Abstract
It was recently reported that inhibitory molecules such as PD-1 were up-regulated on CD8+ T cells during acute Friend retrovirus infection, and that the cells were prematurely exhausted and dysfunctional in vitro. The current study confirms that most activated CD8+ T cells up-regulated expression of PD-1 during acute infection and revealed a dichotomy of function between PD-1hi and PD-1lo subsets. More PD-1lo cells produced anti-viral cytokines such as IFNγ and TNFα, while more PD-1hi cells displayed characteristics of cytotoxic effectors such as production of granzymes and surface expression of CD107a. Importantly, CD8+ T cells mediated rapid in vivo cytotoxicity and were critical for control of acute Friend virus replication. Thus direct ex vivo analyses and in vivo experiments revealed high CD8+ T cell functionality and indicate that PD-1 expression during acute infection is not a marker of T cell exhaustion.
Introduction
CD8+ T cells, which produce cytokines and cytotoxic molecules to fight virus-infected cells, are crucial for the control of virus replication in many virus infections. However, in several chronic virus infections, like HIV or HCV infection in humans, virus-specific CD8+ T cells become functionally exhausted, which likely contributes to the inability of the host to eliminate the pathogen (1, 2). One mechanism leading to CD8+ T cell dysfunction is the ligation of inhibitory receptors that induce T cell exhaustion (3–5). Blocking such receptor-ligand interactions partially restores T cell function and reduces viral loads in chronically infected animals (3, 6). The inhibitory receptor that has been studied in most detail is programmed death 1 (PD-1), which is one of the prototype inhibitory receptors described as a potent mediator of T cell exhaustion in chronic viral infection (3). It has been shown in recent studies that effector T cells already express PD-1 during acute infections. This was found in acute virus infections of humans with Epstein Barr virus (EBV) (7), Hepatitis C virus (HCV) (8), and Hepatitis B virus (HBV) (9) and monkeys with Simian Immunodeficiency virus (SIV) (10) and SIV-HIV hybrid virus (SHIV) (11). The SIV study provided evidence that T cell receptor stimulation is inducing the PD-1 expression on CD8+ T cells (10). However, the functional role of enhanced PD-1 expression on CD8+ T cells during acute infections is still poorly understood and remains controversial. Whereas some studies showed a correlation between the expression levels of PD-1 and reduced CD8+ T cell functions (11, 12) others did not find any association between PD-1 expression and CD8+ T cell responses (7). In addition, PD-1 expression by virus-specific CD8+ T cells seems to correlate with the clinical outcome of acute hepatitis B (9) but not acute HCV infection (8). Results are also controversial when comparing studies on acute infections in which the PD-1 signaling was blocked by using PD-L1 knockout mice or PD-L1-specific antibodies. In these experiments all possible outcomes, from enhanced to unchanged or even decreased CD8+ T cell responses and infection levels were observed (7, 13–16). Not surprisingly, Brown et al. (17) wrote in their recent review that the precise role of PD-1 during acute infections remains to be defined.
A recent study (18) reported that PD-1 up-regulation on virus-specific CD8+ T cells during acute Friend retrovirus (FV) infection of mice was associated with premature exhaustion rendering the CD8+ T cell response ineffectual. Such a severe T cell dysfunction during acute virus infection was not reported in any of the studies mentioned above and is thus far unique to FV. In addition, the results contradicted previous results from the FV model (19, 20) so it was of interest to investigate this finding further. We used the same FV mouse model as Takamura et al. to study the phenotypic and functional properties of effector CD8+ T cells during acute retroviral infection. Most importantly, we examined the in vivo efficacy of the antiviral CD8+ T cell response. Our results indicated that while PD-1 was indeed up-regulated during acute infection, the FV-specific CD8+ T cells were not prematurely exhausted or dysfunctional. Quite to the contrary we observed potent and rapid CD8+ T cell cytotoxicity in vivo, and dependence on the CD8+ T cell response for survival from acute FV infection.
Materials and Methods
Mice
Inbred C57BL/6 (B6) mice were maintained under pathogen free conditions. Experiments were performed using C57BL/6 (B6) and (B10 x A.BY)F1 (Y10) mice. The relevant FV resistance genotype of B6 and B10 mice is H-2b/b, Fv1b/b, Fv2r/r, Rfv3r/r. The F1 mice are more susceptible to FV infection with a resistance genotype of H-2b/a, Fv1b/b, Fv2r/s, Rfv3r/s. The B6 mice were obtained from Charles River Laboratories. All mice were females of 8–16 weeks of age at the onset of the experiments.
Virus and viral infection
The FV stock used in these experiments was FV complex containing B-tropic Friend murine leukemia helper virus (F-MuLV) and polycythemia-inducing spleen focus-forming virus and was free of lactate dehydrogenase-elevating virus (21, 22). The stock was prepared as a 10% spleen cell homogenate from BALB/c mice infected 14 days previously with 3,000 spleen focus-forming units of non-cloned virus stock. Experimental mice were injected intravenously with 0.5ml of PBS containing 20,000 spleen focus-forming units of FV. This virus was prepared from the same stock that was supplied to the Miyazawa lab by KJH.
Cell surface and intracellular staining by flow cytometry
Cell surface staining was performed using Becton Dickinson or eBioscience reagents. T cell antibodies were: anti-CD4 (RM4-5), anti-CD8 (53-6.7) anti-CD43 (1B11), anti-CD44 (Ly-24), anti-CD122 (TM-β1), anti-CD62L (MEL-14), anti-CD127 (A7R34), anti-Bcl2 (3F11), anti-2B4 (eBio244F4), anti-BTLA (6F7), anti-PD-1 (J43), anti-LAG3 (eBioC987W), anti-CD107a (1D4B), and anti-PD-L1 (MIH-5), anti-Tim-3 (8B.2C12). Dead cells (propidium iodide positive) were excluded from analyses. Intracellular granzyme A (polyclonal rabbit-anti-mouse granzyme A IgG, protein A purified) and granzyme B (monoclonal anti-human granzyme B (GB11), Invitrogen, Darmstadt, Germany) staining was performed as described (23). For intracellular cytokine staining, spleen cells were incubated with plate-bound anti-CD3 (145-2C11) and soluble anti-CD28 ( 37.51) (2 μg/ml) for 5 h at 37°C in the presence of brefeldin A (2 μg/ml). Cells were washed twice, incubated with anti-Fcγ 2/3 receptor (2.4G2) to block Fc receptors, and stained with anti-CD8, anti-CD43, and anti-PD-1 in round-bottom 96-well plates. The cells were then washed, permeabilized using the Cytofix/Cytoperm intracellular staining kit (Becton Dickinson), and reacted with monoclonal antibodies specific for IL-2 (JES6-5H4), gamma interferon (IFNγ) (XMG1.2), and tumor necrosis factor alpha (TNFα) (MP6-XT22). Data were acquired on a LSR II flow cytometer (Becton Dickinson) from 100,000–300,000 lymphocyte-gated events per sample. Analyses were done using FlowJo (Treestar) and FACSDiva software (Becton Dickinson).
Tetramers and tetramer staining
For detection of Db-GagL-specific CD8+ T cells spleen cells were stained with APC labelled anti-CD8 (Ly-2), FITC labelled CD43 (1B11) and PE labelled MHC class I H2-Db tetramers specific for FV GagL peptide (24, 25) (Beckman Coulter, Marseille, France).
In vivo cytotoxicity assay
The in vivo CTL assay described by Barber et al. (26) was modified to measure cytotoxicity in FV-infected mice. Splenocytes from naive mice were loaded with 1–5 μM DbGagL peptide (24, 25). The loaded cells were then stained with 200 nM CFSE (Molecular Probes, Eugene, OR). As control, spleen cells without peptide were stained with 2 nM CFSE. Splenocytes (0.5–1 × 107 cells of each population) were transferred i.v. into naïve or FV-infected mice. Two hours after the adoptive transfer, spleens from the recipient mice were harvested and cell suspensions were prepared. Target cells were distinguished from recipient cells and from one another based on CFSE staining. The percentage of killing was calculated as follows: 100 - ([(% peptide pulsed in infected/% unpulsed in infected)/(%peptide pulsed in uninfected/% unpulsed in uninfected)] × 100).
PDL-1 and Tim-3 blockade and CD8-depletion
Y10 mice were infected with FV by i.v. injection of 0.5 mL phosphate-buffered balanced salt solution containing 6000 spleen focus forming units of FV complex. 250 ug of anti-PDL-1 (10F.9G2) (BioXCell) or anti-Tim-3 (RMT3-23) (BioXCell) or control rat IgG (BioXCell) was given i.p. at the time of infection and every other day for a total of 5 injections. For CD8+ T cell depletions mice were given a total of 3 i.p. injections every other day of 0.5 mL supernatant harvested from hybridoma cell line 169.4 cultures. Mice were then infected with FV 2 days following the last treatment.
Infectious centers assay
Dilutions of splenocytes were incubated at 37°C and 5% CO2 on Mus dunni cells for 2 days. Cells were then fixed with 95% ethanol, stained with F-MuLV envelope-specific mAb 720 and then viral foci developed with peroxidase-conjugated goat anti-mouse IgG and substrate.
Statistical Analysis
Statistics comparing two groups were done using the non-parametric t-test. When more than two groups were compared a one-way ANOVA was used with a Tukey post-test (GraphPad Prism software; GraphPad Software Inc., San Diego, USA).
Results
PD-1 expression on activated virus-specific CD8+ T cells during acute FV infection
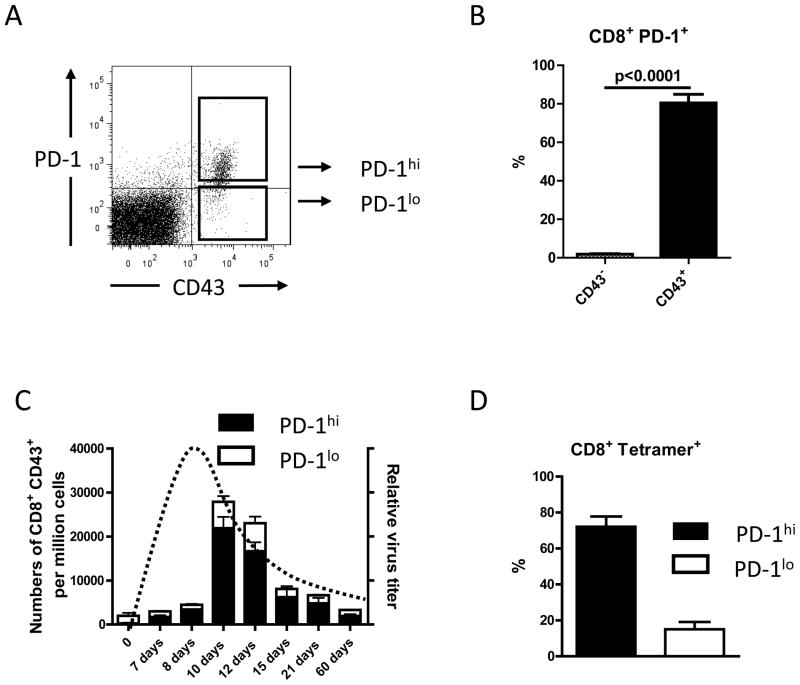
Since Takmura et al. showed up-regulation of the PD-1 inhibitory molecule already at 2 weeks post FV infection, we first sought to replicate this finding and determine the kinetics of PD-1 expression on activated CD8+ T cells throughout the course of FV infection. Figure 1A shows a representative dot plot of activated (CD43+) CD8+ cells from mice infected with FV for 10 days, which demonstrates the gating strategy for PD-1hi and PD-1lo cells. The expression of PD-1 was strongly correlated with the activation status of the cells (Fig.1B). The kinetic analysis revealed that the proportion of PD-1hi cells was already increased at 7 days post-infection (dpi) and remained elevated during both the expansion and contraction phases of the CD8+ T cell response. At the peak of the CD8+ T cell response at 10 dpi more than 80% of the activated CD8+ T cells were PD-1hi (Fig. 1B). PD-1 expression on the immunodominant subset of tetramer positive CD8+ T cells (specific for the immunodominant H-2Db-restricted F-MuLV glycosylated gag epitope (24, 25)) was similar to the overall population of activated CD8+ T cells (Fig. 1C).
Figure 1. Expression of PD-1 on activated CD8+ T cells during acute FV infection.
CD8+ T cells from FV infected B6 mice were stained for the activation-associated glycoform of CD43 and PD-1 to detect the expression of PD-1 on the total population of activated CD8+ T cells at different time points after FV infection. CD8+ T cells positive for CD43 during acute FV infection expressed several other effector T cell markers, like CD44 and CD69, and were negative for CD62L (data not shown (23)).
A. A representative dot plot shows how gated CD8 T cells were stained for the activation-associated glycoform of CD43 and PD-1 to analyze the two different populations of activated CD8+ T cells. B. The percentages of PD-1 positive CD8+ T cells expressing the activation marker CD43 (black bar) or were CD43 negative (white bar) are shown for a group of 6 – 10 mice on day 10 post FV infection. Data were pooled from two independent experiments with similar results. Each column represents the mean percentage plus SEM. Statistically significant differences between the groups are indicated by a P value. C. Kinetic analysis of the expansion of activated (CD43+) CD8+ T cells and their relative expression of PD-1 (hi=high; lo=low). Each column represents mean numbers of CD43+ CD8+ T cells per one million nucleated cells for a group of 6 – 10 mice. Black bars show the numbers of PD-1hi CD43+ CD8+ cells plus SEM and white bars show numbers of PD-1lo CD8+ CD43+ T cells plus SEM. Data were pooled from two independent experiments with similar results. The kinetics of the relative virus titer is indicated by a dotted line. D. The percentages of DbGagL class I tetramer reactive virus-specific CD8+ T cells, which were PD-1hi (black bar) or PD-1lo (white bar) are shown for a group of 5 – 10 mice on day 10 post FV infection. Data were pooled from two independent experiments with similar results. Each column represents the mean percentage plus SEM of CD8+ tetramer+ T cells.
Phenotypic characteristics of PD-1hi and PD-1lo CD8+ T cells during acute FV infection
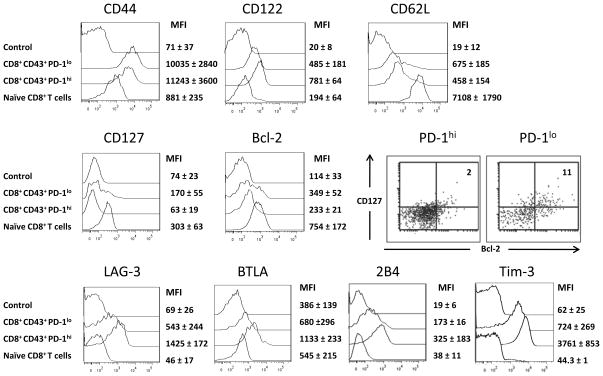
Both the PD-1hi and PD-1lo subsets were analyzed by multi-parameter flow cytometry to determine if other phenotypic differences distinguished them. Splenocytes from mice at peak expansion (10 dpi) were gated on CD8+ CD43+ PD-1hi or PD-1lo cells, and analyzed for the expression of characteristic CD8+ T cell markers. Both subsets expressed similar levels of the effector cell marker CD44 (hyaluronic acid receptor) and down-regulated CD62L (L-selectin). The PD-1hi subset displayed the phenotype of short-lived effector cells (27) with increased expression of CD122 (IL-2s receptor) and CD127 (IL-7 receptor) (Fig. 2). As expected for short-lived effectors, they also down-regulated the anti-apoptotic protein Bcl-2 (28)(Fig. 2). Analysis of the PD-1lo CD8+ T cells revealed a small subset with increased expression of CD127 and/or BCL-2 (Fig. 2). Dual staining showed that a proportion of the PD-1lo CD8+ T cells were double positive for CD127 and Bcl-2 (Fig. 2), characteristic of memory precursor effector cells, which are important for the development of effector and central memory cells (29). As shown by Takmura et al., PD-1hi CD8+ T cells were also positive for the inhibitory receptor LAG-3 (Fig. 2). Expression of inhibitory receptors BTLA, 2B4 (6), and Tim-3 (18) was also observed. In contrast, PD-1lo cells were predominantly low for LAG-3, BTLA, 2B4, and Tim-3.
Figure 2. Phenotype of activated PD-1hi and PD-1lo CD8+ T cells from acutely FV infected mice.
C57BL/6 mice were infected with FV and CD8+ T cells were harvested at 10 dpi.
A. Multi-parameter flow cytometry was used to compare activated (CD43+) PD-1hi and PD-1lo CD8+ T cells for expression of CD44, CD122, CD62L, CD127, and intracellular Bcl-2. Staining with control antibody and staining of CD8+ T cells from naive mice are shown as negative controls. Dual staining of gated CD8+ CD43+ T cells for CD127 and Bcl-2 is shown in a representative dot plot (numbers represent the percentage of double positive cells). The expression of the inhibitory receptors LAG-3, BTLA, 2B4, and Tim-3 on activated PD-1lo or PD-1hi CD8+ T cells was analyzed. 6 – 9 mice per group were analyzed in three independent experiments. Representative histogram or dot plots and the mean fluorescence intensities plus SEM for each group are shown. Significant differences between the two cell populations were found for P< .01: CD122 and CD127; P< .001: Bcl-2; P< .0001: LAG-3, BTLA, 2B4, and Tim-3.
Functional properties of PD-1hi and PD-1lo CD8+ T cells during acute FV infection
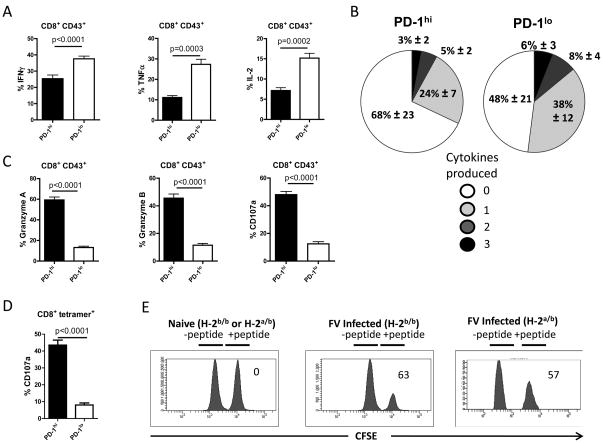
To analyze whether PD-1hi and PD-1lo effector T cells had different functional properties during acute FV infection, the production of cytokines, cytotoxic molecules, and surface expression of the degranulation marker CD107a (30, 31) was analyzed. At 10 dpi the mean percentage of PD-1lo CD43+ CD8+ T cells that produced the cytokines IFNγ, TNFα, or IL-2 was significantly higher than that of PD-1hi cells (Fig. 3A). Since CD8+ T cells producing multiple cytokines have been uniquely associated with non-progression in HIV infection (32), we measured the number of cytokines produced by individual T cells of the PD-1hi versus PD-1lo T cell population. Figure 3B shows that PD-1lo cells were more effective in producing multiple cytokines than PD-1hi cells. To analyze the cytotoxic potential of the subpopulations intracellular expression of the granzymes A and B and surface expression of the degranulation marker CD107a was measured. Although most of the PD-1lo cells produced cytokines, only a small percentage of them expressed granzymes or CD107a (Fig. 3C). In contrast, approximately half of the PD-1hi cells were positive for markers associated with cytotoxicity. Analysis of CD107a expression in the PD-1hi and PD-1lo subsets of tetramer+ virus-specific CD8+ T cells produced similar results to the general population of activated CD8+ T cells (Fig. 3D). In absolute numbers per spleen these percentages calculated to approximately 3×106 tetramer+ cells of the PD-1hi subset expressing CD107a compared to less than 105 cells of the PD-1lo subset (data not shown). Thus the direct ex vivo analysis of CD8+ T cells from acutely infected mice showed no evidence of exhaustion or dysfunction.
Figure 3. Functional properties of activated PD-1hi and PD-1lo CD8+ T cells during acute FV infection.
C57BL/6 mice were infected with FV and CD8+ T cells were harvested at 10 dpi.
Multi-parameter flow cytometry was used to compare activated (CD43+) PD-1hi and PD-1lo CD8+ T cells for intracellular production of the cytokines IFNγ, TNFα, and IL-2 and the production of granzymes or the expression of the degranulation marker CD107a. A. Mean percentages plus SEM of CD43+ CD8+ T cells producing IFNγ, TNFα, or IL-2 are shown. B. Mean percentages plus SEM of individual CD43+ CD8+ T cells producing no, 1, 2, or 3 different cytokines at the same time. The figure shows PD-1hi (left) versus PD-1lo (right) cells using data pooled from two independent experiments (n=8) with similar results. C. Mean percentages plus SEM of activated PD-1hi and PD-1lo CD8+ T cells expressing granzyme A, granzyme B or CD107a. D. Mean percentages plus SEM of PD-1hi or PD-1lo tetramer+ CD8+ T cells expressing the degranulation marker CD107a. Each column represents the mean percentage plus SEM for a group of 5 – 10 mice. Data were pooled from two independent experiments with similar results. Differences were analyzed by the unpaired t-test. Statistically significant differences between the groups are indicated by P values.
E. Representative histograms showing killing of target cells loaded with the FV DbGagL peptide (CFSE high; +peptide) or control cells without peptide (CFSE low; -peptide) in spleens of naïve or FV infected mice with an H-2b/b (C57BL/6) or H-2a/b ((B10.A x A.BY)F1) genetic background. Numbers in the figure indicate the mean percentage of killing for the whole group. Data from one representative mouse of a group of 7 mice are shown. Two independent experiments with similar results were performed.
Rapid in vivo killing during acute FV infection
As the elimination of virus-infected cells is one of the most critical functions of CD8+ T cells in vivo CTL assays were next performed at 10 dpi both in resistant C57BL/6 (H-2b/b) mice and more susceptible Y10.A (H-2a/b)F1 mice. Splenocytes loaded with the same viral peptide recognized by tetramer+ CD8+ T cells were injected intravenously into FV-infected or non-infected mice. In C57BL/6 mice average of 63% of peptide-loaded target cells were eliminated in infected mice within 2 hours with equivalent killing in H2a/b F1 mice (Fig. 3E). This rapid killing demonstrated the high cytotoxic activity of CD8+ T cells during acute FV infection. These data indicate potent antiviral effector functions of CD8+ T cells inconsistent with premature exhaustion and dysfunction.
Blocking the PD-1/PDL-1 and Tim-3 pathways during acute FV infection
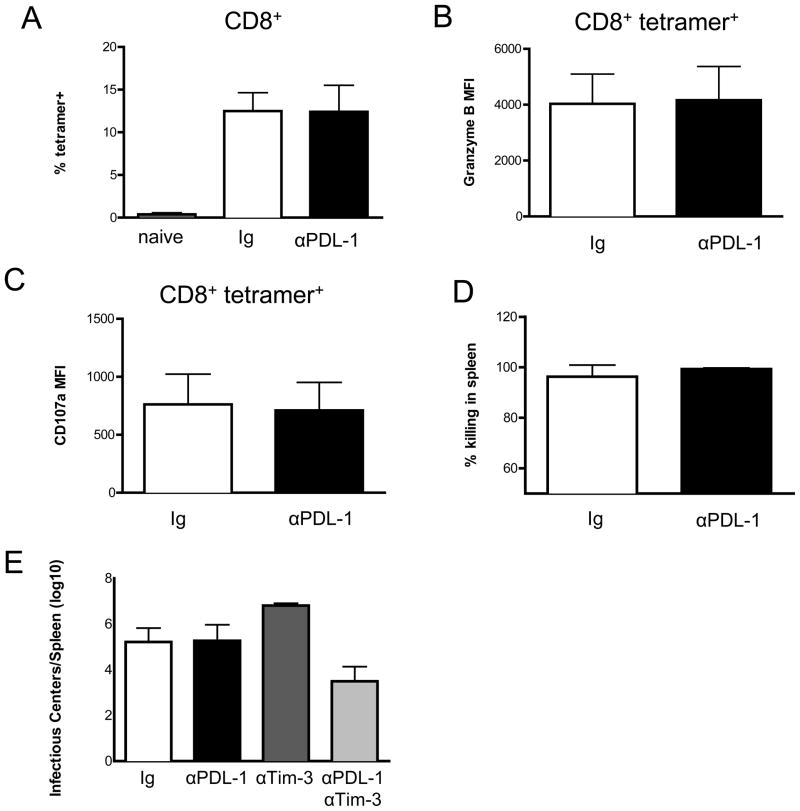
Although several studies have shown that blockade of PD-1 signaling by treatment with anti-PDL-1 antibodies during chronic infections can reactivate exhausted CD8+ T cells to reduce chronic viral loads, studies on acute infections are limited and somewhat contradictory (17). To determine the effects of PD-1/PDL-1 signaling blockade during acute FV infection, C57BL/6 mice were treated with anti-PDL-1 blocking antibody during the first 10 days of infection. No significant increase of the mean percentage of virus-specific (tetramer+) CD8+ T cells was found compared to acutely infected mice that were treated with an irrelevant isotype control antibody (Fig. 4A). Functional analysis of the tetramer+ CD8+ T cells also revealed no effects on either granzyme B expression (Fig. 4B) or CD107a expression (Fig. 4C). Finally, in two independent in vivo CTL experiments mice treated with control antibody showed over 90% killing and anti-PDL-1 blockade produced no significant enhancement of killing (Fig. 4D). Furthermore anti-PDL-1 blockade produced no reduction in spleen virus levels (black bar, Fig. 4E). Thus blocking the PD-1-PDL-1 pathway during acute FV infection produced no significant enhancement of CD8 responses. Since no effect on CTL killing or infection levels was observed from PDL-1 blockade, we wanted to analyze the effect of a combined blockage of two inhibitory receptors during acute FV infection. Takamura et al. found that PD-1 signaling blockade combined with blockade of Tim-3 produced a pronounced effect on virus levels. We also observed a significant reduction in spleen virus titers when PDL-1 blockade was combined with Tim-3 blockade (light gray bar, Figure 4E) but it was not as dramatic as reported by Takamura et al.. Dual blockade did not significantly increase in vivo CTL killing (data not shown), but again, that is likely because killing was already above 90% without therapeutic intervention. Interestingly, Tim-3 blockade by itself resulted in paradoxically increased spleen virus titers, suggesting that it might play a positive role in anti-FV immunity in the presence of intact PD-1 signaling. Contrasting roles for Tim-3 in regulating immune responses have previously been reported, predominantly affecting type 1 T helper responses (33). These results suggest that some negative signaling of immune responses occurs during acute FV infection, but that it requires more than one signal and does not induce premature exhaustion of CD8+ T cells.
Figure 4. Inhibitory signal blockade during acute FV infection.
Mice were treated with blocking antibodies at the time of infection and every other day for a total of 5 injections. A. CD8+ splenocytes from naïve mice (gray bar) or mice treated with anti-PDL-1 (black bars) or isotype-matched control IgG (white bars) at 10 days post-infection were analyzed by flow cytometry for FVgagL tetramer binding. Gated CD8+ tetramer+ T cells were analyzed for B. Intracellular expression of granzyme B and C. surface expression of CD107a. Bars depict the mean fluorescent intensity (MFI). D. An in vivo cytotoxicity assay was performed using adoptive transfer of CellTrace Violet labeled control splenocytes and CFSE-labeled FVgagL peptide loaded targets into the anti-PDL-1 or control IgG treated mice at 10 dpi. Two hours after transfer, the spleens were analyzed by flow cytometry for the percentage of target cell killing. Data are combined from two independent experiments with similar results and mean values with standard deviation are shown (n=8 mice per group). E. Spleen infectious centers at 10dpi from mice treated as indicated. All columns of data were compared to the Ig control using a one-way ANOVA with Tukey’s post test. All columns were significantly different (P < .0001) except the control versus anti-PDL-1 (n=8 for the control and anti-PDL-1 groups and n=4 for anti-Tim-3 and dual treatment groups).
CD8+ T cell depletion abrogates acute virus control
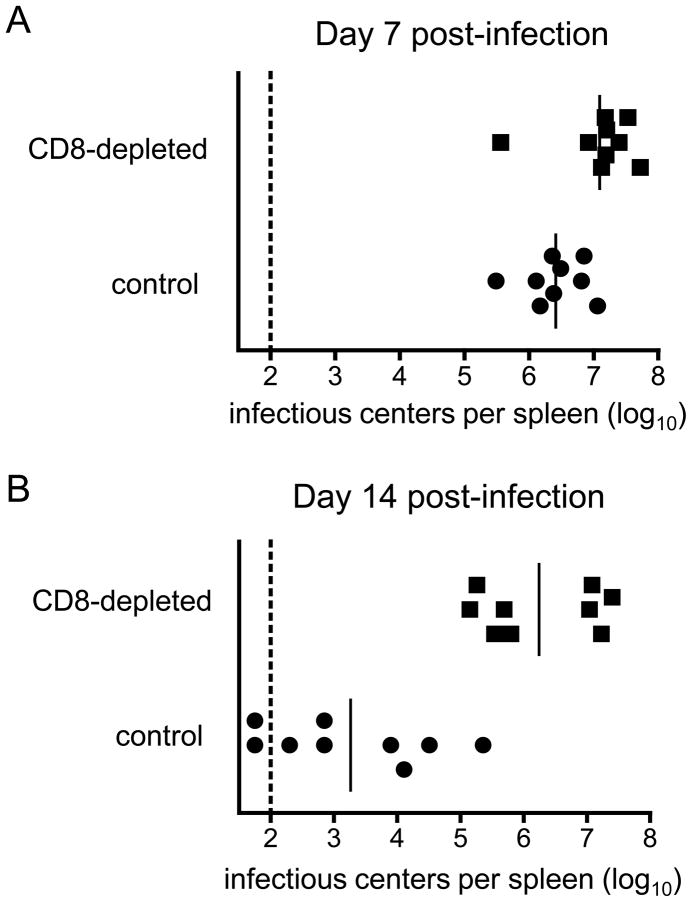
Ultimately the functionality of the CD8+ T cell response during acute FV infection is measured by its ability to control virus replication in vivo. H-2b/b mice were depleted of CD8+ T cells, infected with FV, and spleen virus titers were measured at 7 and 14 dpi. At 7dpi CD8-depeleted mice had an approximately half log10 increase in virus spleen virus titers (Figure 5A). By 14 dpi, when most CD8-depleted mice were recovering from acute infection, virus titers remained very high in the CD8-depleted mice (Fig. 5B). Thus CD8+ T cells not only displayed numerous hallmarks of functionality directly ex vivo and mediated rapid killing of CTL targets but were absolutely critical for recovery from acute FV infection.
Figure 5. CD8+ T cell depletion during acute FV infection.
Mice were depleted of CD8+ T cells as described the methods section and remained depleted to less than 1% of splenocytes in through 14 dpi. Data on viral loads are combined from two separate experiments. The dotted line represents the detection limit of the assay. A. Infectious centers at 7 dpi. Differences between the groups were statistically significant (P= .0192). B. Infectious centers at 14 dpi. Differences between the groups were statistically significant (P< .0001).
Discussion
The current studies provide convincing evidence that the CD8+ T cell response to FV infection is not only vigorous and effective, but is critical for virus control. As in the Takamura et al. study, we found that expression was increased in a large proportion of effector CD8+ T cells during acute FV infection. However, in contrast to their results from in vitro re-stimulation experiments, which they interpreted as premature exhaustion of the PD-1hi CD8+ T cells, our in vivo and direct ex vivo studies showed high cytolytic potential of PD-1 expressing CD8+ T cells and rapid in vivo killing of viral peptide-loaded targets. Interestingly, the function of the activated CD8+ T cells in terms of cytokine production versus cytotoxic potential corresponded to relative PD-1 expression levels. For example, figure 3 shows that approximately half of the activated PD-1lo cells made one or more cytokines, while approximately half of the PD-1hi cells expressed granzymes and the degranulation marker CD107a. These results and the phenotypic analysis shown in Fig.2 strongly suggest that the minor PD-1lo subset was a potent producer of cytokines and contained a significant proportion of effector memory precursors, whereas the PD-1hi CD8+ T cells were terminally differentiated effector cells that were the main mediators of cytotoxic activity during acute FV infection. This lytic activity was clearly demonstrated in in vivo CTL assays using target cells labeled with the same FV epitope peptide that we and Takamura et al. used to detect virus-specific CD8+ T cells. Rapid and efficient FV-specific killing was detected in both FV-resistant C57BL/6 and FV-susceptible Y10.A mice indicating that CD8+ T cell cytotoxicity was not influenced by the Fv-2 genetic background of mice, as had been discussed in two letters to the Journal of Immunology (34). The results from the in vivo CTL assays also indicate that high expression of granzyme B is not, as suggested by Takamura et al., an indicator of excessive T cell activation and exhaustion, but an indicator of cytolytic potential. In fact, it is the loss of granzyme expression that has been defined as a hallmark of CD8+ T cell exhaustion (reviewed in (3, 35)).
Although PD-1 has been defined as a major inhibitory molecule on exhausted CD8+ T cells during chronic infections, and blockade of the PD-1 signaling pathway can revitalize exhausted cells, the mere up-regulation of PD-1 and other inhibitory receptors on CD8+ T cells did not correlate with an exhausted phenotype during acute infection. This finding is consistent with recent studies supporting the notion that the effect of PD-1 signalling varies between acute immunological responses and situations of chronic antigen exposure (36). Although PD-1 signalling may indeed provide an inhibitory signal, the biological effect is dependent on the overall strength of that signal in the context of other signals in the cell, both positive and negative. A comparable system of activating and inhibitory receptors controls the function of NK cells (37). During acute infections the combination of strong TCR signalling, co-stimulation, and cytokine signalling may outweigh the inhibitory effects of PD-1 on CD8+ T cells. As the infection is brought under control the signalling milieu changes from overall positive to negative such that the response contracts to prevent overwhelming cytotoxic CD8+ T cell activity that could cause severe immunopathology. This is in line with our current results showing that blockade of either PD-1 and TIM3 alone did not improve CD8+ T cell function, but blocking both at the same time resulted in a significant reduction in acute FV viral loads (Fig.4). Similar findings have been made by other groups (6) suggesting a requirement for an orchestrated inhibitory signaling machinery composed of multiple receptors to suppress T cell responses. Such a system should ensure that effector CD8+ T cells have enough time to control pathogens before they are shut down in their activity. However, our PD-1 and TIM3 blocking experiments indicate that although CD8+ T cells expressing these receptors mediate vigorous virus-specific cytotoxic responses during acute FV infection, some degree of inhibition is already initiated during this phase.
For some viruses the immune system is not able to completely clear the infection and transition to chronic infection occurs. After chronic infection has been established, signaling through inhibitory receptors sustains and contributes to the progressive functional exhaustion of the remaining effector CD8+ T cells (38). Thus, inhibitory receptors, like PD-1, are excellent markers for the identification and tracking of exhausted T cells during the course of many chronic infections (3, 12, 39) but do not indentify dysfunctional T cells during acute infections (7,8,14,15). In addition, in healthy adult humans most PD-1hi CD8+ T cells are effector memory cells rather than exhausted cells (40). During chronic FV infection virus-specific CD8+ T cells do develop functional exhaustion, which is associated with loss of granzymes, CD107a surface expression, and undetectable target cell killing in the in vivo CTL assay (41). We previously showed that this exhaustion is in large part mediated by regulatory T cells, which suppress the proliferation and function of virus-specific CD8+ T cells. Taken together, our current results from ex vivo and in vivo experiments preclude premature exhaustion of CD8+ T cells during acute FV infection. This is also strongly supported by several CD8+ T cell depletion studies, which show that CD8+ T cells are absolutely critical to control initial FV replication and disease progression in FV-resistant as well as FV-susceptible mouse strains (Fig. 5 and (42)).
To our knowledge this is the first study delineating PD-1hi and PD-1lo effector CD8+ T cell subsets with different functions during acute infection. We show several lines of evidence indicating that activated PD-1hi CD8+ T cells are terminally differentiated cytotoxic cells. In contrast, few activated PD-1lo CD8+ T cells produced cytotoxic molecules but most were efficient producers of cytokines, with significant proportions producing multiple cytokines, a characteristic demonstrated to mediate potent antiviral effects in several virus infections (43). Interestingly, activated PD-1lo CD8+ T cells were low in expression of inhibitory receptors indicating that this suppressive pathway does not seem to play a major role in their control. Hence, inhibitory receptors may be predominantly associated with the control of the cytotoxic function of effector CD8+ T cells. Also of import, a significant proportion of the activated PD-1lo CD8+ T cells did not have the phenotype of short-lived terminally differentiated effector cells, but had phenotypic markers indicative of effector memory precurser cells, a population of long-lived effector cells that develop into central or effector memory T cells (29). This finding implies that memory precurser T cells do not up-regulate inhibitory receptors during acute infections, most likely because their effector functions are not associated with severe immunopathology and their survival is critical for the establishment of an immunological memory. This is in line with a recent report showing that defects in PD-1 signaling results in enhanced central memory CD8+ T cell responses after an acute vaccinia virus infection (44). Thus, blocking the PD-1/PD-L1 pathway during vaccination against pathogens can increase protective memory responses (45). Understanding the role of inhibitory receptors on effector T cells may therefore be important for the design of effective vaccines against virus infections as well as new treatments for acute infections.
Acknowledgments
This work was supported by a grant to U.D. and G.Z. from the Deutsche Forschungsgemeinschaft (TRR60 project B4) and by the Division of Intramural Research at the National Institute of Allergy and Infectious Diseases, NIH (KJH and LM).
References
- 1.Pantaleo G, Soudeyns H, Demarest JF, Vaccarezza M, Graziosi C, Paolucci S, Daucher M, Cohen OJ, Denis F, Biddison WE, Sekaly RP, Fauci AS. Evidence for rapid disappearance of initially expanded HIV-specific CD8+ T cell clones during primary HIV infection. Proc Natl Acad Sci U S A. 1997;94:9848–53. doi: 10.1073/pnas.94.18.9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kantzanou M, Lucas M, Barnes E, Komatsu H, Dusheiko G, Ward S, Harcourt G, Klenerman P. Viral escape and T cell exhaustion in hepatitis C virus infection analysed using Class I peptide tetramers. Immunol Lett. 2003;85:165–71. doi: 10.1016/s0165-2478(02)00224-9. [DOI] [PubMed] [Google Scholar]
- 3.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 4.Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, Hanson HL, Steinberg JP, Masopust D, Wherry EJ, Altman JD, Rouse BT, Freeman GJ, Ahmed R, Grakoui A. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. Journal of Virology. 2007;81:2545–53. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249–58. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenough A, Shaheen SO, Shennan A, Seed PT, Poston L. Respiratory outcomes in early childhood following antenatal vitamin C and E supplementation. Thorax. 2010;65:998–1003. doi: 10.1136/thx.2010.139915. [DOI] [PubMed] [Google Scholar]
- 8.Kasprowicz V, Schulze Zur Wiesch J, Kuntzen T, Nolan BE, Longworth S, Berical A, Blum J, McMahon C, Reyor LL, Elias N, Kwok WW, McGovern BG, Freeman G, Chung RT, Klenerman P, Lewis-Ximenez L, Walker BD, Allen TM, Kim AY, Lauer GM. High level of PD-1 expression on hepatitis C virus (HCV)-specific CD8+ and CD4+ T cells during acute HCV infection, irrespective of clinical outcome. J Virol. 2008;82:3154–60. doi: 10.1128/JVI.02474-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z, Zhang JY, Wherry EJ, Jin B, Xu B, Zou ZS, Zhang SY, Li BS, Wang HF, Wu H, Lau GKK, Fu YX, Wang FS. Dynamic programmed death 1 expression by virus-specific CD8 T cells correlates with the outcome of acute hepatitis B. Gastroenterology. 2008;134:1938–49. doi: 10.1053/j.gastro.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 10.Petrovas C, Price DA, Mattapallil J, Ambrozak DR, Geldmacher C, Cecchinato V, Vaccari M, Tryniszewska E, Gostick E, Roederer M, Douek DC, Morgan SH, Davis SJ, Franchini G, Koup RA. SIV-specific CD8+ T cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood. 2007;110:928–36. doi: 10.1182/blood-2007-01-069112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santosuosso M, Righi E, Hill ED, Leblanc PR, Kodish B, Mylvaganam HN, Siddappa NB, Stevceva L, Hu SL, Ghebremichael M, Chenine AL, Hovav AH, Ruprecht RM, Poznansky MC. R5-SHIV induces multiple defects in T cell function during early infection of rhesus macaques including accumulation of T reg cells in lymph nodes. PLoS One. 2011;6:e18465. doi: 10.1371/journal.pone.0018465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–92. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talay O, Shen CH, Chen L, Chen J. B7-H1 (PD-L1) on T cells is required for T-cell-mediated conditioning of dendritic cell maturation. Proc Natl Acad Sci U S A. 2009;106:2741–6. doi: 10.1073/pnas.0813367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowe JH, Johanns TM, Ertelt JM, Way SS. PDL-1 blockade impedes T cell expansion and protective immunity primed by attenuated Listeria monocytogenes. J Immunol. 2008;180:7553–7. doi: 10.4049/jimmunol.180.11.7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lafon M, Megret F, Meuth SG, Simon O, Velandia Romero ML, Lafage M, Chen L, Alexopoulou L, Flavell RA, Prehaud C, Wiendl H. Detrimental contribution of the immuno-inhibitor B7-H1 to rabies virus encephalitis. J Immunol. 2008;180:7506–15. doi: 10.4049/jimmunol.180.11.7506. [DOI] [PubMed] [Google Scholar]
- 16.Green KA, Okazaki T, Honjo T, Cook WJ, Green WR. The programmed death-1 and interleukin-10 pathways play a down-modulatory role in LP-BM5 retrovirus-induced murine immunodeficiency syndrome. J Virol. 2008;82:2456–69. doi: 10.1128/JVI.01665-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown KE, Freeman GJ, Wherry EJ, Sharpe AH. Role of PD-1 in regulating acute infections. Curr Opin Immunol. 2010;22:397–401. doi: 10.1016/j.coi.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takamura S, Tsuji-Kawahara S, Yagita H, Akiba H, Sakamoto M, Chikaishi T, Kato M, Miyazawa M. Premature terminal exhaustion of Friend virus-specific effector CD8+ T cells by rapid induction of multiple inhibitory receptors. J Immunol. 2010;184:4696–707. doi: 10.4049/jimmunol.0903478. [DOI] [PubMed] [Google Scholar]
- 19.Hasenkrug KJ. Lymphocyte deficiencies increase susceptibility to friend virus-induced erythroleukemia in Fv-2 genetically resistant mice. J Virol. 1999;73:6468–73. doi: 10.1128/jvi.73.8.6468-6473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robertson MN, Spangrude GJ, Hasenkrug K, Perry L, Nishio J, Wehrly K, Chesebro B. Role and specificity of T-cell subsets in spontaneous recovery from Friend virus-induced leukemia in mice. J Virol. 1992;66:3271–7. doi: 10.1128/jvi.66.6.3271-3277.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robertson SJ, Ammann CG, Messer RJ, Carmody AB, Myers L, Dittmer U, Nair S, Gerlach N, Evans LH, Cafruny WA, Hasenkrug KJ. Suppression of acute anti-friend virus CD8+ T-cell responses by coinfection with lactate dehydrogenase-elevating virus. J Virol. 2008;82:408–18. doi: 10.1128/JVI.01413-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lilly F, Steeves RA. B-tropic Friend virus: a host-range pseudotype of spleen focus-forming virus (SFFV) Virology. 1973;55:363–70. doi: 10.1016/0042-6822(73)90176-1. [DOI] [PubMed] [Google Scholar]
- 23.Zelinskyy G, Kraft AR, Schimmer S, Arndt T, Dittmer U. Kinetics of CD8+ effector T cell responses and induced CD4+ regulatory T cell responses during Friend retrovirus infection. Eur J Immunol. 2006;36:2658–70. doi: 10.1002/eji.200636059. [DOI] [PubMed] [Google Scholar]
- 24.Schepers K, Toebes M, Sotthewes G, Vyth-Dreese FA, Dellemijn TA, Melief CJ, Ossendorp F, Schumacher TN. Differential kinetics of antigen-specific CD4+ and CD8+ T cell responses in the regression of retrovirus-induced sarcomas. J Immunol. 2002;169:3191–9. doi: 10.4049/jimmunol.169.6.3191. [DOI] [PubMed] [Google Scholar]
- 25.Chen W, Qin H, Chesebro B, Cheever MA. Identification of a gag-encoded cytotoxic T-lymphocyte epitope from FBL-3 leukemia shared by Friend, Moloney, and Rauscher murine leukemia virus-induced tumors. J Virol. 1996;70:7773–82. doi: 10.1128/jvi.70.11.7773-7782.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barber DL, Wherry EJ, Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J Immunol. 2003;171:27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
- 27.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–40. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–45. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–8. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 30.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 31.Rubio V, Stuge TB, Singh N, Betts MR, Weber JS, Roederer M, Lee PP. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med. 2003;9:1377–82. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- 32.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–50. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 33.Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, Chandwaskar R, Karman J, Su EW, Hirashima M, Bruce JN, Kane LP, Kuchroo VK, Hafler DA. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318:1141–3. doi: 10.1126/science.1148536. [DOI] [PubMed] [Google Scholar]
- 34.Hasenkrug KJ, Dittmer U. Comment on “Premature terminal exhaustion of Friend virus-specific effector CD8+ T cells by rapid induction of multiple inhibitory receptors”. J Immunol. 2010;185:1349. doi: 10.4049/jimmunol.1090058. author reply - 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zelinskyy G, Robertson SJ, Schimmer S, Messer RJ, Hasenkrug KJ, Dittmer U. CD8+ T-cell dysfunction due to cytolytic granule deficiency in persistent Friend retrovirus infection. J Virol. 2005;79:10619–26. doi: 10.1128/JVI.79.16.10619-10626.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–45. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 37.Long EO. Negative signaling by inhibitory receptors: the NK cell paradigm. Immunol Rev. 2008;224:70–84. doi: 10.1111/j.1600-065X.2008.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ha SJ, West EE, Araki K, Smith KA, Ahmed R. Manipulating both the inhibitory and stimulatory immune system towards the success of therapeutic vaccination against chronic viral infections. Immunol Rev. 2008;223:317–33. doi: 10.1111/j.1600-065X.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- 39.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–4. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 40.Duraiswamy J, Ibegbu CC, Masopust D, Miller JD, Araki K, Doho GH, Tata P, Gupta S, Zilliox MJ, Nakaya HI, Pulendran B, Haining WN, Freeman GJ, Ahmed R. Phenotype, function, and gene expression profiles of programmed death-1(hi) CD8 T cells in healthy human adults. J Immunol. 2011;186:4200–12. doi: 10.4049/jimmunol.1001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dietze KK, Zelinskyy G, Gibbert K, Schimmer S, Francois S, Myers L, Sparwasser T, Hasenkrug KJ, Dittmer U. Transient depletion of regulatory T cells in transgenic mice reactivates virus-specific CD8+ T cells and reduces chronic retroviral set points. Proc Natl Acad Sci U S A. 2011;108:2420–5. doi: 10.1073/pnas.1015148108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gibbert K, Dietze KK, Zelinskyy G, Lang KS, Barchet W, Kirschning CJ, Dittmer U. Polyinosinic-polycytidylic acid treatment of Friend retrovirus-infected mice improves functional properties of virus-specific T cells and prevents virus-induced disease. J Immunol. 2010;185:6179–89. doi: 10.4049/jimmunol.1000858. [DOI] [PubMed] [Google Scholar]
- 43.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–9. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allie SR, Zhang W, Fuse S, Usherwood EJ. Programmed death 1 regulates development of central memory CD8 T cells after acute viral infection. J Immunol. 2011;186:6280–6. doi: 10.4049/jimmunol.1003870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song MY, Park SH, Nam HJ, Choi DH, Sung YC. Enhancement of vaccine-induced primary and memory CD8(+) T-cell responses by soluble PD-1. J Immunother. 2011;34:297–306. doi: 10.1097/CJI.0b013e318210ed0e. [DOI] [PubMed] [Google Scholar]