Abstract
Glucose homeostasis requires the coordinated actions of various organs and is critically dependent on the proper functioning of the various cell types present in the pancreatic Langerhans islets. Here we report that chromatin architectural protein HMGN3 is highly expressed in all pancreatic endocrine islet cells, and that Hmgn3−/− mice which have a mild diabetic phenotype, have reduced glucagon levels in their blood. To elucidate the mechanism leading to altered glucagon secretion of Hmgn3−/− mice, we tested whether HMGN3 affect glucagon synthesis and secretion in αTC1-9 cells, a glucagon secreting cell line that is used to study pancreatic α-cell function. We find that in these cells deletion of either HMGN3 or other HMGN variants, does not significantly affect glucagon gene expression or glucagon secretion. Our studies demonstrate a link between HMGN3 and glucagon blood levels that is not directly dependent of the function of pancreatic α-cells.
Keywords: HMGN PROTEINS, GLUCAGON SECRETION, PANCREATIC ISLETS, DIABETES
Maintenance of proper glucose homeostasis is essential to preventing diabetes mellitus, one of the most prevalent metabolic disorders. Glucose homeostasis requires the coordinated actions of various organs and is critically dependent on the proper functioning of the various cell types present in the pancreatic Langerhans islets [Gromada et al., 2007]. The pancreatic islets contain several distinct polypeptide-secreting cell types known to affect glucose homeostasis: α-cells secreting glucagon, β-cells secreting insulin, δ-cells secreting somatostatin, pancreatic polypeptide (PP)-secreting cells and ghrelin producing cells [Gromada et al., 2007].
We recently reported that the chromatin-binding protein HMGN3 is highly expressed in the pancreatic islets and studied its role in β-cell functions [Ueda et al., 2009] HMGN3 is a member of the HMGN nucleosome binding protein family. Members of this protein family bind specifically to the 147 bp nucleosome core particle, the building block of the chromatin fiber, and modulate the structure and activity of chromatin [Bustin, 2001]. The interaction of HMGNs with nucleosomes alters the levels of posttranslational modifications in the tail of the core histone [Lim et al., 2004] and affects DNA related processes such as repair, replication and transcription [Vestner et al., 1998; Bustin, 2001; Kim et al., 2009]. Misregulation of HMGN expression has been shown to affect cellular phenotype [Rubinstein et al., 2005].
Hmgn3−/− mice are viable and appear normal; however, loss of HMGN3 protein leads to a mild diabetic phenotype [Ueda et al., 2009]. Studies with MIN6 cells, a mouse insulinoma cell line that is used for functional studies of β-cells, indicated that HMGN3 affects the expression of several genes involved in insulin secretion and that loss of the protein affects insulin secretion from these cells. Among the genes most affected was GLUT2, a major transporter of glucose into these cells. The rate of glucose import into β-cells affects insulin secretion; however, several studies suggested that a partial down regulation of GLUT2 levels in pancreatic β-cells does not impact significantly insulin secretion [Johnson et al., 1990; Valera et al., 1994; Guillam et al., 1997, 2000]. Thus, the partial down regulation of GLUT2 in Hmgn3−/− mice does not fully explain their diabetic phenotype. Therefore, we examined whether HMGN3 affects the function of other cells in the pancreatic islets which are known to regulate insulin levels and glucose homeostasis.
We now show that compared to exocrine cells, the expression of HMGN3 is elevated not only in the insulin secreting β-cells but also in all the other endocrine cells present in Langerhans pancreatic islets. Since the glucagon-insulin balance plays a major role in glucose homeostasis and in the onset of Type 2 diabetes [Sloop et al., 2005], and since glucagon release is critical for the prevention or rapid correction of hypoglycemia, we focus on the possible role of HMGN3 in regulating glucagon levels. We find that in Hmgn3−/− mice, loss of HMGN3 reduces the levels of glucagon in the plasma. However, analysis of αTC1-9 cells, a cell line that is used for studies on pancreatic α-cell function, did not reveal a major role for HMGN3 in glucagon synthesis and secretion. Our results indicate that HMGN3 reduces the levels of glucagon in the plasma of mice without affecting glucagon gene expression.
MATERIALS AND METHODS
CELL CULTURE, siRNA MEDIATED KNOCKDOWN OF HMGN PROTEIN AND CELL VIABILITY ASSAY
αTC1-9 cells (purchased form ATCC) were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 16.6 mM Glucose, 15 mM HEPES, 0.1 mM NEAA, 0.02% BSA and 1 mM Sodium Pyruvate. siRNA for HMGN3 was purchased from Qiagen. ON-target plus SMART pool siRNA for HMGN1, HMGN2 or control siRNA were purchased from Dharmacon. The siRNA (final concentration is 20 nM) was transfected into αTC1-9 cells using DharmaFECT4 transfection reagent (Dharmacon) according to manufacture’s instruction. Cells were collected and analyzed after 4 days of transfection. Efficiency of HMGNs knock down was monitored by Western blot (protein level) and quantitative RT-PCR (RNA level). Cell viability was monitored by CellTiter-Blue Cell Viability Assay (Promega) according to manufacture’s instruction.
IMMUNOSTAINING OF PANCREATIC SECTIONS
Immunofluorescence was performed as previously described [Furusawa et al., 2006]. Primary antibodies used were: rabbit anti-HMGN3, prepared in our laboratory and monoclonal anti-Glucagon (Clone K79bB10, SIGMA), polyclonal Rabbit anti-Somatostatin antibody (ZYMED), and polyclonal Rabbit anti-Pancreatic Polypeptide antibody (ZYMED) Secondary antibodies, AlexaFluor488 or AlexaFluor568 goat anti-rabbit IgG or donkey anti-goat IgG (Molecular Probe) were used at 8 μg/ml.
GLUCAGON MEASUREMENTS
Cells were washed twice with KRBH (25 mM HEPES, pH7.4, 125 mM NaCl, 1.3 mM CaCl2, 5 mM NaHCO3, 5.9 mM KCl, 1.2 mM MgCl2, 0.1% (w/v) BSA) containing 10 mM glucose. Then cells were pre-cultured in KRBH for 1 h. After 1 h, the cells were washed twice with KRBH, and cultured in KRBH for 30 min. After 30 min, supernatants were collected. To measure total cellular glucagon, cells were collected with glycine-BSA (100 mM glycine, 0.25% BSA). Cells were disrupted by sonication and kept on ice for 15 min. After centrifugation at 14,000g for 30 min at 4°C, the supernatants were collected and stored at −80°C for assay. Plasma glucagon levels of mice and glucagon of αTC1-9 cells were measured with Glucagon RIA kit (Millipore) according to manufacturer’s instruction. Plasma was prepared from blood that was collected directly into Lithium heparin/PST gel containing tubes (BD catalogue 365958).
QUANTITATIVE RT-PCR ANALYSIS
Total RNA from αTC1-9 cells were purified by RNeasy mini plus kit (Qiagen). cDNA was prepared from purified RNA with iScript cDNA synthesis kit (Bio-Rad). Quantitative RT-PCR analysis was performed using ABI PRISM 7900 system and Power SYBER Green PCR master mix (Applied Biosystems) according to manufacturer’s recommendations. The following primer sequences were used:
Primers used for Quantitative RT-PCR of mRNA
| Forward | Reverse | |
|---|---|---|
| 18S rRNA | GCGGATCTTCTCTGTG-GTTCGTCCTTC | CGGCTACCACATCCAAGGAA |
| HMGN3 | GGAAATTTCCATC-ATCCTCAAGTCACGC | CTCAGTAAAAACACCACAGTCTGCACAG |
| HMGN2 | GCGAAGAAGGGA-GAGAAGGT | CCTGGTCTGTTTTGGCATCT |
| HMGN1 | CGGGAAAGGATA-AAGCATCA | TGGACTCTGGTTTTCCGTCT |
| Glucagon | AACAACATTGCCA-AACGTCA | AGCAATGGCGACTTCTTCTG |
WESTERN BLOT ANALYSIS
Cell lysates were fractionated on SDS–PAGE, transferred to PVDF membrane (Millipore), and then stained with each antibody. Primary antibodies used were: anti-HMGN1, anti-HMGN2, anti-HMGN3 and anti-Histone H3. Bound antibody was detected HRP conjugated 2nd antibody (Pierce) and Immobilon reagent (Millipore).
RESULTS AND DISCUSSION
SPECIFIC EXPRESSION OF HMGN3 IN PANCREATIC ENDOCRINE CELLS
We recently reported that HMGN3 is enriched in pancreatic β-cells and affects insulin secretion from pancreatic β-cells [Ueda et al., 2009]. To investigate whether HMGN3 is also expressed in other pancreatic endocrine cells, we performed double immunofluorescence with antibodies to HMGN3 and with antibodies specific to each of the other pancreatic islet endocrine cell types. Immunostaining reveals that HMGN3 is expressed in all pancreatic endocrine cells (Fig. 1A,B). Magnified images reveal that the cells expressing either Glucagon, Somatostatin or Pancreatic polypeptide in the cytoplasm, contain HMGN3 in their nucleus (Fig. 1B). These results raise the possibility that HMGN3 affects the function of these cells thereby impacting glucose homeostasis. Given the important role of glucagon in glucose homeostasis [Gromada et al., 2007] we first focused on the possible role of HMGN3 in the function of α-cells.
Fig. 1.
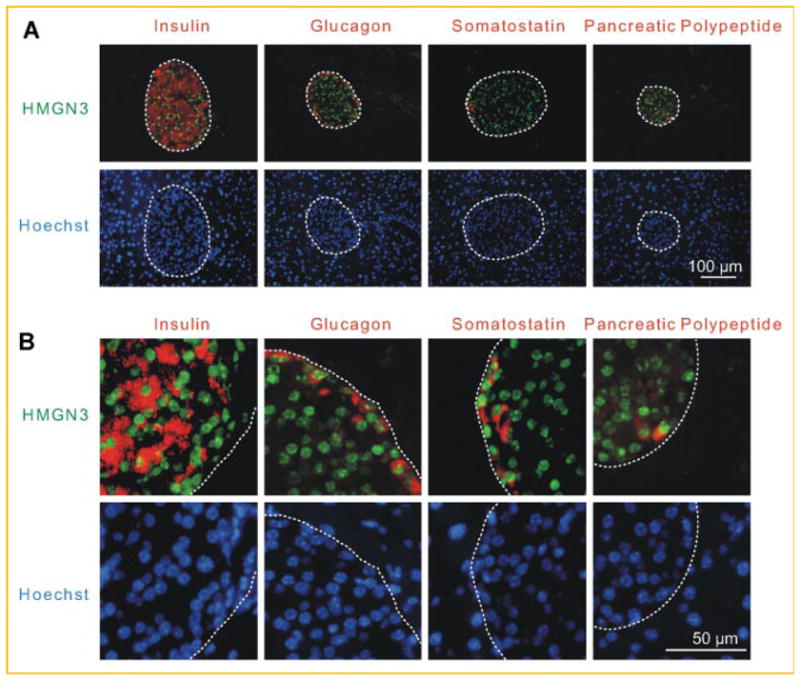
Elevated levels of HMGN3 in pancreatic endocrine cells. A: Immunostaining of HMGN3 or pancreatic peptide hormones of wild type mice. The red color demonstrates immunolocalization of each pancreatic peptide hormones in the cytoplasm and the green color visualizes the localization of HMGN3 in the nucleus. The nuclei are visualized by Hoechst. B: A section from the upper panels was magnified in lower panel. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DECREASE GLUCAGON LEVELS IN THE PLASMA OF Hmgn3−/− MICE
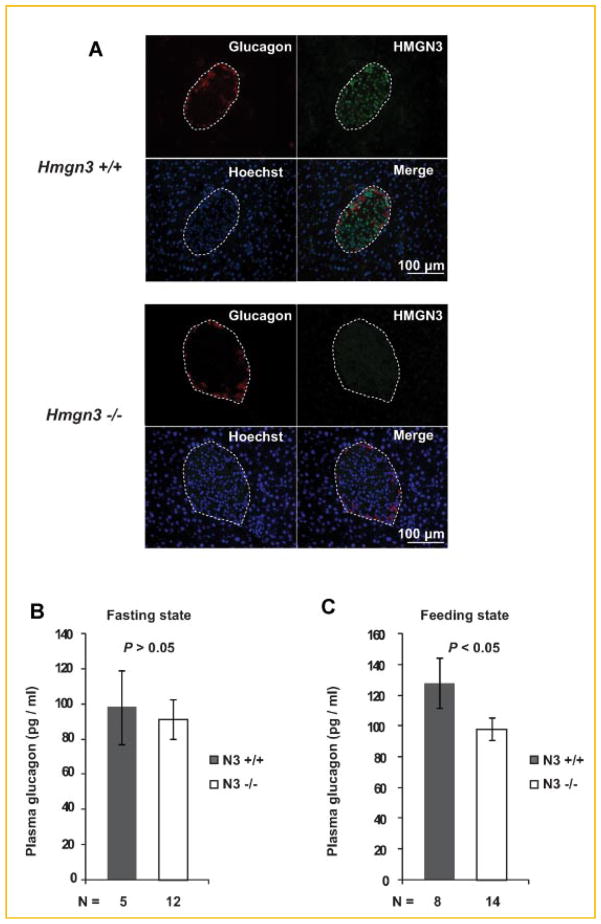
The high level of HMGN3 in the glucagon producing cells of the pancreatic islets raises the possibility that this nucleosomal binding protein regulates pancreatic α-cell function. To investigate this possibility we used the Hmgn3−/− mice that we generated [Ueda et al., 2009] to examine whether loss of HMGN3 affects the glucagon synthesis in pancreatic islets. Immunohistological analysis clearly reveals loss of HMGN3 expression in the pancreatic islets of Hmgn3−/− mice. In contrast, the signal from the glucagon specific antibody indicates that loss of HMGN3 did not affect the relative levels of glucagon, the overall shape of the islets, or the location of the α-cells in the mantle of the pancreatic islets (Fig. 2A).
Fig. 2.
Abnormal glucagon secretion in Hmgn3−/− mice under feeding state. A: Upper panel shows the global organization of HMGN3 and glucagon in Hmgn3+/+ mice. The red color demonstrates immunolocalization of glucagon in the cytoplasm and the green color visualizes the localization of HMGN3 in the nucleus. Lower panel shows immunostaining of HMGN3 and glucagon of Hmgn3−/− mice. Note complete absence of HMGN3 but no obvious gross morphological change in glucagon. B: No statistical difference was detected between Hmgn3−/− mice and Hmgn3+/+ mice for plasma glucagon levels after 16 h of fasting. C: Reduced plasma glucagon levels under fed condition whereas Hmgn3−/− mice displayed. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Since glucagon synthesis and secretion is one of the main known functions of pancreatic α-cells, we examined whether loss of HMGN3 affects the glucagon levels in mice. To this end we prepared plasma from the blood of either continuously feeding Hmgn3+/+ and Hmgn3−/− mice, or from mice that have been fasted for 16 h, and measured the concentration of glucagon by radioimmuno assay (RIA). Under fasting condition, we could not see significant difference of blood glucagon concentration between Hmgn3+/+ and Hmgn3−/− mice (Fig. 2B). On the other hand in feeding mice, the blood glucagon concentration of Hmgn3−/− mice are reduced significantly (P <0.05, Fig. 2C). These data suggest that HMGN3 affects glucagon secretion in feeding, but not in fasting mice. These results are surprising since we found that in feeding Hmgn3−/− mice the levels of insulin are significantly reduced, as compared to their Hmgn3+/+ littermates [Ueda et al., 2009]. Since insulin inhibits, while loss of insulin stimulates glucagon secretion [Kawamori et al., 2009], we expected an increase in glucagon concentration in the feeding Hmgn3−/− mice. However, opposite to our expectation, both the insulin levels and the blood glucagon levels are reduced in Hmgn3−/− mice, as compared to their Hmgn3+/+ littermates.
HMGN3 DOES NOT AFFECT VIABILITY OF αTC1-9 CELLS
To examine the molecular mechanism underlying the altered levels of glucagon in Hmgn3−/− mice, we examined the possible role of HMGN3 in glucagon synthesis and secretion using the mouse glucagonoma αTC1-9 cells, which have been extensively used to study the properties of pancreatic α-cells.
To ensure that αTC1-9 cells are indeed suitable for studies on HMGN3 function, and representative of the in vivo situation, we first evaluated the relative amount of HMGN3 in the cells. To this end we used western analyses and compared the content of HMGN proteins of αTC1-9 cells to that in mouse insulinoma MIN6 cells, which has typical properties of pancreatic β-cells. We previously demonstrated that MIN6 cells have an elevated content of HMGN3, just like the β-cells in the islets isolated from the pancreas of mice [Ueda et al., 2009]. Quantitative RT-PCR analyses reveal that the relative abundance of HMGN3 transcripts in αTC1-9 cells is similar to that of MIN6 cells (Fig. 3A). Likewise, western analysis demonstrates that the protein content of both HMGN3 splice variants, HMGN3a and HMGN3b in αTC1-9 cells is very similar to that of MIN6 (Fig. 3B). Thus, the αTC1-9 cells are suitable for studies on the potential role of HMGN3 in glucagon synthesis and secretion from pancreatic α-cells.
Fig. 3.
A: HMGN3 is highly expressed in αTC1-9 cells. The mRNA expression level of Hmgn3 was compared between αTC1-9 cells and MIN6 cells (mouse insulinoma cell line) by quantitative RT-PCR. B: Western blot was performed using total cell lysate from αTC1-9 cells or MIN6 cells. Histone H3 indicate equal loading.
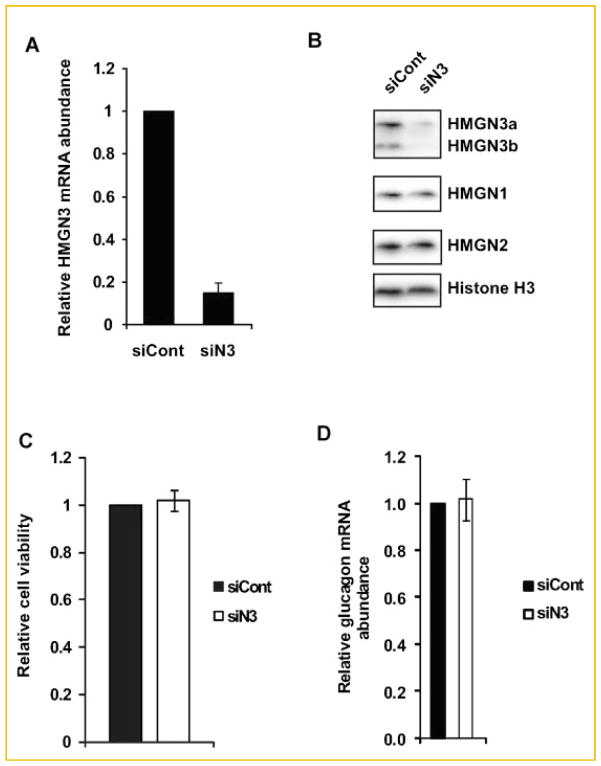
To test for a possible role of HMGN3 in the function of αTC1-9 cells we treated the cells with siRNA targeted to Hmgn3 (siN3). Quantitative RT-PCR revealed that the treatment reduced the levels of Hmgn3 transcripts by over 80% (Fig. 4A). Western analysis revealed a similar reduction in the protein levels of both HMGN3a and HMGN3b variants (Fig. 4B). Importantly, this down regulation was specific to HMGN3 since the levels of HMGN1 and HMGN2 variants were not affected by the loss of HMGN3. Because HMGN3 is a chromatin binding protein that could affect the structure and activity of chromatin [Bustin, 2001], we tested whether loss of this protein affected the viability of the transfected cells. Cell titer blue assay showed that siRNA mediated depletion of HMGN3 did not affect cell viability of αTC1-9 cells (Fig. 4C), a finding that is consistent with the observation of normal α-cells in the pancreas of Hmgn3−/− mice (Fig. 2A). Furthermore, depletion of HMGN3 did not significantly affect the cell growth rate as determined by the number of cells growing in culture (Table I). Taken together, these data suggest that HMGN3 does not affect cell viability and proliferation of pancreatic α-cells.
Fig. 4.
Loss of HMGN3 does not affect cell viability or glucagon transcription. A: Specific siRNA mediated down regulation Hmgn3 transcript in αTC1-9 cells. B: Western blot analysis demonstrated specific down regulation of HMGN3 protein levels in siN3-treatd cells. C: siRNA mediated decrease in HMGN3 protein does not affect cell viability as measured by the CellTiter-Blue Cell Viability Assay. D: siRNA mediated decrease in HMGN3 protein does not affect the expression of glucagon gene. αTC1-9 cells were collected 4 days after transfection of siRNA.
TABLE I.
Glucagon Release and Intracellular Content of HMGN3 Knock Downed αTC1-9 Cells
| Viable cells (×106) | Medium (ng/30 min) | Content (ng) | Total (ng) | Total (ng/106 cells) | Medium/total (%) | |
|---|---|---|---|---|---|---|
| siCont | 2.80 ± 0.23* | 3.43 ± 0.15* | 10.09 ± 0.16* | 13.52* | 4.83* | 25.37* |
| siN3 | 2.95 ± 0.15* | 3.22 ± 0.14* | 9.91 ± 0.3* | 13.13* | 4.45* | 24.52* |
Depletion of HMGN3 does not have effects on glucagon secretion in αTC1-9 cells. Glucagon release and intracellular content of cells 4 days after transfection of siRNA. Logarithmically growing cells were collected by centrifugation, resuspended in fresh growing medium for 30 min and sedimented. The glucagon content in the medium and in the collected cells was determined by radioimmuneassay.
Values are means ± SD.
P >0.05.
HMGN3 DOES NOT AFFECT GLUCAGON GENE EXPRESSION AND SECRETION IN α-CELL LINE
HMGN proteins have been shown to regulate and facilitate various DNA-related activities such as transcription, replication, recombination and repair, but only in the context of chromatin [Bustin, 2001]. Previous analyses of genetically altered mice lacking various HMGN variants suggested that these proteins affect transcription by modulating the levels of histone modifications in gene regulatory regions [Lim et al., 2004]. We therefore tested whether HMGN3 is involved in glucagon gene expression and performed quantitative RT-PCR in αTC1-9 cells that were treated either with control siRNA or with siN3, which down regulated the levels of HMGN3. The data indicate that an 80% reduction in the levels of HMGN3 did not affect the levels of glucagon transcripts in αTC1-9 cells (Fig. 4D). The results suggest that the altered levels of glucagon in the serum of Hmgn3−/− mice are not due to altered levels of glucagon gene transcription in pancreatic α-cell.
HMGN3 may affect the plasma glucagon levels not only by regulating glucagon synthesis but also by affecting its secretion from pancreatic α-cells. To examine a possible effect of depletion of HMGN3 on glucagon secretion, logarithmically growing cells that were treated with either control- or Hmgn3-specific siRNA were sedimented, the medium was replaced with KRBH Buffer and after 30 min resedimented. The levels of glucagon in the medium and in the sedimented cells were determined by RIA. We find that for both siRNA treatments the cells secreted about 25% of their glucagon and that the levels of glucagon in the medium and in the cells were not significantly affected by loss of HMGN3 (Table I). In summary, in this α-cell line, HMGN3 does not significantly affect the levels of glucagon or its secretion.
HMGN VARIANTS DO NOT AFFECT GLUCAGON GENE EXPRESSION IN α-CELL LINE
One possible explanation for the lack of HMGN3 effect on glucagon synthesis and secretion is that other HMGN variants compensate for loss of HMGN3. Indeed, our analyses of the HMGN content of αTC1-9 cells indicated high levels of both HMGN1 and HMGN2 in these cells (Fig. 3B) and previous studies raised the possibility of functional redundancy among HMGN variants [Furusawa et al., 2006; Postnikov et al., 2006]. The possible functional redundancy is based on two major findings: first, that the binding of all HMGNs to chromatin is highly dynamic [Ueda et al., 2008]. The proteins bind only temporarily to any specific nucleosome and they continuously move throughout the entire nucleus. Thus, potentially every nucleosome is associated with each type of HMGN variant at some of the time. Second, all HMGNs seem to have similar physical properties and bind to similar sites on nucleosomes [Ueda et al., 2008]. Therefore, it is possible that the loss of one HMGN variant type would not have a significant affects on a specific cell function. On the other hand, loss of HMGN1 leads to distinct changes in chromatin and to specific phenotypes and loss of HMGN3 results in a mild diabetic phenotype [Ueda et al., 2009]. The emerging picture suggests that the various HMGNs do not fully compensate, but rather dampen the deleterious effects of the absence of an HMGN variant.
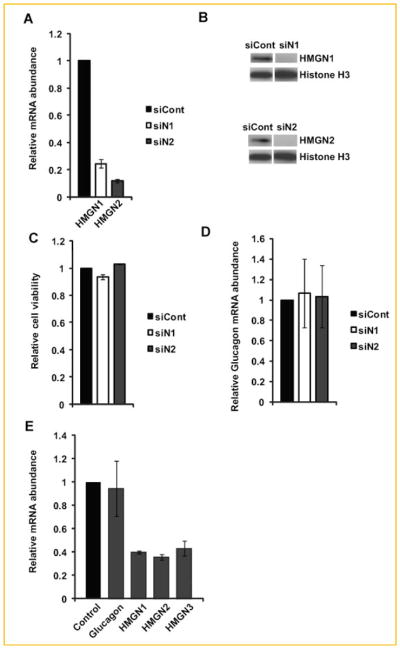
To investigate possible effects of the HMGN1 and HMGN2 variants on glucagon gene expression, we depleted the levels of these proteins in αTC1-9 cells by treating them with specific siRNAs. We already demonstrated that these siRNAs are specific for their respective variants [Ueda et al., 2009] in αTC1-9 cells, treatment with siN1 reduced the levels of Hmgn1 transcript by 80% and the protein levels by over 90% (Fig. 5A,B). Likewise, treatment with siN2 reduced the levels of Hmgn2 transcript by almost 90%, and the protein was not detectable in the cells (Fig. 5A,B). Down regulation of either HMGN1 or HMGN2 did not affect cell viability (Fig. 5C) or the levels of glucagon transcripts (Fig. 5D). Thus, by themselves neither HMGN1 nor HMGN2 has significant effects on the transcription of the glucagon gene. Finally, to test for the possibility of functional redundancy even more rigorously we treated the cells with a combination of siRNAs against all the HMGN variants. In these cells the transcript levels were reduced by over 70%, but neither cell viability nor the levels of glucagon transcripts were significantly affected (Fig. 5E). The results suggest that HMGNs do not play a major role in the regulating the viability of phenotype of αTC1-9 cells and that the reduction of glucagon in the serum of Hmgn3−/− is not due to a direct effect of HMGN3 on the function of pancreatic α-cells.
Fig. 5.
HMGN variants are not involved in glucagon gene expression in αTC1-9 cell line. A: Specific siRNA mediated down regulation of Hmgn1 and Hmgn2 transcripts in αTC1-9 cells. B: Western blotting analysis shows effective siRNA mediated depletion of HMGN1 and HMGN2 protein. C: siRNA mediated depletion of HMGN1 or HMGN2 protein does not affect cell viability of αTC1-9 cells. D: siRNA mediated depletion of HMGN1 or HMGN2 protein does not affect the expression of glucagon gene. E: Triple knock down of HMGN1, HMGN2 and HMGN3 do not affect the expression of glucagon gene in αTC1-9 cells.
In summary, we find that HMGN3 is expressed in all pancreatic endocrine cells in mouse. Hmgn3−/− mice have a mild diabetic phenotype, an indication that HMGN3 protein plays a role in glucose homeostasis. Indeed, the mice have abnormal insulin levels in their blood [Ueda et al., 2009] and low levels of glucagon in their plasma (Fig. 2). The low levels of insulin in the blood could be attributed to disruption of the transcriptional network of pancreatic β-cells. On the other hand the down regulation of glucagon in the plasma of these mice cannot be attributed directly to the low levels of insulin or to dysfunction of pancreatic α-cells since in our model system the glucagon synthesis and secretion was not affected by loss of HMGN3. We note however that the αTC1-9 cells were generated by transformation with SV40 and therefore it is possible that their properties are not identical to wild type pancreatic cells. Nevertheless, these cells have been shown to be a good model for pancreatic α-cells function and therefore it is reasonable to conclude that our observation truly reflect the fact that HMGN3 does not have a major function in α-cells. We also note that HMGN3 is expressed in all the endocrine cells of the islets and that in Hmgn3−/− mice all these cells lack the protein. Therefore it is possible that the blood levels of somatostatin or pancreatic polypeptide are also changed in Hmgn3−/− mice. Indeed, it is well established that somatostatin is a potent inhibitor of glucagon and insulin secretion [Sakurai et al., 1974; Luft et al., 1978]. In a recent manuscript we demonstrated that Hmgn3−/− have a mild diabetic phenotype which can be linked to transcriptional changes in β-cells [Ueda et al., 2009]. Finally, it is also possible that the altered levels of glucagon in the plasma of Hmgn3−/− are due to the abnormalities in the function of other organs known to affect glucose homeostasis such as the brain [Rosen and Spiegelman, 2006]. We have demonstrated that HMGN3 is indeed expressed in brain tissue [Ito and Bustin, 2002].
Although by itself HMGN3 does not have detectable effects on the function of α-cells it is still possible that in conjunction with additional genetic events HMGN3 may play a role in these cells. Indeed, we found that cells lacking the HMGN1 variant are hypersensitive to various stresses such as UV irradiation [Birger et al., 2005], gamma irradiation [Birger et al., 2005], and heat shock [Belova et al., 2008].
Acknowledgments
This research was supported by a JSPS research fellowship from the Japanese Biomedical and Behavioral Research at NIH to T.K., and by the Center for Cancer Research, the intramural program of the National Cancer Institute, NIH.
References
- Belova GI, Postnikov YV, Furusawa T, Birger Y, Bustin M. Chromosomal protein HMGN1 enhances the heat shock-induced remodeling of Hsp70 chromatin. J Biol Chem. 2008;283:8080–8088. doi: 10.1074/jbc.M709782200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birger Y, Catez F, Furusawa T, Lim JH, Prymakowska-Bosak M, West KL, Postnikov YV, Haines DC, Bustin M. Increased tumorigenicity and sensitivity to ionizing radiation upon loss of chromosomal protein HMGN1. Cancer Res. 2005;65:6711–6718. doi: 10.1158/0008-5472.CAN-05-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin M. Chromatin unfolding and activation by HMGN(*) chromosomal proteins. Trends Biochem Sci. 2001;26:431–437. doi: 10.1016/s0968-0004(01)01855-2. [DOI] [PubMed] [Google Scholar]
- Furusawa T, Lim JH, Catez F, Birger Y, Mackem S, Bustin M. Down-regulation of nucleosomal binding protein HMGN1 expression during embryogenesis modulates Sox9 expression in chondrocytes. Mol Cell Biol. 2006;26:592–604. doi: 10.1128/MCB.26.2.592-604.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromada J, Franklin I, Wollheim CB. α-Cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev. 2007;28:84–116. doi: 10.1210/er.2006-0007. [DOI] [PubMed] [Google Scholar]
- Guillam MT, Hummler E, Schaerer E, Yeh JI, Birnbaum MJ, Beermann F, Schmidt A, Deriaz N, Thorens B. Early diabetes and abnormal postnatal pancreatic islet development in mice lacking Glut-2. Nat Genet. 1997;17:327–330. doi: 10.1038/ng1197-327. [DOI] [PubMed] [Google Scholar]
- Guillam MT, Dupraz P, Thorens B. Glucose uptake, utilization, and signaling in GLUT2-null islets. Diabetes. 2000;49:1485–1491. doi: 10.2337/diabetes.49.9.1485. [DOI] [PubMed] [Google Scholar]
- Ito Y, Bustin M. Immunohistochemical localization of the nucleosome-binding protein HMGN3 in mouse brain. J Histochem Cytochem. 2002;50:1273–1275. doi: 10.1177/002215540205000914. [DOI] [PubMed] [Google Scholar]
- Johnson JH, Ogawa A, Chen L, Orci L, Newgard CB, Alam T, Unger RH. Underexpression of beta cell high Km glucose transporters in noninsulin-dependent diabetes. Science. 1990;250:546–549. doi: 10.1126/science.2237405. [DOI] [PubMed] [Google Scholar]
- Kawamori D, Kurpad AJ, Hu J, Liew CW, Shih JL, Ford EL, Herrera PL, Polonsky KS, McGuinness OP, Kulkarni RN. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab. 2009;9:350–361. doi: 10.1016/j.cmet.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Gerlitz G, Furusawa T, Catez F, Nussenzweig A, Oh KS, Kraemer KH, Shiloh Y, Bustin M. Activation of ATM depends on chromatin interactions occurring before induction of DNA damage. Nat Cell Biol. 2009;11:92–96. doi: 10.1038/ncb1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JH, Catez F, Birger Y, West KL, Prymakowska-Bosak M, Postnikov YV, Bustin M. Chromosomal protein HMGN1 modulates histone H3 phosphorylation. Mol Cell. 2004;15:573–584. doi: 10.1016/j.molcel.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Luft R, Efendic S, Hokfelt T. Somatostatin—Both hormone and neuro-transmitter? Diabetologia. 1978;14:1–13. doi: 10.1007/BF00429702. [DOI] [PubMed] [Google Scholar]
- Postnikov YV, Belova GI, Lim JH, Bustin M. Chromosomal protein HMGN1 modulates the phosphorylation of serine 1 in histone H2A. Biochemistry. 2006;45:15092–15099. doi: 10.1021/bi0613271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein YR, Furusawa T, Lim JH, Postnikov YV, West KL, Birger Y, Lee S, Nguyen P, Trepel JB, Bustin M. Chromosomal protein HMGN1 modulates the expression of N-cadherin. FEBS J. 2005;272:5853–5863. doi: 10.1111/j.1742-4658.2005.04980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai H, Dobbs R, Unger RH. Somatostatin-induced changes in insulin and glucagon secretion in normal and diabetic dogs. J Clin Invest. 1974;54:1395–1402. doi: 10.1172/JCI107886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloop KW, Michael MD, Moyers JS. Glucagon as a target for the treatment of Type 2 diabetes. Expert Opin Ther Targets. 2005;9:593–600. doi: 10.1517/14728222.9.3.593. [DOI] [PubMed] [Google Scholar]
- Ueda T, Catez F, Gerlitz G, Bustin M. Delineation of the protein module that anchors HMGN proteins to nucleosomes in the chromatin of living cells. Mol Cell Biol. 2008;28:2872–2883. doi: 10.1128/MCB.02181-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T, Furusawa T, Kurahashi T, Tessarollo L, Bustin M. The nucleosome binding protein HMGN3 modulates the transcription profile of pancreatic β-cells and affects insulin secretion. Mol Cell Biol. 2009;29:5264–5276. doi: 10.1128/MCB.00526-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera A, Solanes G, Fernandez-Alvarez J, Pujol A, Ferrer J, Asins G, Gomis R, Bosch F. Expression of GLUT-2 antisense RNA in beta cells of transgenic mice leads to diabetes. J Biol Chem. 1994;269:28543–28546. [PubMed] [Google Scholar]
- Vestner B, Bustin M, Gruss C. Stimulation of replication efficiency of a chromatin template by chromosomal protein HMG-17. J Biol Chem. 1998;273:9409–9414. doi: 10.1074/jbc.273.16.9409. [DOI] [PubMed] [Google Scholar]