Abstract
A case-control epidemiological study was conducted to determine whether an increased diagnostic rate for group A rotavirus in swine herds in Ontario was associated with specific management factors. The number of new herds tested per year and the proportion of new positive herds increased between 1994 and 1997. Herd size was larger and weaning age was younger in rotavirus-positive herds compared with rotavirus-negative herds. Pigs raised in all-in all-out nurseries were 3.4 times more likely to have a positive group A rotavirus diagnosis than pigs in continuous flow facilities. This study demonstrates that the changes seen in group A rotavirus disease herd status in Ontario are associated with changes in farm management practices, including farm expansion, early weaning, and all-in all-out production.
Introduction
The common causes of infectious diarrhea in nursing and nursery pigs are Escherichia coli, transmissible gas troenteritis (TGE) virus, rotavirus, Isospora suis, and Clostridium perfringens (1,2,3). These agents can cause mild to moderate to severe diarrhea, with potentially high morbidity and mortality, and the etiology of disease or clinical signs cannot be differentiated clinically. Diagnosis depends on examination of newly affected, untreated pigs presented alive for postmortem exami nation, and includes tests designed specifically to identify each pathogen.
Rotaviruses are nonenveloped, segmented, double-stranded RNA viruses classified in the family Reoviridae. The rotaviruses that affect pigs are differentiated as group A, B, and C on the basis of the group-specific inner capsid protein (4). Group A rotaviruses cause diarrhea in pigs, both before and after weaning (5), and are reported to account for 53% of preweaning and 44% of postweaning rotavirus diarrhea in swine (2). A more recent report attributes 89% of all rotavirus diarrhea in commercial pig operations to group A rotavirus (6).
Group A rotaviruses are further divided into 11 sero types, depending on the identity of 2 surface proteins, VP4 and VP7 (4). Swine may be infected with serotypes 3, 4, and 5 (7). Multiple serotypes of group A rotaviruses may occur in the same herd, and both group A and nongroup A rotaviruses can be found in the same herd (8). Virulence may be strain specific (9).
Rotaviruses survive in the environment for long periods of time and are transmitted via the fecal-oral route. Replication occurs in the epithelial cells at the tips of the small intestinal villi, destroying enterocytes, especially in the jejunum and ileum, and causing villous atrophy (10,11), which is succeeded by a compensatory hyperplasia of crypt epithelial cells (11). The severity of the disease depends on the percentage of absorptive or digestive cells that are affected (12). Eventually, the mature columnar epithelial cells on the villi are replaced by immature cuboidal enterocytes that are unable to produce digestive enzymes and have lost their absorptive capabilities. Intestinal content cannot be digested or absorbed and severe malabsorption may occur (13). Loss of sodium and chloride into the intestinal lumen contributes to increased osmotic pressure, drawing more fluid into the intestine. Ultimately, net fluid loss exceeds absorption, and diarrhea results.
In field situations, affected pigs have yellow-white diarrhea and may become dehydrated. The clinical disease lasts for up to 3 d, resulting in low weaning weights and poor average daily gain in both colostrum-deprived and colostrum-fed piglets (13), with as much as a 59% decrease in body weight (14) by 9 d postinfection (15). Morbidity may be 20%, with mortality as high as 15% (16). Three-day-old pigs recover from the intesti nal lesions in 6 to 10 d, while 21-day-old pigs recover in 2 to 4 d (16). Litters that have had preweaning rotavirus diarrhea are more likely than unaffected litters to experience postweaning diarrhea, accompanying skin and respiratory problems, and reduced weight gain (14,17). In natural infections, rotavirus is excreted in both normal and diarrheic feces (5).
Age resistance to clinical rotavirus disease occurs in swine and is associated with factors other than development of an age-dependent resistance of enterocytes to infection (18). Older pigs are more likely to have developed active immunity due to natural exposure, also the rate of regeneration of villi is increased and the volume of milk or feed intake increases, which results in reduced severity and duration of clinical signs (18). Protection depends mainly on milk immunoglobulin (Ig) A anti body. Piglets acquire all of their maternal antibodies post natally, via colostrum; therefore, the quantity and quality of colostrum received is critical. Colostrum-fed pigs show less severe clinical disease (10), with circu lating maternal antibody being significant in mitigating clinical disease, depending on the antibody titer (19), for at least the first 1 or 2 wk after birth (20). In previous studies, after oral challenge with virulent virus, the duration of viral shedding and the disease severity were significantly less in neonatal pigs with high serum levels of maternally derived rotavirus antibodies than in those without (19). The active immune response to rotaviruses is largely serotype specific, but cross- reacting epitopes may induce a heterotypic response (4).
Personnel in the Ontario provincial veterinary diagnostic laboratory (Animal Health Laboratory, Laboratory Services Division, University of Guelph) noted an increase in the diagnostic rate for porcine rotavirus group A between 1994 and 1998 (unpublished data). There had been no change in the diagnostic test used (rotavirus latex agglutination) or in management of the virology labo ratory. The purposes of this project were to determine the number of swine herds submitting samples to be tested for rotavirus during these 4 y, then to determine whether the number of positive herds had increased during this time and, if so, to identify farm management factors asso ciated with the increase. It was hypothesized that factors such as herd size, commingling of pigs from different sow herds, and early weaning might be associated with the increased diagnostic rate for group A rotavirus.
Materials and methods
Cases for this study were selected from the 1994 to 1998 submissions to the Animal Health Laboratory, Laboratory Services Division, University of Guelph, Guelph, Ontario (formerly the Veterinary Laboratory Services Branch, Ontario Ministry of Agriculture, Food, and Rural Affairs). All swine submissions tested for porcine rotavirus group A by the rotavirus latex agglu tination test were selected from the laboratory data base. The number of different herds represented was determined, and the submissions positive for group A rotavirus were identified. Herds that submitted more than one sample to the laboratory during the 4-year period were included only once in the data set. A case-control epidemiological study was performed by selecting 3 control herds for every case herd. Cases and controls were matched by the age of onset of diarrhea (categorized as 0 to 13 d, 14 to 24 d, or greater than 24 d).
Management data were collected from the labora tory submission form. Missing data were collected by sending a survey form to the herd veterinarian for com pletion. If the survey was not completed within 2 wk, the veterinarian was telephoned. If the veterinarian could not be reached, the producer was contacted. Management fac tors included herd management type (farrow-to-finish or multisite production); herd size, as measured by the number of sows (in the case of off-site early weaning pro duction, this was indicated by the number of sows that fed the nurseries); average weaning age; commingling of pigs in the nursery from multiple sow farms; con tinuous flow of pigs into the nursery; and rotavirus vaccination of the sows. Multisite production described a unit where pigs were weaned from the sow unit and then moved to an off-site nursery. The association between dichotomous management factors and the diagnosis of group A rotavirus was analyzed by using the chi-square statistic (21). The odds of being positive for the rotavirus test were calculated for all significant associations (P < 0.05). The association between rotavirus diagnosis and herd size or weaning age was tested by using a 2-sample t-test (21). The association between all-in all-out management and weaning pigs before 21 d was tested by using the chi-square statistic.
For the purposes of this study, commingling was defined as the use of more than 1 herd source to fill a nursery. Continuous flow was defined as the absence of all-in all-out management.
Diagnosis of group A rotavirus was made by using a RotaScreen Test (Kalyx Biosciences, Nepean, Ontario) according to the manufacturer's instructions. Briefly, latex particles coated with polyclonal rabbit antibodies to group A rotavirus were mixed with feces or gut scrapings. Specimens were also mixed with latex beads coated with an unrelated antibody to control for non specific agglutination. When rotavirus antigens were present in a fecal sample, agglutination occurred.
Results
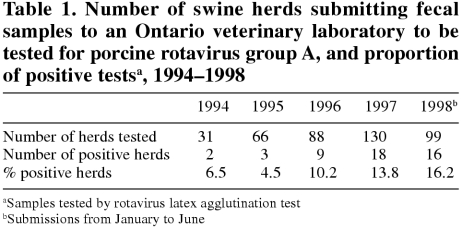
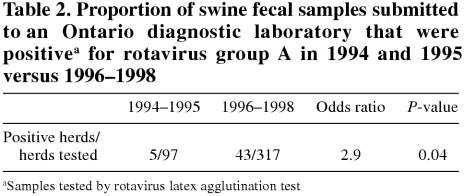
The number of new herds tested per year increased from 31 in 1994 to 130 in 1997 (Table 1), and the pro portion of positive herds increased from 4.5% in 1995 to 13.8% in 1997. Data are presented for only the first 6 mo of 1998, but the trend of increasing numbers of positive herds is evident. Producers submitting samples after 1995 were 2.9 times more likely (P = 0.04) to receive a positive diagnosis for group A rotavirus than were producers submitting samples in 1994 or 1995 (Table 2).
Table 1.
Table 2.
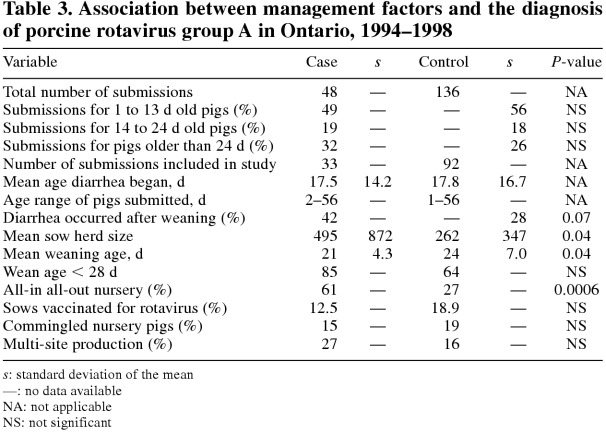
A total of 48 herds were positive for rotavirus group A. An attempt was made to match each positive herd with 3 rotavirus-negative control herds selected from the same data base within the same year; 136 control herds were identified from the 366 negative herds. The pro portion of pigs submitted for diarrhea in each age category was approximately the same for case and control herds (Table 3). Management data information was compiled for 33 of the case herds and 92 of the control herds (Table 3). Herds were excluded from the study if the producer was out of business, the veterinarian did not respond, or the producer was identified by last name only and this name was not a unique identifier in the labo ratory records.
Table 3.
The average age of the pigs when the diarrhea began from herds that tested positive for group A rotavirus was 17.5 d (range 2 to 56 d), while the average age for pigs when the diarrhea began from herds that tested negative was 17.8 d (range 1 to 63 d) (Table 3). Case herds tended to submit more pigs postweaning (42%) than did control herds (28%) (P = 0.07). Herd size was larger (P = 0.04) in rotavirus-positive herds (495 ± 872 sows) than in rotavirus-negative herds (262 ± 347 sows) (Table 3). Pigs were weaned earlier (P = 0.04) in rotavirus-positive herds (21 ± 4.3 d) than in control herds (24 ± 7 d). Pigs raised in all-in all-out facilities were 3.4 times (95% CI 1.56, 7.28) more likely to have a positive group A rotavirus diagnosis than were pigs raised in continuous flow facilities (P = 0.0006). There were no differences in the proportions of group A-positive herds that used the rotavirus vaccine (12%) compared with the negative herds that used the rotavirus vaccine (18%). Farms with a group A rotavirus diagnosis were no more likely to commingle pigs or to use multisite production than were farms without the diagnosis.
Herds with an average weaning age less than 28 d were 3.14 times (95% CI 1.1, 8.9) more likely to have a positive group A rotavirus diagnosis than were herds with a higher weaning age (P = 0.03). Overall, herds submitting a pig with postweaning diarrhea tended to be more likely to have a positive group A rotavirus diagnosis (34%) than were herds submitting a pig for preweaning diarrhea (21%) (P = 0.13).
Producers who used multisite production weaned pigs from 14.5 d to 28 d. The average weaning age for these herds was 17.8 d and the median was 17 d. For pro ducers not using multisite production, the weaning age ranged from 11.5 d to 46 d, with an average of 24.7 d and a median of 21.5 d.
All-in all-out management and early weaning age were confounded. Herds using all-in all-out management were 5.2 times (95% CI 2.3, 11.7) more likely to wean pigs before 21 d than were herds using continuous flow management. Although the majority of multisite units used all-in all-out production (63%), 30% of farrow-to-finish operations also used all-in all-out production.
Discussion
The rate of positive group A rotavirus diagnosis in Ontario increased during this 5-year period. Producers, veterinarians, or both submitting samples after 1995 were 2.9 times more likely (P = 0.04) to receive a positive diagnosis for group A rotavirus than were those sub mitting samples in 1994 or 1995 (Table 2).
Although the average age of pigs submitted because of diarrhea in these group A rotavirus positive herds was 17.5 d, the producers indicated that pigs were affected from 2 to 56 d of age. Another study reported the average age of onset of rotavirus diarrhea to be 19 d (22). Others have shown that after the initial infection of 1 or 2 pigs in a litter, every pig in the litter was infected within 4 to 10 d (23).
Rotavirus-positive herds had more sows, in agreement with an earlier report on traditionally managed swine herds, where the incidence of preweaning diarrhea increased as herd size increased, and decreased with increasing parity (24). As with other diseases, levels of exposure and immunity to rotavirus become less uniform in sows as herd size increases. Indeed, IgA antibody in milk increases significantly for 3rd litter sows compared with gilts and 2nd litter sows (25). In a previous study, gilt litters had a higher incidence of rotavirus diarrhea (14). The incidence of preweaning diarrhea has also been shown to increase with increasing litter size and in piglets housed on solid floors with loose sows (24). We may, therefore, hypothesize that in these cases, uneven protection was provided to the piglets by their dams. This could be related to the recent expansion of many of these herds, which has resulted in a higher proportion of gilts and young parity sows. As farm sizes continue to grow larger across Ontario, we may expect to see a continuing increase in the diagnosis of rotavirus.
Case herds tended to have a higher proportion of postweaned pig submissions than did control herds. Diarrhea occurring postweaning tended to be more likely to have a group A rotavirus diagnosis than did diar rhea occurring preweaning. Pigs were weaned earlier in rotavirus-positive herds than in rotavirus-negative herds. This is in agreement with an earlier report where the inci dence of rotavirus shedding was greater in litters weaned at 2 wk of age compared with litters weaned at 3 wk (16). Lactogenic immunity is important in the control of porcine rotavirus diarrhea. Pigs weaned early do not have the benefit of protective antibodies from milk and are probably more susceptible to all diarrheal diseases, including rotavirus. Sows shed rotavirus as they approach parturition, and their piglets are exposed in the farrowing crate (19). Clinical disease develops if milk con taining maternal antibody is withdrawn before the piglet has developed active immunity. Because of the high prevalence of rotavirus in pigs weaned at 2 wk, and the high morbidity and mortality when these piglets are affected, it has been recommended that pigs should not be weaned before 3 wk of age or below a body weight of 6 to 7 kg (14).
Herds using continuous flow facilities for the nurseries were less likely to have a rotavirus diagnosis than were herds using all-in all-out production systems (P = 0.0006). It may be that nursing pigs exposed to rotavirus in the environment were able to develop an active immune response under the partial protection of maternal antibody. In this study, all-in all-out was associated with early weaning; therefore, the diagnosis of group A rotavirus was associated with a combination of early weaning and all-in all-out production.
Rotavirus diagnosis was not associated with farm type (farrow-to-finish or multisite), nor was it associated with the use of rotavirus vaccines. The reason for using the vaccine was not included in the survey. It may be that producers used the vaccine because they had had a diagnosis of rotavirus. The performance of rotavirus vaccines in studies has had mixed results (14). However, at the time of the diagnosis, rotavirus vaccine was used in only 12% of rotavirus-positive herds and 18% of the rotavirus-negative herds. A controlled vaccine field trial would be a more appropriate scientific study of the value of rotavirus vaccines.
Although we must be cognizant of submission biases to laboratories, we believe this study provides a useful examination of trends in the submission rate and the diag nosis rate for rotavirus in a 4-year period. Similarly, the associations between rotavirus diagnosis and management factors provide an indication of why the increase in the rate of diagnosis may be occurring in the swine industry in Ontario. These associations deserve further investigations under controlled research conditions.CVJ
References
- 1.Driesen SJ, Garland PG, Fahy VA. Studies on preweaning piglet diarrhea. Aust Vet J 1993;70:259–262. [DOI] [PubMed]
- 2.Fitzgerald GR, Barker T, Welter MW, Welter CJ. Diarrhea in young pigs: comparing the incidence of the five most common infectious agents. Vet Med Food Anim Pract 1988;1:80–86.
- 3.Liebler EM, Pohlenz JF, Whipp SC. Digestive system. In: Leman AD, Straw BE, Mengeling WL, D'Allaire S, Taylor DJ, eds. Diseases of Swine. 7th ed. Ames, Iowa: Iowa State Univ Pr, 1992:12–20.
- 4.Paul PS, Lyoo YS. Immunogens of rotaviruses. Vet Microbiol 1993;37:299–317. [DOI] [PubMed]
- 5.Gatti MS, Ferraz MM, Racz ML, de Castro AF. Rotavirus excretion in naturally infected pigs with and without diarrhea. Vet Microbiol 1993;37:187–190. [DOI] [PubMed]
- 6.Will LA, Paul PS, Proescholdt TA, et al. Evaluation of rotavirus infection in diarrhea in Iowa commercials pigs based on an epi demiologic study of a population represented by diagnostic laboratory cases. J Vet Diagn Invest 1994;6:416–422. [DOI] [PubMed]
- 7.Saif LJ, Theil KW. Viral diarrheas of man and animals. Boca Raton, Florida: CRC, Pr, 1990:45–47.
- 8.Geyer A, Sebata T, Peenze I, Steele A. A molecular epidemio logical study of porcine rotaviruses. J S Afr Vet Assoc 1995;66: 202–205. [PubMed]
- 9.Collins JE, Benfield DA, Duimstra JR. Comparative virulence of two porcine group-A rotavirus isolates in gnotobiotic pigs. Am J Vet Res 1989;50:827–835. [PubMed]
- 10.Shaw DP, Morehouse LG, Solorzano RF. Experimental rotavirus infection in three-week old pigs. Am J Vet Res 1989;50:1961–1965. [PubMed]
- 11.Moon HW. Comparative histopathology of intestinal infections. Adv Exp Med Biol 1997;412:1–19. [DOI] [PubMed]
- 12.Saif LJ. Enteric viral infections in pigs and strategies for induction of mucosal immunity. Adv Vet Med 1999;41:429–446. [DOI] [PMC free article] [PubMed]
- 13.Svensmark B, Askaa J, Wolstrup C, Nielsen K. Epidemiological studies of piglet diarrhea in intensively managed Danish sow herds. IV. Pathogenicity of porcine rotavirus. Acta Vet Scand 1989;30:71–76. [DOI] [PMC free article] [PubMed]
- 14.Svensmark B, Nielsen K, Dalsgaard K, Willeberg P. Epidemio logical studies of piglet diarrhea in intensively managed Danish sow herds. III Rotavirus infection. Acta Vet Scand 1989;30:63–70. [DOI] [PMC free article] [PubMed]
- 15.Zijlstra RT, Donovan SM, Odle J, Gelberg HB, Petschow BW, Gaskins HR. Protein-energy malnutrition delays small- intestinal recovery in neonatal pigs infected with rotavirus. J Nutrition 1997;127:1118–1127. [DOI] [PubMed]
- 16.Paul PS, Stevenson GW. Rotavirus and reovirus. In: Leman AD, Straw BE, Mengeling WL, D'Allaire S, Taylor DJ, eds. Diseases of Swine. 7th ed. Ames, Iowa: Iowa State Univ Pr, 1992:331–348.
- 17.Svensmark B, Nielsen K, Willeberg P, Jorsal SE. Epidemiological studies of piglet diarrhea in intensively managed Danish sow herds. II. Post-weaning diarrhea. Acta Vet Scand 1989;30:55–62. [DOI] [PMC free article] [PubMed]
- 18.Gelburg HB. Studies on the age resistance of swine to group A rotavirus infection. Vet Pathol 1992;29:161–168. [DOI] [PubMed]
- 19.Ward LA, Rich ED, Besser TE. Role of maternally derived cir culating antibodies in protection of neonatal swine against porcine group A rotavirus. J Infect Dis 1996;174:276–282. [DOI] [PubMed]
- 20.Fu ZF, Hampson DJ, Wilks CR. Transfer of maternal antibody against group A rotavirus from sows to piglets and serological responses following natural infection. Res Vet Sci 1990;48: 365–373. [PubMed]
- 21.Snedecor GW, Cochrane WG. Statistical Methods. 7th ed. Ames, Iowa: Iowa State Univ Pr, 1980;19:35–37, 349–364.
- 22.Fu ZF, Hampson DJ. Group A rotavirus excretion patterns in naturally infected pigs. Res Vet Sci 1987;43:297–300. [PubMed]
- 23.Fu ZF, Hampson J. Natural transmission of group A rotavirus within a pig population. Res Vet Sci 1989;46:312–317. [PubMed]
- 24.Svensmark B, Jorsal SE, Nielsen K, Willeberg P. Epidemiological studies of piglet diarrhea in intensively managed Danish sow herds. I. Pre-weaning diarrhea. Acta Vet Scand 1989;30:43–53. [DOI] [PMC free article] [PubMed]
- 25.Askaa J, Bloch B, Bertelsen G, Rasmussen KO. Rotavirus- associated diarrhoea in nursing piglets and detection of antibody against rotavirus in colostrum, milk and serum. Nord Vet Med 1983;35:441–447. [PubMed]