neither postulate requires fat oxidation rate to be low, as it may be that higher intermediate concentrations overcome the effects of the defect in overall pathway flux. However, whether the enzyme defects that might be responsible for diacylglycerol and whether triglyceride accumulation would lead to decreases in mitochondrial capacity and function in vivo and ex vivo is not clear (see addendum for information on a recently published article that addresses this question). For mitochondrial oxygen consumption or ATP production ex vivo, the answer will depend on where the hypothesized defects are and the experimental conditions (e.g., nature of oxidizable substrate). 31P MRS measurements of postexercise PCr recovery are also complicated by questions of fuel selection: there are no good data on how much postexercise PCr recovery is driven by oxidation of fat after the relatively short exercise typical in 31P MRS experiments.
SUMMARY: THE INTERPRETATION OF MT EXPERIMENTS
The underlying problem with the interpretation of MT experiments has been that many have assumed that the method is measuring net chemical flux, whereas the experiment is formally equivalent to an isotope-exchange experiment (42,71). The relationship between the flux measured by MT and the metabolically relevant net chemical flux will depend on the enzyme’s mechanism (i.e., the order of substrate binding). For example, in the reaction catalyzed by creatine kinase, which has a random order equilibrium mechanism with rate-limiting interconversion of the ternary complexes (E.MgADP.PCr and E.MgATP.Cr), it is reasonable to equate the PCr→ATP flux measured by MT with overall flux through the reaction (71,72). However, at low ADP concentrations in vitro at pH 7.0, the ATP↔ADP exchange fluxes measured by MT are only half of the PCr↔ATP exchange fluxes, which are, however, similar to the isotope exchange fluxes of the 14C label between ATP and ADP and of the 15N label between Cr and PCr (71), as expected for this reaction mechanism. In other words, the MT experiment underestimates the creatine kinase-catalyzed ATP↔ADP exchange flux. This might be due to loss of magnetization in an on-enzyme intermediate, although this is considered theoretically unlikely (71). This discrepancy has received renewed attention with the recent demonstration that the ADP→ATP flux measured by MT in muscle can be explained by a transferred nuclear Overhauser effect between free and bound ATP, with only a very small contribution from chemical exchange between ATP and ADP (53). The authors of this study suggest that a large pool of ADP in muscle is bound to a large macromolecular assembly (e.g., actin), in which it is difficult to saturate the magnetization [see Brindle (42) for a discussion of this issue] and that this leads to underestimation of the ATP↔ADP exchange flux. However, if this exchange proceeds via a pool of free ADP, then this should be saturable and the exchange measurable, although if the very pool is very small and turning over very rapidly, then again it may be difficult to saturate (71). Balaban and Koretsky (41) have discussed recently the effects of metabolic exchange involving small pools of metabolites. Alternative explanations are direct transfer of the bound ADP to creatine kinase or possibly, more likely, a change in the enzyme mechanism in vivo such that dissociation of ADP from the enzyme becomes very slow, although still allowing rapid exchange between the ternary complexes and thus between ATP and PCr (71,72).
To summarize, in skeletal muscle at rest, the Pi→ATP flux measured by 31P MRS magnetization transfer is many times larger than the net rate of oxidative ATP synthesis (Fig. 1A). Thus, 31P MRS MT measurements of mitochondrial ATP turnover in skeletal muscle can only be made reliably in working muscle, where the GAPDH/PGK-catalyzed exchange is a smaller fraction of the total measured flux. This point was made in the 1989 article by Brindle et al. (17), which is often cited in support of the resting muscle measurement. The 2000 article by Jucker et al. (34), which has been cited as a method reference by many subsequent articles, is fundamentally flawed due to its failure to consider adequately the implications of this substantial GAPDH/PGK exchange in resting muscle. Even if the Pi→ATP flux measured by MT were a reliable measure of oxidative ATP synthesis in resting muscle, this would not be any guide to mitochondrial capacity. The Pi→ATP flux is increased by insulin and tends to be lower in insulin-resistant (IR) states than controls. There is some evidence from other methods (Fig. 2) that oxidative synthesis rate is increased by insulin (the net result of an opposing increase in glucose oxidation and decrease in fat oxidation) and that it is decreased in IR states. However, the apparent congruence between these relative changes may simply be an epiphenomenon.
ADDENDUM
A recently published review addresses the question of how products of lipid oxidation might impair insulin action in muscle: Watt MJ, Hoy AJ. Lipid metabolism in skeletal muscle: generation of adaptive and maladaptive intracellular signals for cellular function. Am J Physiol Endocrinol Metab 2012;302:E1315–1328.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
G.J.K. and K.M.B. drafted parts of the text, and both edited the final manuscript. G.J.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Sleigh A, Raymond-Barker P, Thackray K, et al. Mitochondrial dysfunction in patients with primary congenital insulin resistance. J Clin Invest 2011;121:2457–2461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmid AI, Schrauwen-Hinderling VB, Andreas M, Wolzt M, Moser E, Roden M. Comparison of measuring energy metabolism by different 31P-magnetic resonance spectroscopy techniques in resting, ischemic, and exercising muscle. Magn Reson Med 2012;67:898–905 [DOI] [PubMed] [Google Scholar]
- 3.Trenell MI, Hollingsworth KG, Lim EL, Taylor R. Increased daily walking improves lipid oxidation without changes in mitochondrial function in type 2 diabetes. Diabetes Care 2008;31:1644–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amara CE, Shankland EG, Jubrias SA, Marcinek DJ, Kushmerick MJ, Conley KE. Mild mitochondrial uncoupling impacts cellular aging in human muscles in vivo. Proc Natl Acad Sci USA 2007;104:1057–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blei ML, Conley KE, Kushmerick MJ. Separate measures of ATP utilization and recovery in human skeletal muscle. J Physiol 1993;465:203–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katz A. G-1,6-P2, glycolysis, and energy metabolism during circulatory occlusion in human skeletal muscle. Am J Physiol 1988;255:C140–C144 [DOI] [PubMed] [Google Scholar]
- 7.Sjöholm H, Gidlöf A, Larsson J, Sahlin K. The effect of long-term circulatory occlusion on pH and energy metabolism of the quadriceps muscle in man. Clin Sci (Lond) 1985;68:597–600 [DOI] [PubMed] [Google Scholar]
- 8.Johannsen DL, Conley KE, Bajpeyi S, et al. Ectopic lipid accumulation and reduced glucose tolerance in elderly adults are accompanied by altered skeletal muscle mitochondrial activity. J Clin Endocrinol Metab 2012;97:242–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanza IR, Tevald MA, Befroy DE, Kent-Braun JA. Intracellular energetics and critical PO2 in resting ischemic human skeletal muscle in vivo. Am J Physiol Regul Integr Comp Physiol 2010;299:R1415–R1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vicini P, Kushmerick MJ. Cellular energetics analysis by a mathematical model of energy balance: estimation of parameters in human skeletal muscle. Am J Physiol Cell Physiol 2000;279:C213–C224 [DOI] [PubMed] [Google Scholar]
- 11.van Beekvelt MC, van Engelen BG, Wevers RA, Colier WN. In vivo quantitative near-infrared spectroscopy in skeletal muscle during incremental isometric handgrip exercise. Clin Physiol Funct Imaging 2002;22:210–217 [DOI] [PubMed] [Google Scholar]
- 12.Befroy DE, Petersen KF, Dufour S, et al. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes 2007;56:1376–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Befroy DE, Petersen KF, Dufour S, Mason GF, Rothman DL, Shulman GI. Increased substrate oxidation and mitochondrial uncoupling in skeletal muscle of endurance-trained individuals. Proc Natl Acad Sci USA 2008;105:16701–16706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lebon V, Dufour S, Petersen KF, et al. Effect of triiodothyronine on mitochondrial energy coupling in human skeletal muscle. J Clin Invest 2001;108:733–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell CS, Savage DB, Dufour S, et al. Resistance to thyroid hormone is associated with raised energy expenditure, muscle mitochondrial uncoupling, and hyperphagia. J Clin Invest 2010;120:1345–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 2003;300:1140–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brindle KM, Blackledge MJ, Challiss RA, Radda GK. 31P NMR magnetization-transfer measurements of ATP turnover during steady-state isometric muscle contraction in the rat hind limb in vivo. Biochemistry 1989;28:4887–4893 [DOI] [PubMed] [Google Scholar]
- 18.Yerby B, Deacon R, Beaulieu V, Liang J, Gao J, Laurent D. Insulin-stimulated mitochondrial adenosine triphosphate synthesis is blunted in skeletal muscles of high-fat-fed rats. Metabolism 2008;57:1584–1590 [DOI] [PubMed] [Google Scholar]
- 19.Petersen KF, Dufour S, Shulman GI. Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Med 2005;2:e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 2004;350:664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laurent D, Yerby B, Deacon R, Gao J. Diet-induced modulation of mitochondrial activity in rat muscle. Am J Physiol Endocrinol Metab 2007;293:E1169–E1177 [DOI] [PubMed] [Google Scholar]
- 22.Choi CS, Befroy DE, Codella R, et al. Paradoxical effects of increased expression of PGC-1alpha on muscle mitochondrial function and insulin-stimulated muscle glucose metabolism. Proc Natl Acad Sci USA 2008;105:19926–19931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szendroedi J, Schmid AI, Chmelik M, et al. Muscle mitochondrial ATP synthesis and glucose transport/phosphorylation in type 2 diabetes. PLoS Med 2007;4:e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prikoszovich T, Winzer C, Schmid AI, et al. Body and liver fat mass rather than muscle mitochondrial function determine glucose metabolism in women with a history of gestational diabetes mellitus. Diabetes Care 2011;34:430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szendroedi J, Schmid AI, Meyerspeer M, et al. Impaired mitochondrial function and insulin resistance of skeletal muscle in mitochondrial diabetes. Diabetes Care 2009;32:677–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tzika AA, Mintzopoulos D, Padfield K, et al. Reduced rate of adenosine triphosphate synthesis by in vivo 31P nuclear magnetic resonance spectroscopy and downregulation of PGC-1beta in distal skeletal muscle following burn. Int J Mol Med 2008;21:201–208 [PubMed] [Google Scholar]
- 27.Padfield KE, Astrakas LG, Zhang Q, et al. Burn injury causes mitochondrial dysfunction in skeletal muscle. Proc Natl Acad Sci USA 2005;102:5368–5373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kacerovsky M, Brehm A, Chmelik M, et al. Impaired insulin stimulation of muscular ATP production in patients with type 1 diabetes. J Intern Med 2011;269:189–199 [DOI] [PubMed] [Google Scholar]
- 29.Szendroedi J, Zwettler E, Schmid AI, et al. Reduced basal ATP synthetic flux of skeletal muscle in patients with previous acromegaly. PLoS ONE 2008;3:e3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kacerovsky-Bielesz G, Chmelik M, Ling C, et al. Short-term exercise training does not stimulate skeletal muscle ATP synthesis in relatives of humans with type 2 diabetes. Diabetes 2009;58:1333–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim EL, Hollingsworth KG, Smith FE, Thelwall PE, Taylor R. Effects of raising muscle glycogen synthesis rate on skeletal muscle ATP turnover rate in type 2 diabetes. Am J Physiol Endocrinol Metab 2011;301:E1155–E1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim EL, Hollingsworth KG, Thelwall PE, Taylor R. Measuring the acute effect of insulin infusion on ATP turnover rate in human skeletal muscle using phosphorus-31 magnetic resonance saturation transfer spectroscopy. NMR Biomed 2010;23:952–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jucker BM, Yang D, Casey WM, et al. Selective PPARdelta agonist treatment increases skeletal muscle lipid metabolism without altering mitochondrial energy coupling: an in vivo magnetic resonance spectroscopy study. Am J Physiol Endocrinol Metab 2007;293:E1256–E1264 [DOI] [PubMed] [Google Scholar]
- 34.Jucker BM, Dufour S, Ren J, et al. Assessment of mitochondrial energy coupling in vivo by 13C/31P NMR. Proc Natl Acad Sci USA 2000;97:6880–6884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jucker BM, Ren J, Dufour S, et al. 13C/31P NMR assessment of mitochondrial energy coupling in skeletal muscle of awake fed and fasted rats. Relationship with uncoupling protein 3 expression. J Biol Chem 2000;275:39279–39286 [DOI] [PubMed] [Google Scholar]
- 36.Cline GW, Vidal-Puig AJ, Dufour S, Cadman KS, Lowell BB, Shulman GI. In vivo effects of uncoupling protein-3 gene disruption on mitochondrial energy metabolism. J Biol Chem 2001;276:20240–20244 [DOI] [PubMed] [Google Scholar]
- 37.Marcinek DJ. Mitochondrial dysfunction measured in vivo. Acta Physiol Scand 2004;182:343–352 [DOI] [PubMed] [Google Scholar]
- 38.Brand MD. The efficiency and plasticity of mitochondrial energy transduction. Biochem Soc Trans 2005;33:897–904 [DOI] [PubMed] [Google Scholar]
- 39.Kemp GJ. The interpretation of abnormal 31P magnetic resonance saturation transfer measurements of Pi/ATP exchange in insulin-resistant skeletal muscle. Am J Physiol Endocrinol Metab 2008;294:E640–E642 [DOI] [PubMed] [Google Scholar]
- 40.From AH, Ugurbil K. Standard magnetic resonance-based measurements of the Pi→ATP rate do not index the rate of oxidative phosphorylation in cardiac and skeletal muscles. Am J Physiol Cell Physiol 2011;301:C1–C11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balaban RS, Koretsky AP. Interpretation of ³¹P NMR saturation transfer experiments: what you can’t see might confuse you. Focus on “Standard magnetic resonance-based measurements of the Pi→ATP rate do not index the rate of oxidative phosphorylation in cardiac and skeletal muscles”. Am J Physiol Cell Physiol 2011;301:C12–C15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brindle KM. NMR methods for measuring enzyme kinetics in vivo. Prog Nucl Magn Reson Spectrosc 1988;20:257–293 [Google Scholar]
- 43.Kemp GJ, Taylor DJ, Thompson CH, et al. Quantitative analysis by 31P magnetic resonance spectroscopy of abnormal mitochondrial oxidation in skeletal muscle during recovery from exercise. NMR Biomed 1993;6:302–310 [DOI] [PubMed] [Google Scholar]
- 44.Brindle KM, Radda GK. 31P-NMR saturation transfer measurements of exchange between Pi and ATP in the reactions catalysed by glyceraldehyde-3-phosphate dehydrogenase and phosphoglycerate kinase in vitro. Biochim Biophys Acta 1987;928:45–55 [DOI] [PubMed] [Google Scholar]
- 45.Brehm A, Krssak M, Schmid AI, Nowotny P, Waldhäusl W, Roden M. Increased lipid availability impairs insulin-stimulated ATP synthesis in human skeletal muscle. Diabetes 2006;55:136–140 [PubMed] [Google Scholar]
- 46.Szendroedi J, Schmid AI, Chmelik M, et al. Skeletal muscle phosphodiester content relates to body mass and glycemic control. PLoS ONE 2011;6:e21846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol 1999;277:E1130–E1141 [DOI] [PubMed] [Google Scholar]
- 48.Mandarino LJ, Consoli A, Jain A, Kelley DE. Differential regulation of intracellular glucose metabolism by glucose and insulin in human muscle. Am J Physiol 1993;265:E898–E905 [DOI] [PubMed] [Google Scholar]
- 49.Mandarino LJ, Consoli A, Jain A, Kelley DE. Interaction of carbohydrate and fat fuels in human skeletal muscle: impact of obesity and NIDDM. Am J Physiol 1996;270:E463–E470 [DOI] [PubMed] [Google Scholar]
- 50.Kelley DE, Mandarino LJ. Hyperglycemia normalizes insulin-stimulated skeletal muscle glucose oxidation and storage in noninsulin-dependent diabetes mellitus. J Clin Invest 1990;86:1999–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kelley DE, Reilly JP, Veneman T, Mandarino LJ. Effects of insulin on skeletal muscle glucose storage, oxidation, and glycolysis in humans. Am J Physiol 1990;258:E923–E929 [DOI] [PubMed] [Google Scholar]
- 52.van den Broek NM, Ciapaite J, Nicolay K, Prompers JJ. Comparison of in vivo postexercise phosphocreatine recovery and resting ATP synthesis flux for the assessment of skeletal muscle mitochondrial function. Am J Physiol Cell Physiol 2010;299:C1136–C1143 [DOI] [PubMed] [Google Scholar]
- 53.Nabuurs C, Huijbregts B, Wieringa B, Hilbers CW, Heerschap A. 31P saturation transfer spectroscopy predicts differential intracellular macromolecular association of ATP and ADP in skeletal muscle. J Biol Chem 2010;285:39588–39596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor DJ, Coppack SW, Cadoux-Hudson TA, et al. Effect of insulin on intracellular pH and phosphate metabolism in human skeletal muscle in vivo. Clin Sci (Lond) 1991;81:123–128 [DOI] [PubMed] [Google Scholar]
- 55.Steenge GR, Lambourne J, Casey A, Macdonald IA, Greenhaff PL. Stimulatory effect of insulin on creatine accumulation in human skeletal muscle. Am J Physiol 1998;275:E974–E979 [DOI] [PubMed] [Google Scholar]
- 56.Holloszy JO. Skeletal muscle “mitochondrial deficiency” does not mediate insulin resistance. Am J Clin Nutr 2009;89:463S–466S [DOI] [PubMed] [Google Scholar]
- 57.Kemp GJ, Radda GK. Quantitative interpretation of bioenergetic data from 31P and 1H magnetic resonance spectroscopic studies of skeletal muscle: an analytical review. Magn Reson Q 1994;10:43–63 [PubMed] [Google Scholar]
- 58.Brand MD. Regulation analysis of energy metabolism. J Exp Biol 1997;200:193–202 [DOI] [PubMed] [Google Scholar]
- 59.Pagel-Langenickel I, Bao J, Pang L, Sack MN. The role of mitochondria in the pathophysiology of skeletal muscle insulin resistance. Endocr Rev 2010;31:25–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johannsen DL, Ravussin E. The role of mitochondria in health and disease. Curr Opin Pharmacol 2009;9:780–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Irving BA, Short KR, Nair KS, Stump CS. Nine days of intensive exercise training improves mitochondrial function but not insulin action in adult offspring of mothers with type 2 diabetes. J Clin Endocrinol Metab 2011;96:E1137–E1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scheuermann-Freestone M, Madsen PL, Manners D, et al. Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation 2003;107:3040–3046 [DOI] [PubMed] [Google Scholar]
- 63.Schrauwen-Hinderling VB, Kooi ME, Hesselink MK, et al. Impaired in vivo mitochondrial function but similar intramyocellular lipid content in patients with type 2 diabetes mellitus and BMI-matched control subjects. Diabetologia 2007;50:113–120 [DOI] [PubMed] [Google Scholar]
- 64.Meex RC, Schrauwen-Hinderling VB, Moonen-Kornips E, et al. Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes 2010;59:572–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bajpeyi S, Pasarica M, Moro C, et al. Skeletal muscle mitochondrial capacity and insulin resistance in type 2 diabetes. J Clin Endocrinol Metab 2011;96:1160–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.De Feyter HM, van den Broek NM, Praet SF, Nicolay K, van Loon LJ, Prompers JJ. Early or advanced stage type 2 diabetes is not accompanied by in vivo skeletal muscle mitochondrial dysfunction. Eur J Endocrinol 2008;158:643–653 [DOI] [PubMed] [Google Scholar]
- 67.Sleigh A, Stears A, Thackray K, et al. Mitochondrial oxidative phosphorylation is impaired in patients with congenital lipodystrophy. J Clin Endocrinol Metab 2012;97:E438–E442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Praet SF, De Feyter HM, Jonkers RA, et al. 31P MR spectroscopy and in vitro markers of oxidative capacity in type 2 diabetes patients. MAGMA 2006;19:321–331 [DOI] [PubMed] [Google Scholar]
- 69.Muoio DM. Intramuscular triacylglycerol and insulin resistance: guilty as charged or wrongly accused? Biochim Biophys Acta 2010;1801:281–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Szendroedi J, Phielix E, Roden M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol 2012;8:92–103 [DOI] [PubMed] [Google Scholar]
- 71.Brindle KM, Radda GK. Measurements of exchange in the reaction catalysed by creatine kinase using 14C and 15N isotope labels and the NMR technique of saturation transfer. Biochim Biophys Acta 1985;829:188–201 [DOI] [PubMed] [Google Scholar]
- 72.Kupriyanov VV, Balaban RS, Lyulina NV, Steinschneider AYa, Saks VA. Combination of 31P-NMR magnetization transfer and radioisotope exchange methods for assessment of an enzyme reaction mechanism: rate-determining steps of the creatine kinase reaction. Biochim Biophys Acta 1990;1020:290–304 [DOI] [PubMed] [Google Scholar]
- 73.Brehm A, Krssák M, Schmid AI, Nowotny P, Waldhäusl W, Roden M. Acute elevation of plasma lipids does not affect ATP synthesis in human skeletal muscle. Am J Physiol Endocrinol Metab 2010;299:E33–E38 [DOI] [PubMed] [Google Scholar]
- 74.Bangsbo J, Graham TE, Kiens B, Saltin B. Elevated muscle glycogen and anaerobic energy production during exhaustive exercise in man. J Physiol 1992;451:205–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gibala MJ, MacLean DA, Graham TE, Saltin B. Tricarboxylic acid cycle intermediate pool size and estimated cycle flux in human muscle during exercise. Am J Physiol 1998;275:E235–E242 [DOI] [PubMed] [Google Scholar]
- 76.Bangsbo J, Gollnick PD, Graham TE, et al. Anaerobic energy production and O2 deficit-debt relationship during exhaustive exercise in humans. J Physiol 1990;422:539–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rådegran G, Hellsten Y. Adenosine and nitric oxide in exercise-induced human skeletal muscle vasodilatation. Acta Physiol Scand 2000;168:575–591 [DOI] [PubMed] [Google Scholar]
- 78.Bangsbo J, Krustrup P, González-Alonso J, Boushel R, Saltin B. Muscle oxygen kinetics at onset of intense dynamic exercise in humans. Am J Physiol Regul Integr Comp Physiol 2000;279:R899–R906 [DOI] [PubMed] [Google Scholar]
- 79.Andres R, Cader G, Zierler KL. The quantitatively minor role of carbohydrate in oxidative metabolism by skeletal muscle in intact man in the basal state; measurements of oxygen and glucose uptake and carbon dioxide and lactate production in the forearm. J Clin Invest 1956;35:671–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Colier WN, Meeuwsen IB, Degens H, Oeseburg B. Determination of oxygen consumption in muscle during exercise using near infrared spectroscopy. Acta Anaesthesiol Scand 1995;107(Suppl.):151–155 [DOI] [PubMed] [Google Scholar]
- 81.Abozguia K, Phan TT, Shivu GN, et al. Reduced in vivo skeletal muscle oxygen consumption in patients with chronic heart failure—a study using Near Infrared Spectrophotometry (NIRS). Eur J Heart Fail 2008;10:652–657 [DOI] [PubMed] [Google Scholar]
- 82.Blomstrand E, Rådegran G, Saltin B. Maximum rate of oxygen uptake by human skeletal muscle in relation to maximal activities of enzymes in the Krebs cycle. J Physiol 1997;501:455–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marcinek DJ, Schenkman KA, Ciesielski WA, Conley KE. Mitochondrial coupling in vivo in mouse skeletal muscle. Am J Physiol Cell Physiol 2004;286:C457–C463 [DOI] [PubMed] [Google Scholar]