Abstract
Bidirectional cell transfer during pregnancy frequently leads to postpartum persistence of allogeneic cells and alloimmune responses in both the mother and in her offspring. The life-long consequences of naturally acquired alloimmune reactivity are probably of importance for the outcome of allogeneic stem cell transplantation. We investigated the presence of CD8pos minor histocompatibility (H) antigen–specific cytotoxic T lymphocytes (TCTL) and CD8pos minor H antigen–specific T regulator cells (TREG) in peripheral blood cells obtained from 17 minor H antigen–disparate mother-offspring pairs. Absence of minor H antigen–specific TREG, as marked by the feasibility to expand TCTL from isolated tetramerpos populations, was observed in 6 mothers and 1 son. The presence of minor H alloantigen–specific TREG was observed in 4 mothers and 5 sons. These TREG were detected within isolated tetramerdim staining fractions and functioned in a CTLA-4–dependent fashion. Our study indicates that both TCTL and TREG mediated alloimmunity against minor H antigens may be present in healthy female and male hematopoietic stem cell donors, potentially influencing graft-versus-host reactivity in different ways.
Introduction
Mismatches between the human leukocyte antigen (HLA)–haploidentical mother and child may lead to mutual priming of alloimmune cells. Although pregnancy frequently results in activation of maternal B cells1 and TCTL directed against fetal inherited paternal alloantigen, such as HLA2 and minor H antigens,3 not all parous women develop cytolytic activity against the latter alloantigens.3 Importantly, long-lasting tolerance may also be induced in offspring exposed to noninherited maternal alloantigen (NIMA), such as rhesus D4 or HLA.5 The latter is illustrated by a failure to generate alloantibodies after reexposure to the relevant alloantigens through pregnancy4 or through multiple blood transfusions.5
The immunologic mechanism(s) involved in these apparent states of naturally acquired allotolerance is still poorly understood. The presence of fetal or maternal microchimeric cells may play a role in the induction and/or maintenance of a tolerant status.6 There is ample evidence for a mutual exchange of mature blood and progenitor cells between the mother and her fetus. Whereas mature blood cells have a limited lifespan, hematopoietic stem cells7 and HLAdim mesenchymal stem cells8 may engraft in the bone marrow, where they remain throughout life. Cells obviously derived from fetal hematopoietic progenitor cells can be detected in the maternal circulation up to several decades after the delivery.9 Likewise, hematopoietic6,10 and nonhematopoietic11 cells from maternal origin may persist into adulthood. The tolerogenic potential of chimerism, established either through bone marrow transplantation (macrochimerism)12 or through pregnancy (microchimerism),7 has been documented in both rodent13,14 and in human transplantation settings.15,16
We earlier described the presence of minor H antigen HA-1–specific TCTL, minor H antigen HA-1–specific TREG, and HA-1+ circulating microchimeric cells in the setting of kidney transplantation.17 The latter cell populations were observed in a long-term tolerant HA-1− patient transplanted with a renal allograft from her HLA identical HA-1+ sister. These TCTL and TREG could be physically separated based on differences in their capacity to bind HLA-A2/minor H peptide tetramers. Although HA-1 tetramerbright staining T cells were found to mediate delayed-type hypersensitivity reactions and produced interferon-γ in response to HA-1 allopeptide, their function was suppressed in the presence of transforming growth factor β and interleukin-10–producing HA-1 tetramerdim staining T cells. Thus, differences in tetramer staining intensity may indicate the presence of functionally different types of T cells.17,18
The dominant presence of minor H antigen–specific alloimmune TREG or TCTL in a hematopoietic stem cell (SC) graft may have differential impacts on the outcome of HLA identical minor H antigen nonidentical stem cell transplantation (SCT). As a first step toward understanding how the minor H antigen alloimmunization status of SC donors may affect SCT outcome, we analyzed whether “natural” exposure to fetal or maternal minor H alloantigens induces functionally different T cells in healthy parous female and male blood donors, respectively. Regarding the latter donors, only firstborn sons were selected, thereby avoiding any confounding effects of transmaternal transfer of earlier born sibling cells.19 Peripheral blood mononuclear cells (PBMCs) from selected minor H antigen mismatched mother-child pairs were collected. and a detailed analysis on the presence of minor H antigen–specific TCTL and TREG was performed.
Methods
Study participants
Familial alloimmunization to the HLA-A2–restricted minor H antigens HA-1, HA-2, HA-8, and HY20 was studied (Table 1). Donors with a history of blood transfusion were excluded from the analysis.21 After receiving written informed consent in accordance with the Declaration of Helsinki, blood samples were obtained either by leukapheresis or by extraction of the buffy coat from whole-blood donations. Approval for this study (P06.008) was obtained from the Institutional Review Boards of the Leiden University Medical Center and of Sanquin Blood Bank South West.
Table 1.
Autosomal minor H antigen genotyping of 17 HLA-A2–sharing mother-offspring pairs included in the study
| Donor and relevant family | Age, y | HA-1 genotype* | HA-2 genotype | HA-8 genotype |
|---|---|---|---|---|
| Mother 1†‡ | 57 | HA-1 H/R | HA-2 M/M | HA-8 R/R |
| Son | 31 | HA-1 R/R | HA-2 V/M | HA-8 R/R |
| Mother 2† | 54 | HA-1 H/R | HA-2 M/M | HA-8 R/R |
| Son | 28 | HA-1 R/R | HA-2 M/M | HA-8 P/R |
| Mother 3† | 57 | HA-1 H/R | HA-2 M/M | HA-8 R/R |
| Son | 33 | HA-1 H/R | HA-2 V/M | HA-8 P/R |
| Son | 27 | HA-1 R/R | HA-2 M/M | HA-8 P/R |
| Mother 4† | 72 | HA-1 R/R | HA-2 V/V | HA-8 R/R |
| Son | 39 | HA-1 R/R | HA-2 V/V | HA-8 P/R |
| Son | 35 | HA-1 H/R | HA-2 V/V | HA-8 P/R |
| Mother 5† | 53 | HA-1 H/H | HA-2 V/V | HA-8 P/R |
| Son | 24 | HA-1 H/H | HA-2 V/V | HA-8 P/P |
| Son | 20 | HA-1 H/H | HA-2 V/V | HA-8 P/R |
| Mother 6† | 43 | HA-1 H/H | HA-2 V/V | HA-8 P/P |
| Son | 13 | HA-1 H/H | HA-2 V/V | HA-8 P/P |
| Mother 7† | 46 | HA-1 R/R | HA-2 V/M | HA-8 P/R |
| Son | 15 | HA-1 R/R | HA-2 V/V | HA-8 R/R |
| Son | 12 | HA-1 R/R | HA-2 V/M | HA-8 P/R |
| Mother 8† | 43 | HA-1 H/R | HA-2 V/M | HA-8 P/R |
| Son | 9 | ND | ND | ND |
| Son | 7 | ND | ND | ND |
| Mother 9† | 40 | HA-1 H/R | HA-2 V/M | HA-8 P/P |
| Son | 6 | HA-1 H/R | HA-2 V/M | HA-8 P/P |
| Son | 4 | HA-1 H/R | HA-2 V/V | HA-8 P/P |
| Mother 10† | 44 | HA-1 R/R | HA-2 V/V | HA-8 P/P |
| Son | 20 | HA-1 H/R§ | HA-2 V/V | HA-8 P/P |
| Daughter | 17 | HA-1 H/R | HA-2 V/V | HA-8 P/P |
| Daughter | 12 | HA-1 H/R | HA-2 V/V | HA-8 P/P |
| mGM | 74 | HA-1 R/R | HA-2 V/V | HA-8 P/P |
| Mother 11† | 38 | HA-1 R/R | HA-2 V/V | HA-8 P/P |
| Daughter | 6 | HA-1 H/R | HA-2 V/V | HA-8 P/P |
| Mother 12 | 72 | HA-1 H/R | HA-2 V/M | HA-8 P/R |
| Son 1† | 46 | HA-1 R/R | HA-2 V/M | HA-8 R/R |
| Mother 13 | 58 | HA-1 H/R | HA-2 V/V | HA-8 P/R |
| Son 2† | 34 | HA-1 R/R | HA-2 V/M | HA-8 P/R |
| Mother 14 | 59 | HA-1 H/R | HA-2 V/V | HA-8 P/R |
| Son 3† | 34 | HA-1 R/R | HA-2 V/V | HA-8 R/R |
| Mother 15 | 73 | HA-1 H/R | HA-2 V/M | HA-8 P/R |
| Son 4† | 44 | HA-1 R/R | HA-2 M/M | HA-8 P/P |
| Mother 16‡ | 57 | HA-1 H/R | HA-2 M/M | HA-8 R/R |
| Son 5† | 31 | HA-1 R/R | HA-2 V/M | HA-8 R/R |
| Mother 17 | 45 | HA-1 R/R | HA-2 V/M | HA-8 P/P |
| Son 6† | 21 | HA-1 R/R | HA-2 M/M | HA-8 P/R |
| Mother 18 | 51 | HA-1 H/R | ND | HA-8 R/P |
| Son 7† | 19 | HA-1 H/R | ND | HA-8 P/P |
ND indicates not determined; and mGM, HLA-A2+ maternal grandmother.
Autosomal minor H antigens are encoded by diallelic loci composed of an immunogenic allele, for example, HA-1H, HA-2V, and HA-8R, and a nonimmunogenic or a “null” allele, for example, HA-1R, HA-2M, and HA-8P. Consequently, persons expressing HA-1H/R are referred to as HA-1+, whereas HA-1R/R persons are designated as HA-1−.
Subjects analyzed in the study.
The same pair.
The autosomal minor H alloantigen under study is indicated in bold. HY is studied in female donors in case no autosomal minor H mismatch exists between the mother and her male offspring.
HLA class I/minor H peptide tetrameric complexes and monoclonal antibodies
Phycoerythrin-conjugated HLA-A2/minor H peptide HY (HYA2), HA-1 (HA-1A2), and HA-2 (HA-2A2) tetramers were generated and validated as previously described.22 The following cell-surface markers and isotype control antibodies (all from BD Biosciences) were used for multicolor flow cytometric (fluorescence-activated cell sorter [FACS]) analysis: allophycocyanin-conjugated CD45RO, peridinin chlorophyll protein–conjugated CD8, and fluorescein isothiocyanate (FITC)–conjugated CD25 and CTLA-4. Intracellular staining for FoxP3 and the corresponding isotype control were performed with FITC-labeled antibodies according to the supplier's manual (eBioscience).
Detection, isolation, and expansion of minor H antigen–specific CD8+ T cells from peripheral blood samples
Detection and isolation of circulating minor H antigen–specific T cells from CD8-enriched PBMCs was performed according to a previously described protocol.3 In short, PBMCs were isolated by Ficoll-Isopaque density gradient centrifugation and depleted for various cell subsets using CD4, CD14, CD16, and CD19 MACS beads according to the manufacturer's instructions (Miltenyi Biotec). The depleted fraction was subsequently stained with the relevant tetramers and CD8 antibodies. Enrichment of CD8+ tetramer+ T cells was performed on a FACSAria cell sorter (BD Biosciences) using the nonstringent “enrich mode.” The enriched population was immediately resorted using the more stringent “normal-R” mode. Double FACS-sorted populations were expanded in the presence of 105 irradiated autologous minor H antigen− PBMCs in Iscove modified Dulbecco medium containing 10% pooled human serum, 1% phytohemagglutinin, and 150 IU/mL recombinant interleukin-2.
Functional assays
The cytolytic capacity was tested in a standard chromium release assay. In brief, 2500 51Cr-labeled target cells were incubated with serial dilutions of T cells as indicated in the figures. Supernatants were harvested after 4 hours of incubation for gamma counting. Percentage-specific lysis was calculated as follows: (experimental release − spontaneous release)/(maximal release − spontaneous release) × 100%. The data are shown as the mean of duplicate samples.
TREG function was tested in the trans-vivo delayed-type hypersensitivity (tvDTH) assay. This assay identifies the presence of a dominant population of TREG, exploiting bystander immune suppression as their main mode of action.17,23 Briefly, 7 to 9 × 106 cryopreserved PBMCs were injected into the footpads of CB17.SCID mice in the presence of phosphate-buffered saline (negative control), 0.25 LF recall antigens tetanus toxoid/diphtheria toxoid (TT/D, positive control), 1 μg minor H allopeptide (HY or HA-1H), or TT/D plus 1 μg minor H allopeptide. Control swelling responses to 1 μg minor H self-peptide (HA-1R, HA-2V or HA-2M) plus TT/D were tested in parallel. Footpad swelling responses were measured 24 hours later using a dial gauge caliper. Values of the response to phosphate-buffered saline were subtracted from test values to determine the net swelling response (NSR), reported in units of 10−4 inches. The percentage of inhibition is calculated as follows: % suppression = 1 − (NSR to coinjection of recall antigens + minor H allopeptide/NSR to recall antigens alone) × 100%. The following classification criteria for DTH phenotypes were used: a DTH regulator phenotype is defined by a low minor H allopeptide–induced swelling response and more than or equal to 50% inhibition of TT/D recall response in the presence of the same minor H allopeptide. A DTH nonregulator phenotype is also defined by a low minor H allopeptide-induced swelling response together with less than 50% inhibition of TT/D recall response in the presence of minor H allopeptide. Where appropriate, the minor H allopeptide–induced bystander suppression was reversed by addition of 1 μg neutralizing CTLA-4 or control IgG antibody (Antibody Solutions).
Detection of circulating Y-chromosome+ and HA-1H microchimeric cells
PBMCs were isolated from the interphase after Ficoll-Isopaque density gradient centrifugation of whole blood samples. Granulocytes were obtained from the remaining cell pellet after lysing red blood cells with NH4CL and KHCO3 containing lysis buffer. PBMCs were subsequently labeled with FITC-, phycoerythrin-, peridinin chlorophyll protein–, or allophycocyanin-conjugated antibodies (all from BD Biosciences) for isolation of CD3+ T cells, CD20+ B cells, CD14+ monocytes, and CD3−CD20−CD14−CD11c+ myeloid dendritic cells (DCs) by FACS sorting. The QIAamp DNA blood minikit (QIAGEN) was used for genomic DNA extraction from all fractions. For the detection of Y-chromosome–specific microchimerism, we adapted the real-time PCR protocol kindly provided by Dr D. W. Bianchi (Tufts-New England Medical Center). To standardize the data, a second PCR detecting the human hematopoietic cell kinase gene was carried out in parallel.24 Table 2 lists the corresponding primers and probes. For the detection of HA-1–positive (HA-1H) microchimeric cells, we adapted an earlier established nested PCR protocol.25 The different primer sets for the first and second PCRs performed in this study are listed in Table 2. The sensitivity of each PCR reaction was routinely determined by incorporating a titration series of a minor H antigen+ Epstein-Barr virus–transformed lymphoblastoid cell line (EBV-LCL) diluted in minor H antigen− EBV-LCL.25 The differences in sensitivity between both methods are the result of significant differences in copy numbers of both genes; whereas the lower threshold of sensitivity in the HA-1H–specific PCR is 1 HA-1+ cell in 104 HA-1− cells, the Y chromosome–specific PCR reliably detects one male cell in 105 female cells.
Table 2.
Primer and probe nucleotide sequences used for the detection of Y chromosome and HA-1H–positive microchimeric cells by real-time PCR
| Primer | Nucleotide sequence | Fragment size, bp |
|---|---|---|
| Forw-DYS1 | 5′-TCCTGCTTATCCAAATTCACCAT-3′ | 86* |
| Rev-DYS1 | 5′-ACTTCCCTCTGACATTACCTGATAATTG-3′ | |
| Y probe | 5′-(FAM)-AAGTCGCCACTGGATATCAGTTCCCTTGT-(TAMRA)-3′ | |
| Forw-HCK | 5′-TATTAGCACCATCCATAGGAGGCTT-3′ | 81* |
| Rev-HCK | 5′-GTTAGGGAAAGTGGAGCGGAAG-3′ | |
| HCK Probe | 5′-(FAM)-TAACGCGTCCACCAAGGATGCGAA-(TAMRA)-3′ | |
| Forw-I-COM | 5′-GACGTCGTCGAGGACATCTCCCATC-3′ | 324† |
| Rev-I HA-1H | 5′-CATCAGATCCTTTAAAAAAAGTGG-3′ | |
| For-II HA-1H | 5′-CTTAAGGAGTGTGTGCTGCA-3′ | 191‡ |
| HA-1H Rev-II | 5′-ACTCCTACACATCCCTCAGA-3′ | |
| For-I HCK | 5′-ACCTCCCCGAAGATTCAGAC-3′ | 381† |
| Rev-I HCK | 5′-TTGGGGGCAAGTTGAGTTTA-3′ | |
| For-II HCK | 5′-TATTAGCACCATCCATAGGAGGCTT-3′ | 81‡ |
| Rev-II HCK | 5′-GTTAGGGAAAGTGGAGCGGAAG-3′ |
Amplification conditions: 95°C for 10 minutes, then 40 cycles at 95°C for 15 seconds, 60°C for 30 seconds, and 72°C for 30 seconds.
Amplification reactions: 5 minutes at 95°C, then 30 cycles at 95°C for 1 minute, 58°C for 1 minute, and 72°C for 1 minute. The cycling was followed by 72°C for 5 minutes.
Amplification conditions: 4.5 minutes at 95°C, then 5 cycles at 95°C for 15 seconds and 66°C for 45 seconds, followed by 35 cycles at 95°C for 15 seconds, 62°C for 45 seconds, and 72°C for 30 seconds.
Results
Isolation, expansion, and functional testing of total tetramer-binding fractions obtained from female and male blood donors
We earlier described the usefulness of HLA-A2/minor H peptide tetramers to visualize22 and isolate minor H antigen–specific CD8+ TCTL from peripheral blood cells for subsequent functional analyses after polyclonal expansion.3,26 The latter protocol was used to study the presence of TCTL within total tetramer-binding fractions isolated from 10 female donors with minor H antigen HY or HA-1 mismatched offspring and from 6 HA-1− male donors (ie, firstborn sons), all delivered by an HA-1+ mother. The minor H genotypes of these mother/offspring combinations are listed in Table 1, and the various routes of donor alloantigen exposure are exemplified in Figure 1.
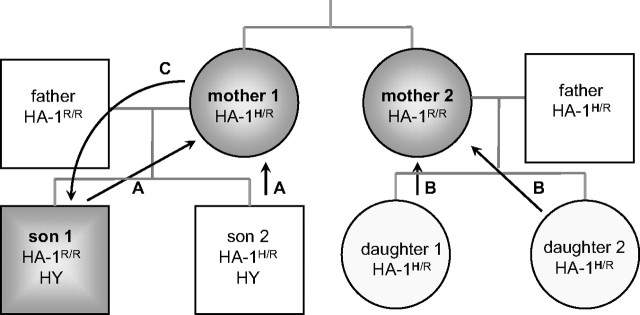
Figure 1.
Bidirectional routes of familial exposure to minor H alloantigens. Pathway C illustrates exposure of the offspring to noninherited maternal minor H alloantigen HA-1H after transfer of cells during pregnancy (indicated by arrow). Maternal exposure to fetal inherited paternal minor H alloantigen HY or HA-1H is illustrated by pathway A and B, respectively.
Female donor analyses.
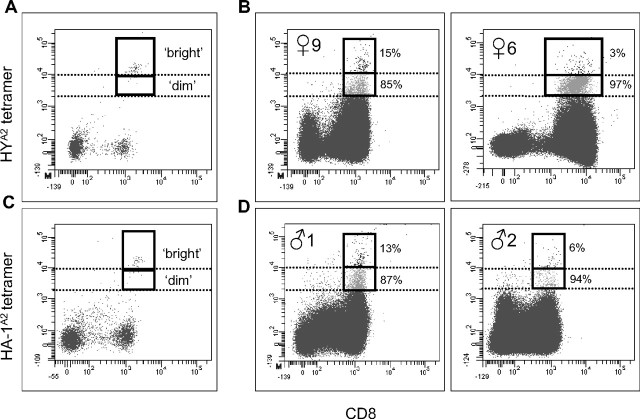
HYA2 (♀1-7 and ♀9) or HA-1A2 (♀10 or 11) tetramer+ populations were isolated, as exemplified in Figure 2A, and cultured in the absence of the relevant minor H alloantigen. Although outgrowth of cytolytic T cells failed in 5 of 10 cases (♀1-5), TCTL were obtained from the 5 other females (Table 3). Figure 2B shows the cytolytic capacity and corresponding tetramer staining plots of 2 representative TCTL populations. TCTL that lysed target cells expressing the natural ligand were obtained from female donor ♀6 (Figure 2B bottom panel) and ♀11 (data not shown). Peptide-specific TCTL were present in TCTL populations expanded from female donor ♀9 (Figure 2B top panel), ♀7, and ♀10 (both; data not shown).
Figure 2.
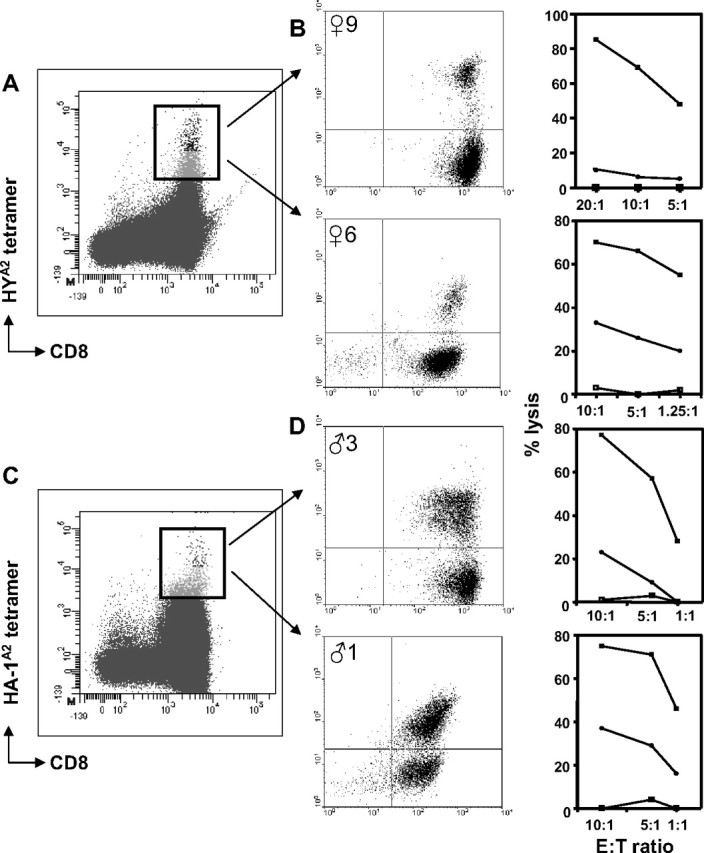
Tetramer staining patterns and cytolytic function of CD8+ minor H antigen–specific T cells obtained from healthy women and men. (A,C) Representative tetramer-binding profiles of HY-specific T cells (A) and HA-1–specific T cells (C) detected ex vivo in CD8-enriched PBMCs after nonstringent FACS sorting. The rectangle indicates the total tetramer+ population isolated during a second round of FACS sorting. (B,D) The cytolytic activity, indicated as percentage lysis on the y-axis, of polyclonally expanded tetramer+ fractions tested against various target cells: □ indicates HLA-A2+, HA-1− female target cells; ■, HLA-A2+, HA-1− female target cells pulsed with HY peptide (B) or HLA-A2+, HA-1− female target cells pulsed with HA-1 peptide (D); and ●, HLA-A2+ HA-1+ male target cells. The effector:target cell ratios (as depicted on the x-axis) were calculated according to the corresponding percentage of HYA2 tetramer-binding T cells as shown in the center plots.
Table 3.
Presence of minor H antigen–specific CD8+ TCTL or minor H antigen–specific CD8+ TREG in healthy blood donors
| Donor | Cytolytic T cells* | tvDTH classification† |
|---|---|---|
| ♀4 | No | Regulator (58%) |
| ♀5 | No | Regulator (60%) |
| ♀1 | No | Regulator (67%) |
| ♀3 | No | Regulator (67%) |
| ♀2 | No | Nonregulator (0%) |
| ♀6 | Yes | Nonregulator (0%) |
| ♀10 | Yes | Nonregulator (0%) |
| ♀7 | Yes | Nonregulator (10%) |
| ♀11 | Yes | Nonregulator (33%) |
| ♀9 | Yes | ND |
| ♂2 | No | Regulator (50%) |
| ♂4 | No | Regulator (50%) |
| ♂6 | No | Regulator (60%) |
| ♂3 | Yes | Regulator (75%) |
| ♂7 | ND | Regulator (75%) |
| ♂5 | Yes | Regulator (80%) |
| ♂1 | Yes | Nonregulator (0%) |
ND indicates not determined.
Total tetramer-binding T cells were isolated by FACS sorting, polyclonally stimulated, and subsequently tested for cytolytic function.
The dominant presence of minor H alloantigen–specific Treg within total PBMCs was measured in the tvDTH assay.17 The percentages indicate the percentage inhibition of recall antigen–induced swelling footpad responses in the presence of the relevant minor H allo peptide.
Male donor analyses.
HA-1A2 (♂1-♂5) or HA-2A2 (♂6) tetramer+ populations were isolated, as exemplified in Figure 2C, and cultured as described for the female donors. TCTL outgrowth occurred in 3 of 6 cases (Table 3). Functional testing revealed the presence of natural ligand-specific TCTL in CD8+ populations obtained from donor ♂1 (Figure 2D bottom plot). Peptide-specific TCTL were present in CD8+ populations generated from donor ♂3 (Figure 2D top plot) and ♂5 (data not shown).
Collectively, these experiments show that minor H antigen–specific TCTL can be identified in approximately half of the analyzed female and male donors.
Minor H antigen–specific T cells with a suppressive function identified in mothers and in firstborn sons
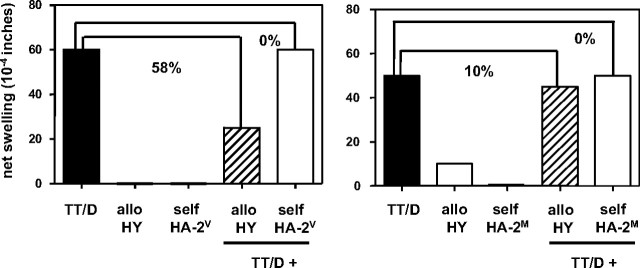
We next questioned whether failure to expand TCTL from total tetramer+ fractions, as observed in the other half of female and male donors tested, could be the result of the copresence of minor H antigen–specific TREG. The tvDTH assay was applied to analyze the putative presence of minor H antigen–specific TREG within total PBMCs.17 These PBMCs were derived from the same blood sample as used for the identification of minor H antigen–specific TCTL. Sixteen blood donors (9 females and 7 males) were selected for these experiments (Table 3). Figure 3 shows the data of 2 mothers with male offspring, representing either the tvDTH regulator phenotype (♀4, left graph), indicating the presence of functional minor H antigen–specific TREG, or the tvDTH nonregulator phenotype (♀7, right graph), indicating the absence of functional minor H antigen–specific TREG. Four of 9 mothers (44%) were classified as a tvDTH regulator, the remaining 5 mothers (55%) as tvDTH nonregulators. The latter group included the 2 females with HA-1 disparate offspring (♀10 and ♀11). Thus, natural exposure of mothers to fetal inherited minor H alloantigens seems to lead to either sensitization or tolerization to minor H alloantigens.
Figure 3.
Presence or absence of minor H antigen–specific regulatory T cells analyzed in the tvDTH assay. Total PBMCs of 2 mothers with male offspring were tested for minor H antigen HY-driven bystander suppression of recall responses. PBMCs were injected together with either a mixture of recall antigens comprising tetanus toxoid (TT) and diphtheria toxoid (D), minor H allopeptide HY (allo HY) or minor H self-peptide HA-2V or HA-2M (self HA-2V, self-HA-2M) alone or with a combination of TT/D + allo- or self-peptide into the footpads of CB17.SCID mice. Footpad swelling, indicated as net swelling on the y-axis, was measured 24 hours later. Percentages indicate the percentage of inhibition of the recall response (■) in the presence of allo- (▨) or self-(□) minor H peptide. Mother ♀4 (left graph) displays a tvDTH regulator phenotype; mother ♀7 (right graph) is classified as a tvDTH nonregulator.
Likewise, 5 HA-1− sons, all delivered by an HA-1+ mother, were tested in the tvDTH assay (Table 3). Only one donor (♂1) could be assigned as a tvDTH nonregulator. Unlike the other male donors, this donor even displayed significant swelling responses (> 25 × 10−4 inches) when his PBMCs were injected with HA-1 allopeptide alone (data not shown), indicating the presence of high numbers of minor H antigen–specific TCTL mediating DTH reactions.17 The remaining 4 males were all classified as tvDTH regulators. Two additional males (♂6 and ♂7), with an HA-2 and HA-8 mismatched mother, respectively, were also tested for regulatory capacity. Both donors displayed profound suppression of recall responses in the presence of HA-2V (60%) and HA-8R (75%) allopeptide, respectively. Thus, 6 of 7 male donors tested could be classified as a tvDTH regulator, indicating that tolerization to noninherited maternal minor H antigens may be more prevalent than sensitization in male donors.
Minor H antigen–specific T-cell populations contain tetramerbright and tetramerdim staining cells
In a previous study, we have identified the presence of minor H antigen HA-1–specific TREG in a renal allograft tolerant patient.17 Dissection of the total tetramer-binding CD8+ T-cell population into tetramerbright and tetramerdim staining T cells proved to be a crucial step in the identification of these TREG in the latter population. Likewise, we dissected the total tetramer-binding populations observed in our healthy subjects. Nine of 11 female donors and 6 of 7 male donors were used for these analyses. Figure 4A and C exemplifies how the distinction between brightly and dimly tetramer staining T cells was made. Two different staining patterns were observed, that is, a dual tetramer staining pattern17 and a pattern dominated by tetramerdim staining T cells. Figure 4B (left panel) shows a representative example of coexisting HYA2 tetramerbright and tetramerdim staining T cells that were observed in donor ♀9; the corresponding ratio bright:dim staining T cells is 1:6. This dual staining pattern was observed in 4 of 7 females who gave birth to sons (Table 4). Predominant tetramerdim staining T cells, ie, a ratio bright:dim staining T cells greater than 1:10, were observed in the 3 other mothers with sons. Female 6 illustrates the latter phenotype; the ratio of bright:dim staining T cells in this donor is 1:32 (Figure 4B right panel). Both dual and predominantly tetramerdim staining profiles were also found in the 2 mothers with HA-1–mismatched offspring, respectively (Table 4). Collectively, dual tetramerbright and tetramerdim staining T cells are observed in 5 of a total of 9 women analyzed in this study. Predominantly tetramerdim staining T cells were observed in the 4 remaining women.
Figure 4.
Dissection of HLA-A2/minor H peptide tetramer staining profiles. (A,C) Definition of tetramerbright and tetramerdim gate settings (as indicated by dotted lines) using a tetramerbright staining cytolytic HY (A) or HA-1 (C) specific T-cell clone titrated into CD8-enriched PBMCs. (B) Analysis of tetramer-binding profiles of 2 female donors (♀9 and ♀6) with male offspring after nonstringent sorting of HYA2 tetramer+ CD8+ cells. (D) Analysis of HA-1A2 tetramer-binding profiles of 2 HA-1R/R male donors (♂1 and ♂2) with a HA-1H/R mother after nonstringent sorting of HA-1A2 tetramer+ CD8+ cells. The solid box represents the total CD8+ tetramer+ population; tetramerbright staining T cells are plotted in black; tetramerdim staining T cells are plotted in gray. Percentages indicate the distribution of each T-cell subset in the total tetramer+ fraction.
Table 4.
Tetramer staining profiles in female and male blood donors with minor H antigen–disparate family members
| Mother anti-child |
Son anti-mother |
|||||
|---|---|---|---|---|---|---|
| HY, mm* | HA-1, mm | Total per profile | HA-1, mm | HA-2, mm | Total per profile | |
| Dual staining pattern† | 4 | 1 | 5 | 2 | 0 | 2 |
| Predominant tetramerdim staining pattern‡ | 3 | 1 | 4 | 3 | 1 | 4 |
| No. of donors analyzed | 7 | 2 | 9 | 5 | 1 | 6 |
Mismatch.
The ratio tetramerbright:tetramerdim is 1: ≤ 10.
The ratio tetramerbright:tetramerdim is 1: > 10.
Likewise, PBMCs from 6 male donors were analyzed for the presence of HA-1A2 or HA-2A2 tetramer bright and/or dim staining T cells (Table 4). Four of 6 males predominantly displayed tetramerdim staining T cells. A representative example is depicted in Figure 4D (right panel), illustrating the ratio of 1:16 bright:dim staining T cells as observed in male donor 2. Donors ♂1 (Figure 4D left panel) and ♂4 displayed dual tetramerbright and tetramerdim staining T cells (Table 4). The corresponding ratios of bright:dim staining T cells in these males are 1:7 (♂1) and 1:5 (♂4). Thus, similar tetramer staining profiles as observed in parous women were detected in the male donors, although the predominant tetramerdim phenotype seems more frequent.
Minor H antigen–specific tetramerdim staining T cells express phenotypic markers typically associated with regulatory function
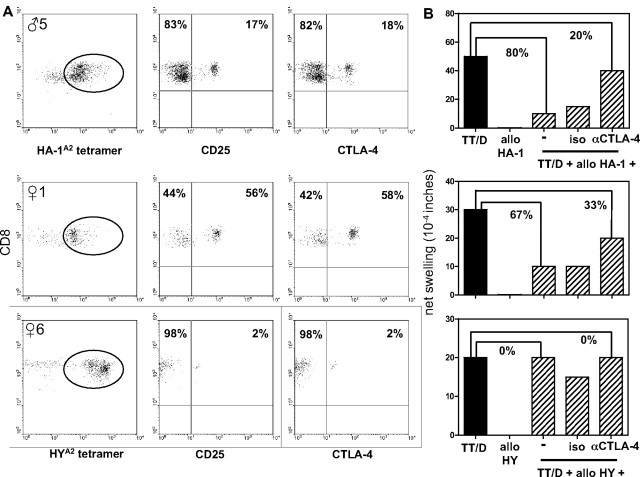
A predominant tetramerdim staining profile was observed in 5 of 8 donors who were classified as strong tvDTH regulators (♀1, ♀3, ♂3, ♂5, ♂6). To further characterize putatively present minor H antigen–specific TREG, we determined the percentages of, respectively, CD25, CTLA-4, and FoxP3 expressing CD8+ T cells within the isolated tetramerdim staining fractions obtained from 6 donors: ♀1 (tvDTH regulator), ♀6 (tvDTH nonregulator), ♂4 (tvDTH regulator), ♂5 (tvDTH regulator), ♀8, and ♀9. The latter 2 donors were not tested for immune regulation in the tvDTH assay because of insufficient numbers of PBMCs. A large variation in CD25 and CTLA-4 expression was observed within these tetramerdim staining populations. The percentages of HYA2 tetramerdim staining T cells expressing these markers varied from 1% to 58%; the highest percentage of these cells were detected in ♀1 (Figure 5A), followed by ♀8 (12% and 13%, respectively), ♀6 (Figure 5A), and ♀9 (both 1%). HA-1A2 tetramerdim staining T cells expressing CD25 and CTLA-4 were also detected in the 2 male donors analyzed. Whereas donor ♂4 only displayed very low numbers of CD25 and CTLA-4–positive cells (both 1%), higher numbers of this phenotype were detected in donor ♂5 (Figure 5A). Neither CTLA-4 nor CD25 was expressed by control CD8+ tetramer− cells analyzed in parallel (data not shown). Furthermore, none of the CD8+ tetramerdim or CD8+ tetramer− populations expressed FoxP3 (data not shown). Thus, minor H antigen–specific tetramerdim staining CD8+ T-cell populations contain variable numbers of T cells expressing CTLA-4, but not FoxP3.
Figure 5.
Phenotypic and functional analysis of minor H alloantigen–specific TREG. Results from 1 male donor (♂5) and 2 female donors (♀1 and ♀6) with minor H antigen disparate family members are shown. (A) Cell-surface expression of CD25 and CTLA-4 by CD8+ HA-1A2 (top graph) or HYA2 (center and bottom graphs) tetramerdim staining T cells (indicated by circle) isolated by 2 consecutive rounds of FACS sorting from CD8-enriched PBMCs. (B) Defining the role of CTLA-4 in minor H alloantigen–driven bystander suppression of recall responses. Total PBMCs, obtained from the same blood sample as shown in panel A, were injected together with the recall antigens tetanus toxoid (TT) and diphtheria toxoid (D), allopeptide (HA-1 for ♂5; HY for ♀1 and ♀6) alone or a combination thereof into the footpads of CB17.SCID mice. Footpad swelling indicated as net swelling is depicted on the y-axis. Uncovering of recall antigen–induced footpad swelling was induced by coinjection of blocking CTLA-4 antibodies (αCTLA-4) or an isotype control antibody (iso). The percentages indicate the percentage of inhibition of the recall response (■) when minor H allopeptide is coinjected with or without blocking CTLA-4 antibody.
Minor H antigen–mediated regulation involves CTLA-4
We next questioned whether CTLA-4 expressed by minor H antigen–specific tetramerdim staining T cells would be functionally involved in the suppression of recall antigen–specific T cells as measured in the tvDTH assay. To address this question, mother ♀1 and her son ♂5 were selected, displaying a distinctive population of CTLA-4+ cells within, respectively, the HY and HA-1 tetramerdim staining fractions (Figure 5A). Addition of CTLA-4 neutralizing antibodies was tested for its expected uncovering of recall antigen–induced footpad swelling responses.17 Both donor ♀1 and her son ♂5 showed partly (50% and 75%, respectively) restored footpad swelling responses to recall antigens when the CTLA-4 antibody was added (Figure 5B). Donor ♀6, classified as a tvDTH nonregulator and displaying only 2% of CTLA-4+ cells (Figure 5A), did not show such an effect on addition of CTLA-4 blocking antibodies. Likewise, addition of an isotype control antibody could not reverse minor H allopeptide–induced suppression of footpad swelling. These first observations suggest that the regulatory effect of the tetramerdim population, provided the presence of sufficient numbers of TREG cells, is partially depending on functional CTLA-4 signaling.
Discussion
Bilateral cell trafficking during pregnancy and delivery induces alloimmunization in mutual direction as illustrated by the presence of minor H antigen–specific TCTL populations in the mother and in the newborn child.3,26,27 In this study, we questioned whether, besides TCTL, also minor H antigen–specific CD8+ TREG are generated and, if so, whether both populations can be demonstrated in adult donors. To this end, we collected PBMCs from 17 minor H antigen–mismatched familial combinations and analyzed the presence of functionally different types of CD8+ minor H antigen–specific T cells. We show, for the first time, the presence of minor H antigen–specific TREG in healthy adult women and men. These TREG seem to coexist with variable numbers of minor H antigen–specific TCTL. Both T-cell subsets arise across HY and autosomal minor H antigen barriers; the latter occurs in mutual direction. The minor H antigen–specific CD8+ TREG coexpress cell-surface CD25 and CTLA-4, but no FoxP3. Although adaptive CD8+CD25+CTLA-4+FoxP3+ TREG have been identified in transplantation patients or in patients with autoimmune or infectious disease,28 not all thus far identified CD8+ TREG express FoxP3.29 Indeed, FoxP3 is not a very specific marker for human TREG given that activated nonregulatory T cells may transiently up-regulate this transcription factor. The CD8+ TREG described in this study were identified within the tetramer dim staining fraction. These results are in agreement with 2 earlier studies describing reduced HLA/peptide multimer binding by TREG.17,18 Low avidity CD8dim staining HY-specific TREG have been described in a murine allograft tolerance model.30 These observations collectively point to incomplete T-cell receptor signaling as a common feature of TREG function. This phenotypic characteristic can however not be used for identification of minor H antigen–specific TREG because some tetramerdim staining fractions also contained minor H antigen–specific TCTL. Indeed, functional testing (data not shown) demonstrated the presence of TCTL in 3 expanded populations derived from isolated tetramerdim staining fractions obtained from donor ♂3, ♂5 (both HA-1), and ♀9 (HY).
Recent observations show that tolerogenic fetal CD4+ CD25high FoxP3+ TREG specific for undefined maternal alloantigen(s) may be primed in utero and persist long after birth.31 In line with this report, our results show that also minor H antigen–specific CD8+ TCTL may be (partly) functionally tolerized in mother and child as a consequence of exposure to minor H alloantigens through pregnancy. The minor H antigen–specific CD8+ TREG identified in our study may, like their CD4+ CD25high counterparts, persist for decades. Indeed, the majority of adult males and approximately half of the women analyzed in our study clearly displayed minor H allopeptide–specific regulatory function in the tvDTH assay. Whereas primary contact with alloantigens during fetal/neonatal life may be a far more favorable setting for the induction of tolerance, (repetitive) exposure to such antigens in adulthood should, theoretically, result in a sensitized phenotype as earlier proposed.32 Although further studies are necessary to determine the effect of more than one pregnancy on the maternal alloimmunization status, the in this study observed T-cell heterogeneity may provide a first clue why donor parity is still a controversial factor affecting HLA identical SCT outcome. Indeed, unequivocal conclusions regarding the effect of donor parity on the graft-versus-host disease (GVHD) risk still exist as reviewed.33 Interestingly, in 8 of 9 females analyzed, a correlation was found between their tvDTH test result, which was generated ex vivo in a blinded fashion, and the outcome of the in parallel performed in vitro experiments on TCTL outgrowth (Table 3). Dissection of tolerized donors from sensitized donors may also be useful in male-to-male SCT settings. Because of the presumed lowest GVHD risk, an HLA-identical male donor is considered as the optimal SC donor for male patients.33 This study indicates the presence of CD8+ TREG directed against the hematopoietic system-restricted minor H antigens HA-1 or HA-2 in male donors and thus potentially also in their hematopoietic SC graft. Such TREG may impair HA-1 or HA-2 TCTL–driven graft-versus-leukemia reactions.34,35 Evidently, the presence of these TREG must also be considered when HA-1– or HA-2–specific TCTL are generated from unselected PBMCs or CD8-enriched populations for the purpose of cellular adoptive immunotherapy.36
Given our current observations that nonfunctional CD8+ T cells, ie, failure to lyse or produce interferon-γ when stimulated with minor H alloantigen, can also be expanded from some of our donors (♀2, ♀3, and ♂2, data not shown), we speculate that naturally established tolerance to minor H antigens may be the result of either the presence of minor H antigen–specific TREG and/or the induction of T-cell anergy. Several experimental transplantation models have demonstrated that the establishment and maintenance of stable allograft tolerance depend on systemically persisting alloantigen-expressing microchimeric cell types, such as lymphocytes,37,38 DCs,39–42 or both.17 These cell types continuously present alloantigen to host T cells under noninflammatory conditions, which may lead to active deletion of host alloreactive T cells37 and/or the induction of alloreactive adaptive TREG.42 Similar observations were recently made in a murine F1 breeding model, in which detectable levels of maternal CD11b+ monocytes or CD11c expressing mDC correlated with the presence of a dominant population of transforming growth factor β–producing CD4+ TREG in NIMA tolerant offspring.43 From 8 donors (6 females and 2 males), sufficient numbers of PBMCs were available to determine the copresence of microchimeric cells expressing the relevant minor H alloantigen. Using DNA extracted from highly purified hematopoietic cell fractions and real-time PCR technology, we were able to demonstrate the presence of minimally one microchimeric cell type in 7 of 8 donors tested (data not shown). It remains to be studied whether or not particular microchimeric cell types are associated with either a sensitized or a tolerized minor H antigen immunization status.
A significant proportion of patients eligible for SCT does not have an HLA-identical family donor or an HLA-matched unrelated donor and may therefore receive an HLA-haploidentical or partially identical SC graft. Several clinical studies suggest that mutual allotolerance, marked by the presence of fetal microchimerism in the maternal SC donor and maternal microchimeric cells in the recipient before SCT, is an important prerequisite for successful outcome of haploidentical SCT using the mother as SC donor.44,45 Although the risk of acute GVHD may be enhanced in the latter transplantation setting,46 event-free survival seems to be significantly better for patients receiving a transplant from their mother compared with grafts donated by their father.47 Moreover, HLA haploidentical sibling SC grafts mismatched for NIMA were shown to induce less acute GVHD compared with noninherited paternal alloantigen-mismatched grafts.46 Such compelling evidence supporting the powerful effects of naturally acquired NIMA-specific tolerance is also accumulating in the field of solid organ transplantation. These studies include the reported prolonged survival of sibling donated haploidentical NIMA-mismatched kidney transplantations, compared with grafts from noninherited paternal alloantigen-mismatched sibling donors.48 Familial alloantigen tolerance induced as a consequence of fetomaternal or transmaternal sibling cell traffic may also account for reduced transplantation-related mortality observed in firstborn patients receiving an HLA-identical SC graft from a younger sibling donor.19 Not only in the HLA-identical but also in the HLA-haploidentical situation, minor H alloreactivities may be induced.3 Thus, pretransplantation minor H genotyping,49 combined with functional testing for donor TCTL or TREG activity as well as determination of the presence of microchimeric cell populations, may be of clinical value, particularly in familial SCT settings.
Acknowledgments
The authors thank G. de Roo, M. van den Hoorn, and J. Stegehuis-Kamp for excellent technical assistance in the FACS sorting experiments; M. Kester for tetramer synthesis; A. Goekoop for collecting blood samples or buccal swabs and the HLA tissue–typing laboratory of the Department of Immunohematology and Blood Transfusion for performing HLA and minor H antigen genotyping; and Prof Dr F. H. Claas, Prof Dr J. J. van Rood, Dr E. Spierings, and Dr L. Hambach for critical reading of the manuscript.
This work was supported in part by the National Blood Foundation, the Macropa Foundation, the Dutch Cancer Society (grant UL 2005-3657), and the Netherlands Organization for Scientific Research (NWO-Spinoza Award).
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.G.S.v.H., W.J.B., and E.G. designed the research; A.G.S.v.H., E.J.-G., A.J, E.B., and J.P. performed experiments and collected data; A.G.S.v.H. analyzed results and prepared figures; and A.G.S.v.H., A.B., W.J.B., and E.G. drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Astrid G. S. van Halteren, Department of Pediatrics (WA-KJC), Immunology Laboratory (P3-P), Leiden University Medical Center, PO Box 9600, 2300 RC Leiden, The Netherlands; e-mail: a.g.s.van_Halteren@lumc.nl.
References
- 1.van Rood JJ, Eernisse JG, van Leeuwen A. Leucocyte antibodies in sera from pregnant women. Nature. 1958;181:1735–1736. doi: 10.1038/1811735a0. [DOI] [PubMed] [Google Scholar]
- 2.van Kampen CA, Versteeg-van der Voort Maarschalk MF, Langerak J, et al. Pregnancy can induce long-persisting primed CTLs specific for inherited paternal HLA antigens. Hum Immunol. 2001;62:201–207. doi: 10.1016/s0198-8859(01)00209-9. [DOI] [PubMed] [Google Scholar]
- 3.Verdijk RM, Kloosterman A, Pool J, et al. Pregnancy induces minor histocompatibility antigen-specific cytotoxic T cells: implications for stem cell transplantation and immunotherapy. Blood. 2004;103:1961–1964. doi: 10.1182/blood-2003-05-1625. [DOI] [PubMed] [Google Scholar]
- 4.Owen RD, Wood HR, Foord AG, Sturgeon P, Baldwin LG. Evidence for actively acquired tolerance to Rh antigens. Proc Natl Acad Sci U S A. 1954;40:420–424. doi: 10.1073/pnas.40.6.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Claas FH, Gijbels Y, van der Velden-de Munck, van Rood JJ. Induction of B cell unresponsiveness to noninherited maternal HLA antigens during fetal life. Science. 1988;241:1815–1817. doi: 10.1126/science.3051377. [DOI] [PubMed] [Google Scholar]
- 6.Ichinohe T, Teshima T, Matsuoka K, Maruya E, Saji H. Fetal-maternal microchimerism: impact on hematopoietic stem cell transplantation. Curr Opin Immunol. 2005;17:546–552. doi: 10.1016/j.coi.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Bianchi DW. Fetomaternal cell traffic, pregnancy-associated progenitor cells, and autoimmune disease. Best Pract Res Clin Obstet Gynaecol. 2004;18:959–975. doi: 10.1016/j.bpobgyn.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 8.O'Donoghue K, Chan J, de la Fuente J, et al. Microchimerism in female bone marrow and bone decades after fetal mesenchymal stem-cell trafficking in pregnancy. Lancet. 2004;364:179–182. doi: 10.1016/S0140-6736(04)16631-2. [DOI] [PubMed] [Google Scholar]
- 9.Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci U S A. 1996;93:705–708. doi: 10.1073/pnas.93.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maloney S, Smith A, Furst DE, et al. Microchimerism of maternal origin persists into adult life. J Clin Invest. 1999;104:41–47. doi: 10.1172/JCI6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson JL, Gillespie KM, Lambert NC, et al. Maternal microchimerism in peripheral blood in type 1 diabetes and pancreatic islet beta cell microchimerism. Proc Natl Acad Sci U S A. 2007;104:1637–1642. doi: 10.1073/pnas.0606169104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wekerle T, Kurtz J, Ito H, et al. Allogeneic bone marrow transplantation with co-stimulatory blockade induces macrochimerism and tolerance without cytoreductive host treatment. Nat Med. 2000;6:464–469. doi: 10.1038/74731. [DOI] [PubMed] [Google Scholar]
- 13.Andrassy J, Kusaka S, Jankowska-Gan E, et al. Tolerance to noninherited maternal MHC antigens in mice. J Immunol. 2003;171:5554–5561. doi: 10.4049/jimmunol.171.10.5554. [DOI] [PubMed] [Google Scholar]
- 14.Molitor-Dart ML, Andrassy J, Kwun J, et al. Developmental exposure to noninherited maternal antigens induces CD4+ T regulatory cells: relevance to mechanism of heart allograft tolerance. J Immunol. 2007;179:6749–6761. doi: 10.4049/jimmunol.179.10.6749. [DOI] [PubMed] [Google Scholar]
- 15.Burlingham WJ, Grailer AP, Fechner JH, Jr, et al. Microchimerism linked to cytotoxic T lymphocyte functional unresponsiveness (clonal anergy) in a tolerant renal transplant recipient. Transplantation. 1995;59:1147–1155. [PubMed] [Google Scholar]
- 16.Alexander SI, Smith N, Hu M, et al. Chimerism and tolerance in a recipient of a deceased-donor liver transplant. N Engl J Med. 2008;358:369–374. doi: 10.1056/NEJMoa0707255. [DOI] [PubMed] [Google Scholar]
- 17.Cai J, Lee J, Jankowska-Gan E, et al. Minor H antigen HA-1-specific regulator and effector CD8+ T cells, and HA-1 microchimerism, in allograft tolerance. J Exp Med. 2004;199:1017–1023. doi: 10.1084/jem.20031012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nolte-'t Hoen EN, Amoroso MG, Veenstra J, et al. Effector and regulatory T cells derived from the same T cell clone differ in MHC class II-peptide multimer binding. Eur J Immunol. 2004;34:3359–3369. doi: 10.1002/eji.200425563. [DOI] [PubMed] [Google Scholar]
- 19.Bucher C, Stern M, Buser A, et al. Role of primacy of birth in HLA-identical sibling transplantation. Blood. 2007;110:468–469. doi: 10.1182/blood-2007-02-076257. [DOI] [PubMed] [Google Scholar]
- 20.Hambach L, Spierings E, Goulmy E. Risk assessment in haematopoietic stem cell transplantation: minor histocompatibility antigens. Best Pract Res Clin Haematol. 2007;20:171–187. doi: 10.1016/j.beha.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Utter GH, Owings JT, Lee TH, et al. Microchimerism in transfused trauma patients is associated with diminished donor-specific lymphocyte response. J Trauma. 2005;58:925–931. doi: 10.1097/01.ta.0000162142.72817.5c. [DOI] [PubMed] [Google Scholar]
- 22.Mutis T, Gillespie G, Schrama E, et al. Tetrameric HLA class I-minor histocompatibility antigen peptide complexes demonstrate minor histocompatibility antigen-specific cytotoxic T lymphocytes in patients with graft-versus-host disease. Nat Med. 1999;5:839–842. doi: 10.1038/10563. [DOI] [PubMed] [Google Scholar]
- 23.Derks RA, Jankowska-Gan E, Xu Q, Burlingham WJ. Dendritic cell type determines the mechanism of bystander suppression by adaptive T regulatory cells specific for the minor antigen HA-1. J Immunol. 2007;179:3443–3451. doi: 10.4049/jimmunol.179.6.3443. [DOI] [PubMed] [Google Scholar]
- 24.Fehse B, Chukhlovin A, Kuhlcke K, et al. Real-time quantitative Y chromosome-specific PCR (QYCS-PCR) for monitoring hematopoietic chimerism after sex-mismatched allogeneic stem cell transplantation. J Hematother Stem Cell Res. 2001;10:419–425. doi: 10.1089/152581601750289028. [DOI] [PubMed] [Google Scholar]
- 25.Wieles B, Pool J, Derks R, Burlingham WJ, Goulmy E. Detection of microchimerism by minor histocompatibility antigen HA-1 allele-specific nested polymerase chain reaction. Biol Blood Marrow Transplant. 2005;11:345–348. doi: 10.1016/j.bbmt.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Piper KP, McLarnon A, Arrazi J, et al. Functional HY-specific CD8+ T cells are found in a high proportion of women following pregnancy with a male fetus. Biol Reprod. 2007;76:96–101. doi: 10.1095/biolreprod.106.055426. [DOI] [PubMed] [Google Scholar]
- 27.Mommaas B, Stegehuis-Kamp JA, van Halteren AG, et al. Cord blood comprises antigen-experienced T cells specific for maternal minor histocompatibility antigen HA-1. Blood. 2004;105:1823–1827. doi: 10.1182/blood-2004-07-2832. [DOI] [PubMed] [Google Scholar]
- 28.Joosten SA, Ottenhoff TH. Human CD4 and CD8 regulatory T cells in infectious diseases and vaccination. Hum Immunol. 2008;69:760–770. doi: 10.1016/j.humimm.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 29.Filaci G, Fenoglio D, Fravega M, et al. CD8+CD28− T regulatory lymphocytes inhibiting T cell proliferative and cytotoxic functions infiltrate human cancers. J Immunol. 2007;179:4323–4334. doi: 10.4049/jimmunol.179.7.4323. [DOI] [PubMed] [Google Scholar]
- 30.Maile R, Pop SM, Tisch R, et al. Low-avidity CD8lo T cells induced by incomplete antigen stimulation in vivo regulate naive higher avidity CD8hi T cell responses to the same antigen. Eur J Immunol. 2006;36:397–410. doi: 10.1002/eji.200535064. [DOI] [PubMed] [Google Scholar]
- 31.Mold JE, Michaelsson J, Burt TD, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Rood JJ, Roelen DL, Claas FH. The effect of noninherited maternal antigens in allogeneic transplantation. Semin Hematol. 2005;42:104–111. doi: 10.1053/j.seminhematol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Loren AW, Bunin GR, Boudreau C, et al. Impact of donor and recipient sex and parity on outcomes of HLA-identical sibling allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12:758–769. doi: 10.1016/j.bbmt.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 34.Marijt WA, Heemskerk MH, Kloosterboer FM, et al. Hematopoiesis-restricted minor histocompatibility antigens HA-1- or HA-2-specific T cells can induce complete remissions of relapsed leukemia. Proc Natl Acad Sci U S A. 2003;100:2742–2747. doi: 10.1073/pnas.0530192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hambach L, Nijmeijer BA, Aghai Z, et al. Human cytotoxic T lymphocytes specific for a single minor histocompatibility antigen HA-1 are effective against human lymphoblastic leukaemia in NOD/scid mice. Leukemia. 2006;20:371–374. doi: 10.1038/sj.leu.2404056. [DOI] [PubMed] [Google Scholar]
- 36.Mutis T, Verdijk R, Schrama E, et al. Feasibility of immunotherapy of relapsed leukemia with ex vivo-generated cytotoxic T lymphocytes specific for hematopoietic system-restricted minor histocompatibility antigens. Blood. 1999;93:2336–2341. [PubMed] [Google Scholar]
- 37.Bonilla WV, Geuking MB, Aichele P, et al. Microchimerism maintains deletion of the donor cell-specific CD8+ T cell repertoire. J Clin Invest. 2006;116:156–162. doi: 10.1172/JCI26565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan WF, Perez-Diez A, Razavy H, Anderson CC. The ability of natural tolerance to be applied to allogeneic tissue: determinants and limits. Biol Direct. 2007;2:10. doi: 10.1186/1745-6150-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomson AW, Lu L, Murase N, et al. Microchimerism, dendritic cell progenitors and transplantation tolerance. Stem Cells. 1995;13:622–639. doi: 10.1002/stem.5530130607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Probst HC, McCoy K, Okazaki T, Honjo T, van den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat Immunol. 2005;6:280–286. doi: 10.1038/ni1165. [DOI] [PubMed] [Google Scholar]
- 41.Steptoe RJ, Ritchie JM, Jones LK, Harrison LC. Autoimmune diabetes is suppressed by transfer of proinsulin-encoding Gr-1+ myeloid progenitor cells that differentiate in vivo into resting dendritic cells. Diabetes. 2005;54:434–442. doi: 10.2337/diabetes.54.2.434. [DOI] [PubMed] [Google Scholar]
- 42.Ochando JC, Homma C, Yang Y, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 43.Dutta P, Molitor-Dart M, Bobadilla JL, et al. Microchimerism is strongly correlated with tolerance to noninherited maternal antigens in mice. Blood. doi: 10.1182/blood-2009-03-213561. Prepublished on August 21, 2009, as DOI 10.1182/blood-2009-03-213561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ichinohe T, Maruya E, Saji H. Long-term feto-maternal microchimerism: nature's hidden clue for alternative donor hematopoietic cell transplantation? Int J Hematol. 2002;76:229–237. doi: 10.1007/BF02982792. [DOI] [PubMed] [Google Scholar]
- 45.Obama K, Takemoto Y, Takatsuka Y, Utsunomiya A. Successful reduced-intensity HLA-haploidentical stem cell transplantation based on the concept of feto-maternal tolerance for an elderly patient with myelodysplastic syndrome. Bone Marrow Transplant. 2004;33:253. doi: 10.1038/sj.bmt.1704350. [DOI] [PubMed] [Google Scholar]
- 46.van Rood JJ, Loberiza FR, Jr, Zhang MJ, et al. Effect of tolerance to noninherited maternal antigens on the occurrence of graft-versus-host disease after bone marrow transplantation from a parent or an HLA-haploidentical sibling. Blood. 2002;99:1572–1577. doi: 10.1182/blood.v99.5.1572. [DOI] [PubMed] [Google Scholar]
- 47.Stern M, Ruggeri L, Mancusi A, et al. Survival after T cell-depleted haploidentical stem cell transplantation is improved using the mother as donor. Blood. 2008;112:2990–2995. doi: 10.1182/blood-2008-01-135285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burlingham WJ, Grailer AP, Heisey DM, et al. The effect of tolerance to noninherited maternal HLA antigens on the survival of renal transplants from sibling donors. N Engl J Med. 1998;339:1657–1664. doi: 10.1056/NEJM199812033392302. [DOI] [PubMed] [Google Scholar]
- 49.Spierings E, Drabbels J, Hendriks M, et al. A uniform genomic minor histocompatibility antigen typing methodology and database designed to facilitate clinical applications. PLoS ONE. 2006;1:e42. doi: 10.1371/journal.pone.0000042. [DOI] [PMC free article] [PubMed] [Google Scholar]