Abstract
West Nile virus (WNV) is a re-emerging pathogen responsible for outbreaks of fatal meningoencephalitis in humans. Previous studies have suggested a protective role for monocytes in a mouse model of WNV infection, but the molecular mechanisms have remained unclear. Here we show that genetic deficiency in Ccr2, a chemokine receptor on Ly6chi inflammatory monocytes and other leukocyte subtypes, markedly increases mortality due to WNV encephalitis in C57Bl/6 mice; this was associated with a large and selective reduction of Ly6chi monocyte accumulation in the brain. WNV infection in Ccr2+/+ mice induced a strong and highly selective monocytosis in peripheral blood that was absent in Ccr2−/− mice, which in contrast showed sustained monocytopenia. When a 1:1 mixture of Ccr2+/+ and Ccr2−/− donor monocytes was transferred by vein into WNV-infected Ccr2−/− recipient mice, monocyte accumulation in the CNS was not skewed toward either component of the mixture, indicating that Ccr2 is not required for trafficking of monocytes from blood to brain. We conclude that Ccr2 mediates highly selective peripheral blood monocytosis during WNV infection of mice and that this is critical for accumulation of monocytes in the brain.
Introduction
West Nile Virus (WNV) is a re-emerging flavivirus that cycles naturally between mosquitoes and birds, with humans and other vertebrates serving as accidental hosts. WNV did not appear in the United States until 1999, where it was identified as the cause of a meningo-encephalitis outbreak in the New York City area. Since then the virus has spread rapidly across North America, causing severe neurologic illness and death in humans and decimating bird populations. There were 29,625 cases of WNV reported to the CDC as of November 2009, including 1173 deaths (4.0%). Neither vaccines nor antiviral therapies are available, and new concepts based on precise understanding of the molecular pathogenesis are needed.
WNV infection remains subclinical in most humans, but ~20–30% may develop symptoms of WNV disease ranging from fever to meningoencephalitis, leading to paralysis or death (1). In both mice and humans, WNV encephalitis is characterized by virus-associated pathological processes, including reaction of the CNS resident cells and infiltration of inflammatory leukocytes in perivascular space and parenchyma, primarily monocytes and T cells (2–5). At the molecular level, leukocyte trafficking is mediated through a multistep process involving chemokines and chemokine receptors, which guide leukocytes to infected tissues. Recently, we identified chemokine receptor CCR5 as a critical host factor for protection against WNV infection in both mice and humans (6). Infection of CCR5 deficient mice was uniformly fatal with significantly fewer T cells and monocytes accumulating in the CNS (2). A similar increase in susceptibility was observed in individuals homozygous for the complete loss-of-function mutation CCR5Δ32; conversely CCR5Δ32 homozygosity was absent in asymptomatic WNV-infected individuals (7–9).
The importance of T cell recruitment and function in the CNS in WNV infection has been demonstrated in studies of mice lacking the Th1-specific chemokine CXCL10 and its receptor CXCR3 (4, 10). Loss of either resulted in decreased CD4+ and CD8+ T cell accumulation in the CNS, increased viral burden and death. Perforin-mediated CD8+ T cell responses are also critical for effective clearance of WNV from infected neurons (11). Consistent with this, enhancing migration of CD8+ T cells into the parenchyma of the WNV-infected CNS has been reported to increase viral clearance and survival (12).
In general, monocytes play an important role during CNS injury as precursors of macrophages and microglia (13). However, their role in WNV infection has not been clearly delineated. In a recent study, lethal intranasal delivery of WNV using a non-neurotropic strain caused monocytes to accumulate in the CNS, >90% of which were Ly6chi and capable of differentiating into both microglia and macrophages (14). Administration of neutralizing anti-Ccl2 antibody delayed migration of these cells into the brain and prolonged survival, suggesting a Ccr2-dependent, pathogenic role for monocytes in the brain in the model. In contrast, monocyte depletion in vivo using clodronate-loaded liposomes resulted in increased mortality of mice challenged i.p. with a neurotropic strain of WNV, supporting a protective role for these cells (15). Thus, monocytes may be beneficial or harmful depending on the context and details of the model.
Two major monocyte subsets have been identified in both humans and mice: ‘resident’ monocytes, which traffic into peripheral tissues under non-inflammatory conditions, and ‘inflammatory’ monocytes, which rapidly respond to tissue injury and inflammation (16). High expression of Ccr2 is associated with the ‘inflammatory’ (Ly6chi) monocyte subset and correlates with their function of rapid recruitment into inflamed tissues. Conversely, ‘resident’ (Ly6clo) monocytes express high levels of Cx3cr1, not Ccr2. Ccr2 is expressed primarily on monocytes and monocyte-derived cells, but is also expressed on a subset of T cells. Ccr2 is activated by the selective ligand Ccl2, and the nonselective ligands Ccl7 and Ccl12. Ccr2-deficient mice are monocytopenic and tissue recruitment of monocytes is impaired in these mice in infectious disease models, including Listeria monocytogenes (17, 18), Cryptococcus neoformans (19, 20), and tuberculosis (21, 22). In the present study, we have addressed the role of Ccr2 in a mouse model of WNV infection.
Materials and Methods
WNV infection model
Mouse studies were carried out in an animal biosafety level 3 facility under protocols approved by the NIAID/NIH Animal Care and Use Committee. Ccr2−/− mice and wild type C57BL6/J control mice were purchased from the Jackson Laboratory (Bar Harbor, Maine). Ccr2−/− mice have been backcrossed onto wild type C57BL/6J mice from Jackson Laboratories for 8 generations. All experiments were initiated using female mice 8–12 weeks of age. WNV strain NY99-35262 was kindly provided by Dr. R. Lanciotti (CDC, Fort Collins, CO). Mice were injected subcutaneously in the scruff of the neck with 102 focus forming units (ffu) WNV-NY99-35262 suspended in 50 μL HBSS (Invitrogen, Carlsbad, California) or with HBSS alone (mock-infected). Mice were monitored visually and weighed daily. Mice were perfused prior to aseptic removal of organs, which were weighed and placed directly in either 2 ml HBSS (for ELISA and viral titer assay) or 5 ml FACS buffer (PBS+ 1% BSA + 0.1% NaN3).
RNA isolation and expression analysis
Total RNA was isolated from brain homogenates using Qiagen Lipid RNeasy kit (Qiagen, Valencia, CA). After DNase I treatment (Qiagen), total RNA was converted to cDNA using a Superscript III Supermix kit (Invitrogen) with random hexamers. Real-time PCR was performed in a total reaction volume of 25 μl using 2 μl of cDNA, 12.5 μl 2X TaqMan PCR Master mix, and 1.25 μl primer-probe mix. All primers and probes used for quantitative PCR were pre-designed by the manufacturer (Applied Biosystems, Inc.) and run using the ABI 7900 Fast Real Time PCR System. Calculated copies were normalized against copies of housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and fold increase was determined using the ΔΔCT method.
Protein quantification
Brains were homogenized into HBSS and centrifuged at 1500g for 25 minutes at 4ºC. The supernatants were aliquoted and frozen at −80°C until further use. Plasma from uninfected or infected mice were collected and frozen at −80°C until further use. Mouse Ccl2 and Ccl12 were quantified using Quantikine Immunoassay kits (R&D systems). Mouse Ccl7 was measured using CCL7 Instant ELISA kit (BenderMed Systems). Mouse Ccl8 was quantified by sandwich ELISA using a kit according to manufacturer’s instructions (Antigenix, America, Inc).
Viral titers
Vero cells were maintained in OptiPro SFM (Invitrogen). For the viral titer assay, the media was supplemented with 2% FBS and 50 μg/ml gentamicin. Focus forming units (ffu) were assayed on confluent Vero cell monolayers in 12 well plates. 200 μl of virus-containing material were diluted and placed on the cells in duplicate. Virus was adsorbed for 1 hour at 37°C, then cells were overlayed with 2 ml Opti-MEM (Invitrogen) + 8 g/l methyl cellulose + 2% FBS + 50 μg/ml gentamicin. Cells were incubated for 2 days at 37°C, then fixed for 20 min with 100% methanol, washed 3 times with PBS and incubated for 1 hour at 37°C with 500 μl of 1:1000 diluted WNV-specific antibodies in hyperimmune mouse ascetic fluid (HMAF, ATCC #VR-82). Samples were then washed 3 times and 500 μl of anti-mouse horseradish peroxidase-labeled polymer (DAKO Cytomation) at a concentration of 1:10 was added. Cells were incubated for 1 hour at 37°C, washed 3 times and WNV ffu were visualized by addition of 1 ml diaminobenzidine chromogen (4.5 mg DAB/10 ml PBS + 4.5 μl 30% H202 in 10 ml PBS). Viral titers are expressed as ffu/gm tissue.
Cell isolation and FACS analysis
Mice were anesthetized prior to perfusion or sacrificed via cervical dislocation prior to aseptic removal of organs. Brains were collected in FACS buffer and homogenized using the back of a 6 cc syringe on ice in a tissue culture plate. The suspension was brought to 7 ml with FACS buffer and 3 ml of 90% Percoll (in PBS) were added. After thorough mixing, the solution was underlayed with 1 ml of 70% Percoll (in PBS). This was centrifuged at 2470 rpm for 30 min at 4°C. Leukocytes at the interphase were isolated and washed 3 times in FACS buffer. Cells were fixed in 100 μl of BD Cytofix (Pharmingen) at 4°C for 30 min and then filtered though a 70-micron mesh screen. 300 μl of peripheral blood were collected into EDTA tubes by mandibular vein puncture or cardiac puncture. Erythrocytes were removed by incubation with 1X PharmLyse buffer (BD Biosciences) according to the manufacturer’s protocol. Cells were washed 3 times with FACS buffer and blocked in 100 μl Fc block (1:200 dilution of CD16/32 Ab [Pharmingen] in FACS buffer). Cells were then incubated with specific antibodies for 30 min at 4°C, washed 3 times and suspended in 400 μl 2% paraformaldehyde buffer. Rat anti-mouse monoclonal antibodies coupled to the following fluorophores and directed against the following surface molecules were used for flow cytometry: FITC-conjugated CD19 (BD Biosciences) and Ly6c (eBiosciences); PE-conjugated CD115 (eBiosciences) and CD3 (BD Biosciences); PE-Cy-7-conjugated F4/80 (eBiosciences) and CD8α (eBiosciences); APC-conjugated CD45 (BD Biosciences) and NK1.1 (BD Biosciences); APC-Cy-7 conjugated CD11b (eBiosciences) and CD4 (eBiosciences). UV live/dead cell staining was used to identify cell death (Invitrogen). FACS data were collected on an LSR II instrument (Becton- Dickinson) and analysis was performed using FlowJo version 8.5.3. Cell numbers were quantified using counting beads (Spherotech, Inc).
Immunohistochemistry
Brains were aseptically removed from mice following cervical dislocation and placed directly into 10% normal buffered formalin (Fisher Scientific, Pittsburgh, PA) for 24 hours. 4–5 μm sections of paraffin-embedded slides were pre-treated using Diva Decloaking solution (Biocare Medical Inc) and blocked with 10% normal goat serum (Vector laboratories). Slides were then incubated with primary rat anti-mouse Mac-2 hybridoma (ATCC, Manassas VA) or with anti-CD3 antibody (DAKO) in the presence of background reducing agent (DAKO Corp). After incubation with anti-mouse polymer-horseradish peroxidase (DAKO Corp), slides were developed with the streptavidin ABC system utilizing biotinylated goat anti-rat IgG (Mouse adsorbed; Biocare Medical, Inc) and a diaminobenzidine chromogen (DAKO Corp) followed by counterstaining with hematoxylin.
In vivo monocyte adoptive transfer
In mice, systemic administration of recombinant Flt3L or injection of B16 melanoma cells expressing Flt3L have both previously been shown to markedly expand dendritic cells in many tissues, including bone marrow (23, 24). We found that injection of mice with Flt3L-expressing B16 cells (source, location) also markedly increased the percentage of monocytes in bone marrow, and we used this observation to generate sufficient monocytes for adoptive transfer studies. 8–12 week old wild type and Ccr2−/− C57BL/6J mice were injected subcutaneously in the axilla with 2×106 B16 cells expressing Flt3L. Tumors grew at the site of cell injection to variable sizes, but were not allowed to grow beyond 2 cm, per protocol. By 2 weeks of growth, monocyte yields typically were increased 3–4 fold over yields from uninjected mice. Bone marrow cells were harvested and enriched for monocytes using positive selection. Briefly, rat anti-mouse CD115-biotinylated monoclonal antibody (eBiosciences) was incubated with bone marrow cells harvested from C57BL/6J or Ccr2−/− mice. Using anti-biotin microbeads (Miltenyi Biotec), cells positive for CD115 were separated using the AutoMACS automatic magnetic cell sorting system. CD115-enriched cells were further purified for the classical monocyte subset by FACS using rat anti-mouse monoclonal antibodies for the following markers: CD4 (APC), CD8α (APC), CD19 (APC), CD11c (APC), Ly6c (FITC), CD11b (APC-Cy7), CD117 (APC), TCRβ (APC); and streptavidin-PE (eBiosciences) were also used. All cells staining for APC were excluded, and cells positive for PE (CD115), high expression of FITC (Ly6c), and positive for APC-Cy7 (CD11b) were collected and used for monocyte labeling and injection. Thus, FACS-purified monocytes from bone marrow of tumor cell-injected mice had the following immunophenotype: CD115+ Ly6Chi CD11bhi CD4- CD8α-CD11c- CD19- NK1.1-CD117-. Tumor cell injection did not affect activation markers on harvested monocytes (no upregulation of MHC-II, CD40, CD80, CD86 or CD69). Monocytes were resuspended at 5×106 cells/ml in RPMI complete media (+10% FBS, 1% L-glutamine, 1% non-essential amino acids, 1% penicillin/streptomycin). CMFDA Cell Tracker Green (Invitrogen) or CMTMR Cell Tracker Orange (Invitrogen) were added at a final concentration of 5 μM and incubated in a water bath at 37°C for 7 minutes. Cells were washed with cold RPMI complete media 3 times and resuspended in sterile PBS at a 1:1 ratio. A total of 2–2.5×106 monocytes were injected intravenously into Ccr2−/− mice on day 7 post-infection. Cell migration was calculated based on the ratio detected in the output organ (CNS or blood) compared to the initial input ratio of labeled cells at the time of cell transfer (Ccr2+/+: Ccr2−/− cells).
Results
WNV infection induces expression of Ccr2 and its ligands in mouse brain
We and others (2, 4) have previously reported that mRNA for most inflammatory chemokines is absent or expressed at very low levels in the brains of wild type C57Bl/6 mice, but can be induced by infection with WNV soon after the virus enters the brain. Here, we extended these results by using quantitative PCR and found that mRNA for all of the Ccr2 ligands was induced in the CNS. At day 7, the rank order was Ccl2>Ccl7>Ccl12>Ccl8. At day 12, the induction was greatly reduced compared to day 7, but mRNA for Ccl2 remained elevated compared to the other ligands, which appeared to be induced equivalently (Fig. 1A, B). We also tested several chemokine receptors, including Ccr5 and Cxcr3, both of which have previously been demonstrated to be important for control of WNV in the CNS and their respective ligands, Ccl3, Ccl4, Ccl5 (Ccr5 ligands), Cxcl9, Cxcl10, and Cxcl11 (Cxcr3 ligands). Relative to these, mRNA for Ccr2 and its ligands were induced by WNV to a similar level in the CNS (2, 10).
Figure 1.
Ccr2 restricts viral burden in the brain and promotes survival in a mouse model of WNV infection. A–C. Expression of RNA for inflammatory chemokines (A, B) and chemokine receptors (C). Data are derived from quantitative PCR and are presented as the mean ± SEM fold-induction in WNV-infected over uninfected Ccr2+/+ mouse brain (n=5 per data point) at (A) day 7 post-infection and (B) day 12 post-infection. Dark grey bars in panels A and B indicate Ccr2 ligands. (D) Survival analysis. Data are the summary of 3 experiments comparing Ccr2+/+ and Ccr2−/− mice infected with 102 FFU of WNV, with a total of 34 and 29 mice per group, respectively. (E, F) Ccr2 dependence of viral load in brain (E) and blood (F). Virus titer in the brains of 10 mice per time point was quantified by FFU assay. Virus load in the plasma was determined by quantitative real-time PCR and data are represented as the mean virus genome copies/ml plasma of 3–10 mice per time point. (* indicates p<0.05).
Genetic deficiency of Ccr2 results in increased mortality and viral load in the CNS of WNV-infected mice
Since Ccr2 and three of its ligands were induced in the CNS following WNV infection, we hypothesized that Ccr2 may be critical for pathogenesis. To test this, we compared survival in Ccr2-deficient (Ccr2−/−) mice compared with wild-type controls (Ccr2+/+ mice). Compared to control mice, where survival was ~65%, Ccr2-deficiency resulted in markedly increased mortality (~20% survival), as shown in Figure 1D. This was associated with increased viral load in the CNS of Ccr2-deficient mice on day 12 post-infection (Fig. 1E). This appeared to be specific to the brain, since viral load in the blood was not elevated in Ccr2−/− mice compared to controls (Fig. 1F).
Ccl2 and Ccl7 selectively accumulate in the CNS of WNV-infected Ccr2−/− mice
To further characterize the involvement of Ccr2 in the CNS and to test whether differences in the Ccr2−/− mice may exist in their intrinsic capacity to produce Ccr2 ligands, we compared the induction of a panel of inflammatory chemokines at the RNA level between the Ccr2+/+ and Ccr2−/− mice, including Ccl2, Ccl7, Ccl8, and Ccl12, on days 7 and 12 post-infection (Fig. 2A–B). No significant differences were observed at either time point, suggesting that the capacity to broadcast pro-inflammatory signals in response to WNV infection in the CNS was not significantly altered in the Ccr2−/− mice. We also tested the accumulation of the Ccr2 ligands at the protein level (Fig. 2C). Ccl2, Ccl7, and Ccl12, but not Ccl8 protein was induced by day 7 post-infection, and this induction was similar for wild type and knockout mice. However, Ccl2 and Ccl7 protein levels were significantly elevated in knockout mouse brain over wild type mouse brain on day 12 post-infection, suggesting that Ccr2 may be involved in virus clearance.
Figure 2.
Genetic deficiency of Ccr2 results in the accumulation Ccl2 and Ccl7 protein in the CNS of WNV-infected mice. (A, B) Ccr2 deficiency does not affect WNV induction of inflammatory chemokine mRNA in brain. Brain tissue from WNV-infected Ccr2+/+ mice was compared to Ccr2−/− mice for expression of RNA for a panel of chemokine ligands on day 7 (A) and day 12 (B) post-infection. Data were generated by quantitative PCR and are presented as mean ± SEM fold-induction of infected over uninfected controls, with 5 mice per time point. (C) Ccr2 deficiency selectively affects WNV induction of Ccr2 ligand protein in brain. Supernatants of brain homogenates of 5 mice per genotype per time post-infection indicated on the x-axis were tested for the presence of Ccr2 ligand proteins by ELISA. Mock, mock-infected control mice. (* indicates a significant difference in the Ccr2 ligand expression in the brain between Ccr2+/+ and Ccr2−/− mice, p<0.05).
Monocyte accumulation in the CNS is Ccr2-dependent
To further examine differences in the CNS that may be involved in viral clearance, we conducted a thorough analysis of leukocyte accumulation following WNV infection. As previously reported (2), uninfected wild type mouse brain contained few leukocytes other than microglia. After infection, no significant leukocyte accumulation was observed in the CNS until day 7, when monocytes, neutrophils, CD4+ and CD8+ T cells, and NK cells began to accumulate in the CNS of Ccr2+/+ mice (Fig. 3). In Ccr2−/− mice, similar levels of CD4+ and CD8+ T cells, and NK1.1+ cells were observed as in control mice (Fig. 3A–C). The one major exception was the inflammatory monocyte population (Ly6chiCD11b+) which was severely deficient at all time points tested in Ccr2−/− mice relative to wild type controls (Fig. 3D). A representative FACS plot at day 11 post infection is shown (Fig. 3F, upper gate). Compared to infected wild type mice, infected Ccr2−/− mice also had a small increase in the number of neutrophils accumulating in the CNS, although the difference did not reach statistical significance (p>0.05; Fig. 3E and F, lower gate). The selective deficiency in monocyte accumulation in knockout mice was further validated by histopathological examination of WNV-infected Ccr2+/+ and Ccr2−/− mice stained for Mac-2 and CD3. Infected Ccr2+/+ mice showed immunoreactivity for Mac-2 throughout the brain, with intense staining in the hippocampus and thalamus, whereas brain from infected Ccr2−/− mice showed markedly fewer immunoreactive cells (Fig. 4). In contrast, the density of CD3-immunostaining cells was similar for CD3 in brain for the infected control and knockout mice, consistent with the FACS data shown in Figure 3. The data indicate that Ccr2 may be specifically involved in the accumulation of inflammatory monocytes in the CNS and are consistent with the known role of Ccr2 as major chemokine receptor on inflammatory monocytes.
Figure 3.
WNV induction of monocyte accumulation in the CNS is selectively and strongly reduced in the absence of Ccr2: FACS analysis. Brain tissue was collected from uninfected Ccr2+/+ or Ccr2−/− mice (day 0) and mice at the indicated time points after infection with WNV. The total numbers of CD4+ T cells (A), CD8+ T cells (B), NK1.1+ cells (C), Ly6chiCD11b+ monocytes (D) and Ly6cintCD11b+ neutrophils (E) accumulating in the CNS were determined by flow cytometry. Representative FACS plots are shown for monocytes and neutrophils on day 11 (F). Data from 2 experiments were compiled and each data point represents the mean +/− SEM of 3 to 13 mice.
Figure 4.
WNV induction of monocyte accumulation in the CNS is selectively and strongly reduced in the absence of Ccr2: immunohistochemistry analysis. Brain tissue was collected from mice with the genotype indicated at the top of each photomicrograph at day 12 post-infection with WNV. Tissue sections were stained with antibodies against Mac-2 (A) or CD3 (B), and representative photomicrographs in the hippocampus and thalamus are shown at 40X magnification.
WNV infection induces an early monocytosis that is entirely Ccr2-dependent
Recent studies have identified a function for Ccr2 in the mobilization of inflammatory monocytes from the bone marrow to the blood under homeostatic conditions (18, 25). Unstressed Ccr2−/− mice are severely monocytopenic, and this is dependent on both Ccl2 and Ccl7 since both Ccl2−/− and Ccl7−/− mice are also monocytopenic (17). Thus, the deficiency of inflammatory monocytes in the CNS of WNV-infected Ccr2−/− mice may be due to monocytopenia or the absence of Ccr2-dependent monocyte trafficking from blood to brain, or a combination of the two. To address these possibilities, we first measured the effect of WNV infection on the levels of monocytes and other leukocyte subsets in the blood over time. In Ccr2+/+ mice, infection resulted in ~5 fold induction of monocyte numbers by day 5 post infection (Fig. 5A). By days 7, 9, and 11, the level of monocytes decreased in the blood of these mice, coincident with their accumulation in the CNS (Fig. 3D). In contrast, before infection Ccr2−/− mice were severely monocytopenic, as has been previously described, and WNV infection failed to induce monocytosis. Representative FACS plots are shown for day 0 (uninfected) and day 7 post-infection for both Ccr2+/+ and Ccr2−/− mice (Fig. 5C). Other leukocyte subsets in the blood remained relatively unaffected by WNV throughout the time course of the infection and levels were similar for Ccr2+/+ and Ccr2−/− mice (Fig. 5B and D). These data indicate that WNV infection induces an early and highly selective monocytosis that is entirely Ccr2-dependent.
Figure 5.
WNV infection induces an early and highly selective monocytosis that is entirely Ccr2-dependent. Blood was collected from Ccr2+/+ and Ccr2−/− mice before and after infection with WNV. The total numbers of Ly6chiCD11b+ monocytes (A) and Ly6cintCD11b+ neutrophils (B) per ml of blood were determined by FACS analysis. Representative FACS plots are shown for day 0 and day 7 (C). Total numbers of T cells, B cell, and NK cells were also calculated as shown in (D). Data from 2 experiments were compiled and each data point represents the mean +/− SEM of 3 to 13 mice. (E) Plasma levels of Ccr2 ligands Ccl2 and Ccl7 are hyper-induced by WNV infection in Ccr2 knockout mice. Data are from one experiment where plasma from a separate group of three mice per time point per genotype were analyzed by ELISA for the indicated Ccr2 ligands.
Since Ccr2-dependent monocytosis is also dependent on Ccl2 and ccl7, but not Ccl12, we measured the induction and accumulation of these ligands in the blood during the course of infection as shown in Figure 5E (17, 25). As measured by ELISA, we found that Ccl2 levels in the blood of Ccr2+/+ mice were low in steady state condition (0.37±0.05 ng/ml), and these levels increased 6-fold at day 7. In Ccr2−/− mice, a higher steady state level was observed (0.69±0.10 ng/ml). However, during infection, Ccl2 increased ~11-fold, peaking at day 9 post infection. An even more profound increase was observed for Ccl7, where protein was undetectable at the steady state of both Ccr2+/+ and Ccr2−/− mice. However, Ccl7 accumulation in the Ccr2−/− mice after WNV infection was measured at 28.10±0.70 ng/ml, which was approximately 10-fold higher than what we observed in Ccr2+/+ mice (2.9±1.65 ng/ml). Analysis of Ccl12 showed no significant differences at any time point between the two strains. Our data is consistent with the idea that these ligands are important for monocytosis in the blood, and without these cells, the ligands may accumulate.
Ccr2 mediates accumulation of monocytes in the CNS during WNV infection by regulating blood monocyte levels, not trafficking from blood to brain
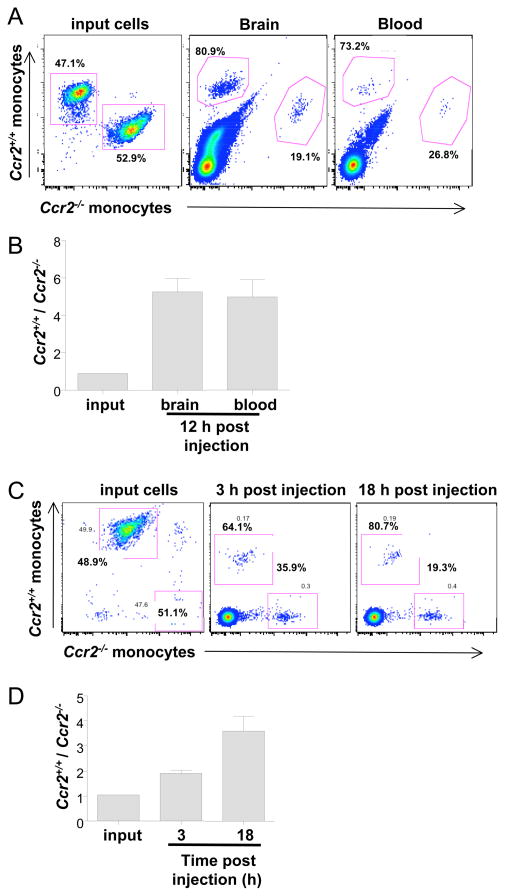
To test the role of Ccr2 in monocyte trafficking from blood to CNS directly, we conducted a competitive repopulation study where equal numbers of Ccr2+/+ and Ccr2−/−Ly6chi monocytes were sorted by FACS from uninfected mice, then differentially labeled, mixed 1:1 and injected into WNV-infected Ccr2−/− recipient mice on day 7 post infection. This time point is after virus has entered the CNS and when chemokine production is highest (Figure 2). We used Ccr2−/− mice as recipients since they are monocytopenic, which would facilitate analysis of donor cell trafficking to brain. Twelve hours after transfer, mice were euthanized and the CNS was analyzed for the presence of the labeled cells. As shown in Figure 6A, the ratio of labeled Ccr2+/+ and Ccr2−/− monocytes prior to injection was approximately 1:1, as intended (left panel). Twelve hours post injection, the ratio of Ccr2+/+:Ccr2−/− monocytes in the CNS was strongly skewed towards Ccr2+/+ monocytes at approximately a 4:1 ratio. However, this preponderance of Ccr2-sufficient cells in the CNS was also observed in blood collected from the same mouse at the same time. This pattern held up for all animals tested (n=9; Fig. 6B), suggesting that the increased proportion of Ccr2+ monocytes in the CNS was not due to a preferential trafficking of Ccr2-expressing monocytes, but rather to the disappearance of the Ccr2-deficient monocytes from the blood.
Figure 6.
Ccr2 mediates accumulation of monocytes in the CNS during WNV encephalitis by regulating blood monocyte levels, not trafficking from blood to brain. Sorted Ly6chiCD11b+ monocytes from uninfected Ccr2+/+ or Ccr2−/− mice were differentially-labeled, mixed at a 1:1 ratio, and analyzed prior to injection of recipient mice (A and C, left-most panels). The mixed donor cells were then injected into Ccr2−/−recipients that had been infected 7 days earlier with WNV (A, B) or not infected (C, D). (A, B) Distribution of labeled Ccr2+/+ and Ccr2−/− donor monocytes in WNV-infected Ccr2−/− recipient mice. Brain tissue (A; middle panel) and blood from the same mouse (A: right panel) were harvested 12 hours after injection of donor cells and analyzed for the presence of labeled cells by FACS. (B) Summary data from 9 mice are shown, represented as the ratio of Ccr2+/+ to Ccr2−/− monocytes in each source. (C, D) Distribution of labeled Ccr2+/+ and Ccr2−/− donor monocytes in uninfected Ccr2−/− recipient mice. Blood was collected from mice at 3 hours and 18 hours post injection and measured for the presence of labeled cells. Representative data from 1 mouse are shown in (C), and summary data from 3 mice are shown in D.
To test whether the unequal distribution of Ccr2+/+ versus Ccr2−/− donor monocytes we observed was caused by WNV infection, we performed the same experiment using uninfected Ccr2−/− mice as recipients (Fig. 6C and D). Blood was collected at 3 and 18 hours post injection, and analyzed for the ratio of differentially labeled Ccr2+/+:Ccr2−/− monocytes by FACS. As shown in Figure 6C (left-most panel), the ratio of Ccr2+/+:Ccr2−/− monocytes prior to injection was approximately 1:1, as intended. At 3 hours post-injection, the ratio of Ccr2+/+:Ccr2−/− cells had increased, indicating an advantage of Ccr2+/+ monocytes for populating the blood. At 18 hours, the ratio had increased further and was similar to that observed when the cells were injected into WNV-infected mice (Fig. 6A). Thus, the skewed repopulation is not due to WNV infection per se. Analysis of spleen tissue from uninfected mice 18 hours after injection showed a skewed distribution of donor cells similar to that found in the blood (data not shown). However, analysis of bone marrow from uninfected Ccr2−/− mice 18 hours after injection of donor cells revealed that Ccr2+/+ and Ccr2−/− monocytes were present at nearly a 1:1 ratio (data not shown). Together these data indicate that monocytes may traffic back to the bone marrow and again accumulate, unable to egress in the absence of Ccr2.
Discussion
The present study demonstrates a critical role for chemokine receptor Ccr2 on Ly6chi inflammatory monocytes for accumulation of these cells in the brain in a mouse model of WNV encephalitis. This extends to the molecular level previous work showing that monocytes accumulate in the brains of WNV-infected mice and humans and are important for survival in the mouse model (2, 3, 5, 14). Consistent with this, we found that Ccr2-deficiency resulted in markedly decreased survival and increased viral load in the brains after WNV infection of mice.
The detailed leukocyte dynamics that we defined in the blood and brain of WNV-infected mice allow important new insights into the role of Ccr2 in WNV pathogenesis, based on several unexpected findings. First, although WNV induces a multifocal mixed leukocyte encephalitis, leukocyte deficiency in the brain in WNV-infected Ccr2 knockout mice appears to be restricted exclusively to monocytes (~90% reduction). This suggests that Ccr2 is most important in monocyte trafficking in mouse brain, however additional work will be needed to define whether there is also a deficiency of T cell subsets and other leukocyte subsets known to express Ccr2, such as highly differentiated Th1 cells that are compensated by subsets that do not express the receptor.
Second, although Ccr2 ligands Ccl2, Ccl7 and Ccl12 are strongly induced by WNV in the brain at the RNA and protein levels, Ccr2 appears to be dispensable for trafficking of monocytes from the blood to the brain of WNV-infected mice. Instead, the driving force appears to be a highly selective, WNV-induced, and entirely Ccr2-dependent monocytosis that precedes accumulation of monocytes in the brain. This conclusion is based in part on competitive repopulation experiments performed using differentially-labeled Ccr2+/+ and Ccr2−/− donor monocytes transferred to monocytopenic WNV-infected Ccr2−/− mice. These studies also suggest that monocytes lacking Ccr2 may traffic back to the bone marrow and preferentially accumulate, unable to egress. Thus the mechanism of monocytopenia in uninfected Ccr2−/− mice may involve both decreased monocyte egress from bone marrow to blood and increased monocyte return from blood to bone marrow, among other possibilities.
Because CCR2 appears not to be involved in the migration of monocytes from blood into the CNS, this suggests that another chemokine receptor may be involved at this level. Our previous studies have shown a dominant role of chemokine receptor CCR5 in migration of monocytes, T cells, and NK cells into the CNS (REF GLASS). However, our data suggest that CCR2 is involved very early after infection with WNV, increasing monocyte numbers in the periphery. Once monocytes are in circulation, it appears that another receptor is critical for their migration into the infected CNS, perhaps CCR5. To address this question, a similar competitive repopulation analysis to the one we conducted in Figure 6 could be performed using monocytes derived from Ccr5+/+ and Ccr5−/− mice.
We are unaware of any other infectious agent that induces a selective monocytosis in any host. As with WNV, other infectious agents have been reported that fail to overcome the monocytopenia in Ccr2-deficient mice, but in these diseases a specific monocytosis has not been described as a typical feature. Additional work will be needed to define whether Ccr2 and its ligands are needed for trafficking, organization and/or activation of monocytes within the brain, once the cells have crossed the blood brain barrier. Although previous work has shown that both Ccl2 and Ccl7 are important determinants of the normal monocyte count in unstressed wild type mice, additional work will be needed to define which Ccr2 ligands induce monocytosis in WNV-infected mice.
Although previous studies have shown that monocytes play an important role in controlling WNV infection in mice, their precise mechanism of action has not been defined and may vary depending on the model. One study using a lethal intranasal model has suggested that monocytes may play a pathogenic role, since delaying migration of these cells prolonged survival. The mechanism may involve macrophage/microglial cell production of substances potentially toxic to tissue, such as superoxide anion, nitric oxide, and pro-inflammatory cytokines (26, 27). In contrast, a second study using a model very similar to ours showed that monocytes may be protective, since depletion using clodronate-loaded liposomes decreased survival of the infected mice (15). This is also plausible, since a beneficial role of macrophages/microglia in CNS recovery has also been demonstrated through the production of neurotrophic factors, removal of debris, and axonal regeneration (28–31). Future approaches for defining functional roles of monocytes in controlling WNV infection, once they reach the CNS, may include 1) histopathologic studies of the correspondence of virus, macrophages and neurons in vivo, 2) ex vivo studies of expression of pro-inflammatory cytokines and effector molecules in monocytes and monocyte-derived cells (macrophages and microglia) purified from infected brain, 3) immunophenotyping the cells that produce Ccr2 ligands after viral entry into the CNS, and 4) in vitro studies of monocyte/macrophage interaction with virally-infected neurons.
At present, there are no data delineating leukocyte dynamics in patients with WNV infection. Our data suggest that factors limiting monocytosis could be associated with poor outcome, and that novel therapeutics able to induce monocytosis, such as GM-CSF and AMD3100 (a CXCR4 antagonist), could be useful in such patients. AMD3100 is currently approved for stem cell mobilization, and can also mobilize monocytes in both humans (32) and in mice (33). In this regard, a study by Klein and colleagues showed that AMD3100 can significantly improve survival from WNV infection in mice, although the mechanism of leukocytosis has not been defined (12).
Monocytosis could also potentially be induced in humans using agonists to CCR2, since the ‘inflammatory’ monocyte subset in humans (CD14+CD16− monocytes) that is analogous to the Ccr2+ Ly6Chi mouse monocyte subset that we studied also uniformly expresses CCR2 (16). At present, CCR2 agonists are not available clinically and are in general limited to chemokine ligands which have very short half-lives. Unless modified, CCR2 ligands may not be ideal for such an application. Conversely, CCR2 antagonists, which are currently being developed by the pharmaceutical industry with anticipated long-term administration for chronic inflammatory diseases, could potentially predispose to poor outcomes in WNV-infected patients.
In previous work, we have identified two genetic determinants of WNV susceptibility in humans, homozygous CCR5Δ32 (7, 8) and OAS1 rs10774671 (34). These mutations were tested as candidates based on work we performed with the corresponding mouse genes in mouse models of WNV (35, 36). A similar genetic approach may also be available for translating our findings on Ccr2 to human WNV disease, since several common polymorphisms have been described for CCR2, CCL2 and CCL7 (37, 38). Understanding the mechanisms of Ccr2 action in monocyte migration during WNV infection in mice is an important first step towards these goals.
In conclusion, our data identify Ccr2 as a critical protective factor in encephalitis caused by WNV in a mouse model. Our data show for the first time that chemokine receptors may function at an entirely different migratory step than previously shown for WNV infection, and that early events following infection may affect outcome of WNV encephalitis. Further studies are needed to investigate the role of Ccr2 ligands, and to further understand the functional role of monocytes in the CNS.
Acknowledgments
The authors would like to thank Dr. Jerrold Ward and Lawrence Faucette for assistance with immunohistochemistry and pathology. This work was supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Disease, National Institutes of Health, USA.
References
- 1.Hayes EB, Gubler DJ. West Nile virus: epidemiology and clinical features of an emerging epidemic in the United States. Annu Rev Med. 2006;57:181–194. doi: 10.1146/annurev.med.57.121304.131418. [DOI] [PubMed] [Google Scholar]
- 2.Glass WG, Lim JK, Cholera R, Pletnev AG, Gao JL, Murphy PM. Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection. J Exp Med. 2005;202:1087–1098. doi: 10.1084/jem.20042530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelley TW, Prayson RA, Ruiz AI, Isada CM, Gordon SM. The neuropathology of West Nile virus meningoencephalitis. A report of two cases and review of the literature. Am J Clin Pathol. 2003;119:749–753. doi: 10.1309/PU4R-76JJ-MG1F-81RP. [DOI] [PubMed] [Google Scholar]
- 4.Klein RS, Lin E, Zhang B, Luster AD, Tollett J, Samuel MA, Engle M, Diamond MS. Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J Virol. 2005;79:11457–11466. doi: 10.1128/JVI.79.17.11457-11466.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sampson BA, Ambrosi C, Charlot A, Reiber K, Veress JF, Armbrustmacher V. The pathology of human West Nile Virus infection. Hum Pathol. 2000;31:527–531. doi: 10.1053/hp.2000.8047. [DOI] [PubMed] [Google Scholar]
- 6.Lim JK, Glass WG, McDermott DH, Murphy PM. CCR5: no longer a “good for nothing” gene--chemokine control of West Nile virus infection. Trends Immunol. 2006;27:308–312. doi: 10.1016/j.it.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Glass WG, McDermott DH, Lim JK, Lekhong S, Yu SF, Frank WA, Pape J, Cheshier RC, Murphy PM. CCR5 deficiency increases risk of symptomatic West Nile virus infection. J Exp Med. 2006;203:35–40. doi: 10.1084/jem.20051970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim JK, Louie CY, Glaser C, Jean C, Johnson B, Johnson H, McDermott DH, Murphy PM. Genetic deficiency of chemokine receptor CCR5 is a strong risk factor for symptomatic West Nile virus infection: a meta-analysis of 4 cohorts in the US epidemic. J Infect Dis. 2008;197:262–265. doi: 10.1086/524691. [DOI] [PubMed] [Google Scholar]
- 9.Lim JK, McDermott DH, Lisco A, Foster GA, Krysztof D, Follmann D, Stramer SL, Murphy PM. CCR5 deficiency is a risk factor for early clinical manifestations of West Nile virus infection but not for viral transmission. J Infect Dis. 201:178–185. doi: 10.1086/649426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang B, Chan YK, Lu B, Diamond MS, Klein RS. CXCR3 mediates region-specific antiviral T cell trafficking within the central nervous system during West Nile virus encephalitis. J Immunol. 2008;180:2641–2649. doi: 10.4049/jimmunol.180.4.2641. [DOI] [PubMed] [Google Scholar]
- 11.Shrestha B, Samuel MA, Diamond MS. CD8+ T cells require perforin to clear West Nile virus from infected neurons. J Virol. 2006;80:119–129. doi: 10.1128/JVI.80.1.119-129.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCandless EE, Zhang B, Diamond MS, Klein RS. CXCR4 antagonism increases T cell trafficking in the central nervous system and improves survival from West Nile virus encephalitis. Proc Natl Acad Sci U S A. 2008;105:11270–11275. doi: 10.1073/pnas.0800898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eltayeb S, Berg AL, Lassmann H, Wallstrom E, Nilsson M, Olsson T, Ericsson-Dahlstrand A, Sunnemark D. Temporal expression and cellular origin of CC chemokine receptors CCR1, CCR2 and CCR5 in the central nervous system: insight into mechanisms of MOG-induced EAE. J Neuroinflammation. 2007;4:14. doi: 10.1186/1742-2094-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Getts DR, Terry RL, Getts MT, Muller M, Rana S, Shrestha B, Radford J, Van Rooijen N, Campbell IL, King NJ. Ly6c+ “inflammatory monocytes” are microglial precursors recruited in a pathogenic manner in West Nile virus encephalitis. J Exp Med. 2008;205:2319–2337. doi: 10.1084/jem.20080421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ben-Nathan D, Huitinga I, Lustig S, van Rooijen N, Kobiler D. West Nile virus neuroinvasion and encephalitis induced by macrophage depletion in mice. Arch Virol. 1996;141:459–469. doi: 10.1007/BF01718310. [DOI] [PubMed] [Google Scholar]
- 16.Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–618. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 17.Jia T, Serbina NV, Brandl K, Zhong MX, Leiner IM, Charo IF, Pamer EG. Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during Listeria monocytogenes infection. J Immunol. 2008;180:6846–6853. doi: 10.4049/jimmunol.180.10.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 19.Huffnagle GB, Traynor TR, McDonald RA, Olszewski MA, Lindell DM, Herring AC, Toews GB. Leukocyte recruitment during pulmonary Cryptococcus neoformans infection. Immunopharmacology. 2000;48:231–236. doi: 10.1016/s0162-3109(00)00222-8. [DOI] [PubMed] [Google Scholar]
- 20.Traynor TR, Kuziel WA, Toews GB, Huffnagle GB. CCR2 expression determines T1 versus T2 polarization during pulmonary Cryptococcus neoformans infection. J Immunol. 2000;164:2021–2027. doi: 10.4049/jimmunol.164.4.2021. [DOI] [PubMed] [Google Scholar]
- 21.Peters W, Scott HM, Chambers HF, Flynn JL, Charo IF, Ernst JD. Chemokine receptor 2 serves an early and essential role in resistance to Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2001;98:7958–7963. doi: 10.1073/pnas.131207398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott HM, Flynn JL. Mycobacterium tuberculosis in chemokine receptor 2-deficient mice: influence of dose on disease progression. Infect Immun. 2002;70:5946–5954. doi: 10.1128/IAI.70.11.5946-5954.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Contractor N, Louten J, Kim L, Biron CA, Kelsall BL. Cutting edge: Peyer’s patch plasmacytoid dendritic cells (pDCs) produce low levels of type I interferons: possible role for IL-10, TGFbeta, and prostaglandin E2 in conditioning a unique mucosal pDC phenotype. J Immunol. 2007;179:2690–2694. doi: 10.4049/jimmunol.179.5.2690. [DOI] [PubMed] [Google Scholar]
- 24.Mach N, Gillessen S, Wilson SB, Sheehan C, Mihm M, Dranoff G. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 2000;60:3239–3246. [PubMed] [Google Scholar]
- 25.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 27.Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol. 1992;149:2736–2741. [PubMed] [Google Scholar]
- 28.Dougherty KD, Dreyfus CF, Black IB. Brain-derived neurotrophic factor in astrocytes, oligodendrocytes, and microglia/macrophages after spinal cord injury. Neurobiol Dis. 2000;7:574–585. doi: 10.1006/nbdi.2000.0318. [DOI] [PubMed] [Google Scholar]
- 29.Richardson PM, Lu X. Inflammation and axonal regeneration. J Neurol. 1994;242:S57–60. doi: 10.1007/BF00939244. [DOI] [PubMed] [Google Scholar]
- 30.Smith ME. Phagocytic properties of microglia in vitro: implications for a role in multiple sclerosis and EAE. Microsc Res Tech. 2001;54:81–94. doi: 10.1002/jemt.1123. [DOI] [PubMed] [Google Scholar]
- 31.Yin Y, Cui Q, Li Y, Irwin N, Fischer D, Harvey AR, Benowitz LI. Macrophage-derived factors stimulate optic nerve regeneration. J Neurosci. 2003;23:2284–2293. doi: 10.1523/JNEUROSCI.23-06-02284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hubel K, Liles WC, Broxmeyer HE, Rodger E, Wood B, Cooper S, Hangoc G, Macfarland R, Bridger GJ, Henson GW, Calandra G, Dale DC. Leukocytosis and Mobilization of CD34+ Hematopoietic Progenitor Cells by AMD3100, a CXCR4 Antagonist. Support Cancer Ther. 2004;1:165–172. doi: 10.3816/SCT.2004.n.008. [DOI] [PubMed] [Google Scholar]
- 33.Capoccia BJ, Shepherd RM, Link DC. G-CSF and AMD3100 mobilize monocytes into the blood that stimulate angiogenesis in vivo through a paracrine mechanism. Blood. 2006;108:2438–2445. doi: 10.1182/blood-2006-04-013755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim JK, Lisco A, McDermott DH, Huynh L, Ward JM, Johnson B, Johnson H, Pape J, Foster GA, Krysztof D, Follmann D, Stramer SL, Margolis LB, Murphy PM. Genetic variation in OAS1 is a risk factor for initial infection with West Nile virus in man. PLoS Pathog. 2009;5:e1000321. doi: 10.1371/journal.ppat.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mashimo T, Lucas M, Simon-Chazottes D, Frenkiel MP, Montagutelli X, Ceccaldi PE, Deubel V, Guenet JL, Despres P. A nonsense mutation in the gene encoding 2′-5′-oligoadenylate synthetase/L1 isoform is associated with West Nile virus susceptibility in laboratory mice. Proc Natl Acad Sci U S A. 2002;99:11311–11316. doi: 10.1073/pnas.172195399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perelygin AA, Scherbik SV, Zhulin IB, Stockman BM, Li Y, Brinton MA. Positional cloning of the murine flavivirus resistance gene. Proc Natl Acad Sci U S A. 2002;99:9322–9327. doi: 10.1073/pnas.142287799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Modi WS, Goedert JJ, Strathdee S, Buchbinder S, Detels R, Donfield S, O’Brien SJ, Winkler C. MCP-1-MCP-3-Eotaxin gene cluster influences HIV-1 transmission. Aids. 2003;17:2357–2365. doi: 10.1097/00002030-200311070-00011. [DOI] [PubMed] [Google Scholar]
- 38.Smith MW, Dean M, Carrington M, Winkler C, Huttley GA, Lomb DA, Goedert JJ, O’Brien TR, Jacobson LP, Kaslow R, Buchbinder S, Vittinghoff E, Vlahov D, Hoots K, Hilgartner MW, O’Brien SJ. Contrasting genetic influence of CCR2 and CCR5 variants on HIV-1 infection and disease progression. Hemophilia Growth and Development Study (HGDS), Multicenter AIDS Cohort Study (MACS), Multicenter Hemophilia Cohort Study (MHCS), San Francisco City Cohort (SFCC), ALIVE Study. Science. 1997;277:959–965. doi: 10.1126/science.277.5328.959. [DOI] [PubMed] [Google Scholar]