Abstract
The access to whole genome sequences has provided the opportunity to study the evolution and organization of immunologically related genes on a large scale. The genes encoding the T cell receptor (TCR) α and δ chains are part of a complex locus that has shown remarkable conserved organization across different amniote lineages. In this study we have examined and annotated the TCRα/δ locus in the chicken (Gallus gallus) and compared it to that of the zebra finch (Taeniopygia guttata) and other avian species using the current available genome data. We also analyzed the expressed chicken TCRα/δ transcript repertoire and compared it with that previously described for zebra finch. The analyses conducted in this study show that the TCRα/δ locus in birds have undergone major rearrangements and expansion of the germ-line repertoire in chicken, compared to zebra finch. A major expansion of the chicken variable gene repertoire appears to be driven by selection for genes from a limited number of subgroups.
Keywords: TCRα/δ, Evolution, avian TCR
Conventional T cell receptors (TCR) are heterodimers composed of α and β chains, or of γ and δ chains, that are expressed on the surface of αβT cells or γδT cells, respectively (Davis and Chein 2008). The genes encoding these four TCR chains have been found in species belonging to all the major lineages of jawed vertebrates, from cartilaginous fish to placental mammals (Rast et al. 1997). As with antibodies, somatic DNA recombination of gene segments is used to assemble the genes encoding the antigen binding variable (V) domains of TCR chains (Davis and Chein 2008). These gene segments are the V, D (diversity) and J (joining) gene segments in the TCRβ and TCRδ chains, or by somatic recombination of V and J in TCRα and TCRγ chains. The number and organization of V, D, and J gene segments available for somatic recombination can vary between species. The genes encoding TCRα and TCRδ chains are interspersed at a single locus in all mammals, birds, and amphibians examined so far, and the organization has been remarkably conserved (Satyanarayana et al. 1988, Koop et al. 1992, Kubota et al. 1999, Uenishi et al. 2003, Reinink and Rhijn 2009, Parra et al. 2008, Parra et al. 2010).
In the past few years, unconventional TCR that appear to be hybrid between TCRδ and Ig heavy chain (IgH) has been described in phylogenetically distant species (Parra et al. 2010, 2012). These unconventional TCR chains are composed of a constant (C) region that is clearly a Cδ, but paired with a V domain that is nearly indistinguishable from V used by IgH chains (VHδ). Such unconventional TCRδ were first reported in an amphibian and have been most recently found in birds. In the zebra finch the genes encoding this unusual TCR are found within the TCRα/δ locus, while in chicken and turkey these genes have been translocated to a different chromosome (Parra et al. 2012). The discovery of such differences among avian species, along with the importance of chickens as an economic and model species, prompted this more detailed examination of the chicken TCRα/δ organization and repertoire.
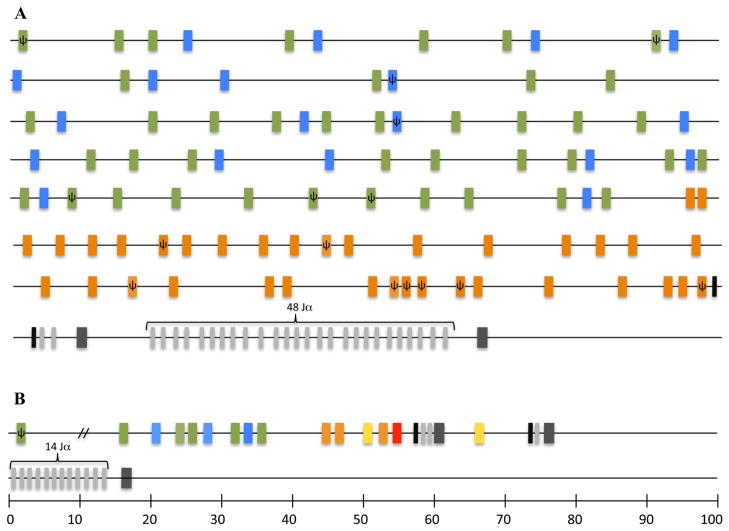
Genomic organization of the TCRα/δ locus in the chicken and finch is highly conserved with that of other tetrapods. In the chicken it spans approximately 800 Kb on chromosome 27, based on the latest assembly of the chicken genome (2.1) (Fig. 1A, International Chicken Genome Sequencing Consortium 2004). There are single Cα and Cδ genes and a total of 96 V gene segments. Chicken Vα/δ genes fall in three distinct groups previously identified as Vα1 (Göbel et al. 1994), Vα2 (Chen et al. 1996) and Vδ1 (Kubota et al. 1999) subgroups, based on phylogenetic analysis (Fig. 1 and 2). The subgroup Vα1 contains 41 V genes, 33 of which appear functional based on having open reading frames (ORF) and identifiable leader and recombination signal sequences in the genomic sequence (Fig. 1A). Subgroup Vα2 contains 19 V genes, 17 of which appear to be functional. Subgroup Vδ1 contains 36 V genes, 32 of which appear functional (Fig. 1A).
Figure 1.
Simplified illustration of chicken and zebra finch TCRα/δ loci. A. distribution of the Vα/δ genes in the Chicken TCRα/δ locus. Vα/δ genes are color coded by group. Vα1 are green, Vα2 are blue, Vδ1 are orange. D genes appear in black, J genes are light gray and C regions are in dark gray. B. Zebra finch gene segments and constant regions are color coded as in the chicken. Vδ genes that belong to a subgroup not found in chicken are shown in yellow. A VHδ gene present in finch is shown in red.
Figure 2.
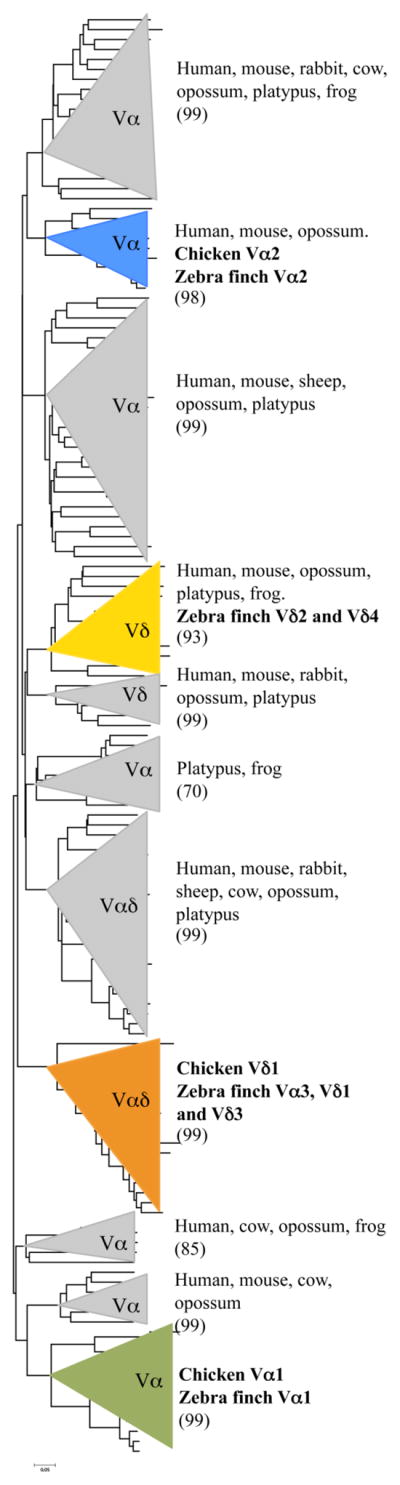
Minimum evolution tree showing the phylogenetic relationship of TCR Vα/δ gene segments from avian species compared to mammals and to an amphibian. Main groups are encased by triangles and species in each group are indicated. Bootstrap values based on 1000 replicates are shown in parenthesis for each group. Avian groups are color coded as in figure 1. Nucleotide sequences of the V regions were aligned using ClustalX (Thompson et al. 1997) and BioEdit (Hall 1999) programs. Phylogenetic analyses were performed using MEGA version 4.1. (Kumar et al. 2004). GenBank numbers for sequences used in this analysis are provided in supplementary table 1.
The chicken TCRα/δ locus is substantially larger than its ortholog in the zebra finch (Parra et al. 2012). The zebra finch TCRα/δ locus is also located in chromosome 27 but is only 100 kb from the most 5′ functional V gene segment to the Cα region at the 3′ end (Fig. 1B). There is an additional V pseudogene (lacking an ORF) approximately 220kb upstream of the functional V genes (Fig. 1B). The chicken locus contains a substantially greater number of V (n = 96) and Jα genes (n = 48) than does the zebra finch (n = 13 Vα/δ; n = 14 Jα) (Fig. 1) (Parra et al. 2012). However, in spite of having fewer numbers of V genes, those in the zebra finch TCRα/δ locus are more diverse than in chickens, based on their distribution in a phylogenetic analysis (Fig. 2). Although there are only 13 zebra finch Vα/δ gene segments they can be classified into seven different subgroups based on nucleotide identity. The zebra finch Vα3, Vδ1 and Vδ3 subgroups cluster with chicken Vδ1, but are distinct subgroups (supplementary figure 1). Zebra finch Vα1 and Vα2 cluster with chicken Vα1 and Vα2, respectively (supplementary figure 1). An in silico search of other available avian transcriptome and whole genome shotgun sequences publically available uncovered Vα/δ genes in turkey, parrot, grouse, and duck. These sequences are located in short-unassembled contigs. Unfortunately the TCRα/δ locus in the turkey contains abundant sequence gaps making it impossible to compare the overall organization (Dalloul et al. 2010, Parra et al. 2012). These other avian Vα/δ sequences also cluster with the three groups found in the chicken (Fig. 2 and supplementary figure 2). Only the zebra finch Vδ2 and Vδ4 genes fell outside these three subgroups and appear unique to this passerine species. When all available avian Vα/δ genes were compared in a phylogenetic analysis to sequences from other tetrapods (amphibians, placentals, marsupials, and monotremes), ten major groups with good bootstrap support were evident (Fig. 2). Two of the groups contain sequences from only avian species (Fig. 2).
Zebra finch Vδ2 and Vδ4 fall in the same group as Vδ sequences from human (Vδ2), mouse (Vδ4), opossum (Vδ2 and Vδ3), platypus (Vδ1 subgroup) and frog (Vδ1.1 and Vδ1.2) (Fig. 2 and supplementary fig. 1). Curiously all the sequences in this group appear to be only used in TCRδ (Triebel et al 1988, Loh et al. 1988, Giudicelli et al. 2005, Parra et al. 2008, Parra et al. 2010). Given the species distribution of these exclusively Vδ genes, it is likely that they were present in a tetrapod common ancestor, but may have been lost in at least some Galliformes and possibly other avian lineages.
As demonstrated previously, chicken Vα1 gene segments are encoded by a single exon, with no intron between the sequence encoding the leader peptide and the sequence encoding the extra-cellular V domain (Göbel et al. 1994). All potentially functional Vα1 gene segments in the chicken TCRα/δ locus have this unusual characteristic. However, the related complete Vα1 gene segments identified in zebra finch and parrot, all contain an intron. Therefore it appears that during avian evolution some members of the Vα1 subfamily lost their intron, and these were expanded in chickens.
Although the various avian lineages analyzed all clearly contain Vα genes from the same three ancient lineages, there has been independent divergence and species-specific expansion of each subfamily. For example, Vα1 members from chicken and zebra finch clearly are derived from a common ancestral gene and share high percent nucleotide identity (55 to 81%). However, phylogenetic analyses revealed that these genes form separate, species-specific subclades, one clade for chicken and one for zebra finch (not shown). Similar results were obtained when comparison between chicken and zebra finch Vα2 genes was performed. Chicken and zebra finch Vα2 gene segments share a percent nucleotide identity that varies from 63 to 78%. However in a phylogenetic tree, chicken and zebra finch Vα2 genes form separate subclades (not shown).
Zebra finch genes that belong to the subgroups Vα3, Vδ1 and Vδ3 share less than 75% identity at the nucleotide level, therefore were classified as distinct subgroups (Parra et al. 2012). Zebra finch genes from these subgroups fall in the same phylogenetic clade as the chicken Vδ1 genes (Fig. 2). Chicken and zebra finch genes from this group share 46 to 68% identity when compared at the nucleotide level. However, V genes from chicken Vδ1 subgroup are more similar to each other than to any of the genes from zebra finch Vα3, Vδ1 and Vδ3 subgroups.
Another major difference between chicken and zebra finch is the presence of a VHδ gene and a second [Dδ-Jδ-Cδ] cluster in the zebra finch TCRα/δ locus, which are both lacking from the chicken ortholog (Parra et al. 2012). An inverted Vδ gene segment found in the TCRα/δ locus in the three mammalian lineages (placentals, marsupials and monotremes) appears to be mammal specific in that it is absent in the zebra finch, chicken and in the amphibian Xenopus tropicalis (Parra et al. 2008, 2010, 2012).
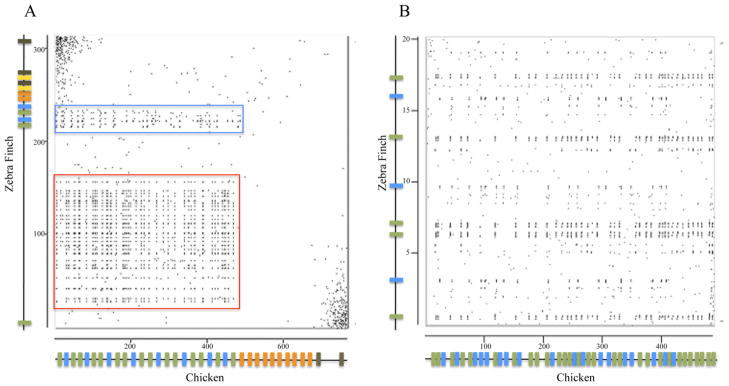
Examination of the whole TCRα/δ loci in chickens and zebra finch suggest that major rearrangements have occurred from the time the two species shared a common ancestor (90–120 MYA, Tuinen and Hedges 2001). Dot plot analysis shows sequence similarities between chicken and finch in the places corresponding to Vα1 and Vα2 groups and in the sequences encoding feather β-keratin genes (Fig. 3). Linked to the TCRα/δ locus in birds is a cluster of feather β-keratin genes that have been described (Greenwold and Sawyer 2010). A comparison of the location of the feather β-keratin genes relative to the location of TCRα/δ was performed. In the chicken some feather β-keratin genes (FK1 to FK39) are interspersed among the Vα1 and Vα2 gene segments (indicated by a red box in Fig. 3). The remaining feather β-keratin genes (FK40-FK61) located in chromosome 27 are found upstream of the TCRα/δ locus in chicken (not shown, Greenwold and Sawyer 2010). In the zebra finch the feather β-keratins are not interspersed with the functional Vα/δ gene segments, but many are found between the TCRα/δ locus and the distal Vα pseudogene (indicated by a red box in Fig. 3). The β-keratin genes linked to TCRα/δ in chicken and finch are not orthologous (Greenwold and Sawyer 2010). Therefore, it is difficult to determine whether the current organizations in chicken and finch represent an expansion of the Vα/δ genes into the β-keratin region in chicken or a loss of Vα/δ genes from this region in finch.
Figure 3.
Dot plot comparison between zebra finch and chicken TCRα/δ loci. A. Dot plot analysis of 314kb region containing the zebra finch TCRα/δ locus (vertical axis) compared to 767kb region that contains the chicken TCRα/δ locus (horizontal axis). Approximate TCR gene locations are shown as colored bars on each axis. Genes are color coded as in figure 1. The red box indicates the regions that contain feather β-keratin genes. The blue box is shown in greater detail in 3B. B. Dot plot analysis of zebra finch (20kb) and chicken (485kb) regions that contains Vα1 and Vα2 subgroups. Genomic sequences were compared using the Spin program of Staden Package (staden.sourceforge.net).
In conclusion, the Vα/δ repertoire observed in Galliformes and Passeriformes appears to be the product of lineage specific deletions and expansions. Chickens have an expanded number of Vα/δ genes, at least relative to the zebra finch, but have not retained necessarily the level of ancient diversity that finches have. A group of V genes exclusively used in TCRδ chains known from mammals, which were thought to be lacking from birds due to their absence in chickens, are in fact ancient in amniotes and have been retained in the passerines. The unusual intronless characteristic of avian Vα1 genes appears to be unique to chickens (Göbel et al. 1994), although examination of Vα1 sequences from other Galliformes is necessary to rule out their presence in other members of this lineage. However Vα1 genes are derived from an ancestral gene that still has its introns in other lineages such as Passeriformes (finches) and Psittaciformes (parrots).
Supplementary Material
Acknowledgments
This article was made possible in part by support from a National Institutes of Health grant no. IP20RR18754 from the Institutional Development Award (IDeA) program and National Science Foundation award IOS-0641382.
References
- Chen CH, Six A, Kubota T, Tsuji S, Kong FK, Göbel TW, Cooper MD. T cell receptors and T cell development. Curr Top Microbiol Immunol. 1996;212:37–53. doi: 10.1007/978-3-642-80057-3_5. [DOI] [PubMed] [Google Scholar]
- Dalloul RA, et al. Multi-platform next-generation sequencing of the domestic turkey (Meleagris gallopavo): genome assembly and analysis. PLoS Biol. 2010;8:e1000475. doi: 10.1371/journal.pbio.1000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MM, Chein YH. T cell antigen receptors. In: Paul WE, editor. Fundamental Immunology. 6. Lippincott; Philadelphia: 2008. pp. 313–345. [Google Scholar]
- Greenwold MJ, Sawyer RH. Genomic organization and molecular phylogenies of the beta (beta) keratin multigene family in the chicken (Gallus gallus) and zebra finch (Taeniopygia guttata): implications for feather evolution. BMC Evol Biol. 2010;10:148. doi: 10.1186/1471-2148-10-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göbel TW, Chen CL, Lahti J, Kubota T, Kuo CL, Aebersold R, Hood L, Cooper MD. Identification of T-cell receptor alpha-chain genes in the chicken. Proc Natl Acad Sci USA. 1994;91:1094–1098. doi: 10.1073/pnas.91.3.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- International Chicken Genome Sequencing Consortium . Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature. 2004;432:695–716. doi: 10.1038/nature03154. [DOI] [PubMed] [Google Scholar]
- Koop BF, Wilson RK, Wang K, Vernooij B, Zallwer D, Kuo CL, Seto D, Toda M, Hood L. Organization, structure, and function of 95 kb of DNA spanning the murine T-cell receptor C alpha/C delta region. Genomics. 1992;13:1209–1230. doi: 10.1016/0888-7543(92)90039-u. [DOI] [PubMed] [Google Scholar]
- Kubota T, Wang J, Göbel TW, Hockett RD, Cooper MD, Chen CH. Characterization of an avian (Gallus gallus domesticus) TCR ad gene locus. J Immunol. 1999;163:3858–3866. [PubMed] [Google Scholar]
- Kumar S, Tamura K, Nei M. MEGA3: Integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief Bioinform. 2004;5:150–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- Loh EY, Cwirla S, Serafini ET, Phillips JH, Lanier LL. Human T-cell-receptor d chain: Genomic organization, diversity, and expression in populations of cells. Proc Natl Acad Sci USA. 1988;85:9714–9718. doi: 10.1073/pnas.85.24.9714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra ZE, Baker ML, Hathaway J, Lopez AM, Trujillo J, Sharp A, Miller RD. Comparative genomic analysis and evolution of the T cell receptor loci in the opossum Monodelphis domestica. BMC Genomics. 2008;9:111. doi: 10.1186/1471-2164-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra ZE, Ohta Y, Criscitiello MF, Flajnik MF, Miller RD. The dynamic TCRδ: TCRδ chains in the amphibian Xenopus tropicalis utilize antibody-like V genes. Eur J Immunol. 2010;40:2319–2329. doi: 10.1002/eji.201040515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra ZE, Mitchell K, Dalloul RA, Miller RD. A Second TCRδ Locus in Galliformes Uses Antibody-like V Domains: Insight into the Evolution of TCRδ and TCRμ Genes in Tetrapods. J Immunol. 2012;188:3912–3919. doi: 10.4049/jimmunol.1103521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rast JP, Anderson MK, Strong SJ, Luer C, Litman RT, Litman GW. Alpha, beta, gamma, and delta T cell antigen receptor genes arose early in vertebrate phylogeny. Immunity. 1997;6:1–11. doi: 10.1016/s1074-7613(00)80237-x. [DOI] [PubMed] [Google Scholar]
- Reinink P, Rhijn IV. The bovine T cell receptor alpha/delta locus contains over 400 V genes and encodes V genes without CDR2. Immunogenetics. 2009;61:541–549. doi: 10.1007/s00251-009-0384-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyanarayana K, Hata S, Devlin P, Roncarolo MG, De Vries JE, Spits H, Strominger JL, Krangel MS. Genomic organization of the human T-cell antigen-receptor a/d locus. Proc Natl Acad Sci USA. 1988;85:8166–8170. doi: 10.1073/pnas.85.21.8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden package. http://staden.sourceforge.net/
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triebel F, Faure F, Mami-Chouaib F, Jitsukawa S, Griscelli A, Genevée C, Roman-Roman S, Hercend T. A novel human Vδ gene expressed predominantly in the TiγA fraction of γ/δ+ peripheral lymphocytes. Eur J Immunol. 1988;18:2021–2027. doi: 10.1002/eji.1830181223. [DOI] [PubMed] [Google Scholar]
- Uenishi H, hiraiwa H, yamamoto R, yasue H, takagaki Y, shiina T, kikkawa E, inoko H, awata T. Genomic structure around joining segments and constant regions of swine T-cell receptor a/d (TRA/TRD) locus. Immunology. 2003;109:515–526. doi: 10.1046/j.1365-2567.2003.01695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tuinen M, Hedges SB. Calibration of avian molecular clocks. Mol Biol Evol. 2001;18:206–213. doi: 10.1093/oxfordjournals.molbev.a003794. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.