Abstract
Background
In breast cancer patients, venous drainage of the breast may contain cells of immunological importance, tumor cells undergoing dissemination, and other biological factors derived from the tumor microenvironment. Collecting axillary venous blood during modified radical mastectomy and thus before dilution in the circulation may allow us to define biological properties of the tumor microenvironment. Aims were to (1) develop a surgical approach to collect blood from the breast tumor microenvironment through tributaries of the axillary vein and (2) characterize and compare immune cells collected from the axillary vein with those in peripheral blood of breast cancer patients.
Materials and Methods
We enrolled 17 women aged 30–50 years and diagnosed with breast cancer by mammography, ultrasound, and biopsy (stages II–III). All patients were, preoperatively, treatment-naive. During routine surgical dissection, blood was collected in heparin tubes, 10 mL from tributaries of the axillary vein and 10 mL from peripheral blood. Mononuclear cells were separated, and percentages of different leukocyte populations were determined by flow cytometry.
Results
We detected a significant increase in the percentage of total T lymphocytes and T helper cells collected from axillary tributaries, but not in the percentages of cytotoxic T cells, monocytes, natural killer, or B cells compared with peripheral blood.
Conclusions
The present study validated using an intra-operative surgical approach to collect leukocytes drained from the tumor microenvironment through axillary tributaries. Our results showed an increase in the infiltration of total T-lymphocytes and T helper cells in the tumor microenvironment, suggesting that they may contribute to tumor pathogenesis.
Breast cancer is the most common cancer in women, with more than 1 million cases and nearly 600,000 deaths occurring worldwide annually. Although breast cancer rates have decreased in the United States over the last few years, studies suggest that the rate of breast cancer in Egypt increased from 29% in 2003 to 37.5% of all reported cancer cases in Egypt in 2007.1–3 Invasive properties and involvement of positive lymph nodes are associated with poor prognosis and low survival rate among Egyptian breast cancer patients.4 In Egypt, modified radical mastectomy (MRM) is the most performed operation with an average of 85% compared with 15% breast conservation surgery.3 This may be attributed to many factors including late presentation of the patients, high costs of neoadjuvant therapy, patient anxiety from chemotherapy, difficulty of patient follow-up, and cultural issues.3 Intraoperative cellular and biochemical characterization of venous blood during MRM and thus before dilution in the circulation may allow us to define critical biological properties of the tumor.5
The significance of collecting venous blood from tumor sites other than breast cancer has been described by different studies. For instance, circulating tumor cells (CTC) detected in venous drainage of colorectal cancer could be used as a prognostic marker and a “mode of staging” of the disease.6 In addition, CTC were detected in most pulmonary venous blood of most lung cancer patients and not in their peripheral blood.7 In breast cancer patients, venous drainage of the breast through internal mammary veins and axillary vein may contain disseminated tumor cells and other factors derived from the tumor microenvironment. Collecting blood from breast tumor site through the axillary vein has been described previously in regard to assessing the level of serum tumor markers (sialic acid, ferritin, and carcinoembryonic antigen [CEA]) in blood collected from axillary vein during breast cancer surgery compared with peripheral blood.8 Although the results revealed no significant difference between the assessed tumor biomarkers in axillary venous blood versus peripheral blood, further studies were recommended to “clarify” the advantages of their surgical method. In addition, this previous study did not look at the immunophenotype of the cells collected at the two sites.8
Immune cells, including macrophages and T lymphocytes, are known to infiltrate various tumors including breast, contributing to high levels of growth factors, hormones, and cytokines in the tumor microenvironment.9,10 A strong association between breast tumor-associated macrophages and poor prognosis has been reported.11,12 Furthermore, infiltration of breast tumors by FOXP3-positive regulatory T cells has been found to be associated with poor prognosis.13 On the other hand, human breast tumor tissues are also infiltrated with dendritic cells, which are often localized in clusters with CD3 + T lymphocytes and thus are indicative of an immune reaction.14
At present, there are no approaches during breast cancer surgery to capture cells migrating from the tumor microenvironment. Therefore, the objectives of the present study are to: (1) develop an innovative surgical approach to collect blood from the breast tumor microenvironment through tributaries of the axillary vein during MRM and (2) characterize and compare the leukocyte composition of blood collected from the tributaries of the axillary vein with that of peripheral blood in breast cancer patients.
MATERIALS AND METHODS
Patients
All human specimens were obtained with informed consent as approved by Ain Shams University Human Research Ethics Committee. A total of 17 women diagnosed with breast cancer by clinical examination, ultrasound, mammography, and confirmed by biopsy (trucut; stages II–III) were enrolled into this study from Ain Shams University Hospitals. Ages ranges from 30 to 50 years old. All patients were preoperatively treatment naive and scheduled to undergo MRM. Samples were collected during MRM for research purposes and diagnosis. All patients were diagnosed as invasive ductal carcinoma stages II–III with positive metastatic lymph nodes. Patients suffering from any viral infection or auto-immune diseases were excluded from our study. Routine pathological diagnosis was assessed for all patients. Pathological diagnosis included: tumor size, histological type, tumor grade, presence and absence of lymphovascular invasion, and level of expression of hormone and growth factor receptors.15
Surgical Procedures to Collect Blood from Axillary Tributary During Modified Radical Mastectomy
MRM operations took an average of 90 min, depending on patient anatomy. During axillary dissection, the axillary adipose tissues were swept laterally from the chest wall and inferiorly from the axillary vein. Superficial axillary tributaries of the axillary vein collecting blood from the tumor site were identified as described elsewhere.16 About 10 ml blood was withdrawn from the identified axillary tributaries in a heparinized syringe with angular needle. We have to note that blood withdrawal was performed during routine axillary dissection and before superficial axillary tributaries ligation. Also, 10 ml control peripheral blood was collected from the antecubital vein in vacutainer heparin tubes.
Isolation of Blood Mononuclear Cells
Blood collected from axillary tributaries and peripheral blood was transferred directly to the laboratory after surgical procedures for isolation of leukocytes. Each type of blood was diluted with an equal amount of phosphate-buffered saline (PBS), pH 7.2 at room temperature. Mononuclear cells were separated by Ficoll-Hypaque density gradient centrifugation (Sigma, USA) at 2000 rev/min as described previously.17 The buffy coat layer containing mononuclear cells was separated and washed twice in PBS. Cells were suspended in RPMI 1640 medium (Invitrogen, USA) supplemented with penicillin G (200 U/mL), streptomycin sulfate (100 μg/ml), L-glutamine (2 mM) and 10% heat-inactivated pooled human AB serum (Hyclone, USA). Mononuclear cells were used fresh for immunophenotyping by flow cytometric analysis or stored in freezing media containing 95% FBS (Hyclone, USA) and 5% DMSO (Sigma, USA) and kept in liquid nitrogen until used for flow cytometric analysis.
Immunophenotyping of Isolated Blood Mononuclear Cells (BMC)
We compared the immunophenotype of mononuclear cells isolated from axillary tributaries with that of peripheral blood of the same patient. Briefly, mononuclear cell suspensions were adjusted to 1 × 106 cells/ml, and the phenotype of the isolated leukocytes was assessed using flow cytometry. T cells were defined by expression of CD3+, whereas NK cells were defined as CD56+ and CD3−. NK T cells were defined as CD3+/CD56+ cells. However, CD4+ T cells were defined by expression of both CD3+ and CD4+ and lack of CD8− expression and CD8+ T cells by expression of both CD3+ and CD8+ and lack of CD4− expression. B cells were defined as CD19+ CD3−and CD14− cells and monocytes/macrophages as CD14+, CD3−, and CD19−. Immunophenotypic characterization was determined using the following fluorochrome-labeled monoclonal antibodies (FITC-CD4, PE-CD3, PerCP-CD3, PerCP-CD8, PerCP-CD19, APC-CD56, and APC-CD14; all from BD Pharmingen, CA). Each sample was stained with a combination of 4 monoclonal antibodies listed before and cell phenotype was detected using 4-color FACSCalibur flow cytometer (BD Biosciences, CA). Briefly, the isolated mononuclear cells were stained with monoclonal antibodies for cell surface markers unique to particular cell types. After an incubation time of 30 min at 4°C in a dark place, the cells were washed twice and resuspended in 500 μl wash buffer and then analyzed with a FACCalibur flow cytometer (Becton Dickinson, San Jose, CA); 50,000 events were acquired on a FACSCalibur flow cytometer and data were analyzed using FlowJo software (Tree Star Inc., CA).
Statistical Analysis
Data are expressed as mean ± standard deviation (SD). Statistical significance was determined using t test with P ≤ 0.05 considered significant.
RESULTS
Patient Clinical and Pathological Characteristics
Clinical and pathological characterization of patients tested is described in Table 1. Patients’ ages ranged from 30 to 50 years (with median age of 47.4 years). Tumor sizes ranged from 3 to 8 cm (median size of 5.5 cm). There were 11 patients diagnosed as tumor grade II and 6 patients diagnosed as tumor grade III. All patients were lymph node positive: 8 patients with ≤4 and 9 patients with>4 positive metastatic lymph nodes. Test for hormone receptor expression revealed that 7 patients were ER positive, 9 patients were PR positive, and 6 patients were HER-2 positive. Invasion of tumor cells into lymphatic vessels was detected in 3 patients.
TABLE 1.
Patients and tumor characteristics (Total 17)
| Characteristics | N (%) |
|---|---|
| Age (years) | |
| Median | 47.4 |
| Range | 30–50 |
| Tumor size (cm) | |
| Median | 5.5 |
| Range | 3–8 |
| Tumor grade | |
| Grade I | 0 (0%) |
| Grade II | 11 (65%) |
| Grade III | 6 (35%) |
| No. of metastatic lymph nodes | |
| ≤4 | 8 (47%) |
| > 4 | 9 (53%) |
| Lymphovascular invasion | |
| Positive | 3 (18%) |
| Negative | 14 (82%) |
| Estrogen receptors | |
| Positive | 7 (41%) |
| Negative | 10 (59%) |
| Progesterone receptors | |
| Positive | 9 (53%) |
| Negative | 8 (47%) |
| HER-2 | |
| Positive | 6 (35%) |
| Negative | 10 (59%) |
| Unknown | 1 (6%) |
Exposure of Axillary Tributaries and Collection of Blood
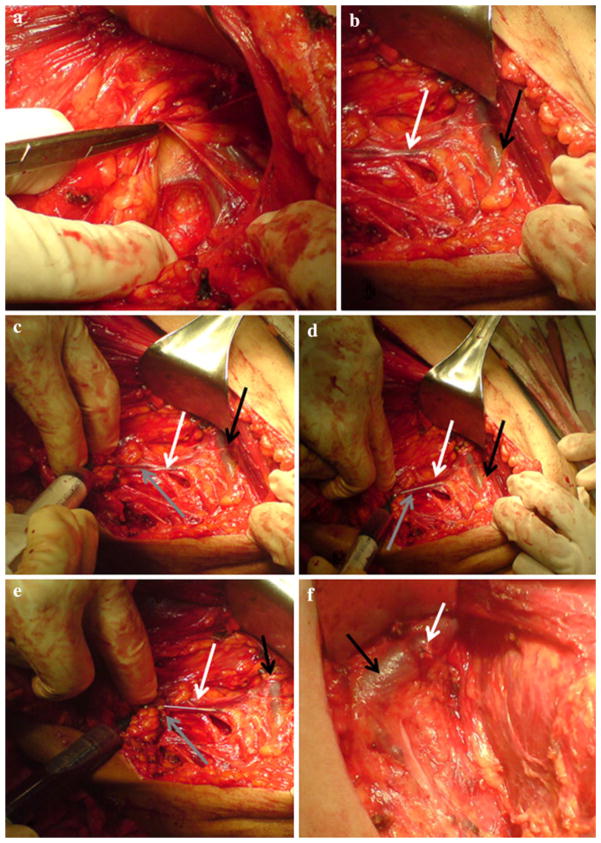
Blood was collected from the superficial tributaries of the axillary vein (Fig. 1a, b). About 10 ml of blood was withdrawn with a heparinized syringe via an angular needle to facilitate blood withdrawal as shown in Fig. 1c–e. Intraoperative blood withdrawal from tributaries of the axillary vein took about 10 min. After blood withdrawal the axillary tributary was ligated (Fig. 1f) before proceeding with a routine MRM operation. During axillary dissection, level I and level II lymph nodes (n) were dissected and sent for pathological diagnosis. The number of positive metastatic lymph nodes (x) was recorded in the pathology report (x/n). Peripheral blood was also collected during surgery for comparative purposes.
FIG. 1.
a, b represent dissection of the axilla and identification of the superficial tributaries of the axillary vein in a female breast cancer patient during modified radical mastectomy. c–e represent a time series showing collection of blood from identified superficial tributary (white arrow) of the axillary vein (black arrow) by angular needle (gray arrow)
Analysis of Mononuclear Cells Collected from Axillary Vein Tributaries and Peripheral Blood in Breast Cancer Patients
Blood mononuclear cells collected from axillary tributaries and peripheral blood were separated and subjected to immunophenotyping using specific monoclonal antibodies to identify the following cells: T cells (CD4+ and CD8+), NK cells (CD56+), B lymphocytes (CD19+), and monocytes (CD14+) as described in the Materials and Methods section. Flow cytometric immunophenotypic data of cells collected from axillary tributaries and peripheral blood of breast cancer patients were expressed as percentage of each cell type and are summarized in Table 2.
TABLE 2.
Immunophenotype of mononuclear cells collected from axillary tributaries and peripheral blood of breast cancer patients (n = 17)
| Cell surface antigens | % of cells in the axillary tributaries | % of cells in peripheral blood |
|---|---|---|
| T cells (CD3+) | 63 ± 8* | 37 ± 5 |
| NK cells (CD56+) | 8 ± 4 | 8 ± 3 |
| CD4+ T cells (CD4+ CD3+) | 44 ± 8* | 21 ± 1 |
| CD8+ T cells (CD3+ CD4−) | 17 ± 4 | 15 ± 6 |
| B cells (CD19+) | 13 ± 4 | 14 ± 4 |
| Monocytes (CD14+) | 7 ± 2 | 6 ± 1 |
Statistically significant difference, P ≤ 0.05
Increased Percentage of T Lymphocytes (CD3+) Cells in Blood Collected from Axillary Tributaries
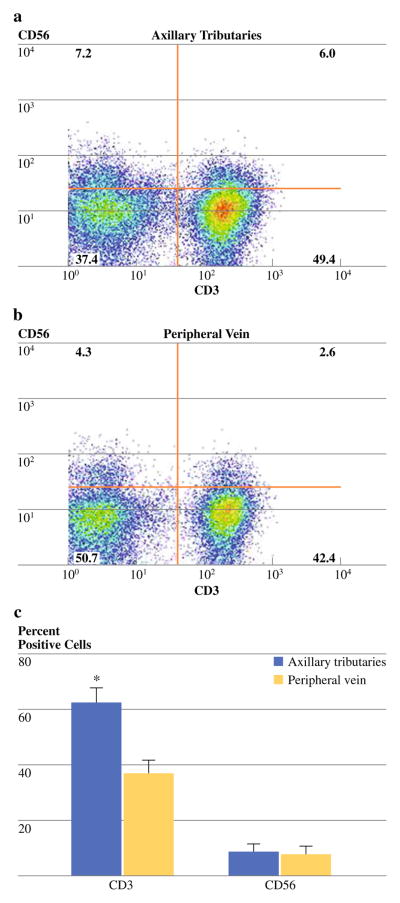
The immunophenotype of mononuclear cells collected from axillary tributaries and isolated from peripheral blood was analyzed by immunofluorescent staining and multi-color flow cytometry and expressed as a percentage of the total cell population (Figure 2a, b). The analysis revealed a significant increase (P < 0.05) in the percentage of T-lymphocytes collected from axillary tributaries (63 ± 8%) compared with peripheral blood (37 ± 5%; Table 2; Fig. 2c). These data suggest that more T cells are present in the venous drainage of the breast than in peripheral blood in breast cancer patients.
FIG. 2.
Representative dual-parameter staining density plots of CD3+ and CD56+ cells from the axillary tributaries (Panel a) and peripheral blood (Panel b). Bars (Panel c) represent cumulative data (n = 17) for the mean percentage of CD3+ and CD56+ cells. Data are shown as the mean ± SD. * P value<0.05 as determined by t test
Predominance of T Helper Cells (CD3+/CD4+) in Blood Collected from Axillary Tributaries
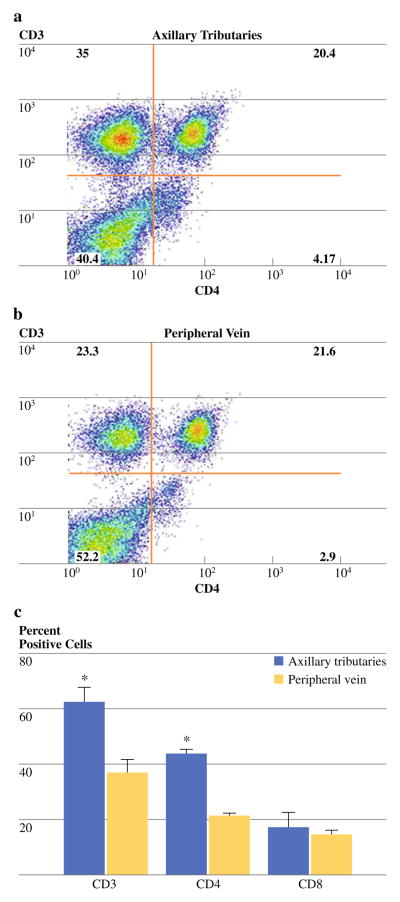
To further phenotype the CD3+ T cells isolated from the tumor microenvironment and those of peripheral blood into T helper (CD4+) and cytotoxic (CD8+) subsets, cells were stained with monoclonal antibodies specific for CD3 and CD4 (Fig. 3a, b). Cytotoxic T cells were further identified using both CD3 and CD8 expression showing the same outcome as CD3+/CD4− staining. Results revealed a significant increase (P < 0.05) in the percentage of CD4+ T cells collected from axillary tributaries (44 ± 8%) versus peripheral blood (21 ± 1%; Table 2; Fig. 3c). However, the average percentage of CD8+ T cells collected from axillary tributaries (17 ± 4%) and peripheral blood (15 ± 6%) was not statistically different (Table 2; Fig. 3c).
FIG. 3.
Representative dual-parameter staining density plots of CD4+ and CD8+ T lymphocytes from axillary tributaries (Panel a) and peripheral blood (Panel b). Bars (Panel c) represent cumulative data (n = 17) for the mean percentage of CD3+ T, CD4+ T, and CD8+ cells. Data are shown as the mean ± SD. * P value <0.05 as determined by t test
Nonsignificant Differences in the Percentage of Natural Killer Cells (CD56+), B Cells (CD19+) and Monocytes (CD14+) Collected from Axillary Tributaries and Peripheral Blood
We stained NK cells with monoclonal antibodies specific for CD56 and CD3. NK cell were defined as CD56+/CD3− (Fig. 2a, b). The percentage of CD56+ collected from axillary tributaries (8 ± 4%) and peripheral blood (8 ± 3%) were not significantly different (Table 2; Fig. 2c).
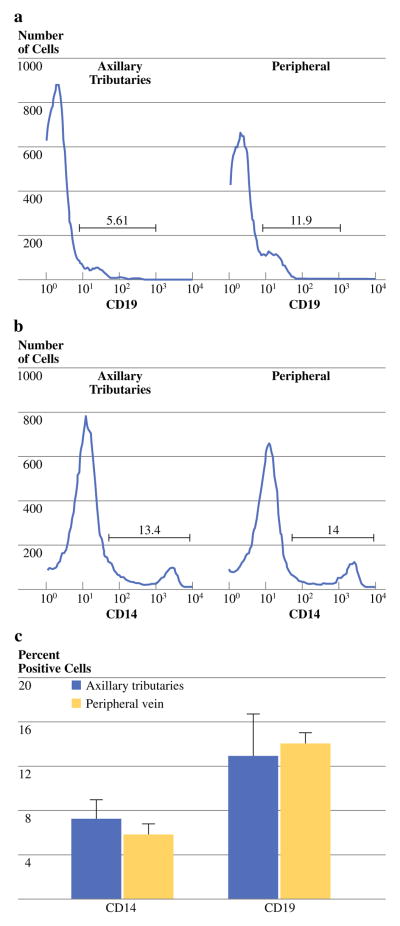
B-lymphocytes and monocytes/macrophages were stained with monoclonal antibodies specific for CD19 and CD14. B-lymphocytes were defined as CD19+/CD14−, while monocytes/macrophages were defined as CD14+/CD19− (Fig. 4a, b). There were no significant difference in the percentage of B-lymphocytes and monocytes/macrophages collected from axillary tributaries versus peripheral blood, where the percentage of B cells in the 2 sites were 13 ± 4% and 14 ± 4%, while the percentage of monocyte/macrophages were 7 ± 2% and 6 ± 1%, respectively (Table 2; Fig. 4c).
FIG. 4.
Representative histogram for cells staining for CD19+ and CD14+, from the axillary tributaries (Panel a) and peripheral blood (Panel b) of the same patient. Cumulative data are shown in panel c for the mean percentage of CD19+ and CD14+ cells in peripheral blood and axillary tributaries of BC patients. Data are shown as the mean ± SD
DISCUSSION
Our study presents a surgical approach to collect cells from the breast tumor microenvironment through venous drainage system of the breast. This was a modification of a previously described method, which suggested that collecting blood during MRM from the internal mammary vein or axillary vein yields an amount of blood sufficient for cellular analyses and for biological studies.5 In the present study, we found that during axillary dissection, we had easy access to the superficial axillary tributaries rather than to the internal mammary and axillary veins. The number of axillary tributaries ranged from 2 to 3 depending on the anatomy of the patient. We concluded that collecting blood from axillary tributaries during MRM for further analysis of different biological properties of the patients is more appropriate than method suggested before.5,8
We analyzed and compared using flow cytometry the immunophenotype of cells collected from axillary tributaries, which represent immune cells leaving the breast tumor microenvironment through the venous circulation, versus cells in peripheral blood of breast cancer patients. Indeed, the surgical approach used may be more efficient in collecting immune cells infiltrating tumor microenvironment than method described by Allinen and colleagues by mince breast tissues into small pieces using proteolytic enzymes and collecting different cellular fractions using the bead collection method.18
Cells collected from axillary tributaries possessed an immunophenotypic composition that was different from those collected from peripheral blood with a significant increase in the percentage of CD3+ T lymphocytes. As a host adaptive immune response, CD3+ T cells are known to infiltrate into the breast tumor microenvironment.19 The presence of T cells in the tumor microenvironment has been shown to predict clinical outcome better than tumor stage and nodal involvement in colon cancer, breast cancer, and cervical cancer.20–22
Since subsets of T cells have opposing actions against cancer cells, we further phenotyped the T-lymphocyte subsets isolated from axillary tributaries and that of peripheral blood into CD4+ T-helper and CD8+ T-cytotoxic subsets. We detected a significant increase in CD4+ cells collected from axillary tributaries; no significant increase was detected in the percentage of CD8+ cells. Macchetti et al. had previously identified CD4+ T-helper cells in breast cancer patients with positive lymph nodes.23 Since all patients enrolled in our study had positive meta-static lymph nodes, our findings recapitulate those of Macchetti et al.23 Indeed, CD4+ T cells play a crucial role in adaptive immunity in early tumor rejection by activating CD8+ tumor specific T-cytotoxic cells.24 It is also possible that different T helper cell subsets have contradictory actions on tumor development. In this regard, T-helper 1 cells producing IFNg activate macrophages and enhance the cytolytic function of tumor-specific T cells.25 Interestingly, a recent study using the MMTV-PyMT mice model shows that T-helper 2 cells producing IL-4 promote tumor growth and metastasis by enhancing macrophages “pro-tumor” properties leading to increased tumor dissemination.26 CD4+ cells can also contribute to immunosuppressive properties within the tumor microenvironment by secreting IL-4 and IL-13 that induce accumulation and differentiation of polarized type II macrophages and T-regulatory cells.27 The increased infiltration of CD4+ cells detected in breast cancer patients with positive metastatic lymph nodes suggests their role in augmentation of invasion and metastasis of breast cancer cells by secreting factors that stimulate tumor progression such as VEGF and matrix metalloproteinases (MMPs).23,28–30
There was no significant difference between percentages of NK cells and B cells collected from tumor microenvironment versus peripheral blood. A “paucity” of tumor-infiltrating B lymphocytes and NK cells in breast cancer tissues has been observed before.23 However, natural killer cells are one of the main arms of the immune system against cancers (innate immunity), where they kill tumor cells and play a significant role in antibody-mediated cytotoxicity promoting immunotherapy such as those of Trastuzumab.31,32 The lack of a significant difference between the percentage of NK cells isolated from the tumor microenvironment and peripheral blood need may be attributed to host tumor microenvironment of the studied group.
Tumor-associated macrophages play a crucial role in tumor invasion and metastasis; here we did not detect a significant difference between monocytes/macrophages (CD14+) isolated from axillary tributaries and peripheral blood. Since macrophages infiltrate the breast tumor microenvironment, their collection from axillary tributaries may not actually reflect their distribution in the breast tissue. In this regard we assessed percentage of CD14+ cells in paraffin tissue samples using immunohistochemistry. We found that CD14+ cells constitute about 30% of tumor tissue (data not shown).
It is also possible that the quality and phenotype of monocytes/macrophages isolated from both sites are different. In this regard, M1 macrophages, which are induced by Th1 cells, can contribute with CD8 T cells in suppressing tumor growth.26,33 However, M2 macrophages induced by Th2 cells and IL4 produce TGF-β, which suppresses antitumor activity, and EGFR ligands, which enhance growth and dissemination of the tumor.26,33
In summary, the data presented in this study provide a new method to collect blood from breast tumor microenvironment through axillary tributaries during MRM. Our results revealed immunophenotypic differences in the cellular composition of blood collected from axillary tributaries versus peripheral blood. This technique may be useful to improve our understanding of critical biological and functional properties of tumors and their cellular infiltrates.
Acknowledgments
The authors were supported by an Avon Foundation grant No. 02-2007-049 (M.M.M., B.F.S.), Cairo University grant No. CU-008-09 (M.M.M), and a Science and Technology Development Fund Egypt (STDF) grant No. 343 (M.M.M.)
References
- 1.Omar S, Khaled H, Gaafar R, et al. Breast cancer in Egypt: a review of disease presentation and detection strategies. East Mediterr Health J. 2003;9:448–63. [PubMed] [Google Scholar]
- 2.Zeeneldin AA, Mohamed AM, Abdel HA, et al. Survival effects of cyclooxygenase-2 and 12-lipooxygenase in Egyptian women with operable breast cancer. Indian J Cancer. 2009;46:54–60. doi: 10.4103/0019-509x.48597. [DOI] [PubMed] [Google Scholar]
- 3.Denewer A, Setit A, Farouk O. Outcome of pectoralis major myomammary flap for post-mastectomy breast reconstruction: extended experience. World J Surg. 2007;31:1382–6. doi: 10.1007/s00268-007-9093-4. [DOI] [PubMed] [Google Scholar]
- 4.Nouh MA, Iamail H, Ali El-Din NH, Bolkainy MN. Lymph node metastasis in breast carcinoma: clinicopathologic correlations in 3747 patients. J Egypt Natl Canc Inst. 2004;16:50–6. [PubMed] [Google Scholar]
- 5.Carroll RG. Arresting metastases during excisional cancer surgery. Lancet Oncol. 2004;5:147–8. doi: 10.1016/S1470-2045(04)01409-3. [DOI] [PubMed] [Google Scholar]
- 6.Katsuno H, Zacharakis E, Aziz O, et al. Does the presence of circulating tumor cells in the venous drainage of curative colorectal cancer resections determine prognosis? A meta-analysis. Ann Surg Oncol. 2008;15:3083–91. doi: 10.1245/s10434-008-0131-8. [DOI] [PubMed] [Google Scholar]
- 7.Okumura Y, Tanaka F, Yoneda K, et al. Circulating tumor cells in pulmonary venous blood of primary lung cancer patients. Ann Thorac Surg. 2009;87:1669–75. doi: 10.1016/j.athoracsur.2009.03.073. [DOI] [PubMed] [Google Scholar]
- 8.Monti M, Catania S, Locatelli E, et al. Axillary versus peripheral blood levels of sialic acid, ferritin, and CEA in patients with breast cancer. Breast Cancer Res Treat. 1990;17:77–82. doi: 10.1007/BF01806287. [DOI] [PubMed] [Google Scholar]
- 9.Aaltomaa S, Lipponen P, Eskelinen M, et al. Lymphocyte infiltrates as a prognostic variable in female breast cancer. Eur J Cancer. 1992;28A:859–64. doi: 10.1016/0959-8049(92)90134-n. [DOI] [PubMed] [Google Scholar]
- 10.Georgiannos SN, Renaut A, Goode AW, et al. The immunophenotype and activation status of the lymphocytic infiltrate in human breast cancers, the role of the major histocompatibility complex in cell-mediated immune mechanisms, and their association with prognostic indicators. Surgery. 2003;134:827–34. doi: 10.1016/s0039-6060(03)00292-7. [DOI] [PubMed] [Google Scholar]
- 11.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–12. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 12.Leek RD, Lewis CE, Whitehouse R, et al. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–9. [PubMed] [Google Scholar]
- 13.Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–80. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 14.Aspord C, Pedroza-Gonzalez A, Gallegos M, et al. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med. 2007;204:1037–47. doi: 10.1084/jem.20061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Genestie C, Zafrani B, Asselain B, et al. Comparison of the prognostic value of Scarff-Bloom-Richardson and Nottingham histological grades in a series of 825 cases of breast cancer: major importance of the mitotic count as a component of both grading systems. Anticancer Res. 1998;18:571–6. [PubMed] [Google Scholar]
- 16.Bland KI, Vezeridis MT. Modified radical mastectomy with early or delayed breast reconstruction. In: Baker RJ, Fischer JE, editors. Mastery of Surgery. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 614–27. [Google Scholar]
- 17.Ibrahim SA, Abdelwahab SF, Mohamed MM, et al. T cells are depleted in HCV-Induced hepatocellular carcinoma patients: possible role of apoptosis and p53. Egypt J Immunol. 2006;13:11–22. [PubMed] [Google Scholar]
- 18.Allinen M, Beroukhim R, Cai L, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Ladoire S, Arnould L, Apetoh L, et al. Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating foxp3 + regulatory T cells. Clin Cancer Res. 2008;14:2413–20. doi: 10.1158/1078-0432.CCR-07-4491. [DOI] [PubMed] [Google Scholar]
- 20.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 21.Kohrt HE, Nouri N, Nowels K, et al. Profile of immune cells in axillary lymph nodes predicts disease-free survival in breast cancer. PLoS Med. 2005;2:e284. doi: 10.1371/journal.pmed.0020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piersma SJ, Jordanova ES, van Poelgeest MI, et al. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007;67:354–61. doi: 10.1158/0008-5472.CAN-06-3388. [DOI] [PubMed] [Google Scholar]
- 23.Macchetti AH, Marana HR, Silva JS, et al. Tumor-infiltrating CD4+ T lymphocytes in early breast cancer reflect lymph node involvement. Clinics. 2006;61:203–8. doi: 10.1590/s1807-59322006000300004. [DOI] [PubMed] [Google Scholar]
- 24.Hung K, Hayashi R, Lafond-Walker A, et al. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188:2357–68. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother. 2005;54:721–8. doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeNardo DG, Barreto JB, Andreu P, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704–15. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 28.Freeman MR, Schneck FX, Gagnon ML, et al. Peripheral blood T lymphocytes and lymphocytes infiltrating human cancers express vascular endothelial growth factor: a potential role for T cells in angiogenesis. Cancer Res. 1995;55:4140–5. [PubMed] [Google Scholar]
- 29.Mor F, Quintana FJ, Cohen IR. Angiogenesis-inflammation cross-talk: vascular endothelial growth factor is secreted by activated T cells and induces Th1 polarization. J Immunol. 2004;172:4618–23. doi: 10.4049/jimmunol.172.7.4618. [DOI] [PubMed] [Google Scholar]
- 30.Stetler-Stevenson M, Mansoor A, Lim M, et al. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in reactive and neoplastic lymphoid cells. Blood. 1997;89:1708–15. [PubMed] [Google Scholar]
- 31.Moretta A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat Rev Immunol. 2002;2:957–64. doi: 10.1038/nri956. [DOI] [PubMed] [Google Scholar]
- 32.Arnould L, Gelly M, Penault-Llorca F, et al. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer. 2006;94:259–67. doi: 10.1038/sj.bjc.6602930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pardoll D. Metastasis-promoting immunity: when T cells turn to the dark side. Cancer Cell. 2009;16:81–2. doi: 10.1016/j.ccr.2009.07.007. [DOI] [PubMed] [Google Scholar]