Abstract
Objective
This study of 236 individuals with bipolar disorders employed longitudinal analyses to determine whether the symptoms of mania and depression can be understood as one dimension (with depression and mania as opposites) or two relatively independent dimensions.
Method
Weekly severity ratings of manic and depression were assessed using the Longitudinal Interval Follow-up Evaluation-II for 72 weeks. The within-subjects correlation of manic and depressive severity was examined using random effects regression.
Results
Contrary to the one-dimension model, mania and depression symptoms were not negatively related. Indeed, the correlations of mania with depressive symptoms were quite small.
Conclusion
The data suggest that depressive and manic symptoms are not opposite poles. Rather depressive and manic symptoms appear to fluctuate relatively independently within bipolar disorder.
Keywords: bipolar disorder, mania, depression
For the past 100 years, models of bipolar disorder have assumed that depression and mania are opposite ends of a single continuum. Even the name “bipolar disorder” implies a single dimension.
This unidimensional model has shaped the search for aetiological variables. For example, one theory has been that manic episodes relate to an over-sensitivity of the dopamine system (1), whereas depressive episodes relate to low function of the dopamine system (2). Similarly, researchers have argued that manic episodes might reflect an over-activity of the behavioural approach system, whereas depressive episodes could reflect an under-activity (3). Hence, the unidimensional model has shaped both the diagnostic nomenclature and aetiological research.
Despite the influence of this model, relatively few studies have tested its core assumption – that manic and depressive symptoms are inversely related. Several researchers have examined the relationship between depressive and manic symptoms in cross-sectional designs. In those studies, factor analyses have failed to support the unidimensional model, suggesting that depressive and manic symptoms are independent (4–6). Cross-sectional studies, though, cannot disentangle a person’s relative propensity towards depression and mania from the relationship of depressive and manic symptoms over time. A better approach would be to test whether depressive and manic symptoms are negatively related over time within persons. That is, if the unidimensional model holds, one would expect that when a person is experiencing manic symptoms, they should be less likely to experience depressive symptoms, and vice versa. Although some researchers have assessed bipolar symptoms longitudinally (7–9), such studies have not examined the two poles model.
Aims of the study
The goal of this study was to test the unidimensional model of bipolar disorder in a large longitudinal dataset. More specifically, we examined whether manic and depressive symptoms are inversely related over time using within person analyses. Support for the unidimensional model would be provided if manic and depressive symptoms were robustly negatively related.
Material and methods
Participants
Data were drawn from the MRC UK multicentre trial of adjunctive cognitive behavioural therapy (CBT) for bipolar disorder: 252 participants were randomly assigned to treatment as usual (TAU– pharmacotherapy and outpatient services) or TAU plus 22 sessions of CBT. Inclusion criteria were age ≥18 years, DSM-IV diagnosis of bipolar disorder, mood episode within 12 months and adult psychiatry services within 6 months. Exclusion criteria were current manic episode, severe borderline personality disorder, four or more episodes in the past year, bipolar disorder secondary to organic causes or substance misuse, current psychological treatment for bipolar disorder, and inability or unwillingness to provide written informed consent. Previous reports have described symptom levels and treatment effects (10, 11).
Measures
At baseline, participants completed the Structured Clinical Interview for DSM-IV (12, 13). Then, the Longitudinal Interval Follow-up Evaluation-II (LIFE-II), a semi-structured interview with well-established reliability (14, 15) and validity in bipolar disorder (16, 17) was completed at face-to- face interviews every 8 weeks for 18 months. At each follow-up, interviewers made LIFE-II ratings to capture depressive and manic symptoms separately for each of the preceding 8 weeks (16 ratings per follow-up). Ratings for both depressive and manic symptoms were made on a six-point scale ranging from 1 “no symptoms” to 3 “clear evidence of symptoms, but not meeting full DSM-IV criteria for the syndrome,” to 6 “major depression or psychotic mania with severe functional impairment.” Details of the training, strong inter-rater reliability and minor modifications to the LIFE-II scoring system are provided elsewhere (10, 11).
Statistical analyses
To examine how depressive and manic symptoms correlated over time within subjects, two parallel models were computed with manic symptoms as the outcome variable and then with depressive symptoms as the outcome variable. We were interested in examining the simultaneous relationship of symptoms, but also the effects of manic symptoms on depressive symptoms over the next 2 weeks (and vice versa). Mixed-effects regression models were used to account for the correlations of symptoms within subjects (18). Analyses were conducted with the lme function of the NLME package in R using maximum likelihood estimation (http://cran.r-project.org/src/contrib/Descriptions/nlme.html). This program accounts for varying number of symptom interviews across individuals. As acute mania at baseline was an exclusion criterion, mean mania scores were low during the first 4 weeks, so our analyses focused on weeks 5–72. For manic symptoms, independent variables included current depression (LIFE-II) scores, and depression scores at 1 -week lag and 2 -week lag. We included simple effects of depression, as well as curvilinear effects (to test whether the influence of depressive symptoms on mania varied with depression severity). Other independent variables included lithium or other mood-stabilizer, antidepressant and antipsychotic equivalent doses; number of weeks since study entry (to control for symptom changes over time); and follow-up interview number (to control for the correlations of ratings within each interview). Autocorrelation terms were estimated, and stationarity was not assumed in calculating these. A parallel analysis was then conducted with depressive symptoms as the outcome variable.
Results
Repeated LIFE ratings were available for 236 participants (a total of 14 045 observations). Because of the large N, statistical power was high enough to detect very small effects, and emphasis was placed on evaluating the effect size. This sample was 64% women (n = 152) and the mean age was 41.2 (SD = 10.95) years; 221 (93.64%) met criteria bipolar I disorder, 200 (84.75%) participants were taking a mood stabilizer. As with other bipolar samples (19), 110 reported a lifetime history of substance misuse. Also parallel with other major longitudinal studies (8), 91.5% of patients reported at least some depressive symptoms, and 69.5% reported at least some manic symptoms during the follow-up period.
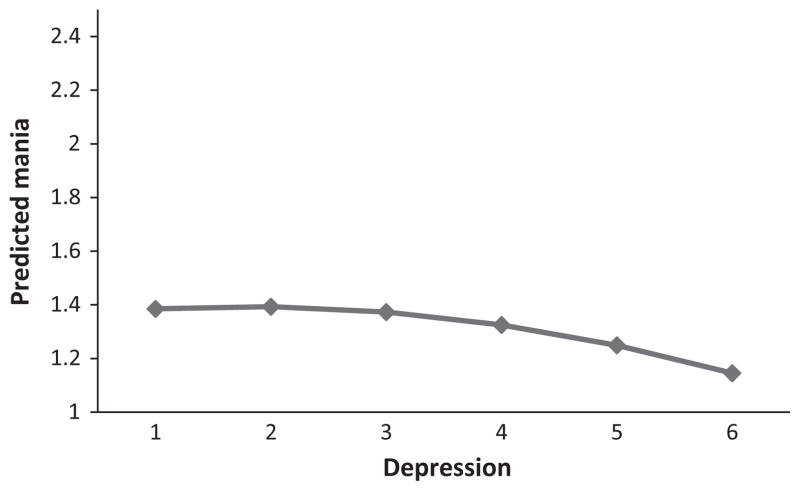
With mania LIFE-II scores as an outcome variable, Phi (the estimated within-subject correlation among mania scores) was 0.72. As shown in Table 1, the intercept term was significant, suggesting that the average mania score was above one. There was a small but significant effect for week; for each week followed, mania scores increased by an average of 0.005 points. For the effect of current depression on mania, there was a significant positive effect that was qualified by a negative curvilinear effect, as shown in Fig. 1. In contrast to the strong inverse relationship assumed by the unidimensional model, depression accounted for very little of the variance in manic scores. Simple effects and curvilinear effects of depression scores at 1-week and 2-week lags were unrelated to mania (all B’s < 0.04, all t’s < 1.66) and so are not displayed in Fig. 1. Medication scores also were unrelated to mania (all B’s < 0.07, all t’s < 1.44). The final model did not include these non-significant effects.
Table 1.
Random effects regression parameters
| B | SE | t | |
|---|---|---|---|
| Individual depression LIFE scores as predictors of individual mania LIFE scores (df = 13 805) | |||
| Intercept | 1.349 | 0.054 | 24.807*** |
| Weeks in the study | 0.005 | 0.002 | 2.305* |
| Current level of depressive symptoms | 0.050 | 0.027 | 1.900* |
| Curvilinear effect of current depressive symptoms | −0.014 | 0.004 | −3.256** |
| Follow-up interview number | −0.048 | 0.017 | −2.762** |
| Individual mania LIFE scores as predictors of individual depression LIFE Scores (df = 13 754) | |||
| Intercept | 1.962 | 0.103 | 18.90*** |
| Weeks in the study | 0.009 | 0.003 | 3.44*** |
| Current level of manic symptoms | 0.102 | 0.038 | 2.68** |
| Curvilinear effect of current manic symptoms | −0.026 | 0.006 | −4.07*** |
| Mania 1 week before depression assessment | 0.078 | 0.039 | 2.01* |
| Curvilinear effect of level of manic symptoms 1 week before | −0.008 | 0.006 | −1.29 |
| Level of manic symptoms two weeks before | 0.109 | 0.039 | 2.84** |
| Curvilinear effect of manic symptoms at 2 weeks before | −0.016 | 0.006 | −2.56 * |
| Follow-up interview number | −0.112 | 0.019 | −5.88*** |
P ≤ 0.05;
P ≤ 0.01;
P ≤ 0.0005.
Fig. 1.
Predicted mania scores as a function of current depression levels.
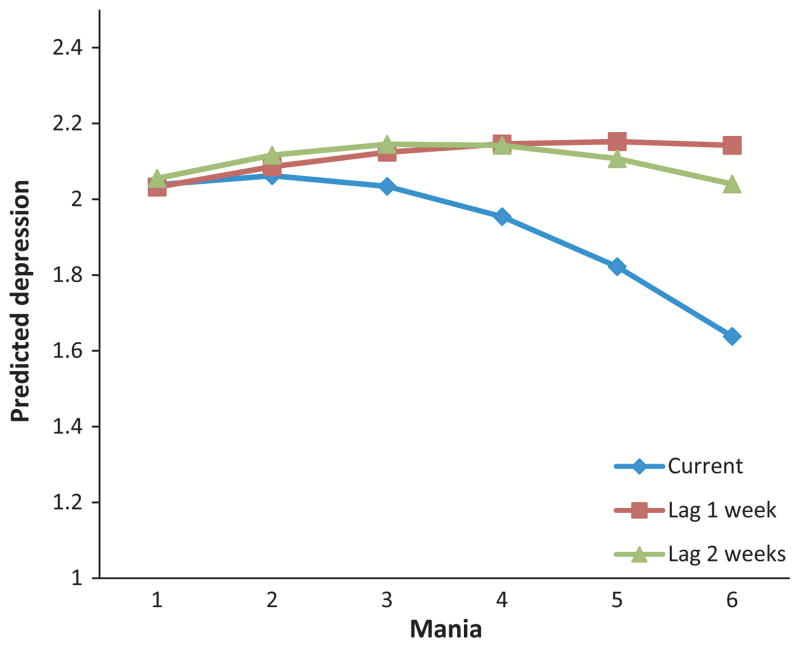
To examine the effects of mania on depression, we computed a parallel model. Phi was 0.80. Focusing on effects of current mania on depression, there was a positive association that was qualified by a negative curvilinear effect. For the effects of mania at 1-week lag, there was a simple effect but no significant curvilinear effect. Considering mania at a 2-week lag, there was a significant positive effect qualified by a negative curvilinear effect. As shown in Fig. 2, findings were not consistent with the hypothesized inverse relationship. As above, effects of mania scores were very small and accounted for very little of the variance in depression scores (All B’s < |0.11|). None of the medication effects were significant.
Fig. 2.
Predicted depression scores as a function of current, 1-week lag, and 2-week lag mania levels.
Parallel analyses were conducted within subgroups. Confining analyses only to persons with bipolar I disorder did not change the pattern of findings nor did separate parallel analyses by gender.
Discussion
It often has been assumed that mania and depression represent two poles of the same dimension. This assumption is embedded in the name of bipolar disorder. This study provides one of the first tests of how manic and depressive symptoms correlate over time within persons. There are limitations in our methods – other patterns might be observed with more frequent assessments, modelling of more specific symptoms or other sampling strategies. The study strengths, though, include the use of psychometrically sound measures, weekly ratings over a 72-week period from interviews with trained independent raters, a large clinically representative sample with heterogeneity in comorbid conditions, and the within-subjects regression analyses that incorporated current and lagged symptom effects as well as medication effects.
The bipolar model suggests that depression and mania would be robustly and negatively correlated. Our findings do not support this view. Correlations between depressive and manic were extremely low. This is consistent with cross-sectional factor-analytic findings that suggest depressive and manic symptoms are independent (cf. 4–6). Our findings also fit with previous findings that severity of depressive symptoms predicts depressive episodes over time, whereas severity of manic symptoms predicts manic episodes over time (20).
Current findings suggest that mania and depression might be conceptualized as two separable symptom dimensions. This two-dimension model fits with the high rates of mixed episodes noted in bipolar disorder (21, 22). That is, one would expect that mania and depression could co-occur if the two syndromes are independently fluctuating.
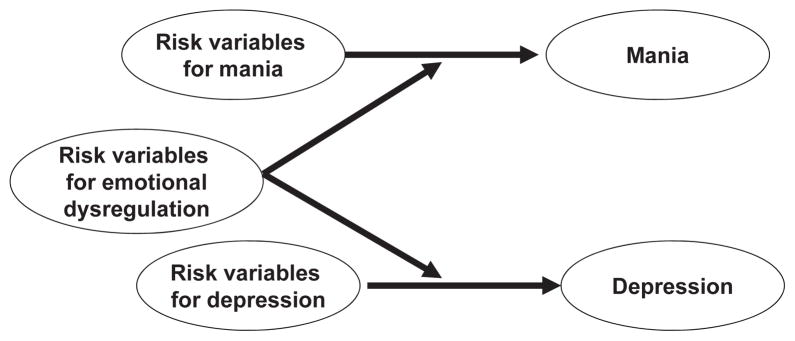
Our finding that depression and mania do not function as opposite poles adds to a growing body of studies questioning the conceptualization of bipolar disorders (23–25). We must acknowledge clear evidence that depression rates are elevated among people with a personal (26) or a family history of mania (23). Taken together, it may be that certain risk factors could operate to increase risks of both mania and depression. As shown in Fig. 3, such variables may enhance a general propensity for dysregulation (27, 28). On the other hand, there may be a set of risk factors that increase the risk for mania, and a different set of risk factors that increase the risk for depression (24, 25, 29). Although preliminary, we hope that our findings stimulate more research on how mania and depression relate within bipolar disorder.
Fig. 3.
Mania and depression as separable dimensions.
Significant outcomes.
Although the very name of the disorder suggests that depression and mania can be considered a single dimension within bipolar disorder, careful statistical analyses suggest that this model is not supported.
Limitations.
More frequent assessments might suggest a different pattern.
We did not examine specific symptom patterns within manic or depressive syndromes.
Acknowledgments
The data for this study came from the database of the MRC UK multi-centre randomized controlled trial for cognitive behaviour therapy for bipolar disorders (Medical Research Council Grant G9721149).
The authors of this paper express their indebtedness to the many patients and researchers who contributed to this study. The authors also thank Hazel Hayhurst and Rosemary Abbott for their contributions to development and management of this dataset, and Michael Edge for his assistance preparing graphs.
Footnotes
Declaration of interest
Sheri Johnson has received funding from the National Cancer Institute and the National Institute of Mental Health. Richard Morris has received speaker’s fees from Lilly and Astra Zeneca and has received grant funding from the UK National Institute for Health Research. Jan Scott has received speaker fees from Jansen-Cilag, AstraZeneca and Eli Lilly and grants from the Medical Research Council and the Research for Patient Benefit Programme. Eugene Paykel has received a speaker fee from Eli Lilly. Ruwanthi Kolamunnage-Dona is funded by Medical Research Council Grant G0400615. Richard Bentall has received grant support from the Medical Research Council, the National Institute of Health Research programme and the Welsh Office of Research and Development. The authors of this paper certify that the publication of findings of this study would not influence their fees, consultancy employment or compensation from any agency.
References
- 1.Strakowski SM, Sax KW. Progressive behavioral response to repeated d-amphetamine challenge: further evidence for sensitization in humans. Biol Psychiatry. 1998;44:1171–1177. doi: 10.1016/s0006-3223(97)00454-x. [DOI] [PubMed] [Google Scholar]
- 2.Naranjo CA, Tremblay LK, Busto UE. The role of the brain reward system in depression. Prog Neuropsychopharmacol. 2001;25:781–823. doi: 10.1016/s0278-5846(01)00156-7. [DOI] [PubMed] [Google Scholar]
- 3.Depue RA. Neurobiological factors in personality and depression. Eur J Pers. 1995;9:413–439. [Google Scholar]
- 4.Dilsaver SC, Chen R, Shoaib AM, Swann AC. Phenomenology of mania: evidence for distinct depressed, dysphoric, and euphoric presentations. Am J Psychiatry. 1999;156:426–430. doi: 10.1176/ajp.156.3.426. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Pinto A, Aldama A, Pinto A, et al. Dimensions of mania: differences between mixed and pure episodes. Eur Psychiatry. 2004;19:307–310. doi: 10.1016/j.eurpsy.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 6.Sato T, Bottlender R, Kleindienst N, Moller HJ. Irritable psychomotor elation in depressed inpatients: a factor validation of mixed depression. J Affect Disord. 2005;84:187–196. doi: 10.1016/S0165-0327(02)00172-6. [DOI] [PubMed] [Google Scholar]
- 7.Judd L, Akiskal HS, Schetteler PJ, et al. The long-term natural history of weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530–537. doi: 10.1001/archpsyc.59.6.530. [DOI] [PubMed] [Google Scholar]
- 8.Post RM, Denicoff KD, Leverich GS, et al. Morbidity in 258 bipolar outpatients followed for 1 year with daily prospective ratings on the NIMH life chart method. J Clin Psychiatry. 2003;64:680–690. doi: 10.4088/jcp.v64n0610. [DOI] [PubMed] [Google Scholar]
- 9.Born C, Seitz NN, Grunze H, et al. Preliminary results of a fine-grain analysis of mood swings and treatment modalities of bipolar I and II patients using the daily prospective life-chart-methodology. Acta Psychiatr Scand. 2009;120:474–480. doi: 10.1111/j.1600-0447.2009.01412.x. [DOI] [PubMed] [Google Scholar]
- 10.Scott J, Paykel ES, Morriss R, et al. Cognitive behaviour therapy plus treatment as usual compared to treatment as usual alone for severe and recurrent bipolar disorders: a randomised controlled treatment trial. Br J Psychiatry. 2006;188:313–320. doi: 10.1192/bjp.188.4.313. [DOI] [PubMed] [Google Scholar]
- 11.Paykel ES, Abbott R, Morriss R, Hayhurst H, Scott J. Subsyndromal and syndromal symptoms in the longitudinal course of bipolar disorder. Br J Psychiatry. 2006;189:118–123. doi: 10.1192/bjp.bp.105.013870. [DOI] [PubMed] [Google Scholar]
- 12.Spitzer RL, Williams JB, Gibbon M, First MB. The structured clinical interview for DSM-III–R (SCID): I. history, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 13.Williams JB, Gibbon M, First MB, et al. The structured clinical interview for DSM-III–R (SCID): II. multisite test-retest reliability. Arch Gen Psychiatry. 1992;49:630–636. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- 14.Keller MB, Lavori PW, Friedman B, Nielsen E. The longitudinal interval follow-up evaluation: a comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- 15.Warshaw MG, Dyck I, Allsworth J, Stout RL, Keller MB. Maintaining reliability in a long-term psychiatric study: an ongoing inter-rater reliability monitoring program using the longitudinal interval follow-up evaluation. J Psychiatr Res. 2001;35:297–305. doi: 10.1016/s0022-3956(01)00030-9. [DOI] [PubMed] [Google Scholar]
- 16.Simon GE, Ludman EJ, Unutzer J, Bauer MS, Operskalski B, Rutter C. Randomized trial of a population-based care program for people with bipolar disorder. Psychol Med. 2005;35:13–24. doi: 10.1017/s0033291704002624. [DOI] [PubMed] [Google Scholar]
- 17.Leon AC, Solomon DA, Mueller TI, et al. A brief assessment of psychosocial functioning of subjects with bipolar I disorder. J Nerv Ment Dis. 2000;188:805–812. doi: 10.1097/00005053-200012000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 19.Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse: results from the epidemiologic catchment area (ECA) study. J Am Med Assoc. 1990;264:2511–2518. [PubMed] [Google Scholar]
- 20.Perlis RH, Ostacher MJ, Patel JK, et al. Predictors of recurrence in bipolar disorder: primary outcomes from the systematic treatment enhancement program for bipolar disorder (STEP-BD) Am J Psychihatry. 2006;163:217–224. doi: 10.1176/appi.ajp.163.2.217. [DOI] [PubMed] [Google Scholar]
- 21.Mcelroy SL, Keck PE, Pope HG, Hudson JI. Clinical and research implications of the diagnosis of dysphoric or mixed mania or hypomania. Am J Psychiatry. 1992;149:1633–1644. doi: 10.1176/ajp.149.12.1633. [DOI] [PubMed] [Google Scholar]
- 22.Bauer MS, Simon GE, Ludman E, Unutzer J. ‘Bipolarity’ in bipolar disorder: distribution of manic and depressive symptoms in a treated population. Br J Psychiatry. 2005;187:87–88. doi: 10.1192/bjp.187.1.87. [DOI] [PubMed] [Google Scholar]
- 23.Mcguffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry. 2003;60:497–502. doi: 10.1001/archpsyc.60.5.497. [DOI] [PubMed] [Google Scholar]
- 24.Joffe RT, Young LT, Macqueen GM. A two-illness model of bipolar disorder. Bipolar Disord. 1999;1:25–30. doi: 10.1034/j.1399-5618.1999.10107.x. [DOI] [PubMed] [Google Scholar]
- 25.Schweitzer I, Maguire K, Ng CH. Should bipolar disorder be viewed as manic disorder? implications for bipolar depression. Bipolar Disord. 2005;7:418–423. doi: 10.1111/j.1399-5618.2005.00246.x. [DOI] [PubMed] [Google Scholar]
- 26.Kessler RC, Rubinow DR, Holmes C, Abelson JM, Zhao S. The epidemiology of DSM-III-R bipolar I disorder in a general population survey. Psychol Med. 1997;27:1079–1089. doi: 10.1017/s0033291797005333. [DOI] [PubMed] [Google Scholar]
- 27.Manji HK, Lenox RH. Signaling: cellular insights into the pathophysiology of bipolar disorder. Biol Psychiatry. 2000;48:518–530. doi: 10.1016/s0006-3223(00)00929-x. [DOI] [PubMed] [Google Scholar]
- 28.Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Arch Gen Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- 29.Cuellar A, Johnson SL, Winters R. Distinctions between bipolar and unipolar depression. Clinic Psychol Rev. 2005;25:307–339. doi: 10.1016/j.cpr.2004.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]