Abstract
Type 1 diabetes involves both T helper (Th)1 and Th17 cells. While the mechanisms underlying the control of Th1 cells are relatively well defined, those operating modulation of Th17 cells remain unknown. Moreover, given that Th17 cells are plastic and can drive disease as stable or convertible T cells, effective approaches to counter type 1 diabetes would have to alter Th17 function under both circumstances. Herein, we genetically incorporated the BDC2.5-reactive p79 mimotope into an Ig molecule, and the resulting Ig-p79 was used to investigate Th17 tolerance. Accordingly, diabetogenic BDC2.5 Th17 cells were transferred into NOD mice under convertible or stable conditions and their fate was evaluated upon induction of tolerance and disease suppression by Ig-p79. The findings show that convertible (Th17 to Th1) cells display downregulation of the chemokine (C-X-C motif) receptor 3 that was associated with diminished T-box transcription factor T-bet expression, retention in the spleen, and inhibition of trafficking to the pancreas. In contrast, stable Th17 cells downregulated orphan nuclear receptor ROR-γt but increased Fas ligand expression and died by apoptosis. Thus, the final signature transcription factor shapes the mechanism of tolerance in plastic Th17 cells. These findings suggest that effective strategies against type 1 diabetes will require regimens that could drive both mechanisms of tolerance to overcome the disease.
T helper (Th)1 cells have always been considered the trigger of type 1 diabetes whether in humans or in the nonobese diabetic (NOD) mouse (1–3). However, when the function of the genes encoding interferon (IFN)-γ (4), IFN-γ receptor (5), or the IFN-γ inducer interleukin (IL)-12 (6) were altered, the disease continued to manifest, suggesting that other effector T-cell subsets can sustain diabetes. Recently, it was shown that diabetic mice display elevated IL-17 expression (7–9) and that neutralization of IL-17 inhibited disease progression (9,10), suggesting that Th17 cells can sustain development of type 1 diabetes (11). Therefore, modulation of both Th1 and Th17 cells would be required to contain the disease. Herein, we used the highly pathogenic T-cell receptor transgenic BDC2.5 T cells (12) and devised an effective Ig-based treatment by expressing the library-defined BDC2.5-reactive p79 mimotope (13,14) on an Ig backbone. The resulting Ig-p79 was then used in defined disease transfer models (7,15) to investigate tolerance of convertible and stable Th17 cells. The use of BDC2.5 transgenic T cells is relevant because they have been shown to react with chromogranin A self-antigen (16). Accordingly, BDC2.5 T cells were polarized in vitro and transferred into mice, and the hosts were treated with Ig-p79. The fate of the transferred T cells was then analyzed upon recovery from disease. The results show that Th17 cells transferred into NOD.scid mice converted into Th1 cells as previously reported (15). It is important that the treatment with Ig-p79 suppressed the transferred diabetes by interfering with the expression of the chemokine (C-X-C motif) receptor CXCR3 on the converted Th1 cells. As was reported (7,17), when the Th17 cells were transferred into nonlymphopenic mice, they were unable to convert to Th1 cells. It is notable that the stable Th17 targets underwent apoptosis upon treatment with Ig-p79. Finally, the different fates of Th1 and Th17 cells were tied to their signature transcription factors: downregulation of T-box transcription factor T-bet led to diminished expression of CXCR3, and modulation of orphan nuclear receptor ROR-γt led to increased Fas ligand (FasL) expression.
RESEARCH DESIGN AND METHODS
Mice.
NOD (H-2g7), NOD.scid, NOD.BDC2.5, and BALB/c.IL-12atm1Jm (IL-12p35−/−) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). NOD.FoxP3GFP.DTR mice were generated by breeding C57BL/6.FoxP3.GFP.DTR mice onto the NOD background for seven backcross generations. The NOD.FoxP3GFP.DTR mice were then bred with NOD.BDC2.5 mice to generate NOD.BDC2.5.FoxP3GFP.DTR mice. NOD.scid.IL-12p35−/− mice were generated by breeding BALB/c.IL-12p35−/− mice with NOD.scid mice. All mice were used according to the guidelines of the University of Missouri Animal Care and Use Committee.
Peptides and Ig chimeras.
p79 peptide corresponds to the mimotope (AVRPLWVRME). Hen egg lysozyme (HEL) peptide corresponds to aa residues 11–25 of HEL. Both peptides were purchased from metabion. Ig-p79 expresses p79, and Ig-HEL incorporates HEL peptide. The genetic engineering of p79 peptide into the heavy chain of the 91A3 anti-arsonate antibody and the production of Ig-p79 were accomplished according to methods used for Ig-HEL (18).
T-cell polarization.
Splenic cells (2 × 106/mL) from age 4 to 6 weeks naïve NOD.BDC2.5.FoxP3GFP.DTR mice were stimulated with p79 peptide (0.5 μmol/L) under Th1 or Th17 conditions for 4 days. For polarization into Th1 cells, the stimulation was carried out in the presence of recombinant (r)IL-12 (10 ng/mL; PeproTech) and anti–IL-4 (10 μg/mL [11B11]). For Th17 polarization, the culture was supplemented with recombinant transforming growth factor-β (3 ng/mL; PeproTech), rIL-6 (20 ng/mL; PeproTech), anti–IFN-γ (10 μg/mL [R4–6A4]), and anti–IL-4 (10 μg/mL [11B11]) antibodies and rIL-23 (20 ng/mL; R&D Systems) (only for the last 2 days).
Purification and adoptive transfer of polarized Th1 and Th17 cells.
Splenic cells were polarized under Th1 or Th17 conditions for 4 days. CD4+ T cells were purified by negative selection using CD4 T-cell isolation kit II (Miltenyi Biotec) and stimulated with phorbol myristic acid (PMA; 50 ng/mL) and ionomycin (500 ng/mL) for 2 h. The cells were then labeled using the mouse IFN-γ (allophycocyanin) and IL-17 (phycoethrin) detection kit from Miltenyi Biotec. Subsequently, the enriched Th17 (IL-17+IFN-γ−FoxP3−) and Th1 (IFN-γ+IL-17−FoxP3−) cells were sorted to at least 98% purity on a Beckman Coulter MoFlo XDP sorter and transferred intravenously into NOD.scid (3 × 106 cells per mouse), NOD, or NOD.FoxP3GFP.DTR mice (10 × 106 cells per mouse).
Treatment with Ig-p79 and neutralizing anti-cytokine antibodies.
Ig-p79 and the control Ig-HEL were injected into mice (300 μg/mouse i.p.) a few hours after cell transfer. For in vivo neutralization of IFN-γ or IL-17, the mice were given anti–IFN-γ (R4–6A4), anti–IL-17 (TC11–18H10), or isotype rat IgG1 control (300 μg/injection i.p.) on day 0, 2, 4, and 6 posttransfer.
Retroviral transduction.
The MSCV-CXCR3-IRES-Thy1.1 and empty MSCV-IRES-Thy1.1 vector used to overexpress CXCR3 in polarized Th17 cells were previously described (19). In brief, 293FT cells (Invitrogen) were transfected with either MSCV-CXCR3-IRES-Thy1.1 or MSCV-IRES-Thy1.1 vector along with the retroviral packaging vector (p-ψECO) by calcium chloride. The culture supernatant containing the retroviruses was used to transduce polarized Thy1.2 BDC2.5 Th17 cells. The CD4+ T cells were then isolated and used for transfer experiments.
Depletion of regulatory T cells.
Diphtheria toxin (DT; Sigma-Aldrich) was given to NOD.FoxP3GFP.DTR mice (300 ng/mouse/injection i.p.) on day −2, −1, 2, 5, 8, 11, and 14 after cell transfer.
Intracellular staining.
For detection of IFN-γ and IL-17 in CD4+ T cells from NOD.scid mice, the cells were stimulated with PMA (50 ng/mL) and ionomycin (500 ng/mL) for 2 h in the presence of Brefeldin A (10 μg/mL). For detection of IFN-γ and IL-17 in T cells from NOD or NOD.FoxP3GFP.DTR mice, total splenic cells were stimulated with p79 mimotope (0.5 μmol/L) for 16 h with addition of Brefeldin A (10 μg/mL) for the last 2 h. For flow cytometry analyses, the cells were then stained for surface markers, fixed in 2% formaldehyde, permeabilized with 0.2% saponin, and stained for intracellular cytokines.
For nuclear detection of T-bet and ROR-γt, the cells were stained for surface markers, treated with Fix/Perm buffer (eBioscience), and then stained with anti–T-bet and anti–ROR-γt. Flow cytometry analyses use the Beckman Coulter CyAn ADP and Summit V4.3 software (Dako).
Histology.
Pancreata were frozen in optimal cutting temperature compound at −80°C. Cryosections of 8 μm thickness were cut 200 μm apart to prevent double counting of the same islet. At least three nonserial sections per pancreas were stained with hematoxylin-eosin and analyzed by light microscopy. Insulitis scoring was performed according to the following criteria: intrainsulitis, severe infiltration within the islet; peri-insulitis, infiltration restricted to the periphery of islets; and no insulitis, absence of cell infiltration.
Statistics.
P values were calculated using the two-tailed Student t test. Details for antibodies, pancreatic cell isolation, and quantitative PCR are listed in the Supplementary Data.
RESULTS
Th17-to-Th1 conversion delays transfer of diabetes by polarized Th17 cells.
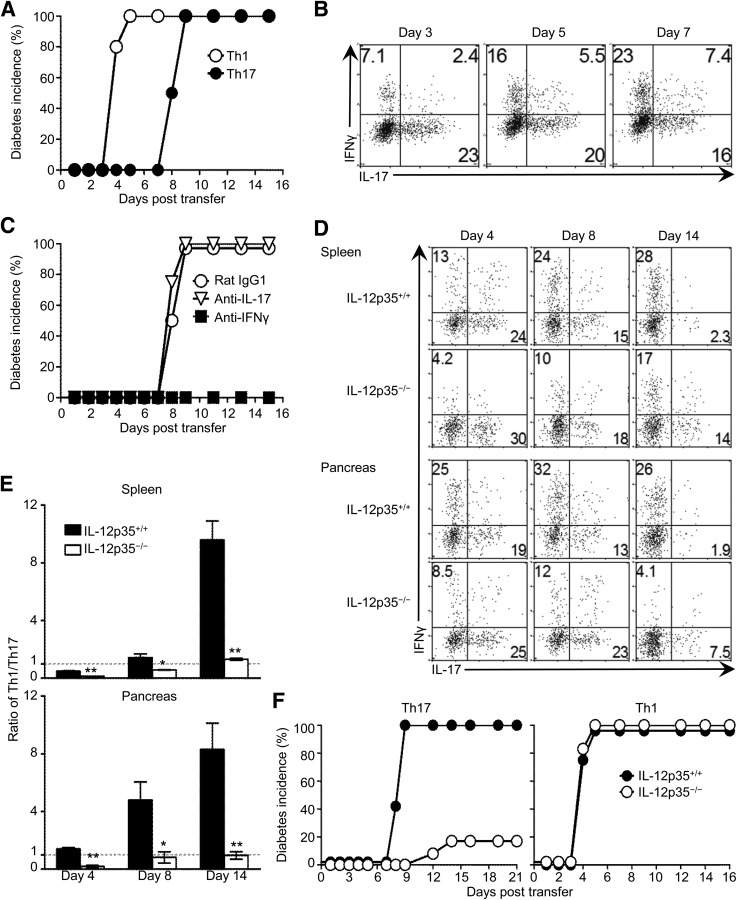
To assess the diabetogenic function of BDC2.5-derived Th1 and Th17 cells, polarized IFN-γ+IL-17− and IL-17+IFN-γ− subsets were sorted (Supplementary Fig. 1) and tested for transfer of diabetes into NOD.scid mice. The results show that Th1 cells induced diabetes within 4 to 5 days posttransfer, while the Th17 cells were delayed and took up to 9 days (Fig. 1A). Since Th17 cells are plastic (7,15,20–22), the time lapse in disease transfer could be related to conversion to Th1 cells. Indeed, despite the thorough depletion of IFN-γ+ contaminants before cell transfer (Supplementary Fig. 1, right panels), a sizable portion of IFN-γ–producing cells was detected in the pancreatic lymph nodes (PLNs) of the host mice on day 3 posttransfer, and the Th1 cells outnumbered Th17 cells (23 vs. 16%, respectively) by day 7 (Fig. 1B). It is important that the Th1 but not the residual Th17 cells are mediating the disease because in vivo neutralization of IFN-γ but not IL-17 abrogated diabetes (Fig. 1C). In fact, conversion from Th17 to Th1 cells is essential for disease development because transfer of the Th17 cells into IL-12p35–deficient (IL-12p35−/−) hosts, an environment that may not favor conversion (15,20,23,24), minimized the frequency of IFN-γ–producing cells and nullified development of diabetes (Fig. 1D–F). Indeed, there was less of a decrease in the frequency of Th17 cells and less of an increase in the percentage of Th1 cells over time in both the spleen and pancreas of IL-12p35−/− mice as compared with IL-12p35+/+ hosts (Fig. 1D), and the Th1-to-Th17 ratio was significantly higher in IL-12p35+/+ versus IL-12p35−/− mice in both organs (Fig. 1E). Moreover, only 20% of the IL-12p35−/− mice developed diabetes post Th17 cell transfer, while 100% of the IL-12p35+/+ littermates developed diabetes after the Th17 cell transfer (Fig. 1F). In contrast, polarized Th1 cells induced a similar pattern of disease in both IL-12p35−/− and IL-12p35+/+ recipients (Fig. 1F). Thus, Th17 cells need to convert to Th1 cells to drive diabetes (7,15), leading to a delayed disease onset.
FIG. 1.
Th17 cells undergo cell conversion to sustain development of type 1 diabetes in NOD.scid mice. A: BDC2.5 T cells were polarized into Th1 or Th17 cells, subsets were highly purified and transferred into NOD.scid mice, and hosts (15 per group) were monitored for incidence of diabetes (blood glucose level of ≥300 mg/dL). B: Kinetics of Th17-to-Th1 conversion as measured by intracellular staining of IFN-γ and IL-17 in CD4+V-β4+ PLN T cells on day 3, 5, and 7 after cell transfer. Numbers indicate the percentage of cells in each quadrant. C: Neutralization of cytokines in vivo by treatment of Th17-recipient NOD.scid mice with anti–IL-17 (n = 8), anti–IFN-γ (n = 6), or rat IgG1 isotype control (n = 8) antibodies. D–F: Polarized BDC2.5 Th17 or Th1 cells were transferred into IL-12p35−/− or IL-12p35+/+ NOD.scid mice, and the hosts were used to monitor Th17-to-Th1 conversion and disease development. D: Kinetics of cell conversion measured by intracellular cytokine staining of splenic and pancreatic CD4+V-β4+ T cells on day 4, 8, and 14 after Th17 cell transfer. The number in the quadrants indicates the percentage of cells producing IFN-γ or IL-17. E: Ratio of Th1 to Th17 (% IFN-γ–producing cells over % IL-17–producing cells) compiled from three independent experiments. Each bar represents the mean ± SEM of six mice. **P < 0.01, *P < 0.05. F: Incidence of diabetes (n = 12) upon transfer of Th17 or Th1 cells. Experiments were performed twice (C) or three times (A, B, D, and E) with consistent results.
Treatment with Ig-p79 interferes with trafficking of converted Th1 cells to suppress diabetes.
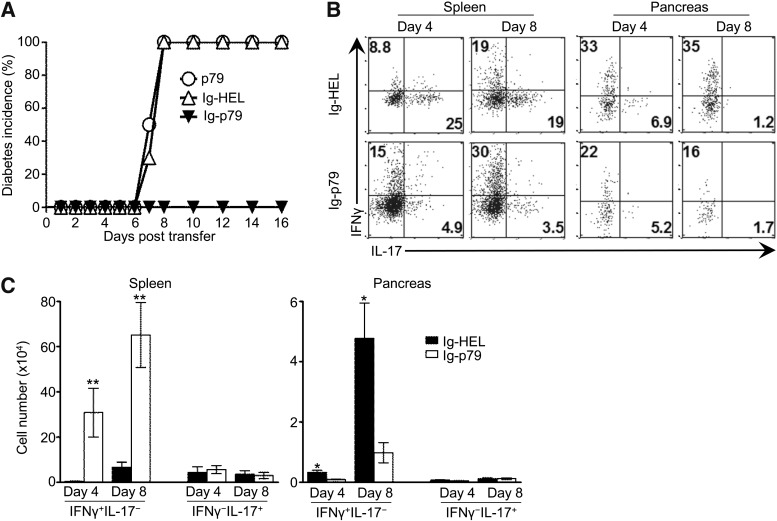
To suppress diabetes mediated by Th17-derived Th1 cells, one would have to interfere with the conversion and/or inactivate the converted Th1 cells. Because delivery of diabetogenic peptides on Igs is effective against diabetes (9,18,25,26), we constructed and used Ig-p79 to define the mechanism for tolerance of convertible Th17 cells. Accordingly, mouse recipients of Th17 cells were given Ig-p79 and monitored for resistance against diabetes. As shown in Fig. 2A, 100% of the mice treated with Ig-p79 were protected against the disease, while all the hosts treated with the control Ig-HEL developed diabetes. As expected, free p79 mimotope was not protective. Furthermore, IFN-γ–producing cells were detected in the spleen on both day 4 and 8, regardless of whether the mice were given Ig-p79 or Ig-HEL, and IL-17–producing cells also were detectable in the spleen, but their percentage was much lower in Ig-p79 versus Ig-HEL treatment (Fig. 2B). These results indicate that conversion from Th17 to Th1 occurs during treatment but to a greater extent with Ig-p79. We then sought to determine the frequency of these cells within the site of inflammation to correlate tolerance with suppression of diabetes. The results show that a very low frequency of Th17 cells relocated to the pancreas in either treatment, most likely as a result of conversion to Th1 cells (Fig. 2B). The absolute number of Th17 cells in the pancreas was also minimal (Fig. 2C). These observations suggest that neither development of diabetes nor suppression of disease involves unconverted Th17 cells in the pancreas. Th1 cells, however, displayed lower percentages (Fig. 2B) and minimal cell numbers (Fig. 2C) in the pancreas but accumulated more efficiently in the spleen by both criteria during treatment with Ig-p79 relative to Ig-HEL. These results suggest that disease development occurs through conversion from Th17 to Th1 cells, but its suppression is mediated by interference with trafficking of the converted Th1 cells to the pancreas.
FIG. 2.
Treatment of Th17-mediated disease with Ig-p79 leads to accumulation of the converted Th1 cells in the spleen and reduced migration to the pancreas. A: NOD.scid mouse recipients of Th17 polarized BDC2.5 cells were treated with p79 peptide (n = 8), Ig-p79 (n = 14), or the control Ig-HEL (n = 16) and then monitored for blood glucose levels for a period of 16 days. B and C: CD4+ T cells were isolated from the spleen and pancreatic islets of Ig-HEL– and Ig-p79–treated mice (n = 6 per group) on day 4 and 8 post Th17 cell transfer and stimulated in vitro with PMA/ionomycin for 2 h in the presence of Brefeldin A; intracellular IFN-γ and IL-17 were measured in CD4+V-β4+ T cells. B: Representative dot plot cytokine measurement with the numbers in each quadrant indicating the percentage of cytokine-positive cells. C: Absolute number of IFNγ+IL-17− or IFNγ−IL-17+ cells in spleen and pancreas. Each bar represents mean ± SEM of 12 mice from four experiments. **P < 0.01, *P < 0.05.
Downregulation of CXCR3 expression on Th1 cells is essential for Ig-p79–mediated interference with cell trafficking to the pancreas.
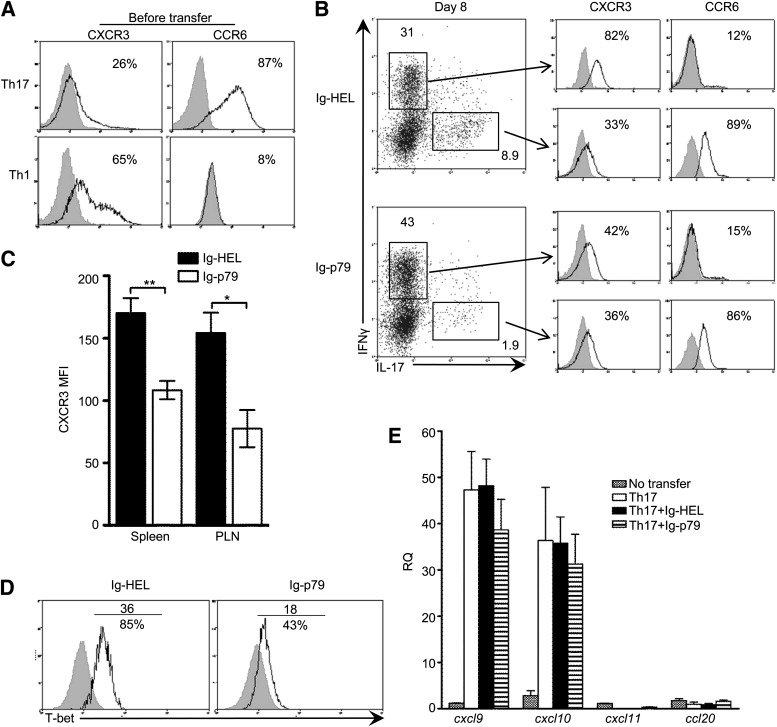
To explore the mechanisms underlying Ig-p79–mediated interference with cell trafficking, we analyzed the expression of the chemokine receptors CCR6 and CXCR3 on Th17 cells before transfer and on both converted Th1 and residual Th17 cells after treatment with Ig-p79. Before transfer, 87% of Th17 cells expressed CCR6, the signature Th17 chemokine receptor (27), and only 26% had the Th1 signature chemokine receptor CXCR3 (28) (Fig. 3A). It is interesting that after transfer and treatment with Ig-HEL, 82% of the converted IFN-γ–producing cells upregulated CXCR3 (mean fluorescence intensity [MFI] = 57) and only 12% retained low CCR6 expression (MFI = 7), while 89% of residual IL-17–producing cells maintained high CCR6 (MFI = 56) and 33% had low CXCR3 expression (MFI = 23) (Fig. 3B). In contrast, with Ig-p79, the number of converted Th1 cells expressing CXCR3 diminished to 42% despite the fact that the number of cells expressing CCR6 went down from 87% before transfer (Fig. 3A) to 15% after treatment (Fig. 3B). Furthermore, the MFI for CXCR3 on the converted Th1 cells was significantly lower in the spleen and PLNs of the Ig-p79–treated mice (Fig. 3C). These results indicate the Th17-to-Th1 conversion is accompanied by the acquisition of CXCR3 expression on the converted Th1 cells. Ig-p79 downregulates CXCR3 expression, leading to retention of the cells in the spleen and, hence, the greater frequency of IFN-γ+ cells relative to Ig-HEL–treated mice (Fig. 3B). Analysis of T-bet expression on day 8 posttransfer indicated a decrease in MFI from 36 with Ig-HEL to 18 with Ig-p79 (Fig. 3D). Similar results were observed at day 4 posttransfer (not shown). These observations bode well with the dependence of CXCR3 on T-bet expression (29,30). Ig-p79 treatment does not significantly interfere with CXCR3 ligand expression in the pancreatic islets because cxcl9 and cxcl10, but not cxcl11, expression increased from 35- to 50-fold upon transfer of Th17 cells and persisted at similar levels after treatment with Ig-HEL or Ig-p79 (Fig. 3E). ccl20, the ligand for CCR6 (31), remained at basal level in all experimental groups (Fig. 3E), suggesting that the absence of ccl20 rather than the treatment with Ig-p79 may be responsible for the lack of relocation of unconverted Th17 cells to the pancreas. In fact, NOD.scid mice have a fivefold decrease in pancreatic CCL20 mRNA relative to NOD mice (not shown). Nevertheless, this may serve as a safeguard against residual Th17 cells. Therefore, these results suggest that downregulation of CXCR3 on the converted Th1 cells is responsible for the diminished migration to the pancreas. In fact, the very few converted Th1 cells that relocated to the pancreas after treatment with Ig-p79 displayed a pattern of cell division similar to those Th1 cells that efficiently migrated to the pancreas under Ig-HEL treatment (Supplementary Fig. 2A). Also, the converted Th1 cells that were retained in the spleen under Ig-p79 treatment had a similar pattern of cell division as the fewer cells within the same site under Ig-HEL treatment (Supplementary Fig. 2A). No difference was observed in cell death analysis in both organs under either treatment (Supplementary Fig. 2B). Thus, proliferation and apoptosis of the converted Th1 cells are not responsible for their increased frequency in the spleen and diminished number in the pancreas, emphasizing the fact that Ig-p79 interferes with trafficking of converted Th1 cells.
FIG. 3.
Treatment with Ig-p79 interferes with CXCR3 expression on converted Th1 cells. A: Expression of CXCR3 and CCR6 on polarized Th1 and Th17 cells. Chemokine receptor expression was analyzed on CD4+V-β4+ gated cells. Results are representative of four independent experiments. Numbers in histograms represent the percentage of cells that are positive for CXCR3 or CCR6 expression. B and C: NOD.scid mice were transferred with Th17 polarized BDC2.5 cells and treated with Ig-HEL or Ig-p79 (3–6 mice per group). The mice were killed on day 8 after treatment, and their splenic and PLN CD4+ T cells were isolated. T cells were then stimulated with PMA/ionomycin for 2 h in the presence of Brefeldin A and stained for surface CXCR3 and CCR6 and for intracellular IFN-γ and IL-17. B: Representative experiment of splenic CD4+V-β4+IFN-γ+IL-17− (converted Th1) and CD4+V-β4+IFN-γ−IL-17+ (unconverted Th17) cells (left) and expression of CXCR3 and CCR6 by each population (right). The numbers in histograms represent the percentages of CXCR3- or CCR6-expressing cells among the converted Th1 and unconverted Th17 cells. C: MFI of CXCR3 expression on splenic and PLN CD4+V-β4+IFN-γ+IL-17− cells on day 8 after treatment with Ig-p79 or Ig-HEL. Each bar represents the mean ± SEM of 10 mice from three independent experiments. **P < 0.01, *P < 0.05. D: T-bet expression by splenic CD4+V-β4+IFN-γ+IL-17− cells on day 8 after Ig-p79 or Ig-HEL treatment. The numbers above the line represent the MFI, and those under the line represent the frequencies of T-bet+ cells. The results are representative of three independent experiments. E: Quantitative PCR analysis of cxcl9, cxcl10, cxcl11, and cxcl20 chemokine expression in pancreatic islets on day 8 following Ig-p79 or Ig-HEL treatment. Each bar represents the mean ± SEM of 7 mice from two independent experiments. RQ, relative quantity.
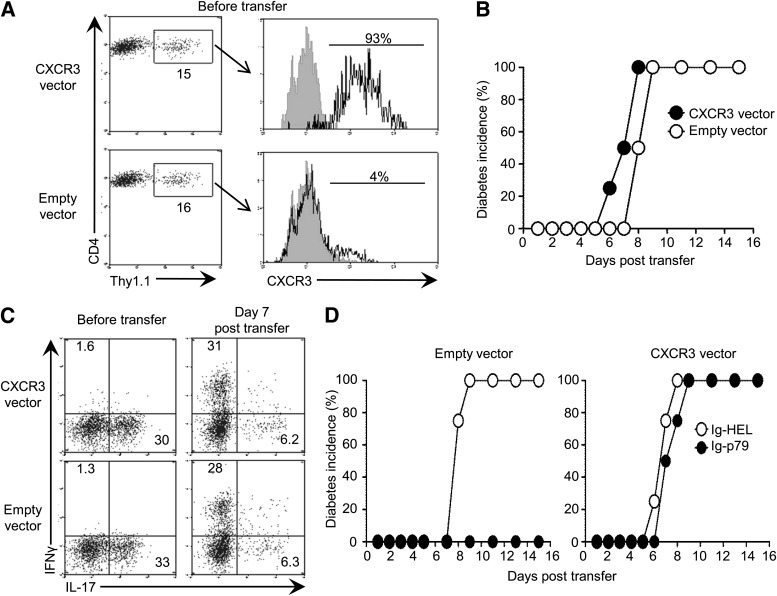
This mechanism of tolerance by Ig-p79 is also operative with polarized or conventional Th1 cells. Indeed, Ig-p79, but not Ig-HEL, suppressed diabetes mediated by transfer of polarized Th1 cells (Fig. 4A). Moreover, while Ig-HEL led to a similar frequency of IFN-γ–producing cells in the spleen and pancreas, Ig-p79 yielded a much higher frequency and absolute cell number in the spleen relative to the pancreas (Fig. 4B and C). In addition, Ig-p79, but not Ig-HEL, downregulated CXCR3 and T-bet expression on splenic Th1 cells (Fig. 4D). The difference was significant as measured by MFI (Fig. 4E). Finally, cxcl9, cxcl10, and cxcl11 gene expression was upregulated upon Th1 transfer, and treatment with Ig-HEL and Ig-p79 did not change such pattern of expression (Fig. 4F). These results indicate that Ig-p79 diminishes CXCR3 expression on polarized Th1 cells, leading to defective trafficking to the pancreas as with converted Th1 cells. In fact, when CXCR3 was overexpressed in the polarized Th17 cells, Ig-p79 was no longer able to suppress the disease despite conversion of the cells into Th1 cells (Fig. 5). Indeed, when polarized Thy1.2 Th17 cells were infected with the mouse stem cell virus (MSCV) carrying CXCR3-IRES-Thy1.1 (19), the transduced cells, which expressed CXCR3 (Fig. 5A), induced diabetes that had a similar kinetic as disease in mouse recipients of empty vector–transduced cells (Fig. 5B). The cells did convert into Th1 cells whether transduced with CXCR3 or empty vector because 31 and 28% of the cells produced IFN-γ, respectively (Fig. 5C). It is interesting that while the mouse recipients of empty vector–transduced Th17 cells did not develop type 1 diabetes when treated with Ig-p79, those recipients of CXCR3-transduced cells did (Fig. 5D). Treatment with Ig-HEL did not protect against the disease, regardless of whether the cells were transduced with CXCR3 or empty vector. These observations indicate that overexpression of CXCR3 nullifies Ig-p79–induced tolerance. Overall, downregulation of CXCR3 is essential for tolerance of Th17-derived Th1 cells and suppression of type 1 diabetes.
FIG. 4.
Polarized, like–converted Th1 cells, downregulate CXCR3 and T-bet upon treatment of the NOD.scid host with Ig-p79. A: Polarized Th1 cells were transferred into NOD.scid mice, and the hosts were treated with p79 peptide (n = 8), Ig-HEL (n = 12), or Ig-p79 (n = 16) and monitored for blood glucose levels. The graph shows diabetes incidence during a 16-day monitoring period. B: Intracellular IFN-γ and IL-17 production by splenic and pancreatic CD4+V-β4+ T cells harvested on day 5 after Th1 transfer. Results are representative of three independent experiments. Numbers indicate the percentage of cells in each quadrant. C: Absolute number of IFN-γ+CD4+V-β4+ T cells in the spleen and pancreas on day 2 and 5 after Th1 transfer. Each point represents the mean ± SEM of 10 mice from three independent experiments. D: CXCR3 and T-bet expression on splenic IFN-γ+CD4+V-β4+ T cells on day 5 after Th1 transfer. Results are representative of four independent experiments. E: MFI of CXCR3 and T-bet expression by Th1 cells described in D. Each bar represents the mean ± SEM of 12 mice. F: Quantitative PCR analysis of cxcl9, cxcl10, cxcl11, and cxcl20 chemokine expression in pancreatic islets on day 5 after Th1 transfer. Each bar represents mean ± SEM of 6 mice from two independent experiments. **P < 0.01. *P < 0.05. RQ, relative quantity.
FIG. 5.
Overexpression of CXCR3 in plastic Th17 cells confers resistance to converted Th1 cells against Ig-p79–induced tolerance. A–C: Polarized Th17 BDC2.5 T cells (Thy1.2) were transduced ex vivo with MSCV-CXCR3-IRES-Thy1.1 or empty MSCV-IRES-Thy1.1 vector and transferred into NOD.scid mice. The hosts were then monitored for blood glucose levels, and the transferred Th17 cells were analyzed for Th17-to-Th1 conversion. A: Expression of Thy1.1 by CD4+V-β4+ polarized Th17 cells and expression of CXCR3 by Thy1.1+CD4+V-β4+ polarized Th17 cells. B: Diabetes incidence of mouse recipients of Th17 cells described in A (n = 4 per group). C: Production of IFN-γ and IL-17 by CXCR3-transduced CD4+V-β4+Thy1.1+ cells before and 7 days after transfer. Results in A–C are representative of three independent experiments. D: NOD.scid mouse recipients of CXCR3- or empty vector–transduced Th17 cells were treated with Ig-HEL or Ig-p79 (n = 4 per group) and monitored for diabetes. Data depict diabetes incidence. Experiment was performed twice with consistent results.
Th17 cells are unable to undergo Th1 conversion upon transfer into NOD mice and require depletion of regulatory T cells to transfer diabetes to the host.
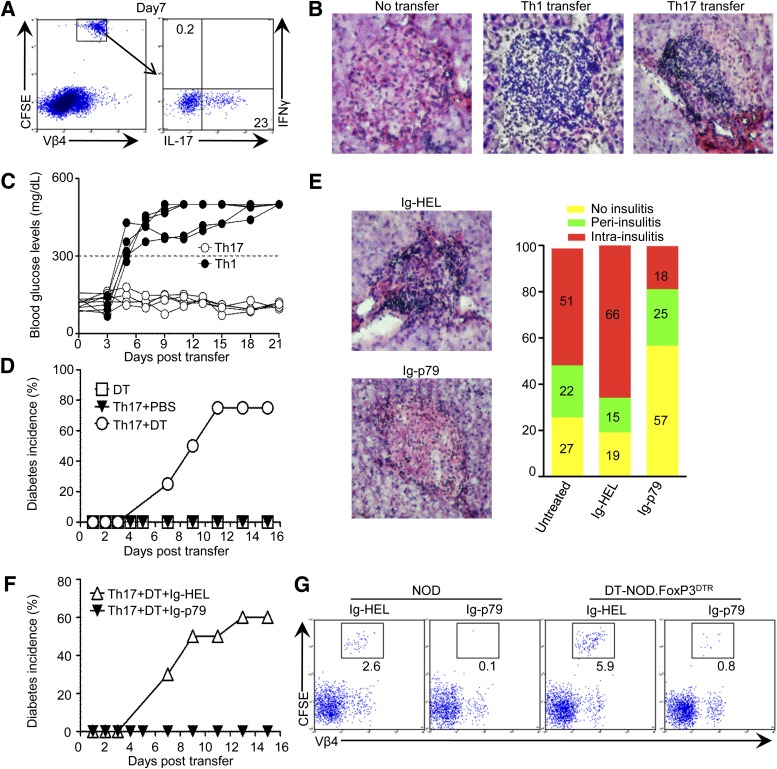
Both neonatal and adult mice do not support prominent Th17-to-Th1 conversion (7,17). Thus, adult NOD mice would provide a model to assess stable Th17 cells for transfer of diabetes. In an initial investigation, we found that upon transfer into NOD mice, Th17 cells (CD4+V-β4+CFSE+) were stable, produced IL-17, and did not convert into IFN-γ–producing cells (Fig. 6A). Histological examination of the pancreas showed that while the animals without transfer had little infiltration, recipients of Th17 cells had a significant cell infiltration comparable with the control Th1 transfer (Fig. 6B). Furthermore, the Th17 recipients had normal blood glucose levels during the 21-day observation period, while those given Th1 cells developed diabetes within 5 days posttransfer (Fig. 6C). Thus, Th17 cells, which usually induce CCL20 in the site of inflammation (32), trigger pancreatic histopathology upon transfer into 4-week-old NOD mice that could not evolve into clinical diabetes. Given the fact that regulatory T cells (Tregs) remain functional in 4-week-old NOD mice (18,25) and can suppress Th17 cells (33–35), it is possible that host Tregs interfere with initiation of inflammation and development of clinical diabetes. In fact, when NOD.FoxP3GFP.DTR mice were depleted of Tregs by injection of DT at age 4 weeks, the transfer of Th17 cells induced diabetes. Indeed, while animals given DT without Th17 transfer had no clinical diabetes, 75% of those given both DT and Th17 cells had diabetes by day 11 posttransfer (Fig. 6D). Overall, stable Th17 cells trigger pancreatic inflammation without clinical diabetes in the NOD mouse, but depletion of Tregs sustains transition to overt diabetes.
FIG. 6.
Ig-p79 ameliorates islet histopathology and clinical diabetes caused by transfer of Th17 cells into NOD mice. A: CFSE-labeled Th17 cells were transferred into 4-week-old female NOD mice and 7 days later, the splenic cells were stimulated with p79 peptide for 16 h. Cells were stained with anti–V-β4 and anti-CD4 antibodies and then stained for intracellular IFN-γ and IL-17 cytokines. The plot shows IFN-γ and IL-17 production by CD4+CFSE+V-β4+ T cells. Results are representative of three independent experiments. B: Hematoxylin-eosin (H-E) staining (×100 magnification) of nonserial pancreatic sections (200 μm apart) from NOD mice that did not receive T-cell transfer (no transfer, n = 3) or mouse recipients of either Th1 or Th17 polarized cells (n = 6 per group). C: Blood glucose levels (mg/dL) of individual NOD mouse recipients of either Th1 or Th17 polarized cells (n = 5 per group). D: Incidence of diabetes in NOD.FoxP3GFP.DTR mouse recipients of Th17 cells (n = 8 per group) that were treated with DT or PBS. A group treated with DT without transfer of Th17 cells (n = 3) was included for control purposes. E: Representative H-E staining and islet infiltration severity scores (bar graph) of NOD mouse recipients of Th17 cells treated with Ig-p79 or Ig-HEL (n = 6 per group). The scoring system is described in research design and methods. Percentages represent the number of islets with a specific score over the total number of islets (30–40 per pancreas). F: Incidence of diabetes in Treg-depleted (by DT) NOD.FoxP3GFP.DTR mouse recipients of Th17 cells treated with Ig-p79 or Ig-HEL (n = 10 per group). G: Frequency of CFSE+CD4+V-β4+ T cells recovered from pancreatic islets of NOD or Treg-depleted NOD.FoxP3GFP.DTR mouse recipients of Th17 transfer treated with Ig-p79 or Ig-HEL. All pancreatic histology and flow cytometry analyses (B, E, and G) were performed on day 7 post T-cell transfer. Results in each panel are representative of two or three independent experiments. (A high-quality digital representation of this figure is available in the online issue.)
Ig-p79 suppresses pancreatic inflammation and diabetes mediated by stable Th17 cells.
To determine whether the stable Th17 cells can be tolerized by Ig-p79, the NOD mouse recipients of Th17 cells (without depletion of Tregs) were given the chimera and assessed for pancreatic inflammation. The results show that the majority (57%) of the islets had minimal infiltration (no insulitis), and the remaining islets had peri-insulitis (25%) or intrainsulitis (18%) (Fig. 6E). In contrast, in untreated and Ig-HEL–treated mice, most of the islets (51 and 66%, respectively) displayed intrainsulitis (Fig. 6E). Furthermore, Ig-p79, but not Ig-HEL, was able to suppress clinical diabetes when given to DT-treated NOD.FoxP3GFP.DTR mouse recipients of Th17 cells (Fig. 6F). Analysis of Th17 relocation to the pancreas showed a minimal frequency of carboxyfluorescein succinimidyl ester (CFSE)-labeled cells in mice treated with Ig-p79 versus Ig-HEL (Fig. 6G). Thus, Ig-p79 is able to suppress both forms of disease.
Stable Th17 cells undergo apoptosis upon treatment with Ig-p79.
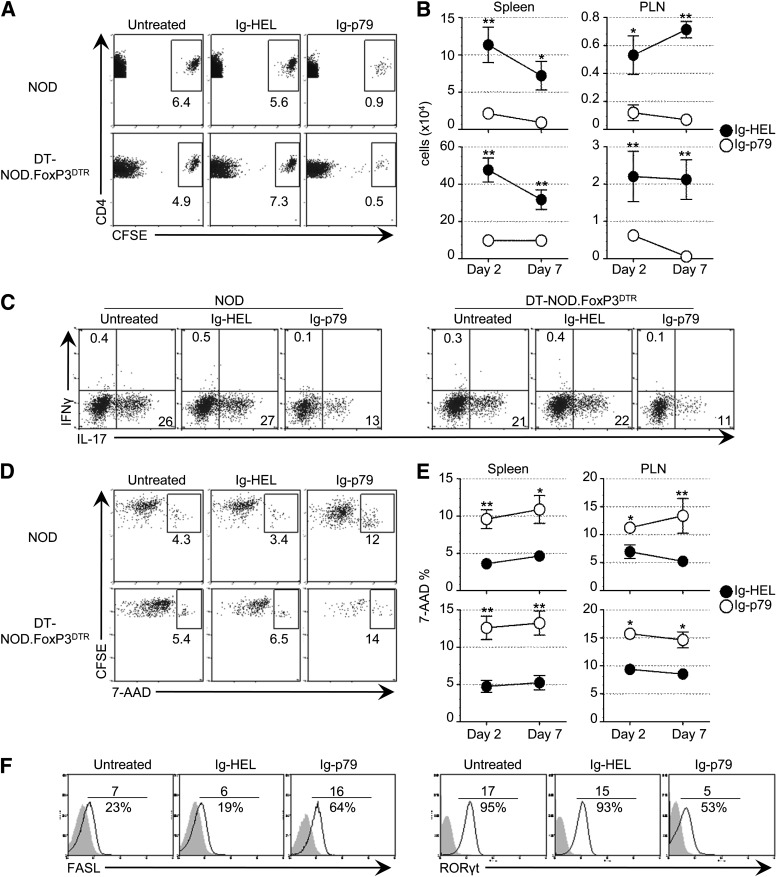
Upon treatment with Ig-p79, the stable Th17 cells were barely found in the pancreas (Fig. 6G). This suggests that the cells were retained and/or died in other organs and could not reach the pancreas. To address this issue, we searched for the Th17 cells in both the spleen and PLNs of Treg-depleted as well as undepleted mice. The results show that very few cells were available in the spleen in Ig-p79–treated relative to untreated or Ig-HEL–treated mice (Fig. 7A). Moreover, by day 2 post Ig-p79 treatment, the number of Th17 cells decreased significantly in the spleen and PLNs relative to Ig-HEL treatment in both strains, and by day 7, the Th17 cells were barely detectable in either organ (Fig. 7B). The treatment with Ig-p79 did not induce conversion because there were no Th1 cells in the PLNs of either strain and the very few residual cells were IL-17–producing cells like the untreated or Ig-HEL–treated mice (Fig. 7C). Overall, Ig-p79 did not induce conversion but reduced the number of Th17 cells in the spleen and PLNs. Since there were very few cells in the pancreas (Fig. 6), it is likely that Ig-p79 induced death of the cells. Indeed, when the splenic stable Th17 cells were analyzed for incorporation of 7 amino-actinomycin D (7-AAD) on day 2 after transfer, there was a significant incorporation of 7-AAD by the Th17 cells in both NOD and DT-NOD.FoxP3GFP-DTR mice, indicating that the cells were undergoing apoptosis (Fig. 7D). The untreated or Ig-HEL–treated mice had much less incorporation of 7-AAD. Like in the spleen, significant 7-AAD incorporation was also observed in the PLNs (Fig. 7E). Incorporation of 7-AAD continued at a similar level on day 7, indicating that the residual cells are undergoing death at a significant rate. Furthermore, in the Ig-p79–treated mice, the cells displayed an increased level of FasL but decreased expression of ROR-γt relative to untreated or Ig-HEL–treated mice (Fig. 7F). Since ROR-γt functions as a negative regulator for FasL (36), it is likely that its downregulation in Th17 cells led to the upregulation of FasL, the trigger of a signaling cascade that sustains apoptosis (37,38). Collectively, these results indicate that upon treatment with Ig-p79, stable Th17 cells undergo apoptosis in the spleen and PLNs, which explains the lack of Th17 cells in the pancreas.
FIG. 7.
Apoptosis of nonconverting Th17 cells represents the mechanism underlying amelioration of clinical and histopathological diabetes by Ig-p79. NOD or Treg-depleted NOD.FoxP3GFP.DTR mouse recipients of CFSE-labeled Th17 cells were treated with Ig-p79 or Ig-HEL and the spleens or PLNs were harvested at the indicated time points. A: Frequency of CD4+V-β4+CFSE+ Th17 cells on day 2 after treatment with Ig-p79 or Ig-HEL (n = 2 for each time). A group of mice that did not receive any treatment (Untreated) is shown for control purposes. B: Absolute cell number of CD4+V-β4+CFSE+ cells in spleen and PLNs on day 2 and 7 posttransfer. Numbers represent the mean ± SEM of six mice. C: Production of intracellular IFN-γ and IL-17 by cells gated on CD4+V-β4+CFSE+ from the PLNs that were harvested on day 7 posttransfer and stimulated with p79 peptide. D: 7-AAD binding by splenic cells from the mice described in A. E: Percentage of 7-AAD+ cells gated on CD4+V-β4+CFSE+ from spleen and PLNs on day 2 and 7 posttransfer of CFSE-labeled Th17 cells. Percentages represent the mean ± SEM of six mice. F: FasL and ROR-γt expression by cells gated on CD4+V-β4+CFSE+ on day 2 for untreated, Ig-p79–, or Ig-HEL– treated mice (n = 2 for each time) post Th17 transfer. Numbers above the line represent the MFI and those under the line represent the frequencies of FasL+ or ROR-γt+ cells. Results in each panel are representative of three independent experiments. **P < 0.01, *P < 0.05.
Polarized Th1 cells downregulate CXCR3 rather than undergo apoptosis upon treatment with Ig-p79.
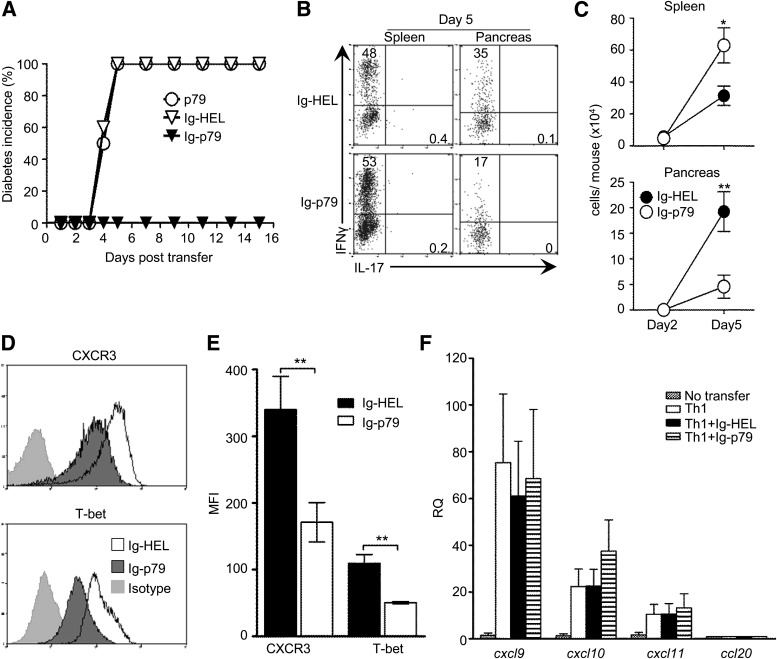
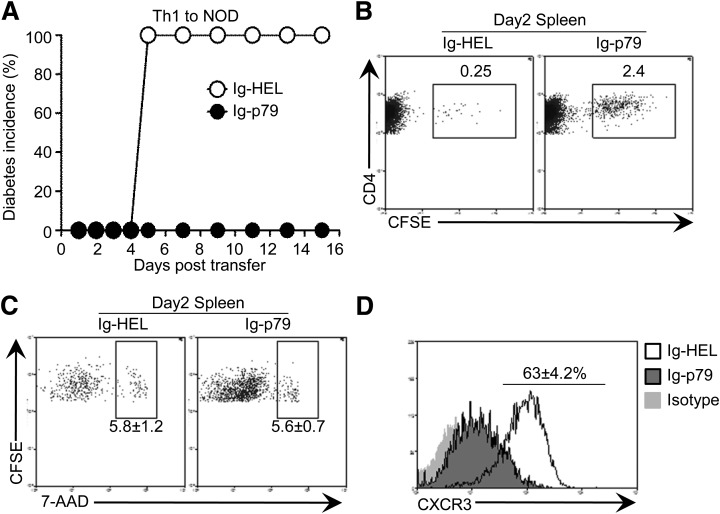
Th1 cells that convert from Th17 cells upon transfer into lymphopenic NOD.scid mice downregulate CXCR3 during suppression of diabetes by Ig-p79 (Figs. 2 and 3). However, Th17 cells that do not convert into Th1 cells upon transfer into nonlymphopenic NOD mice undergo apoptosis during similar disease suppression by Ig-p79 (Figs. 6 and 7). To determine whether the differential mechanism of tolerance among converted Th1 and stable Th17 cells is intrinsic to the target cell or dictated by the host environment, we transferred polarized Th1 cells into nonlymphopenic NOD mice and delineated the mechanism of tolerance during suppression of diabetes by Ig-p79. The results show that Ig-p79, but not Ig-HEL, suppresses diabetes transferred to the NOD hosts by polarized Th1 cells (Fig. 8A). Furthermore, treatment with Ig-p79, but not Ig-HEL, led to retention of the Th1 cells in the spleen (Fig. 8B). Indeed, during treatment with Ig-p79, 2.4% of the Th1 cells remained in the spleen, while only 0.25% of the cells were found in the same organ during treatment with Ig-HEL. Moreover, there were only 1.4 ± 0.6 × 102 cells in the pancreas relative to 6.2 ± 1.8 × 104 cells in Ig-HEL–treated mice. It is interesting that while the rate of apoptosis was low with either treatment (Fig. 8C), expression of CXCR3 was downregulated by Ig-p79 relative to treatment with Ig-HEL (Fig. 8D). Thus, stable Th1 cells downregulate CXCR3 during suppression of diabetes with Ig-p79.
FIG. 8.
Polarized Th1 cells transferred into NOD mice downregulate CXCR3 expression upon treatment with Ig-p79. CFSE-labeled polarized Th1 cells were transferred into NOD mice and 24 h later, the hosts were given Ig-p79 or the control Ig-HEL. A: Incidence of diabetes in both groups of mice (n = 6 per group). B–D: The frequency of splenic CD4+V-β4+CFSE+ cells (B), their apoptosis (C), and CXCR3 expression (D) was analyzed on day 2 after T-cell transfer. The numbers indicate the mean ± SEM of six mice. Each panel is representative of three independent experiments.
DISCUSSION
Although interference with costimulation (39,40), inhibition of cell metabolism (41), decreased chemotaxis (42), or suppression by Tregs (29) have been proven effective against Th1 cells, to date it is not clear whether these strategies would be effective against Th17 cells. Herein, we engineered and used the tolerogen Ig-p79 to define the mechanism by which Th1 and Th17 cells undergo tolerance. Specifically, we investigated Treg-independent mechanisms of Th17 tolerance under conversion-permissive and nonpermissive environments. Our findings demonstrate that upon transfer into NOD.scid mice, Th17 cells convert into Th1 cells in an IL-12–dependent manner, but the hosts develop a delayed diabetes relative to transfer of Th1 cells. It is interesting that when the hosts are treated with Ig-p79, both pancreatic infiltration and disease subsided. However, we observed retention of Th1 cells in the spleen, as was the case for the treatment of spontaneous diabetes with Ig-GAD2 (9). Since Th1 cells upregulate CXCR3 to traffic to the site of inflammation (29), we envisioned that the splenic retention during Ig-p79 treatment could be related to interference with CXCR3 expression within this organ. This was indeed the case, for the splenic Th1 cells had diminished CXCR3 expression. Given the fact that the expression in the pancreas of the ligands for CXCR3 was unaffected by the treatment, it is likely that interference with CXCR3-mediated trafficking is responsible for suppression of diabetes. In fact, overexpression of CXCR3 by viral transduction nullifies disease suppression by Ig-p79 despite effective Th17-to-Th1 conversion. This mechanism seems to be operative with polarized Th1 cells as well. The finding that T-bet is downregulated by Ig-p79 treatment provides support to the diminished expression of CXCR3 because the two molecules usually display coordinated expression in both Th1 cells (30) and Tregs, providing a means for the latter to track their Th1 targets in the site of inflammation (29).
On the other hand, when the Th17 cells were transferred into NOD mice, there was no conversion into Th1 cells, and the hosts displayed islet infiltration without progression into diabetes unless the mice were depleted of their Tregs. In this case, treatment with Ig-p79, which still suppressed diabetes, did not drive conversion of the Th17 cells but reduced their frequency in the pancreas, the PLNs, and even the spleen. Unlike Th1 cells, the stable Th17 cells showed expression of apoptotic markers and upregulated FasL. Apoptosis was restricted to Th17 cells because treatment of NOD host recipients of Th1 transfer with Ig-p79 did not drive death of the cells. It is interesting that Th1 and Th17 cells targeted with the same antigen-specific treatment undergo tolerance by different mechanisms: interference with trafficking for Th1 cells and apoptosis for Th17 cells. More intriguing, even Th1 cells originating from conversion of Th17 cells use the trafficking rather than the apoptotic mechanism. This may reflect a change in the cell signaling networks when the transcription program shifts from IL-17 to IFN-γ. Given that the expression of CXCR3 is regulated by T-bet in both Th1 cells and Tregs (29,30) and that Ig-p79 downregulates T-bet expression, it is logical that CXCR3 expression diminishes regardless of the origin of Th1 cells. For stable Th17 cells, however, despite the fact that Ig-p79 signaling induced downregulation of ROR-γt, the cells upregulated FasL and underwent apoptosis. Again, given that ROR-γt negatively controls FasL expression (36), it is understandable that Ig-p79 drives death of the Th17 cells. Overall, this study shows that Ig-p79 antigen-specific therapy of type 1 diabetes targets the specific signature transcription factor to terminate the pathogenic function of Th1 and Th17 cells according to the pathways controlled by these specific factors. This is therapeutically significant because the strategy will be able to overcome the plasticity associated with pathogenic Th cells.
Supplementary Material
ACKNOWLEDGMENTS
J.A.C. and D.M.T. were supported by Life Sciences Fellowships from the University of Missouri. H.Z. was supported by National Institutes of Health grants RO1-DK-65748, R21-AI-68746, and RO1-NS-057194 and the J. Lavenia Edwards endowment.
R.J. is employed by Merck Research Laboratories. No other potential conflicts of interest relevant to this article were reported.
X.W. performed the experiments and data analysis. F.B.G. designed cell sorting and assisted with flow cytometry analysis. A.M.V. assisted with analysis of diabetes. L.M.R. purified the Ig chimeras and assisted with analysis of diabetes. R.J. performed genetic engineering of Ig-p79. C.L.H., J.A.C., M.D., C.M.H., and D.M.T. assisted in the design of experiments and reviewed data. H.Z. designed and supervised the study and wrote the manuscript. H.Z. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Mohamed Oukka, University of Washington Seattle Children’s Hospital, for providing the C57BL/6.FoxP3.GFP.DTR mice and Mehrhad Matloubian, University of California, San Francisco, for providing the MSCV-CXCR3-IRES-Thy1.1 and empty MSCV-IRES-Thy1.1 vector.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-1723/-/DC1.
REFERENCES
- 1.Katz JD, Benoist C, Mathis D. T helper cell subsets in insulin-dependent diabetes. Science 1995;268:1185–1188 [DOI] [PubMed] [Google Scholar]
- 2.Rabinovitch A. Immunoregulatory and cytokine imbalances in the pathogenesis of IDDM. Therapeutic intervention by immunostimulation? Diabetes 1994;43:613–621 [DOI] [PubMed] [Google Scholar]
- 3.Bach JF. Insulin-dependent diabetes mellitus as an autoimmune disease. Endocr Rev 1994;15:516–542 [DOI] [PubMed] [Google Scholar]
- 4.Hultgren B, Huang X, Dybdal N, Stewart TA. Genetic absence of gamma-interferon delays but does not prevent diabetes in NOD mice. Diabetes 1996;45:812–817 [DOI] [PubMed] [Google Scholar]
- 5.Serreze DV, Post CM, Chapman HD, Johnson EA, Lu B, Rothman PB. Interferon-gamma receptor signaling is dispensable in the development of autoimmune type 1 diabetes in NOD mice. Diabetes 2000;49:2007–2011 [DOI] [PubMed] [Google Scholar]
- 6.Trembleau S, Penna G, Gregori S, et al. Pancreas-infiltrating Th1 cells and diabetes develop in IL-12-deficient nonobese diabetic mice. J Immunol 1999;163:2960–2968 [PubMed] [Google Scholar]
- 7.Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol 2009;39:216–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradshaw EM, Raddassi K, Elyaman W, et al. Monocytes from patients with type 1 diabetes spontaneously secrete proinflammatory cytokines inducing Th17 cells. J Immunol 2009;183:4432–4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain R, Tartar DM, Gregg RK, et al. Innocuous IFNgamma induced by adjuvant-free antigen restores normoglycemia in NOD mice through inhibition of IL-17 production. J Exp Med 2008;205:207–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emamaullee JA, Davis J, Merani S, et al. Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes 2009;58:1302–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honkanen J, Nieminen JK, Gao R, et al. IL-17 immunity in human type 1 diabetes. J Immunol 2010;185:1959–1967 [DOI] [PubMed] [Google Scholar]
- 12.Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell 1993;74:1089–1100 [DOI] [PubMed] [Google Scholar]
- 13.Judkowski V, Pinilla C, Schroder K, Tucker L, Sarvetnick N, Wilson DB. Identification of MHC class II-restricted peptide ligands, including a glutamic acid decarboxylase 65 sequence, that stimulate diabetogenic T cells from transgenic BDC2.5 nonobese diabetic mice. J Immunol 2001;166:908–917 [DOI] [PubMed] [Google Scholar]
- 14.You S, Chen C, Lee WH, et al. Detection and characterization of T cells specific for BDC2.5 T cell-stimulating peptides. J Immunol 2003;170:4011–4020 [DOI] [PubMed] [Google Scholar]
- 15.Bending D, De la Peña H, Veldhoen M, et al. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest 2009;119:565–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stadinski BD, Delong T, Reisdorph N, et al. Chromogranin A is an autoantigen in type 1 diabetes. Nat Immunol 2010;11:225–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nurieva R, Yang XO, Chung Y, Dong C. Cutting edge: in vitro generated Th17 cells maintain their cytokine expression program in normal but not lymphopenic hosts. J Immunol 2009;182:2565–2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gregg RK, Jain R, Schoenleber SJ, et al. A sudden decline in active membrane-bound TGF-beta impairs both T regulatory cell function and protection against autoimmune diabetes. J Immunol 2004;173:7308–7316 [DOI] [PubMed] [Google Scholar]
- 19.Hu JK, Kagari T, Clingan JM, Matloubian M. Expression of chemokine receptor CXCR3 on T cells affects the balance between effector and memory CD8 T-cell generation. Proc Natl Acad Sci USA 2011;108:E118–E127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee YK, Turner H, Maynard CL, et al. Late developmental plasticity in the T helper 17 lineage. Immunity 2009;30:92–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity 2009;30:646–655 [DOI] [PubMed] [Google Scholar]
- 22.Hirota K, Duarte JH, Veldhoen M, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol 2011;12:255–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lexberg MH, Taubner A, Albrecht I, et al. IFN-γ and IL-12 synergize to convert in vivo generated Th17 into Th1/Th17 cells. Eur J Immunol 2010;40:3017–3027 [DOI] [PubMed] [Google Scholar]
- 24.Bending D, Newland S, Krejcí A, Phillips JM, Bray S, Cooke A. Epigenetic changes at Il12rb2 and Tbx21 in relation to plasticity behavior of Th17 cells. J Immunol 2011;186:3373–3382 [DOI] [PubMed] [Google Scholar]
- 25.Tartar DM, VanMorlan AM, Wan X, et al. FoxP3+RORgammat+ T helper intermediates display suppressive function against autoimmune diabetes. J Immunol 2010;184:3377–3385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregg RK, Bell JJ, Lee HH, et al. IL-10 diminishes CTLA-4 expression on islet-resident T cells and sustains their activation rather than tolerance. J Immunol 2005;174:662–670 [DOI] [PubMed] [Google Scholar]
- 27.Singh SP, Zhang HH, Foley JF, Hedrick MN, Farber JM. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J Immunol 2008;180:214–221 [DOI] [PubMed] [Google Scholar]
- 28.Syrbe U, Siveke J, Hamann A. Th1/Th2 subsets: distinct differences in homing and chemokine receptor expression? Springer Semin Immunopathol 1999;21:263–285 [DOI] [PubMed] [Google Scholar]
- 29.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol 2009;10:595–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beima KM, Miazgowicz MM, Lewis MD, Yan PS, Huang TH, Weinmann AS. T-bet binding to newly identified target gene promoters is cell type-independent but results in variable context-dependent functional effects. J Biol Chem 2006;281:11992–12000 [DOI] [PubMed] [Google Scholar]
- 31.Reboldi A, Coisne C, Baumjohann D, et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol 2009;10:514–523 [DOI] [PubMed] [Google Scholar]
- 32.Hirota K, Yoshitomi H, Hashimoto M, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med 2007;204:2803–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaudhry A, Rudra D, Treuting P, et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science 2009;326:986–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaudhry A, Samstein RM, Treuting P, et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity 2011;34:566–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huber S, Gagliani N, Esplugues E, et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3⁻ and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity 2011;34:554–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He YW, Deftos ML, Ojala EW, Bevan MJ. RORgamma t, a novel isoform of an orphan receptor, negatively regulates Fas ligand expression and IL-2 production in T cells. Immunity 1998;9:797–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Xu G, Zhang L, Roberts AI, Shi Y. Th17 cells undergo Fas-mediated activation-induced cell death independent of IFN-gamma. J Immunol 2008;181:190–196 [DOI] [PubMed] [Google Scholar]
- 38.Fang Y, Yu S, Ellis JS, Sharav T, Braley-Mullen H. Comparison of sensitivity of Th1, Th2, and Th17 cells to Fas-mediated apoptosis. J Leukoc Biol 2010;87:1019–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Powell JD, Lerner CG, Schwartz RH. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J Immunol 1999;162:2775–2784 [PubMed] [Google Scholar]
- 40.Vanasek TL, Khoruts A, Zell T, Mueller DL. Antagonistic roles for CTLA-4 and the mammalian target of rapamycin in the regulation of clonal anergy: enhanced cell cycle progression promotes recall antigen responsiveness. J Immunol 2001;167:5636–5644 [DOI] [PubMed] [Google Scholar]
- 41.Zheng Y, Delgoffe GM, Meyer CF, Chan W, Powell JD. Anergic T cells are metabolically anergic. J Immunol 2009;183:6095–6101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brogdon JL, Xu Y, Szabo SJ, et al. Histone deacetylase activities are required for innate immune cell control of Th1 but not Th2 effector cell function. Blood 2007;109:1123–1130 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.