Abstract
Both sphingosine and sphingosine 1-phosphate (S1P) were able to protect the ex vivo rat heart from ischemia reperfusion injury when added to the perfusion medium at the time of reperfusion after a 40 min ischemia (postconditioning). Inhibitor studies revealed distinct mechanisms of protection, with S1P employing a G-protein coupled receptor pathway and sphingosine a cyclic nucleotide dependent protein kinase pathway. However, both restored ischemia-induced depletion of phospho-AKT. Extending the ischemia to 75 min reduced protection by both S1P and sphingosine, but protection could be enhanced by employing them in combination. Extending the time of ischemia further to 90 min almost eliminated cardioprotection by S1P or sphingosine; and their combination gave only modest protection. However, when S1P plus sphingosine was combined with a novel ramped ischemic postconditioning regimen, left ventricle developed pressure recovered by 66% and there was only a 6% infarct size. The data indicate that detrimental changes are accumulating during protracted ischemia but for up to 90 min this damage is not irreversible and hearts can still recover with proper treatment.
Keywords: Cardioprotection, Ischemia, Postconditioning, Sphingosine, Sphingosine 1-phosphate
Introduction
The loss of coronary blood flow for brief periods of time is well tolerated but extended reductions of perfusion lead to ischemic damage and cardiomyocyte death [1,2]. Cell death can result from periods of ischemia exceeding 20 min (1) and this damage occurs following the restoration of coronary blood flow [1–4]. Such ischemia reperfusion injury ultimately results in cell death due to both necrosis and apoptosis [5,6]. However, it has been found that the heart can be treated in ways that greatly diminish the damage associated with moderate periods of ischemia and subsequent reperfusion [7–9]. Treatments that precede the index ischemia are referred to as preconditioning [7,8] while treatments instituted at the time of reperfusion are referred to as postconditioning [9]. Preconditioned. Ischemic postconditioning is achieved by instituting brief cycles of ischemia/reperfusion after the index ischemia and just prior to full reperfusion (9). When a postconditioned heart is then exposed to full reperfusion, the loss of myocardial function and subsequent infarct size is substantially reduced [9]. It has also been found that pharmacologic agents can induce pre- and post-conditioning (8,9).
The lipid mediator sphingosine-1-phosphate (S1P) is an important cell signaling molecule with pro-survival effects (10). It has been found to be a potent cardioprotectant that is effective as both a pharmacologic pre- and post-conditioning agent [11–14]. Recently, we have shown [14] that sphingosine, which is the precursor to S1P, also has potent cardioprotective effects as both a preconditioning and postconditioning agent. Further, we found that the mechanism by which sphingosine preconditions hearts is completely different from that of S1P [14]. In the current study, we report that the effects of S1P and sphingosine as postconditioning agents are also mediated by separate cell signaling pathways and that their protective mechanisms are additive. We used these agents to test the hypothesis that combining known approaches to postconditioning would reduce ischemia reperfusion injury after long term ischemia. We demonstrate that combining both S1P and sphingosine with a novel form of ischemic postconditioning provides a potent cardioprotection that supports the recovery of hearts from prolonged periods of ischemia extending up to 90 minutes.
Materials and Methods
Materials
Triphenyltetrazolium chloride (TTC) and wortmannin were obtained from Sigma. D-erythro-sphingosine (sphingosine), and D-erythro-sphingosine-1-phosphate (S1P), were obtained from Biomol Research Laboratories. The protein kinase A (PKA) inhibitor PKA-I 14–22 amide myristoylated, the protein kinase C (PKC) inhibitor GK109203X (bisindolylmaleimide), and the protein kinase G (PKG) inhibitor KT5823 were obtained from Calbiochem. The receptor inhibitor VPC 23019 was obtained from Avanti Polar Lipids. The rabbit phospho-Akt (ser473) and caspase-3 antibodies were obtained from Cell Signal Technology.
Langendorff Ex Vivo Perfused Heart
This study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Academic Press, Washington DC, 1996). Hearts from 250g rats were removed under pentobarbital anesthesia and mounted on a Langendorff apparatus as described previously [15]. Hearts were perfused at a pressure of 90 mm Hg with oxygenated (95/5 O2:CO2) Krebs-Henseleit solution at 37°C. Left ventricular developed pressure (LVDP) was measured using a Millar micromannometer-tipped catheter. To measure infarct size, hearts were sectioned, stained with TTC and the infarct area determined by computer analysis [11].
The protocol for non-conditioned hearts consisted of continuous perfusion for 20 min after mounting the heart on the Langendorff apparatus. Sustained ischemia (index ischemia) was then induced by halting perfusion for indicated lengths of time. During the index ischemia the heart is lowered into a thermostated chamber that maintains an ambient temperature of 37°. This was followed by the reperfusion stage in which flow was again initiated for 40 min. Pharmacologic postconditioning consisted of adding either S1P or sphingosine or both to the reperfusion medium for the 40 min of reperfusion. To administer S1P, a stock solution of 2.67 mM was prepared in DMSO and 90 μl (for 0.4 μM final S1P concentration) was added per 600 ml of perfusion buffer. To administer D-erythro-sphingosine, a stock solution of 20 mM was prepared in ethanol and added directly to the perfusion buffer at a final concentration of 0.4 μM. For ramped ischemic postconditioning, at the end of the index ischemia, reperfusion was first initiated with 3 cycles of alternating 10 sec of reperfusion /20 sec of ischemia, followed by 15 sec of reperfusion/20 sec of ischemia and finally by 20 sec of reperfusion/20 sec of ischemia . In experiments using combined treatments, the perfusate used for ischemic postconditioning contained optimized concentrations of sphingosine and S1P (0.2 μM each of S1P and D-erythro-sphingosine was more consistent than 0.4 μM of each).
Western Analysis
Hearts were homogenized in 0.13M KCl, 20 mM HEPES pH 7.4, 1 mM EGTA, 1 μg/l leupeptin, 0.25 μg/l each of aprotinin and pepstatin A. The homogenate was filtered through two layers of cheesecloth to remove debris. Homogenates were centrifuged at 100,000 xg to generate a particulate and a cytosolic fraction. The cytosolic fraction was washed once with the isolation buffer. Western analysis was conducted using a rabbit antibody as described previously [14]. Protein concentration was determined using the detergent compatible DC Protein Assay kit from Bio-Rad and used to equalize the protein concentration in all samples. Each lane was loaded with 10 μg of protein.
Statistical Analysis
Statistical data are expressed as the mean ± SD. Numerical data were compared using Students’s t test for paired observations. A P value of <0.05 was considered significant.
Results
The Langendorff ex vivo heart model was used to study ischemia reperfusion injury in rat hearts. The ex vivo hearts were equilibrated for 20 min and then exposed to global ischemia followed by 40 min of reperfusion. The recovery of hemodynamic function was followed by continuous monitoring of the pressure developed by contraction of the left ventricle (LVDP). When this was done for increasing periods of ischemia, we found that ischemia exceeding 25 min began to cause failure of recovery during reperfusion and that a 40 min period of ischemia caused 100% of the hearts to fail (only 8 ± 2% recovery of LVDP). Further, in agreement with previous work [14.15], when the hearts exposed to 40 min of ischemia and then 40 min of reperfusion were stained with TTC, large areas of infarction were seen covering 45 ± 1% of the heart (n=5)..
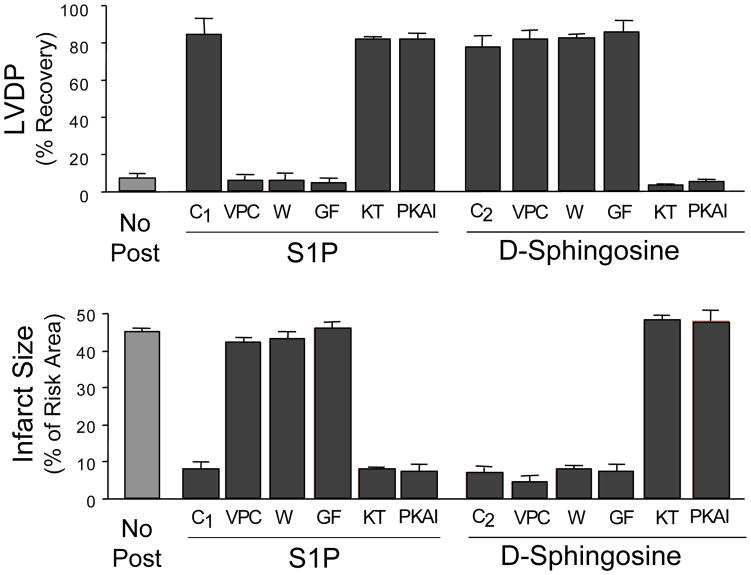
We previously reported that sphingosine and S1P were effective pharmacologic pre- and post-conditioning agents in the ex vivo rat heart model [14]. Characterization of the mechanism of preconditioning demonstrated that S1P and sphingosine utilized separate signaling pathways [14]. To determine if they also use separate pathways for postconditioning, we used inhibitors of cell signaling pathways to characterize postconditioning by both S1P and sphingosine. Following the 40 min index ischemia, reperfusion was commenced with media containing either sphingosine or S1P. The optimum protection for each was achieved with 0.2 to 0.4 μM. Protection was evidenced by a dramatically improved recovery of LVDP during reperfusion (Fig. 1A) and the near elimination of areas of infarction (Fig. 1B). When an antagonist of S1P1 and 3 G-protein coupled receptor function, VPC23019 [16], at a concentration of 1 μM was added to the postconditioning medium along with 0.4 μM S1P, it completely blocked the postconditioning by S1P. This is consistent with the known reliance of S1P on GPCR signaling. However, when 1 μM VPC23019 was added to the postconditioning medium along with the 0.4 μM sphingosine, there was no statistical difference between recovery in the presence or absence of VPC23019 (p>0.05). VPC23019 by itself was without effect. This indicates that postconditioning by sphingosine does not involve S1P-GPCRs.
Figure 1. The Effect of Postconditioning with either S1P or Sphingosine on the Recovery of LVDP and on Infarct Size in Hearts Reperfused after 40 Min of Ischemia, and the Effect of Inhibitors.
Ex vivo hearts were equilibrated for 20 minutes and then exposed to 40 min of global ischemia. This was followed by 40 min of reperfusion in the presence of either 0.4 μM S1P or 0.4 μM sphingosine in the presence or absence of inhibitors. Recovery of LVDP (A) is expressed as a percentage of the pre-index ischemia value. The infarct size (B) is expressed as a percentage of the area at risk determined at the end of the 40 min reperfusion. The data represent the mean and the error bars reflect the standard deviation (n≥4). The vehicle control (No Post) consisted of reperfusion in the absence of any addition. Reperfusion was done with S1P alone (C1) and sphingosine alone (C2), or with the further addition of the inhibitors: 1 μM VPC23019 (VPC), 50 nm GF109203X (GF), 0.1 μM wortmanin (W), 0.2 μM KT5823 (KT), or 0.1 μM PKA-I 14–22 amide-myristoylated (PKAI).
Additional inhibitors were used to further characterize the mechanisms of pharmacologic postconditioning by S1P and sphingosine. Inhibition of protein kinase C (PKC) with 50 nM GF109203X completely blocked postconditioning by S1P (Fig. 1). In contrast, co-treatment with 50 nM GF109203X did not affect postconditioning by 0.4 μM sphingosine (Fig. 1). Likewise, co-treatment with the PI3 kinase inhibitor wortmannin (at 0.1 μM) blocked postconditioning by S1P, but postconditioning by sphingosine was not affected by 0.1 μM wortmannin (Fig. 1).
By contrast, inhibition of protein kinase G with 0.2 μM KT5823 had no effect on S1P driven postconditioning (Fig 1), but completely eliminated postconditioning by 0.4 μM sphingosine (Fig. 1). Also, co-treatment with the protein kinase A inhibitory peptide 14–22 amide myristoylated at 0.1 μM eliminated postconditioning by sphingosine, but had little effect on postconditioning by S1P (Fig. 1). None of the inhibitors tested alone revealed any adverse effects on hemodynamics or induction of infarcts nor were they capable of cardioprotection. Thus, cyclic nucleotide dependent protein kinases were important for postconditioning by sphingosine but not for S1P.
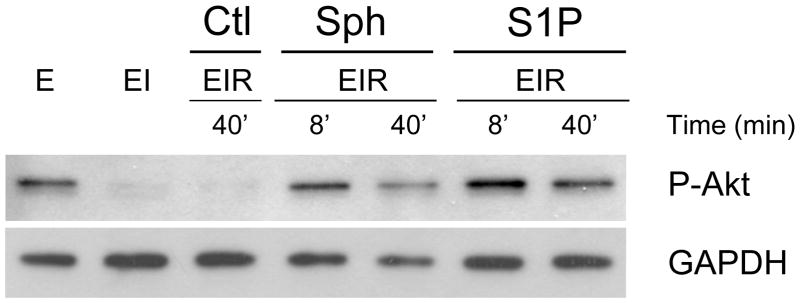
To further characterize postconditioning by the two sphingolipids, we studied the activation of AKT, a key pro-survival signaling molecule [17]. Activation of AKT by phosphorylation of serine 473 was measured by western analysis (Fig. 2). As reported previously [14], hearts normally have a substantial level of phospho-AKT that is severely decreased in response to 40 min of ischemia. However, upon postconditioning with either S1P or sphingosine, phospho-AKT levels recover rapidly and returned to normal by 8 min of reperfusion. This recovery is rapid enough to be a cause of protection associated with the two sphingolipids. If the heart is not postconditioned, the loss of phospho-AKT suffered during ischemia does not recover during reperfusion.
Figure 2. Effect of Postconditioning with S1P and Sphingosine on the Phosphorylation of Akt.
Ex vivo hearts were either equilibrated for 20’ (E), equilibrated followed by 40 min of ischemia (EI), or equilibrated followed by 40 min of ischemia followed by reperfusion (EIR). For the vehicle control (Ctl), reperfusion was for 40 min with medium containing vehicle only (Ctl EIR-40’). For postconditioning, after 40 min of global ischemia (EI), hearts were reperfused with medium containing either 0.4 μM S1P or 0.4 μM sphingosine for either 8 min or 40 min (EIR-8’and EIR-40’). After 40 min of reperfusion, the hearts were collected, homogenized and separated into cytosolic and particulate fractions. The Figure shows the western analysis for the cytosolic fraction using an antibody to phospho-AKT (ser473). Each lane was loaded with 10 μg of protein. The lanes were loaded as follows, lane 1- equilibrated; lane 2-equilibrated plus ischemia; lane 3-equilibrated plus ischemia plus vehicle reperfusion for 40’: lane 4- 8’ of reperfusion in the presence of sphingosine, lane 5– 40’ of reperfusion in the presence of sphingosine,, lane 6– 8’ of reperfusion in the presence of S1P, lane 7– 40’ of reperfusion in the presence of S1P.
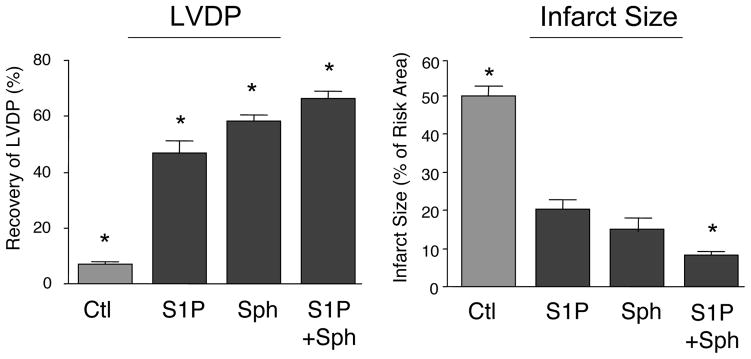
Since cardioprotection by both sphingosine and S1P involves maintenance of phospho-AKT levels, it was considered that their distinct pathways of protection might mutually support one another if necessitated by conditions of greater ischemic stress. To test this hypothesis, we extended the period of ischemia to 75 min. As can be seen in Fig. 3 (as compared to Fig. 1), a 75 min period of ischemia significantly reduces the extent of the protection afforded by both S1P and sphingosine relative to 40 min of ischemia. Under these conditions, it was possible to demonstrate that using both S1P plus sphingosine at optimized concentrations (0.2 μM each), there was a statistically significant (p<.05) improvement in the recovery of LVDP (68 ± 3% for both vs 47 ± 6% and 57 ± 4%, respectively for S1P and sphingosine) and a significant reduction in infarct size (7 ± 1% for both vs 20 ± 4% and 19 ± 1%, respectively for S1P and sphingosine).
Figure 3. Effect of Combined S1P and Sphingosine Postconditioning on Recovery from 75 Min of Ischemia.
Ex vivo hearts were equilibrated for 20 minutes and then exposed to 75 min of global ischemia. This was followed by 40 min of reperfusion in the presence of either vehicle (Ctl), 0.4 μM S1P, 0.4 μM sphingosine, or 0.2 μM S1P plus 0.2 μM sphingosine. Recovery of LVDP (A) is expressed as a percentage of the pre-index ischemia value. The infarct size (B) is expressed as a percentage of the area at risk determined at the end of the 40 min reperfusion. The data represent the mean and the error bars reflect the standard deviation (n≥4). Statistical significance (p<0.05) is indicated by (*)
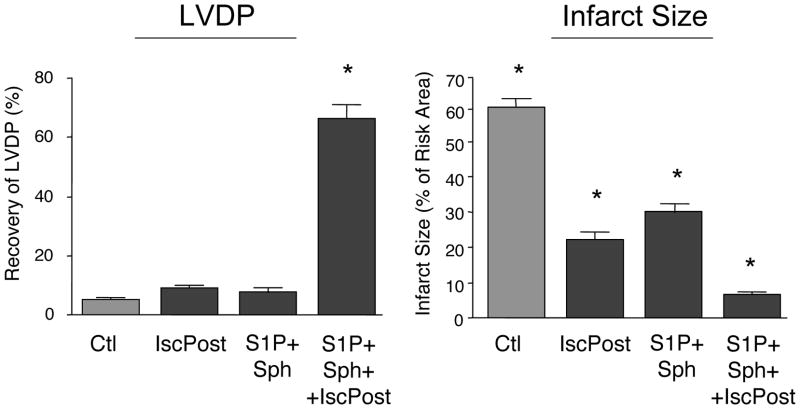
We added further stress to the hearts by increasing the time of ischemia to 90 min. After 90 min of ischemia, the combination of 0.2 μM S1P plus 0.2 μM sphingosine was not able to consistently provide postconditioning (Fig 4). The recovery of LVDP was 7 ± 2% and the infarct size from 30 ± 4%. We next postconditioned with medium containing both S1P and sphingosine and in addition initiated reperfusion with our standard ischemic postconditioning protocol of 4 cycles of 15” reperfusion/15” ischemia. Of 7 hearts studied, 4 recovered (66 ± 6% LVDP with a small 7 ± 1% infarct size), but 3 hearts recovered poorly (7–30% recovery of LVDP with 20–45% infarct size). However, we could achieve consistent recovery (n=5) by modifying the ischemic postconditioning protocol using a ramped ischemic postconditioning regimen of 3-cycles of 10” reperfusion/20” ischemia, 1-cycle of 15” reperfusion/20” ischemia, and finally 1-cycle of 20” reperfusion/20” ischemia. This consistently produced recovery of LVDP (66 ± 7%) with very little infarct (6 ± 1%). Ramped ischemic postconditioning by itself did not support hemodynamic recovery but did result in a modest reduction of infarct size (Fig. 4). Pharmacologic postconditioning with just S1P or sphingosine was ineffective. We have tested longer intervals of ischemia with this multiple conditioning regimen but protection could not be consistently demonstrated.
Figure 4. Effect of Combined S1P and Sphingosine and Ischemic Postconditioning on Recovery from 90 Min of Ischemia.
Ex vivo hearts were equilibrated for 20 minutes and then exposed to 90 min of global ischemia. This was followed by 40 min of reperfusion in the presence of 0.2 μM S1P plus 0.2 μM sphingosine (S1P+Sph) either alone or with the addition of an ischemic postconditioning regimen of 4 cycles of 15” reperfusion/15” ischemia (S1P+Sph+IscPost). The control (Ctl) received no postconditioning and ischemic postconditioning alone (IscPost) received only ischemic postconditioning in the absence of S1P or sphingosine. Recovery of LVDP (A) is expressed as a percentage of the pre-index ischemia value. The infarct size (B) is expressed as a percentage of the area at risk determined at the end of the 40 min reperfusion. The data represent the mean and the error bars reflect the standard deviation (n≥4). Statistical significance (p<0.05) is indicated by (*)
Discussion
Postconditioning is able to restore function to hearts following an index ischemia. This reveals that irreversible damage does not occur during the period of ischemia. We were interested in determining the maximum length of ischemia that constituted the point of no return for recovery. We first observed that as the period of ischemia was increased in length, more potent forms of postconditioning were required but recovery was still possible. We found that, if necessary, different types of postconditioning could be combined to provide more potent postconditioning. The most potent form of postconditioning we could develop consisted of enhancing ischemic postconditioning by incorporating a ramped reperfusion protocol; and then combining this with pharmacologic postconditioning using a GPCR dependent agent (S1P) plus an agent utilizing a cyclic nucleotide dependent pathway (sphingosine). Use of this maximized postconditioning protocol revealed that the heart can still be rescued following periods of ischemia as long as 90 min. This greatly extends the known time of ischemia from which the heart can recover.
It is interesting that the longer the time of ischemia the more potent the postconditioning must be to obtain recovery. This indicates that deleterious changes are gradually accruing that ultimately reach a threshold, i.e., a pointof no return. The increase in deleterious changes with increasing ischemia is evidenced by the increase in infarct size in non-conditioned hearts (45% to 50% to 61% as ischemia increases from 40’ to 75’ to 90’).
We previously showed that S1P and sphingosine given separately are very effective cardiac preconditioning agents, and that they utilize distinct protective pathways (14). In this study we showed that both of these observations are also true for postconditioning. Thus, S1P postconditioning of the ex vivo heart could be blocked by the S1P1/S1P3 GPCR antagonist VPC 23019, by inhibition of PI3 kinase with wortmannin or by inhibition of PKC (Fig 1). Thus, postconditioning by S1P is triggered by S1P binding to GPCRs activating a signaling pathway that includes PI3 kinase and protein kinase C, and it was demonstrated that this leads to the phosphorylation of AKT. This is identical to the findings for the response of the heart to preconditioning by S1P [14] indicating that similar pathways are responsible for both pre- and post-conditioning. Postconditioning by S1P was unaffected by inhibitors of PKA and PKG, and this was also previously found for preconditioning [14].
Sphingosine was also found to provide excellent cardioprotection as a postconditioning agent. It supports 78% recovery of LVDP and reduces the infarct size to 7% of the risk area. Interestingly, sphingosine postconditioning was found to be mediated by a signaling pathway distinct from S1P mediated protection. Thus, the GPCR antagonist VPC23019, the PI3 kinase inhibitor wortmannin and the PKC inhibitor GF 109203X were ineffective in blocking postconditioning by sphingosine. This indicates a lack of involvement of a GPCR signaling pathway and PI3 kinase involvement. In agreement with studies of preconditioning (14), we found that postconditioning by sphingosine could be blocked by inhibitors of either PKA or PKG. Thus, sphingosine appears to utilize the same cyclic nucleotide dependent pathways involving PKA and PKG for both pre- and post-conditioning. This is a distinct pathway from S1P cardioprotection.
The fact that S1P and sphingosine postconditioning were utilizing separate pathways led us to test their ability to provide better protection in combination than either alone. Fig. 3 shows that they are indeed complementary. Protection by these two agents could be further improved by combining them with a ramped ischemic postconditioning to achieve a presumed maximal level of protection. This maximal protection supported recovery of hearts from 90 min of ischemia with only a 6% infarct size and a 66% recovery of hemodynamic function
Although sphingosine and S1P effects are driven by different pathways, for preconditioning they seem to converge at a key pro-survival regulatory component, phosphorylation of AKT [14]. We have found that this holds true also for postconditioning. During ischemia, the level of phospho-AKT was dramatically depleted and does not return during reperfusion in the absence of postconditioning. This loss of phospho-AKT during ischemia might be expected to contribute to the initiation of apoptosis (17). However, lack of phospho-AKT during ischemia does not prevent recovery with postconditioning. S1P and sphingosine driven postconditioning pathways appear to overlap in that they both mediate a rapid recovery of phospho-AKT level upon reperfusion. This suggests that AKT phosphorylation is a major determinant of myocyte survival.
In conclusion, we have found that S1P and sphingosine are effective post-conditioning agents that employ completely different mechanisms. S1P utilizes the same G-protein coupled receptors pathway that activates preconditioning while in contrast, sphingosine utilizes a cyclic nucleotide-dependent protein kinase pathway identical to its mechanism in preconditioning. The S1P and sphingosine pathways were additive and when both were utilized in conjunction with ischemic postconditioning; even more potent postconditioning is achieved. This enabled us to determine for the first time that effective cardioprotection can still be established after 90 min of ischemia, thus extending the known limits of recovery in this rat model. Our results demonstrating that combination therapy is cardioprotective even after protracted ischemia have implications for the effective use of postconditioning in patients with acute ischemic heart disease.
Acknowledgments
This work was supported by grants from the Medical Service of the Department of Veterans Affairs (DAV), and NIH Grant No. 1PO1 HL68738 (JSK). The authors acknowledge the assistance of Norman Honbo in the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Citations
- 1.Jennings RB, Reimer KA. The Cell Biology of Acute Myocardial Ischemia. Ann Revs Med. 1991;42:225–246. doi: 10.1146/annurev.me.42.020191.001301. [DOI] [PubMed] [Google Scholar]
- 2.Buja LM, Entman ML. Modes of myocardial cell injury and cell death in ischemic heart disease. Circulation. 1998;98:1355–1357. doi: 10.1161/01.cir.98.14.1355. [DOI] [PubMed] [Google Scholar]
- 3.Hansen PR. Myocardial reperfusion injury: experimental evidence and clinical relevance. Eur Heart J. 1995;16:734–740. doi: 10.1093/oxfordjournals.eurheartj.a060991. [DOI] [PubMed] [Google Scholar]
- 4.Maxwell SR, Lip GY. Reperfusion injury: a review of the pathophysiology, clinical manifestations and therapeutic options. Int J Cardiol. 1997;58:95–117. doi: 10.1016/s0167-5273(96)02854-9. [DOI] [PubMed] [Google Scholar]
- 5.Zhao ZQ, Vinten-Johansen J. Myocardial apoptosis and ischemic preconditioning. Cardiovasc Res. 2002;55:438–455. doi: 10.1016/s0008-6363(02)00442-x. [DOI] [PubMed] [Google Scholar]
- 6.Gill C, Mestril R, Samali A. Losing heart: the role of apoptosis in heart disease – a novel therapeutic target? FASEB J. 2002;16:135–146. doi: 10.1096/fj.01-0629com. [DOI] [PubMed] [Google Scholar]
- 7.Murry CE, Richard VJ, Reimer KA, Jennings RB. Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ Res. 1990;66:913–931. doi: 10.1161/01.res.66.4.913. [DOI] [PubMed] [Google Scholar]
- 8.Deutsch E, Berger M, Kussmaul WG, Hirshfeld JW, Hermann HC, Laskey WK. Adaptation to ischemia during percutaneous transluminal coronary angioplasty: clinical hemodynamic, and metabolic features. Circulation. 1990;82:2044–2051. doi: 10.1161/01.cir.82.6.2044. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Z-Q, Vinten-Johansen J. Postconditioning: Reduction of reperfusion-induced injury. Cardiovasc Res. 2006;70:200–211. doi: 10.1016/j.cardiores.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 10.Payne S, Milstein A, Spiegel S. Sphingosine-1-phosphate: dual messenger functions. FEBS Lett. 2002;531:54–57. doi: 10.1016/s0014-5793(02)03480-4. [DOI] [PubMed] [Google Scholar]
- 11.Jin Z-Q, Zhou H-Z, Zhu P, Honbo N, Mochly-Rosen D, Messing RO, Goetzl EJ, Karliner JS, Gray MO. Cardioprotection mediated by sphingosine-1-phosphate and ganglioside GM-1 in wild-type and PKCε knockout mouse hearts. Am J Physiol Heart Circ Physiol. 2002;282:H1970–H1977. doi: 10.1152/ajpheart.01029.2001. [DOI] [PubMed] [Google Scholar]
- 12.Lecour S, Smith RM, Woodward B, Opie LH, Rochette L, Sack MN. Identification of a novel role for sphingolipid signaling in TNFa and ischemic preconditioning mediated cardioprotection. J Mol Cell Cardiol. 2002;34:509–518. doi: 10.1006/jmcc.2002.1533. [DOI] [PubMed] [Google Scholar]
- 13.Jin Z-Q, Zhang J, Huang Y, Hoover HE, Vessey DA, Karliner JS. A sphingosine kinase 1 mutation sensitizes the myocardium to ischemia/reperfusion injury. Cardiovasc Res. 2007;76:41–50. doi: 10.1016/j.cardiores.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 14.Vessey DA, Li L, Kelley M, Karliner JS. Sphingosine can pre- and postcondition heart and utilizes a different mechanism from sphingosine 1-phosphate. J Biochem Molec Toxicol. 2008;22:113–118. doi: 10.1002/jbt.20227. [DOI] [PubMed] [Google Scholar]
- 15.Vessey DA, Kelley M, Li L, Huang Y, Zhou H-Z, Zhu BQ, Karliner JS. Role of sphingosine kinase activity in protection of heart against ischemia reperfusion injury. Med Sci Monitor. 2006;12:BR318–324. [PubMed] [Google Scholar]
- 16.Davis MD, Clemens JJ, Macdonald TL, Lynch KR. Sphingosine 1-phosphate analogs as receptor antagonists. J Biol Chem. 2005;280:9833–9841. doi: 10.1074/jbc.M412356200. [DOI] [PubMed] [Google Scholar]
- 17.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]