Abstract
Interpretation of clinical trials to alter the decline in β-cell function after diagnosis of type 1 diabetes depends on a robust understanding of the natural history of disease. Combining data from the Type 1 Diabetes TrialNet studies, we describe the natural history of β-cell function from shortly after diagnosis through 2 years post study randomization, assess the degree of variability between patients, and investigate factors that may be related to C-peptide preservation or loss. We found that 93% of individuals have detectable C-peptide 2 years from diagnosis. In 11% of subjects, there was no significant fall from baseline by 2 years. There was a biphasic decline in C-peptide; the C-peptide slope was −0.0245 pmol/mL/month (95% CI −0.0271 to −0.0215) through the first 12 months and −0.0079 (−0.0113 to −0.0050) from 12 to 24 months (P < 0.001). This pattern of fall in C-peptide over time has implications for understanding trial results in which effects of therapy are most pronounced early and raises the possibility that there are time-dependent differences in pathophysiology. The robust data on the C-peptide obtained under clinical trial conditions should be used in planning and interpretation of clinical trials.
The natural history of type 1 diabetes (T1D) is arguably better understood than that of many other autoimmune diseases, with decades of studies describing the disease course before and after diagnosis. In addition to providing the backdrop for studies to understand the etiopathology of T1D, such information is critical for the design and interpretation of clinical trials to alter the progression of the disease.
The generally understood picture postdiagnosis is of an inevitable fall in β-cell function within a few years after diagnosis, and many studies point to variables that affect the rate of fall, such as glycemic control, HLA type, age at diagnosis, and BMI.
There are several reasons to reexamine the natural history of β-cell function. In recent years, changes in diabetes management and consequent improvement of glycemic control have occurred. In addition, several studies point to a changing HLA distribution in patients with diabetes (1,2), and the overall population generally has had an increase in BMI (3,4). More provocative, there are increasing reports of persistence of C-peptide in individuals long after diagnosis (5) and emerging information from autopsy studies (6) that suggest that the current paradigm should be reconsidered. Furthermore, careful scrutiny of placebo groups from published clinical trials demonstrates major inconsistencies in changes in C-peptide over time between studies, which may be attributable to characteristics of the study populations, procedures used to assess β-cell function, and/or analytic methods used for reporting data.
Type 1 Diabetes TrialNet is an international, multicenter clinical trial network designed to alter the course of T1D, either before diagnosis (prevention) or after clinical diagnosis, with the aim of prolonging β-cell functional survival. As of 2011, TrialNet had acquired data up to 24 months from diagnosis from three clinical trials in individuals with recently diagnosed T1D (7–9). These studies all had similar entry criteria, standardized approaches to diabetes management, and defined protocols for assessment of β-cell function that are highly reproducible (10). In this analysis, we take advantage of this robust dataset to describe the natural history of β-cell function in individuals through 2 years postrandomization, assess the degree of variability between patients, and investigate factors that may be related to C-peptide at and postdiagnosis.
RESEARCH DESIGN AND METHODS
Data from 191 subjects were included in this analysis, including all subjects from one study in which the intervention had no effect on β-cell function (7) and the placebo-treated subjects from two other studies (8,9). Inclusion of the actively treated subjects from the negative study had no appreciable impact on the results (see below). All subjects or their parents gave written informed consent and assent as appropriate prior to participation in these studies. Pertinent study entry criteria for randomization include peak C-peptide during mixed-meal tolerance test (MMTT) ≥0.2 pmol/mL, positive for at least one diabetes autoantibody, diagnosis of T1D within the previous 100 days, and age 7–45 years. To ensure metabolic stability, no MMTTs were performed before 21 days after diagnosis. The mean (median) time from diagnosis to MMTT was 79 (55) days. The study protocol called for subjects to be contacted by the study team every 2 weeks to evaluate and make recommendations to ensure tight glycemic control. The mean HbA1c at 1 and 2 years was 7.3 (±1.5) and 7.6% (±1.5), respectively.
MMTT.
As previously described (10), subjects underwent a 2- or 4-h MMTT under the following circumstances: tests were started before 10 a.m., fasting glucose was required to be 70–200 mg/dL, and long-acting insulin was permitted but no short acting insulin (including no pump bolus) was allowed within 2 h before start of test. A standard preparation of fat, carbohydrate, and protein (Boost-HP; Nestle Health Care Nutrition, Inc.) was used at a dose of 6 mg/kg to a maximum of 360 mL.
Analysis.
Full details of analyses are presented in the Supplementary Material. Key points are summarized here.
C-peptide values recorded as below “lower limit of detection” (LLD) were assigned the value of one-half the LLD. C-peptide area under the curve (AUC) was calculated using the trapezoidal method. Areas were then divided by the time period of the test, 120 or 240 min as indicated. Undetectable C-peptide on MMTT was defined as all timed values on the MMTT below the LLD.
Maintenance of C-peptide.
Subjects were classified as having maintained C-peptide over time if there was no change from baseline to each of the time points after baseline. To account for statistical variation in C-peptide measurements, we used three definitions of maintained C-peptide that are suggested by two published studies. All three definitions are similar in that they each consider no change or an increase from baseline to represent a positive response. They differ in the amount of decrease they allow for a subject to still be classified as having maintained C-peptide:
“Percentage Change” definition of maintenance of C-peptide: Subject whose follow-up C-peptide value is no more than 7.5% below baseline. This was defined as “responder” to therapy in work published by Herold et al. (11).
“Inter-test Variability” definition of maintenance of C-peptide: The MMTT/GST (glucagon stimulation test) (10) was a test-retest study comparing C-peptide measurements between the MMTT and the GST. In the MMTT/GST study, individuals underwent two MMTT within a 1-month period. We used the MMTT data from that study to estimate inter-test variability of C-peptide determined by MMTT. In the current analysis, someone whose change from baseline was either nonnegative or if negative, was no more than 1 inter-test SD below baseline, was defined as having maintained C-peptide.
“CV” definition of maintenance of C-peptide: Using MMTT data from the MMTT/GST study, the median coefficient of variation (CV) among the subjects in that study was determined. In the current analysis, the CV for each subject was computed between the baseline and each of the time points (see Supplementary Material for details). A subject was then classified as having maintained C-peptide whenever his or her change from baseline was either nonnegative or if negative, represented a CV less than the median CV found in the MMTT/GST study.
We also evaluated the occurrence of an increase in C-peptide that is beyond the amount expected from simple inter-test variability (i.e., an increase of at least 1 inter-test SD from baseline).
Associations between C-peptide measures with baseline covariates were assessed with Spearman correlation for continuous covariates and with ANOVA for categorical covariates. The time course of the proportion of subjects with detectable C-peptide values and the proportion of subjects with peak C-peptide ≥0.2 pmol/mL were estimated with the Kaplan-Meier method.
The relationship of C-peptide across time with baseline covariates was evaluated with mixed linear models and, thus, slopes relating C-peptide and time were estimated for each individual subject. Slopes of C-peptide versus time were estimated for subgroups using the mean slope of individuals in that group and the estimates displayed in fan plots. The joint influence of baseline covariates on the longitudinal change in C-peptide was assessed with multivariable models. Only covariates that were found significant (P < 0.05) in univariable analysis were included in the multivariable models. Piecewise linear regression was used to test the data-driven hypothesis that the slope of C-peptide AUC was the same before and after 1 year postbaseline (12). Slopes unadjusted for covariates were compared between the three end points (fasting, peak, and AUC) using the multivariate analysis described in the Supplementary Material.
Our study combined data from placebo- and active-treated subjects from the negative Type 1 Diabetes TrialNet MMF/DZB study to increase the number of observations. To evaluate whether inclusion of the actively treated MMF/DZB subjects significantly altered our findings, we replicated the multivariate analyses using a variable to indicate treatment in the MMF/DZB study. This variable was never statistically significant at the P < 0.05 level. In addition, the novel observation of a biphasic fall of C-peptide (described below) was present with or without the actively treated subjects.
RESULTS
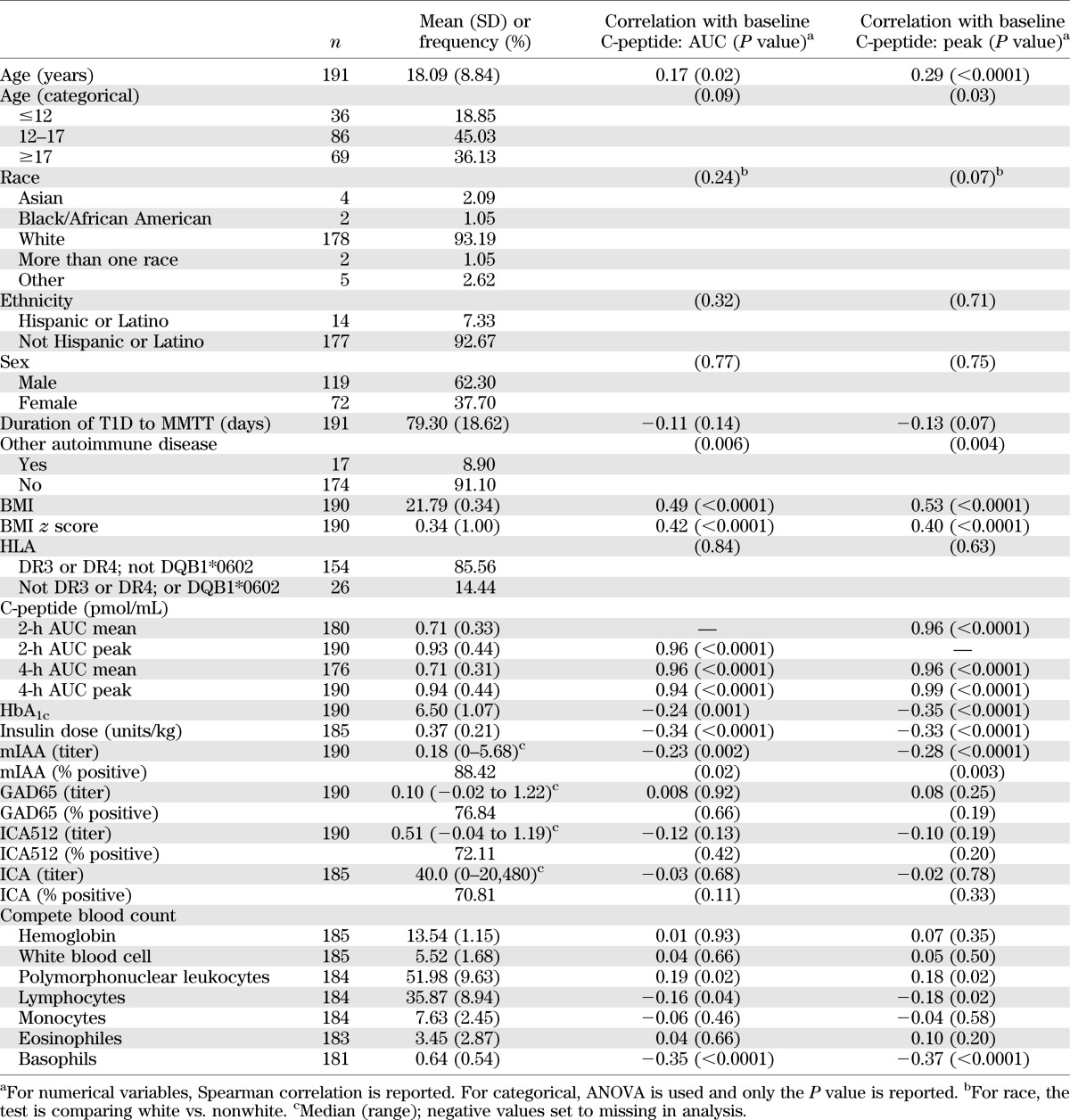
The demographic and baseline characteristics of the 191 subjects included in this analysis are shown in Table 1. Correlations of baseline characteristics with the peak and AUC C-peptide values from their baseline MMTT are also noted in Table 1. Baseline variables that were significantly associated with both peak and AUC C-peptide included age, presence of another autoimmune disease, HbA1c, insulin dose (units/kg/day), BMI and BMI z score, micro–insulin autoantibody (mIAA) positivity, mIAA titer, and basophils.
TABLE 1.
Demographic and baseline characteristics and correlations with peak and AUC C-peptide from baseline MMTT
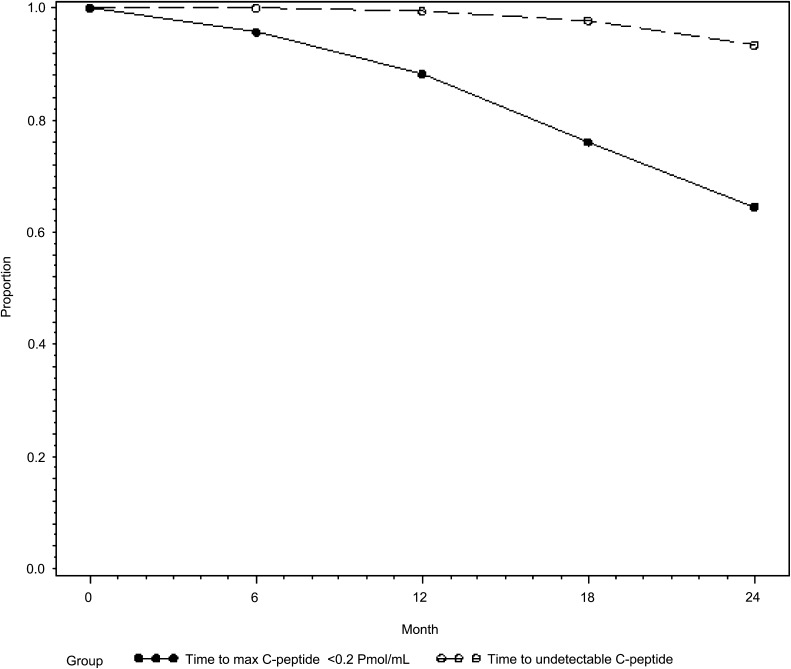
At entry, all individuals were required to have a peak C-peptide ≥0.2 pmol/mL. Over time, as shown in Fig. 1, only 1% of individuals had undetectable C-peptide at 12 months after study entry and 7% had undetectable C-peptide at 24 months. Post hoc analysis of Diabetes Control and Complications Trial (DCCT) data has suggested that individuals with a peak C-peptide on MMTT ≥0.2 pmol/mL were less likely to have progression of retinopathy and severe hypoglycemia than those with less than that value (13). We therefore evaluated the time until individuals reached this value. As shown in Fig. 1, 88% of individuals continued to have peak C-peptide ≥0.2 pmol/mL at 12 months of follow-up and 66% at 24 months.
FIG. 1.
Percent of individuals with detectable C-peptide and C-peptide ≥0.2 pmol/mL over time.
We also evaluated changes of three end points from the MMTT (fasting C-peptide, peak C-peptide, and AUC C-peptide) during the 2 years of the study. Comparisons between peak, AUC, and change from baseline using values from the 4- and 2-h tests showed extremely high concordance (Supplementary Table 1). Since, by protocol, there were fewer 4-h tests performed, results from the 2-h MMTT were used in subsequent analyses. The slope of the regression of each end point on time, measured in months from the start of the study, was determined. Fasting C-peptide fell at a rate of −0.0076 ± 0.0007, peak C-peptide at −0.0222 ± 0.0010, and AUC C-peptide at −0.0172 ± 0.0007 pmol/mL/month. These slopes were each significantly different from each other (P < 0.001), with fasting C-peptide having the smallest change over time (Supplementary Table 2).
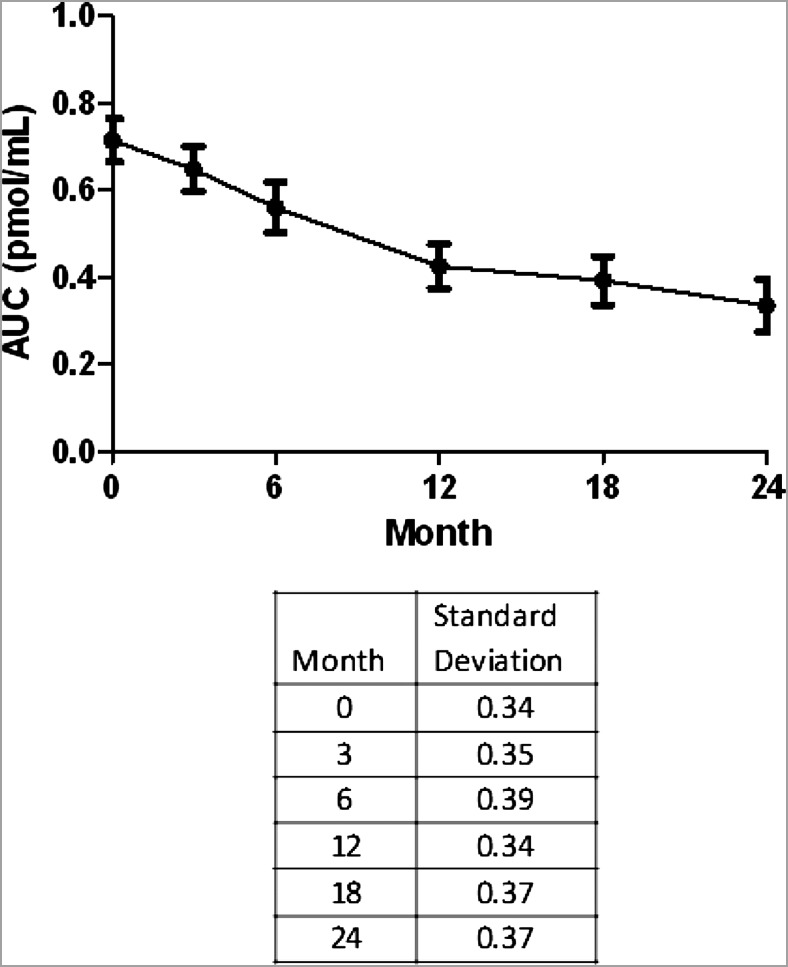
Examination of the plots of the mean AUC C-peptide over time suggested a biphasic decline with differences in the slope before and after 12 months (Fig. 2). This difference was not explained by inclusion of individuals who lost C-peptide during the study period because truncating either those subjects or their data points below the LLD did not affect this pattern. We further explored this data-driven hypothesis using piecewise linear regression and found that the decline after 12 months was significantly slower than that seen during the first 12 months and that this was not affected by age. The slope of the AUC C-peptide before 12 months was estimated to be −0.0245 pmol/mL/month (95% CI −0.0271 to −0.0215) as compared with a slope of −0.0079 (−0.0113 to −0.0050) from 12 to 24 months (P < 0.001). In a similar manner, the slope of the peak C-peptide before 12 months was −0.0318 (−0.0357 to −0.0278) as compared with a slope of −0.0089 (−0.0136 to −0.0046) from 12 to 24 months (P < 0.001). The slope of fasting C-peptide before 12 months was −0.03322 (−0.03984 to −0.02659) and after 12 months was −0.00867 (−0.01626 to −0.00108) (P < 0.001).
FIG. 2.
Biphasic decline of mean AUC C-peptide over time.
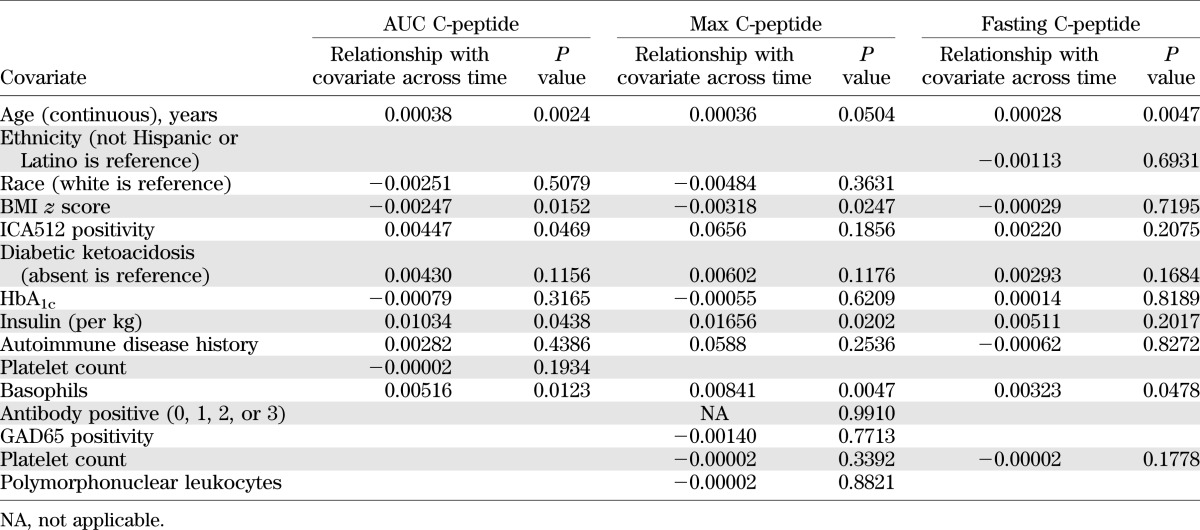
Inspection of each individual’s C-peptide data over time shows the variability in slopes between individuals (Supplementary Fig. 1). We then evaluated baseline factors that may contribute to these differences in C-peptide slope (Supplementary Table 3). Notable variables not found to be associated with C-peptide over time included Tanner stage and class II HLA type categorized as DR3 or DR4 and not DQB1*0602 or otherwise. Those variables found to be significantly associated were then evaluated in a multivariable model. As shown in Table 2, only age and number of basophils impacted C-peptide over time whether AUC, peak, or fasting C-peptide was used. Additional baseline variables significantly associated with stimulated measures only (AUC and peak) were BMI z score and insulin dose.
TABLE 2.
Multivariable analysis of baseline characteristics associated with fasting, peak, and AUC C-peptide over time
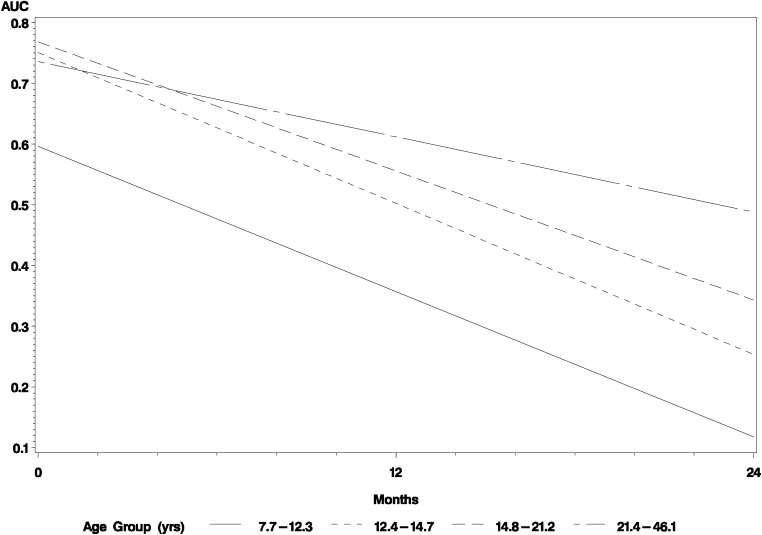
We further explored the effect of age on C-peptide over time by grouping individuals according to quartiles according to age, taking the mean slope estimated by the multivariable model of AUC C-peptide. As shown in Fig. 3, the youngest children (aged 7.7–12.3 years) started with lower C-peptide values at their initial MMTT compared with the other subjects. The three older quartiles (aged 12.4–46.1 years) were not different at their initial MMTT. In contrast, the slopes of C-peptide over time showed remarkable concordance in children among the three youngest groups, distinct from that of subjects in the oldest quartile.
FIG. 3.
Model-based estimates of average slopes of AUC C-peptide over time according to age quartiles.
Additional exploratory analyses, such as considering the C-peptide in relationship to the simultaneous glucose value, neither improved the model nor changed the impact of previously identified variables on the outcome measure.
We then evaluated time-related patterns of C-peptide preservation, using the classification of subjects as having maintained C-peptide or not, separately for each of the three definitions introduced in research design and methods. As shown in Table 3, large numbers of subjects could be considered as having maintained C-peptide by any definition if all tests are taken into consideration. When limited to comparing the baseline with the 1- or 2-year values alone, 33 (17%) and 21 (11%) maintained C-peptide as defined by the CV definition, respectively, with similar values for the other definitions as described in research design and methods. Multivariable analysis found that age significantly increases, and the presence of islet cell antigen (ICA)512 antibody significantly decreases, the probability of having maintained C-peptide by the CV definition at 2 years (odds ratio age 1.067 [95% CI 1.026–1.109]; ICA512 0.329 [0.162–0.667]).
TABLE 3.
Maintenance of C-peptide
In a similar way, we also investigated time-related patterns of subjects who had strictly an increase in C-peptide of at least 1 inter-test difference SD. There were 55 of 191 subjects who experienced at least one such increase during the 2-year period. In a multivariable model, baseline predictors that enhanced the probability of this increase were increased age, GAD65 antibody positivity, and Tanner stage. Those that decreased the probability of an increase in C-peptide were insulin dose and mIAA and ICA512 positivity and titers.
DISCUSSION
Loss of insulin secretion is the fundamental defect of T1D, with natural history studies showing a decline before and continuing after clinical diagnosis until a complete absence of endogenous secretion occurs. This natural history serves as the underlying rationale for studies to halt β-cell destruction. Slowing or stopping this loss of insulin secretion prior to diagnosis would delay or prevent clinical onset of disease. Retention of endogenous insulin secretion after diagnosis has been associated with clinical benefits as shown by the DCCT, in which those with preserved function obtained the benefit of intensive therapy (e.g., reduction of retinopathy) with less risk of severe hypoglycemia (14), and in islet transplant studies, in which there is a reduction in hypoglycemia even when insulin independence is not achieved (15).
Our data show that 93% of individuals who started with C-peptide ≥0.2 pmol/mL have detectable C-peptide 2 years from diagnosis, and ∼66% are above the DCCT C-peptide threshold associated with clinical benefit. Furthermore, in contrast to both peak and AUC measures, there is limited change in fasting C-peptide during 2 years from study entry, suggesting that use of nonstimulated C-peptide as an outcome in clinical trials would make it a difficult end point to demonstrate benefit of an intervention therapy.
Most important, our data indicate that the rate of fall is not constant from study entry during the next 2 years. Our data show a biphasic fall during this time; however, with the limited number of fixed time points studied, we cannot exclude the possibility that the true relationship of C-peptide over time is polyphasic. In this regard, it is worthwhile to recall that the baseline MMTT in TrialNet studies is not performed until at least 21 days after diagnosis and that the median time from diagnosis for the initial studies was 55 days; thus, we have no information about the rate of fall from the time of diagnosis to the start of our study. The rate of fall in C-peptide from ∼2 months until 14 months from diagnosis (e.g., 0–12 months from study start) is distinct from the rate of fall from 14 to 26 months after diagnosis (e.g., 12–24 months after study start). This data suggests that there may be metabolic or immunological factors that differ soon after diagnosis rather than later. This hypothesis would need to be explored further. With the caveat that MMTT- and oral glucose tolerance test (OGTT)-stimulated C-peptide are not equivalent (16), it is of interest to note that Diabetes Prevention Trial–Type 1 (DPT-1) data show little change in OGTT-stimulated C-peptide during the 30 months before diagnosis, while a marked fall in peak C-peptide occurred during the peridiagnosis period (17,18). Further insights are likely to come from measuring C-peptide responses under standardized conditions in individuals as they progress from pre- to postdiagnosis. It is also important to note that for these studies, virtually all patients had T1D diagnosed in the community versus the diagnosis of usually asymptomatic T1D from surveillance OGTTs every 6 months in DPT-1 and TrialNet Natural History and Prevention studies (19–21). How the fall in C-peptide would compare after these two very different methods of diagnosis is unknown but of major interest.
It is important to consider these data in the context of results of clinical trials that have preserved β-cell function. In these studies, the differences between the slopes (i.e., rate of fall) of C-peptide between placebo and treatment groups were most pronounced early, with the slopes appearing similar after this initial period (8,9,22,23). The data presented in our analysis raise two hypotheses: 1) the intervention may only affect the metabolic or immunologic factors soon after diagnosis or 2) the rate of fall further from diagnosis may be too flat to detect an effect of intervention.
Our data also emphasize the variation in the rate of fall of C-peptide over time and demonstrate that more than one-third of subjects were “nonprogressors” experiencing an increase, no change, or fall within the median CV of the MMTT at least once in the 2 years of study. Approximately 1 in 6 subjects meet this definition at 1 year and >1 in 10 subjects at 2 years after randomization. As a consequence, one must be cautious about interpretation of small trials or in drawing clinical conclusions from a single phase 2 study.
Similar to previous studies, we found that age, insulin use at baseline, and BMI z score at baseline are significantly associated with AUC and peak C-peptide over time in multivariable analysis (24–26). It is notable that variables such as class II HLA type as defined with three categories, Tanner stage, and antibodies (number, positive or not, and titer) that have been reported in previous studies to influence rate of β-cell function decline (27–30) were not significantly associated with AUC or peak C-peptide over time in our multivariable model. Further work to define the role of genotype in disease progression is ongoing, including more detailed analysis of HLA class I and II.
We were particularly interested in age both as a consideration for future study design and to explore the question about whether there are differences in disease progression across the ages studied. The rate of decline of C-peptide was similar in individuals aged 7 to 21 and greater than in subjects older than 21 years. However, the youngest subjects (aged 7 to 12) started with lower C-peptide than older subjects aged 12 to 46 years. This observation is consistent with clinical impressions that younger individuals frequently have low levels of C-peptide but does not support the idea that disease progression is different in younger versus older children or that pubertal status influences the disease process. A major caveat to this conclusion, however, is that the youngest subjects in this study were age 7. Thus, our data do not permit any conclusions about younger children. The different baseline C-peptide level in the youngest group is consistent with the use of an age-dependent percentile determination of intravenous glucose tolerance test data that was used for assessing risk in DPT-1 (31). Unfortunately, little information is available on normal stimulated C-peptide values in young children, a gap that limits our ability to interpret the data in individuals with disease. Our data clearly suggest differences in disease progression between children and adults, pointing to areas ripe for further investigation and also emphasizing that a larger sample size may be required to demonstrate a clinical effect of a drug if only adults are studied. These older patients were diagnosed as T1D by their providers. How their decline in β-cell function would compare with so-called late autoimmune diabetes in adult patients is unknown but also very important for potential intervention trials.
The novel observation of the association of basophils with change in C-peptide over time was unexpected. Indeed, we initially evaluated white blood cell differentials to examine how lymphopenia might relate to clinical course. While historically conceptualized as related to mast cells whose release of interleukin (IL)-4 and IL-13 may affect allergy and protection from parasites, as recently reviewed (32), basophils have multiple roles in the immune response, including antibody production, serving as antigen presenting cells, and amplifying the memory response among others. Since higher basophil levels were associated with a less rapid fall in C-peptide over time, it is tempting to speculate that this association is based on a compensatory response to autoimmunity—attempting to deviate cells via IL-4 cytokine release. However, this association may also have occurred by chance and requires replication in another dataset.
TrialNet studies were conducted at a limited number of clinical centers, with close monitoring of glycemic control, using a standardized approach to MMTT (with regard to meals, insulin dosing, baseline glucose value, and time of day), and centralized laboratories with quality assurance programs. As such, combined data from these studies provide a contemporaneous description of the natural history of C-peptide under clinical trial conditions during the first 2 years after randomization in a context by which effects of intervention can be considered and future studies designed. Since hyperglycemia itself has been associated with poor β-cell function (14), the rate of fall seen in these studies in which individuals had a mean HbA1c of 6.5% on entry and 7.6% at 2 years may not reflect the natural history of disease under usual clinical care. In addition, many of the observations reported must be considered hypothesis generating and require confirmation in additional datasets. To this end, TrialNet will soon use data from two additional studies: one using GAD65-alum (33) and the other involving canakinumb to test the associations observed in the trials used for the current analyses. These studies are being conducted under identical circumstances with the notable exception that subjects as young as age 3 are included in the GAD65 study, thus providing comparative data in this important age-group.
Supplementary Material
ACKNOWLEDGMENTS
The sponsor of the trial was the Type 1 Diabetes TrialNet Study Group. Type 1 Diabetes TrialNet Study Group is a clinical trials network funded by the National Institutes of Health (NIH) through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, through Cooperative Agreements U01-DK-061010, U01-DK-061016, U01-DK-061034, U01-DK-061036, U01-DK-061040, U01-DK-061041, U01-DK-061042, U01-DK-061055, U01-DK-061058, U01-DK-084565, U01-DK-085453, U01-DK-085461, U01-DK-085463, U01-DK-085466, U01-DK-085499, U01-DK-085505, U01-DK-085509, and Contract HHSN267200800019C, the National Center for Research Resources, through Clinical Translational Science Awards UL1-RR-024131, UL1-RR-024139, UL1-RR-024153, UL1-RR-024975, UL1-RR-024982, UL1-RR-025744, UL1-RR-025761, UL1-RR-025780, UL1-RR-029890, UL1-RR-031986, and General Clinical Research Center Award M01-RR-00400, the Juvenile Diabetes Research Foundation International (JDRF), and the American Diabetes Association (ADA).
No potential conflicts of interest relevant to this article were reported.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH, JDRF, or ADA.
All authors are members of the Type 1 Diabetes TrialNet Study Group and, as such, contributed to data used in this article. C.J.G. and C.A.B. wrote the manuscript. D.B., S.E.G., P.A.G., K.C.H., J.M.L., P.M., J.P.P., M.D.P., H.K.-S., J.S.S., and J.M.S. contributed to discussion and reviewed and edited the manuscript. C.J.G. and C.A.B. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-1538/-/DC1.
†Deceased.
A complete list of the Type 1 Diabetes TrialNet Study Group can be found in the Supplementary Data online.
REFERENCES
- 1.Fourlanos S, Varney MD, Tait BD, et al. The rising incidence of type 1 diabetes is accounted for by cases with lower-risk human leukocyte antigen genotypes. Diabetes Care 2008;31:1546–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hermann R, Knip M, Veijola R, et al. FinnDiane Study Group . Temporal changes in the frequencies of HLA genotypes in patients with type 1 diabetes—indication of an increased environmental pressure? Diabetologia 2003;46:420–425 [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll M. Prevalence of overweight, obesity, and extreme obesity among adults: United States, trends 1960–1962 through 2007–2008 [article online], 2010. Available from www.cdc.gov/NCHS/data/hestat/obesity_adult_07_08/obesity_adult_07_08.pdf. Accessed 25 June 2011
- 4.Ogden CL, Carroll M. Prevalence of obesity among children and adolescents: United States, trends 1963–1965 through 2007–2008 [article online], 2010. Available from www.cdc.gov/nchs/data/hestat/obesity_child_07_08/obesity_child_07_08.pdf. Accessed 25 June 2011
- 5.Keenan HA, Sun JK, Levine J, et al. Residual insulin production and pancreatic β-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes 2010;59:2846–2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atkinson MA, Gianani R. The pancreas in human type 1 diabetes: providing new answers to age-old questions. Curr Opin Endocrinol Diabetes Obes 2009;16:279–285 [DOI] [PubMed] [Google Scholar]
- 7.Gottlieb PA, Quinlan S, Krause-Steinrauf H, et al. Type 1 Diabetes TrialNet MMF/DZB Study Group . Failure to preserve beta-cell function with mycophenolate mofetil and daclizumab combined therapy in patients with new-onset type 1 diabetes. Diabetes Care 2010;33:826–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orban T, Bundy B, Becker DJ, et al.; Type 1 Diabetes TrialNet Abatacept Study Group. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet 2011;378:412–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. Type 1 Diabetes TrialNet Anti-CD20 Study Group . Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med 2009;361:2143–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenbaum CJ, Mandrup-Poulsen T, McGee PF, et al. Type 1 Diabetes Trial Net Research Group. European C-Peptide Trial Study Group . Mixed-meal tolerance test versus glucagon stimulation test for the assessment of beta-cell function in therapeutic trials in type 1 diabetes. Diabetes Care 2008;31:1966–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herold KC, Gitelman SE, Masharani U, et al. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes 2005;54:1763–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muggeo VM. Estimating regression models with unknown break-points. Stat Med 2003;22:3055–3071 [DOI] [PubMed] [Google Scholar]
- 13.Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care 2003;26:832–836 [DOI] [PubMed] [Google Scholar]
- 14.The Diabetes Control and Complications Trial Research Group . Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. Ann Intern Med 1998;128:517–523 [DOI] [PubMed] [Google Scholar]
- 15.Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006;355:1318–1330 [DOI] [PubMed] [Google Scholar]
- 16.Greenbaum CJ, Buckingham B, Chase HP, Krischer J, Diabetes Prevention Trial, Type 1 Diabetes (DPT-1) Study Group . Metabolic tests to determine risk for type 1 diabetes in clinical trials. Diabetes Metab Res Rev 2011;27:584–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sosenko JM, Palmer JP, Greenbaum CJ, et al. Patterns of metabolic progression to type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 2006;29:643–649 [DOI] [PubMed] [Google Scholar]
- 18.Sosenko JM, Palmer JP, Rafkin-Mervis L, et al. Glucose and C-peptide changes in the perionset period of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 2008;31:2188–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diabetes Prevention Trial—Type 1 Diabetes Study Group . Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 2002;346:1685–1691 [DOI] [PubMed] [Google Scholar]
- 20.Mahon JL, Sosenko JM, Rafkin-Mervis L, et al. The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes 2009;10:97–104 [DOI] [PubMed] [Google Scholar]
- 21.Skyler JS, Krischer JP, Wolfsdorf J, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial—Type 1. Diabetes Care 2005;28:1068–1076 [DOI] [PubMed] [Google Scholar]
- 22.Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 2002;346:1692–1698 [DOI] [PubMed] [Google Scholar]
- 23.Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 2005;352:2598–2608 [DOI] [PubMed] [Google Scholar]
- 24.Petrone A, Galgani A, Spoletini M, et al. Residual insulin secretion at diagnosis of type 1 diabetes is independently associated with both, age of onset and HLA genotype. Diabetes Metab Res Rev 2005;21:271–275 [DOI] [PubMed] [Google Scholar]
- 25.Greenbaum CJ, Anderson AM, Dolan LM, et al. SEARCH Study Group . Preservation of beta-cell function in autoantibody-positive youth with diabetes. Diabetes Care 2009;32:1839–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmer JP. C-peptide in the natural history of type 1 diabetes. Diabetes Metab Res Rev 2009;25:325–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klipper-Aurbach Y, Wasserman M, Braunspiegel-Weintrob N, et al. Mathematical formulae for the prediction of the residual beta cell function during the first two years of disease in children and adolescents with insulin-dependent diabetes mellitus. Med Hypotheses 1995;45:486–490 [DOI] [PubMed] [Google Scholar]
- 28.Komulainen J, Knip M, Lounamaa R, et al. The Childhood Diabetes in Finland Study Group . Poor beta-cell function after the clinical manifestation of type 1 diabetes in children initially positive for islet cell specific autoantibodies. Diabet Med 1997;14:532–537 [DOI] [PubMed] [Google Scholar]
- 29.Martin S, Pawlowski B, Greulich B, Ziegler AG, Mandrup-Poulsen T, Mahon J. Natural course of remission in IDDM during 1st yr after diagnosis. Diabetes Care 1992;15:66–74 [DOI] [PubMed] [Google Scholar]
- 30.Törn C, Landin-Olsson M, Lernmark A, et al. Prognostic factors for the course of beta cell function in autoimmune diabetes. J Clin Endocrinol Metab 2000;85:4619–4623 [DOI] [PubMed] [Google Scholar]
- 31.Chase HP, Cuthbertson DD, Dolan LM, et al. First-phase insulin release during the intravenous glucose tolerance test as a risk factor for type 1 diabetes. J Pediatr 2001;138:244–249 [DOI] [PubMed] [Google Scholar]
- 32.Karasuyama H, Mukai K, Obata K, Tsujimura Y, Wada T. Nonredundant roles of basophils in immunity. Annu Rev Immunol 2011;29:45–69 [DOI] [PubMed] [Google Scholar]
- 33.Wherrett DK, Bundy B, Becker DJ, et al.; Type 1 Diabetes TrialNet GAD Study Group. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet 2011;378:319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.