Abstract
Annexin A1 (ANXA1) inhibits NF-κB, a key regulator of inflammation, the common pathophysiological mechanism of inflammatory bowel diseases (IBD). MC-12, an ANXA1-based tripeptide, suppresses NF-κB activation. Here, we determined the efficacy of MC-12 in the control of IBD. Mice with colitis induced by dextran sodium sulfate (DSS) or 2,4,6-trinitro benzene sulfonic acid (TNBS) were treated with various doses of MC-12 administered intraperitoneally, orally or intrarectally. We determined colon length and the histological score of colitis, and assayed: in colon tissue the levels of TNF-α, IFN-γ, IL-1β, IL-6 and IL-10 by RT-PCR; prostaglandin E2 (PGE2), cytoplasmic phospholipase A2 (cPLA2) and myeloperoxidase by immunoassay; and COX-2 and NF- κB by immunohistochemistry; and in serum the levels of various cytokines by immunoassay. In both models MC-12: reversed dose-dependently colonic inflammation; inhibited by up to 47% myeloperoxidase activity; had a minimal effect on cytoplasmic phospholipase A2; reduced significantly the induced levels of TNF-α, IFN-γ, IL-1β, IL-6 and IL-10, returning them to baseline. DSS and TNBS markedly activated NF-κB in colonic epithelial cells and MC-12 decreased this effect by 85.8% and 72.5%, respectively. MC-12 had a similar effect in cultured NCM460 normal colon epithelial cells. Finally, MC-12 suppressed the induction of COX-2 expression, the level of PGE2 in the colon and PGE2 metabolite in serum. In conclusion, MC-12, representing a novel class of short peptide inhibitors of NF-κB, has a strong effect against colitis in two preclinical models recapitulating features of human IBD. Its mechanism of action is complex and includes pronounced inhibition of NF-κB. MC-12 merits further development as an agent for the control of IBD.
Introduction
Inflammatory bowel diseases (IBD) are a set of complex, life-long and for some patients devastating diseases, for which there is no satisfactory treatment [1]–[3]. There are two distinct clinical entities, ulcerative colitis and Crohn’s disease. A shared clinical manifestation of them is ulcerations in the intestinal mucosa; Crohn’s disease can affect the entire digestive tract while ulcerative colitis affects only the colon. The treatment of IBD has been based on anti-inflammatory medications, with steroids having been the mainstay of treatment for years [4], [5]. The prime limitations of anti-inflammatory medications are variable efficacy and side effects, which, in the case of steroids, can limit dosing or duration of treatment or force physicians to altogether discontinue them. The recently introduced biological agents have also significant limitations, and, in addition, high cost [6]. It is clear that there is a pressing need for new agents for the control of the clinical manifestations of IBD.
Inflammation is the underlying theme in IBD (hence the word inflammatory in their name). A key regulator of inflammation is NF-κB, a transcription factor that is normally sequestered in the cytoplasm [7]. When activated, NF-κB translocates into the nucleus where it regulates the expression of a multitude of genes related to inflammation. We have recently unraveled the connection between glucocorticoids and NF-κB and have proposed a novel mechanism by which they act [8]. Briefly, we have demonstrated that glucocorticoids induce the expression of annexin A1 (ANXA1), which then binds to the p65 subunit of NF-κB, inhibiting its activation. There is a nearly perfect correlation between the anti-inflammatory potency of the various steroids and the induction of ANXA1, on one hand, and the suppression of NF-κB on the other. Short (around 20 amino acids) C-terminal fragments of ANXA1 are known to have many of its biological activities [9], [10].
We have synthesized MC-12, a tripeptide based on the structure of ANXA1 that is as effective as ANXA1 in suppressing NF-κB activation. This peptide is representative of several such ANXA1 peptides that inhibit NF-κB [8]. We assessed its potential efficacy in colitis using two mouse models of IBD, one based on the administration of dextran sulfate sodium (DSS) and the other on trinitrobenzene sulfonic acid (TNBS) [11], [12]. Our data demonstrate that administration of MC-12 reverses in a dose-dependent manner the inflammatory reaction of the colon and prevents the development of ulcerations. There were no apparent side effects from the administration of MC-12 to mice. MC-12 modulates several inflammatory mediators, including NF-κB and several cytokines.
Results
MC-12 Reduces Experimental Colitis in Mice
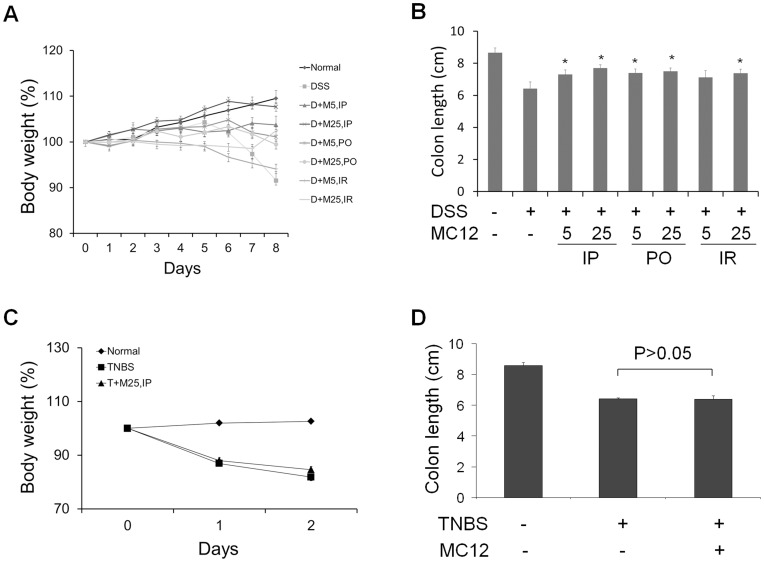
By day 8, mice receiving DSS lost on average 8.4% of their baseline body weight (Fig. 1A). MC-12 given by IP and PO at both doses and by IR at the higher dose prevented such weight loss in a dose-dependent manner, with those receiving the highest IP dose of MC-12 (25 mg/kg), showing 7.7% increase in their body weight compared to baseline. Of note, MC-12 was more effective in preventing weight loss when given IP than PO. Fig. 1B shows the length of the colon of these mice. Compared to normal control, the colon of DSS-treated mice was shorter by 2.3 cm (8.7±0.29 vs. 6.4±0.42 cm; mean ± SEM, for this and all subsequent values; p<0.01). Administration of MC-12 prevented dose-dependently most of this reduction in colon length, with IP, PO and IR administration producing essentially identical results; under treatment, the length of the colon ranged between 7.3±0.29 and 7.7±0.20 cm (p<0.05 for all differences from vehicle control). Macroscopically, shortened colons showed wall edema and fewer feces in the lumen. We also studied colitis induced by TNBS in SJL/J mice. The optimal dose of TNBS for our study was 100 µl of a 2.5% ethanolic solution instilled intracolonically. As shown in Fig. 1C, TNBS reduced the body weight of the animals on day 3 by 18.2% and shortened the length of the colon by 25% (Fig. 1D), compared to baseline. MC-12 25 mg/kg failed to prevent the weight loss (84.7±1.1 vs. 81.8±1.3% in vehicle-treated controls, p>0.05) and the shortening of the colon (6.4±0.20 vs. 6.4±0.07 in vehicle-treated controls, p>0.05).
Figure 1. The effect of MC-12 on DSS- and TNBS-induced colitis in mice.
Mice (7/group) received 2% DSS in drinking water or 2.5% TNBS intra-colonically to induce colitis and were treated with vehicle or MC-12 given IP, PO or IR. A: The body weight of mice of DSS model during treatment by IP, PO or IR, expressed as percentage of baseline (day 0). B: The colon length of DSS model with MC-12 treatments by IP, PO or IR. C: The body weight of mice of TNBS model during treatment, expressed as percentage of baseline (day 0). D: The colonic length of mice of TNBS model in three treatment groups. These studies were repeated at least once giving similar results. Values are mean ± SEM. *, statistically significant difference from the vehicle-treated group.
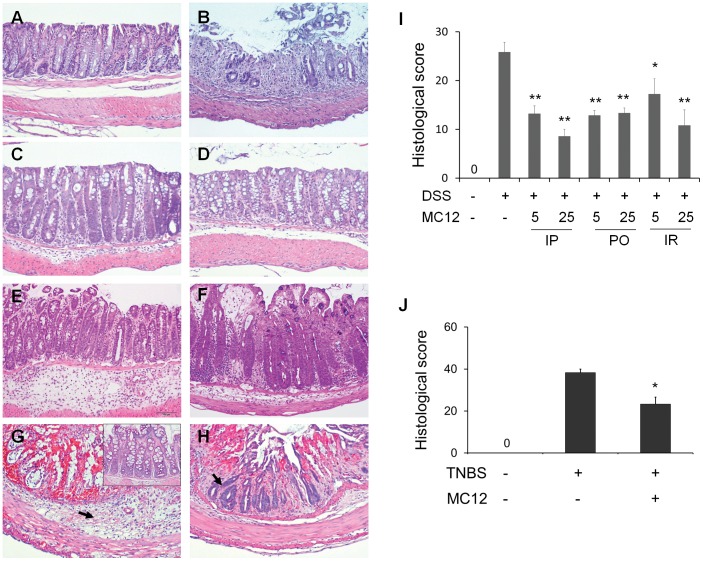
As expected, DSS induced colitis in these mice. The histological sections shown in Fig. 2A–F demonstrate changes in the colonic mucosa by DSS, including significant inflammation, accumulation of mucus and development of ulcers. The granulocytes present in the mucosa establish the development of acute inflammation. Treatment with MC-12 reduced the degree of inflammation, essentially restoring the integrity of the mucosa. After treatment with DSS the histological colitis score became 25.8±2.01 from 0 in normal controls (Fig. 2I). MC-12 reduced the histological colitis score dose-dependently, regardless of its route of administration. Compared to vehicle-treated controls, MC-12 given IP reduced the histological score by 48.9% at 5 mg/kg (13.2±1.62 vs. 25.8±2.01, p<0.01) and 66.8% at 25 mg/kg (8.6±1.43 vs. 25.8±2.01, p<0.01). The dose of 25 mg/kg showed a lower histological score than the dose of 5 mg/kg (8.6±1.43 vs. 13.2±1.62; p<0.05). Given orally, MC-12 was slightly less effective, reducing this score by 50.2% at 5 mg/kg (12.9±1.01 vs. 25.8±2.01, p<0.01) and 48.4% at 25 mg/kg (13.3±1.05 vs. 25.8±2.01, p<0.01). Similarly, the IR administration of MC-12 decreased the histological score by 33.3% (p<0.05) at the dose of 5 mg/kg and 58.1% (p<0.01) at the dose of 25 mg/kg compared to DSS control group. Regardless of its lacking effect on body weight and colon length in the TNBS model, MC-12 had a significant anti-inflammatory effect on the colonic mucosa as shown in Fig. 2G–H, reducing the histological score by 39.1%, compared to TNBS control as shown in Fig. 2J (38.3±1.7 vs. 23.3±3.3; p<0.01).
Figure 2. MC-12 ameliorates colitis induced by both DSS and TNBS.
Paraffin sections of colonic tissues were stained with hematoxylin & eosin and their histological scores were determined as in Methods. A: Normal mucosa of the C57BL/6 mouse. B: Severe inflammation including infiltration by inflammatory cells, edema, loss of crypts and ulcerations are seen in a DSS + vehicle-treated mouse. C, D: MC-12, 5 or 25 mg/kg, IP, significantly decreased DSS-induced colonic inflammation. E: Treatment with MC-12, 25 mg/kg PO, decreased DSS-induced colonic inflammation. F: Treatment with MC-12, 25 mg/kg, IR, markedly reduced DSS-induced colitis. G: Colonic mucosa from a TNBS vehicle-treated mouse, showing severe crypt loss and inflammatory cell infiltration (arrow). Inset: normal mucosa of a healthy SJL/J mouse. H: Treatment with MC-12 25 mg/kg for 2 days decreased the inflammatory cell infiltration and crypt loss (arrow indicates remaining crypts). I, J: The histological score of the various study groups of both DSS- and TNBS-induced colitis. Values are mean ± SEM. H&E staining; magnification 100x.
MC-12 is not Cytotoxic to Normal Colonic Epithelial Cells
MC-12 failed to induce cytotoxicity to the NCM460 normal colonic epithelial cell line. The 24-h IC50 value of MC-12 was higher than 5 mM; higher concentrations were not studied. This concentration far exceeds concentrations of MC-12 that inhibited, for example, the activation of NF-κB (low µM range; shown below).
The Constituent Amino Acids of MC-12 Have No Effect on Colitis
Since peptides are subject to hydrolytic cleavage of their peptide bonds, especially when administered orally, we examined whether MC-12 acts against colitis after its potential degradation to its three constituent amino acids. To this end, we treated mice with DSS-induced colitis using a solution containing the three amino acids of MC-12 (Ac-Gln, Ala and Trp) at equimolar concentrations. We treated mice with DSS-colitis following the same protocol as in the previous studies. The amino acid solution was given ip at a dose equivalent to 25 mg/kg of intact MC-12. The amino acid solution had no significant effect on any of the three parameters that we evaluated: body weight (98.8% vs. 96.7%), colon length (6.5±0.13 vs. 6.2±0.17) and histological score (19.9±2.0 vs. 24.8±1.9); all differences were statistically not significant.
MC-12 Reduces DSS- and TNBS-induced Inflammation in Colonic Mucosa: Effects on MPO, cPLA2 and Cytokines
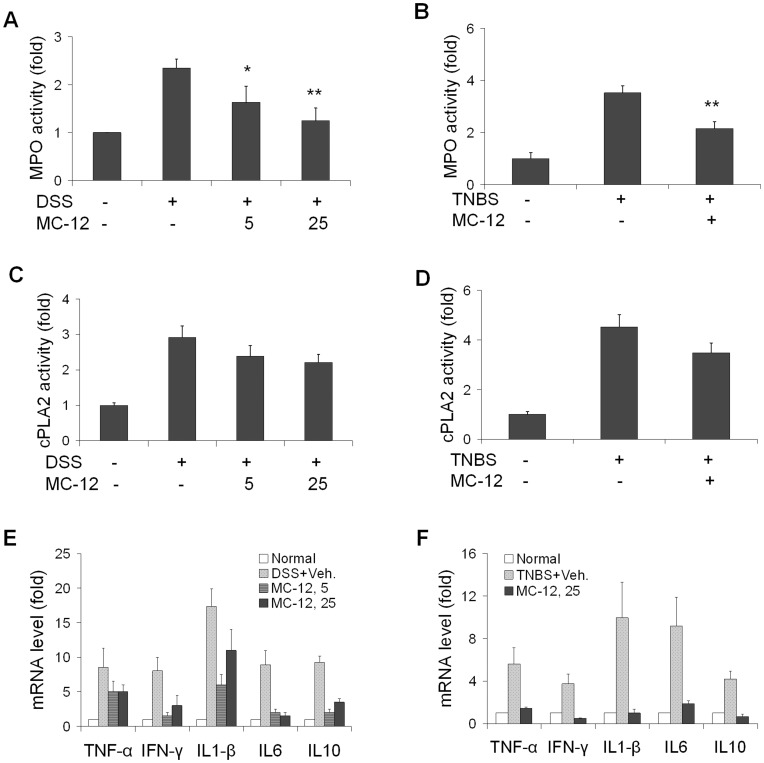
To assess the effect of MC-12 on the inflammatory changes associated with experimental colitis, we determined in colon tissue samples the activity of myeloperoxidase and cytosolic phospholipase A2 (cPLA2) as well as the response of five inflammatory cytokines. MPO activity is an indicator of the degree of acute inflammation in a given tissue [13]. cPLA2, a phospholipase recognizing the sn-2 acyl bond of phospholipids, releases lysophospholipid and arachidonic acid, which can then be converted to prostaglandins and leukotrienes, both inflammatory mediators [14]–[16]. Finally, the pro-inflammatory cytokines TNF-α, IFN-γ, IL-1β and IL-6, and anti-inflammatory cytokine IL-10 have been implicated in experimental and human colitis [17]. Indeed, cytokines are thought to orchestrate the development, recurrence and exacerbation of IBD [18].
Fig. 3 summarizes our findings. DSS and TNBS increased MPO activity 2.4- and 3.5-fold compared to normal controls. In both models, MC-12 inhibited the enhanced MPO activity significantly and in a dose-dependent manner. This reduction was 30% (p<0.05) and 47% (p<0.01) at 5 mg/kg and 25 mg/kg, respectively, in the DSS model and 39% (p<0.01) in the TNBS model.
Figure 3. The effect of MC-12 on parameters of inflammation.
MPO (A, B), cPLA2 (C, D) activity and the mRNA levels of proinflammatory and anti-inflammatory cytokines (E, F) were measured in colon tissue samples of both DSS- and TNBS-induced colitis mouse models. In both models MC-12 significantly inhibited MPO activity and the mRNA levels of all cytokines but not cPLA2 activity. Values are mean ± SEM. *p<0.05 compared to vehicle-treated group, **p<0.01 compared to vehicle-treated group.
Similar to MPO, the activity of cPLA2 in colon mucosa was increased 2.9-fold by DSS and 4.5-fold by TNBS compared to normal mice. The effect of MC-12 on cPLA2 activity was modest and statistically not significant; in the three MC-12 treatment groups, the reduction in cPLA2 activity ranged between 18% and 24%.
We also determined the response of the cytokines TNF-α, IFN-γ, IL-1β, IL-6 and IL-10 in the colon by measuring their corresponding mRNA levels using real-time PCR. As shown in Fig. 4E–F, compared to controls, DSS increased the mRNA levels of all these cytokines by 9- to 17-fold and TNBS by 4- to 10-fold. Treatment with MC-12 at either dose significantly reduced the mRNA levels of TNF-α, IL-1β, IFN-γ, IL-6 and IL-10,
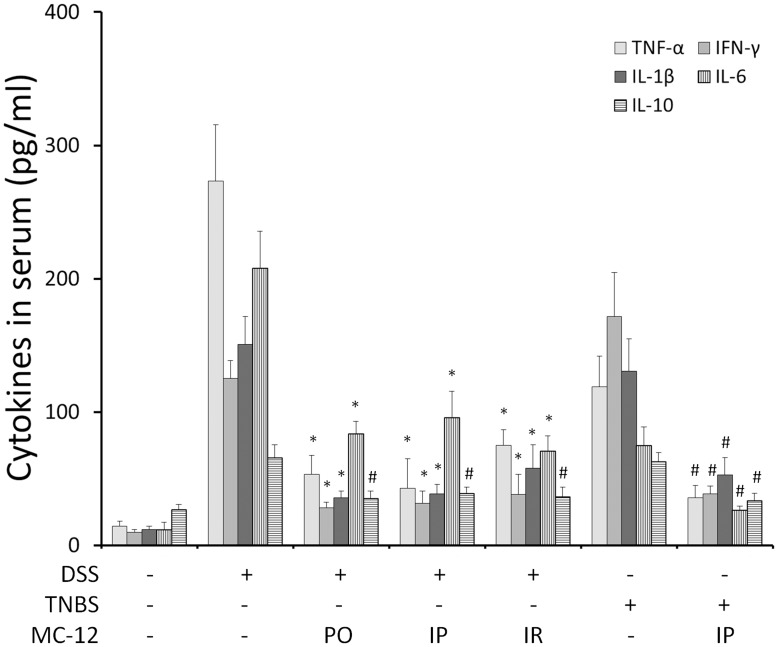
Figure 4. MC-12 reduces the serum levels of cytokines.
The serum levels of TNF-α, IFN-γ, IL-1β, IL-6 and IL-10 were measured by ELISA as in Methods. Both DSS and TNBS induced their levels and treatments with MC-12, 25 mg/kg administered PO, IP or IR reduced these levels 1.6- to 5.1-fold. *p<0.01, #p<0.05 compared to vehicle-treated controls.
Next, we measured the levels of these cytokines in the serum of the experimental animals. As shown in Fig. 4, DSS or TNBS induced the TNF-α, IFN-γ, IL-1β, IL-6, and IL-10 by 2.4- to 19-fold. Treatment with MC-12 administered PO, IP or IR significantly reduced by 1.7- to 6.4-fold the levels of all these cytokines.
MC-12 Inhibits the Activation of NF-κB in vivo and in vitro
The chronic mucosal inflammation in IBD is characterized by hyperactivation of effector immune cells, which produce high levels of pro-inflammatory cytokines, resulting in colonic tissue damage. NF-κB, the master regulator of all inflammation [19], [20], has been identified as a key regulator in this immunological setting. Its activation, markedly induced in IBD patients, strongly influences the course of mucosal inflammation [21], [22]. NF-κB is also the molecular target of the activity of MC-12 [8]. Therefore, we determined by immunohistochemistry the level of NF-κB activation in the colonic mucosa of our mice, using an antibody recognizing the phosphorylation of ser276 of NF-κB’s p65 subunit. As a methodological control, we also showed marked activation of NF-κB in colon samples from patients with ulcerative colitis using the same primary antibody and method (Figure S1) [23].
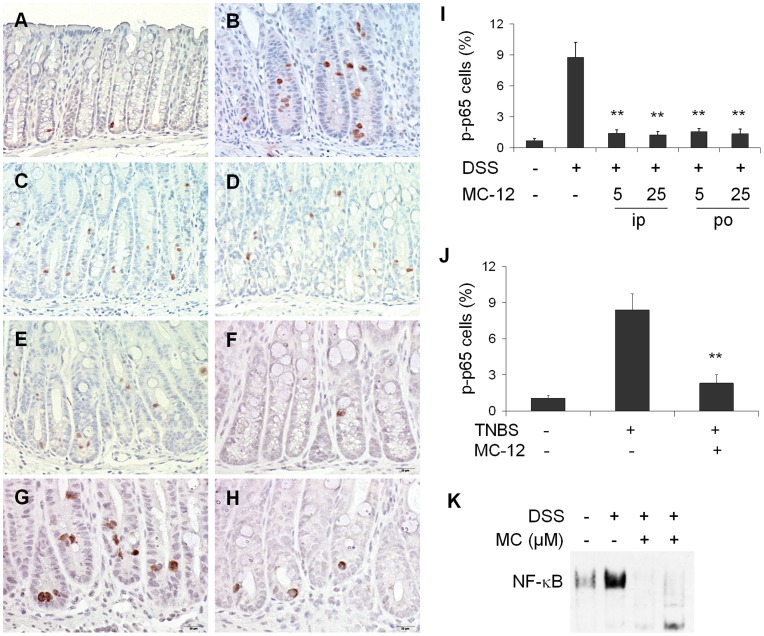
As shown in Fig. 5A–E, there was minimal baseline activation of NF-κB in the colon of normal animals. DSS activated NF-κB in colonic epithelial cells (0.7±0.20 vs. 8.8±1.45, p<0.01), in agreement with previous reports [24]. MC-12 reversed this effect nearly completely (Fig. 5I), regardless of its route of administration: at the highest dose (25 mg/kg) MC-12 decreased this effect of DSS by 85.8% (8.8±1.45 vs. 1.2±0.32; p<0.01). Fig. 5F–H demonstrates that TNBS activated NF-κB in the colonic epithelium; as shown in Fig. 5J, the percentage of cells with activated NF-κB is 8.4±1.32 in TNBS-treated mice vs. 1.1±0.25 in controls (p<0.01). Treatment with MC-12 inhibited the TNBS-induced NF-κB activation by 72.5% (2.3±0.70 vs. 8.4±1.32, p<0.01).
Figure 5. MC-12 inhibits NF-κB activation induced by DSS and TNBS in vivo and in vitro.
Colon tissue sections were immunohistochemically stained with anti-phospho-NF-κB p65 antibody. Normal colon mucosa from C57BL/6 mice (A) or from SJL/J mice (F) shows a few cells with p-p65 nuclear positive staining. Colon mucosa with DSS (B) or TNBS (G) shows increased NF-κB nuclear-positive cells, most of which are crypt epithelial cells. Markedly less p-p65 nuclear translocation was shown in colon mucosa from DSS model treated with MC-12, 5 mg/kg ip (C), 25 mg/kg ip (D) or 25 mg/kg po (E) or from TNBS model treated with MC-12 25 mg/kg ip (H). Changes in p-p65 nuclear positive, evident in the photos are quantified (I, J). DSS increased NF-κB-DNA binding in NCM460 cells determined by EMSA, and MC-12 30 and 300 µM significantly blocked this effect (K). Values are mean ± SEM. **p<0.01 compared to vehicle-treated group. IHC staining; magnification 200x.
The inhibitory effect of MC-12 on NF-κB was also documented in cultured NCM460 cells (normal colon epithelial cells). As expected [25]–[27], DSS significantly increased NF-κB-DNA binding activity in the NCM460 cells. MC-12 at concentrations of 30 µM and 300 µM essentially eliminated this NF-κB activation (Fig. 5K).
MC-12 Inhibits the Induction of COX-2 and Decreases PGE2 Levels in vivo
The eicosanoid cascade seems to be involved in the pathogenesis of IBD but there is some controversy regarding its specific role. COX-2 is induced in experimental colitis [28]–[30] whereas NSAIDs are thought to exacerbate colitis in humans [31]. Thus we evaluated the effect of MC-12 on COX-2 expression and PGE2 levels in colonic tissue and its metabolite 13,14-dihydro-15-keto prostaglandin E2 in the serum of mice.
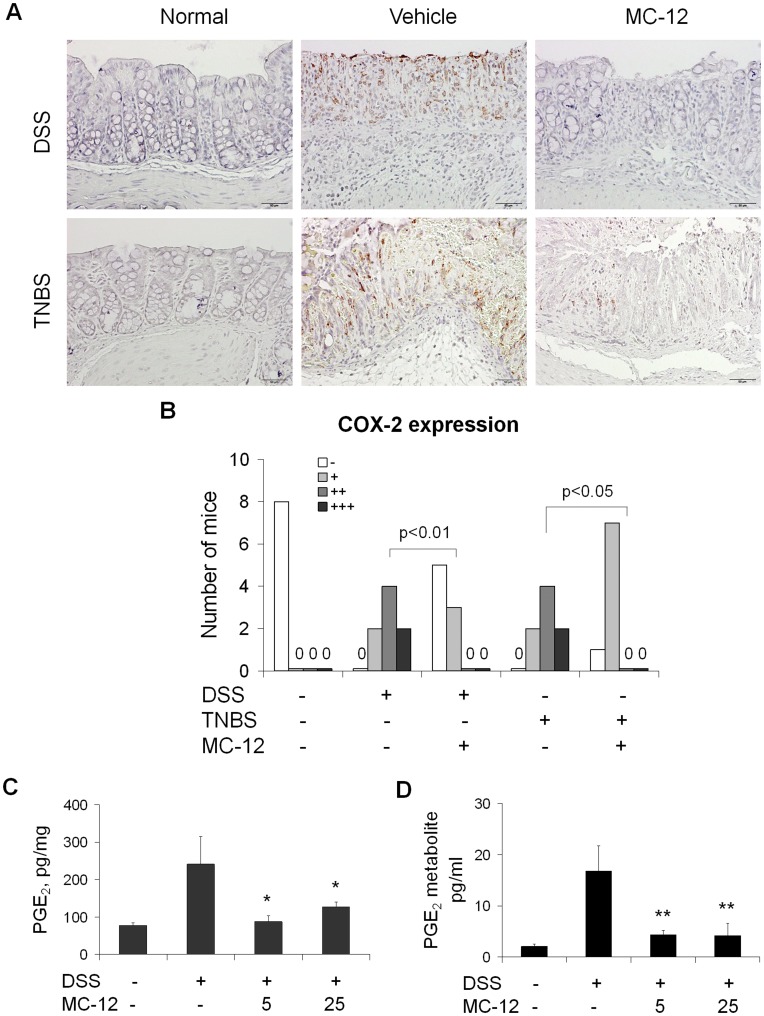
As shown in Fig. 6, in both models of experimental colitis MC-12 markedly reduced the expression of COX-2 in the colonic mucosa (Fig. 6A and B). In addition it decreased the levels of PGE2 in colonic mucosa (87.9±15.8 or 127.0±12.5 vs. 241.4±73.7, p<0.05, Fig. 6C) and PGE2 metabolite in serum (4.3±0.9 or 4.2±2.4 vs. 16.8±4.9, p<0.01, Fig. 6D).
Figure 6. MC-12 inhibits COX-2 induction and decreases PGE2 levels in DSS- and TNBS-induced colitis. A:
Representative photomicrographs of tissue sections with COX-2 immunohistochemical staining on colon tissue from normal (no treatment), vehicle- or MC-12-treated mice from the DSS (upper panels) or TNBS (lower panel) groups. MC-12 treatment: 25 mg/kg ip. B: The COX-2 expression scores in the various groups of animals (n = 8/group). Differences were evaluated by Pearson’s χ2 method. C, D: MC-12 decreased the levels of PGE2 in colonic mucosa and of PGE2 metabolite in serum. Values are mean ± SEM. **p<0.01 compared to vehicle-treated group, *p<0.05 compared to vehicle-treated group.
Discussion
Our data demonstrate the strong anti-inflammatory effect of the novel tripeptide MC-12 in two models of experimental colitis. MC-12, designed as an inhibitor of NF-κB based on a recently unraveled mechanism of NF-κB control, had a profound inhibitory effect on NF-κB activation and on a circuitry of dependent inflammatory mediators.
Experimental models of colitis enable us to not only study its pathogenetic components during the various phases of colitis but also to assess the therapeutic efficacy of experimental agents [12]. The two murine models of colitis that we employed in these studies represent two different entities. The DSS model, technically a model of chemical injury to the colon, recapitulates features of ulcerative colitis, including severe leukocyte infiltration, crypt damage, and tissue edema often accompanied by severe ulceration. The TNBS model, on the other hand, has many of the characteristic features of Crohn’s disease in humans such as severe transmural inflammation. Both are associated with weight loss and gastrointestinal manifestations.
As expected, in both models we observed significant weight loss and decreased colon length, both of which were reversed by MC-12 in DSS-treated mice, either totally or mostly, depending on MC-12’s dose. Interestingly, MC-12 failed to reverse these changes in the TNBS model. Regardless, however, of its effects on body weight and colon length, MC-12 displayed a strong anti-inflammatory effect in both models. The inhibition of inflammation reached nearly 70% in DDS-treated mice and almost 40% in TNBS-treated mice. The dose of MC-12 was relevant as was its route of administration. In the DSS model in which two drug doses and two routes of administration were evaluated, the dose effect was unmistakable and the ip route was modestly better than the oral route. The latter may well reflect a greater chance to hydrolyze MC-12 as it travels down the alimentary canal. Our data confirmed that fully hydrolyzed, MC-12 is rendered ineffective; in a reconstitution experiment the three amino acids of MC-12 were administered orally but had no effect on colitis. Indirect as this result may be, one cannot fail to surmise that properly formulated the MC-12 tripeptide may have even higher efficacy in colitis.
Even simple inspection of the tissue sections of the colon makes it clear that MC-12 acts as an anti-inflammatory agent; our data have objectively and quantitatively confirmed this. In addition to histological scoring, in both models MC-12 significantly reduced MPO activity in the colonic mucosa. MPO, an enzyme contained in lysosomes of neutrophils and, to a much lesser extent, of monocytes and tissue macrophages, has been used as a neutrophil and monocyte marker to evaluate the presence and extent of inflammation in colonic mucosa [13], [32].
We explored the mechanism of the anti-inflammatory action of MC-12. The a priori molecular target of MC-12 is NF-κB. MC-12 is, however, a peptide derived from ANXA1. Since ANXA1 inhibits phospholipase A2 activity as part of its anti-inflammatory action [33], [34] and PLA2 activity is increased in IBD colonic mucosa [35], we studied the potential effect of MC-12 on PLA2. Although we demonstrated 3–5 fold enhanced activity of PLA2 in the colitic mucosa, MC-12 did not affect it, ruling out such a mechanism of action. It is likely that the anti-PLA2 property of ANXA1 lies outside the area corresponding to the tripeptide.
NF-κB plays a critical role in the pathophysiology of IBD. NF-κB is markedly activated in the inflamed gut, especially in macrophages and epithelial cells, to the point that the degree of NF-κB activation correlates with the severity of intestinal inflammation [36]. Through its extensive transcriptional activity, NF-κB is thought to trigger proinflammatory loops involving cytokines and eicosanoids. Indeed, given its strategic position in the inflammatory process of IBD, NF-κB is considered an excellent target for the development of pharmacological agents for IBD [21].
Our data, showing profound inhibition of NF-κB by MC-12 confirmed our initial expectation [8]. Indeed, MC-12 inhibited the activation of NF-κB by 86% in the DSS model and by 72% in the TNBS model. In addition, the in vitro study using normal colonocytes in which NF-κB was activated by DSS generated congruent results, leaving little doubt as to the specificity of the effect of MC-12. NF-κB is apparently very relevant to the pathogenesis if IBD. Several studies have documented in both animal models of colitis and in humans with IBD the brisk activation of NF-κB; our control experiment with human samples generated equally impressive results.
MC-12 had a dual effect on the eicosanoid cascade. First, it suppressed the expression of COX-2, which was induced in both models of colitis. And, second, it reduced the production of PGE2; it is unclear if the reduced PGE2 levels reflect exclusively the suppressive effect of MC-12 on COX-2 expression, the enzyme that catalyzes a crucial step in prostaglandin biosynthesis. The effect of MC-12 on PGE2 is consistent with its general role in inflammation. Whether the effect of MC-12 on COX-2 is a result of its effect on NF-κB or a direct effect is uncertain; NF-κB is known to transcriptionally regulate the expression of COX-2 [37], [38].
In summary, MC-12 markedly suppressed inflammation in both DSS- and TNBS-induced colitis in mice predominantly or exclusively by inhibiting NF-κB activation. Given the magnitude of its effect, the plausibility of its proposed mechanism of action and early evidence for safety, MC-12 merits further development as an agent for the control of IBD.
Materials and Methods
Reagents and Cell Culture
MC-12 (Ac-Gln-Ala-Trp) was custom-synthesized by GenScript (Piscataway, NJ). NCM460 cell line (purchased from Incell Corporation, LLC, San Antonio, TX), which was derived from normal human colon mucosal epithelium, was grown in M3:10A media (Incell Corporation, LLC, San Antonio, TX). Cells (3×106) were seeded in 100 mm dishes and after 24 h test compounds were added into the media for another 24 h, when cell nuclei were isolated for EMSA analysis.
Cell Viability
NCM460 cells were seeded into 96-well plates at 2×104 cells per well. After overnight incubation, they were treated for 24 h with various concentrations of MC-12 (25 µM – 5 mM). Cell viability was determined by a modified colorimetric assay using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT). Briefly, the culture medium was removed and replaced with 100 µl of complete medium containing 0.5 mg/ml MTT. After a 4-h incubation at 37°C, 100 µl of a solution containing 10% SDS and 0.01 N HCl were added. The plate was incubated until MTT formazan crystals were dissolved. Absorbance at 570 nm was measured on a microplate reader and the IC50 was calculated after subtraction of blank values.
Experimental Animals
Female C57BL/6 and SJL/J mice (Taconic, Hudson, NY), 7–9 weeks old, were kept under controlled temperature (25°C) with a 12/12-hour light-dark cycle and free access to a standard diet and drinking water. The mice were allowed to acclimate for 7 days before the start of experiments. Our studies were approved by the Institutional Animal Care and Use Committee of Stony Brook University.
DSS- and TNBS-induced Colitis, MC-12 Treatment, and Histological Evaluation
DSS model
Forty nine C57BL/6 mice were divided into 7 groups (7 mice per group). The mice received 2% dextran sulfate sodium (DSS, MW 36,000 to 50,000, MP Biomedicals, Solon, OH) in drinking water for 8 days [39]; control mice received regular drinking water. During the period when DSS was administered, mice were given MC-12, 5 or 25 mg/kg, by intraperitoneally (IP), oral gavage (PO) or intrarectally (IR), whereas the control group was given normal saline. The mice were weighed and monitored daily for rectal bleeding or prolapse. All mice were euthanized at the end of the study period. Blood samples were collected and colons were dissected and their length was measured. The colon was frozen for molecular analyses except for the distal 3 cm, which were fixed in 4% neutralized formalin, cut into six equal fragments, dehydrated and embedded into paraffin. Cross sections of the colon were stained with hematoxylin and eosin (H&E). In these sections, we determined the histological score by the degree (0–3) and extent (0–3) of inflammation, crypt damage (0–4) and the area involved (0–4) as described by Dieleman et al [40]. The score of each of the first three parameters was multiplied by the fourth and the sum of these three multiples was the final score (ranging from 0 to 40).
TNBS model
Total 21 SJL were divided into 3 groups, 7 mice per group. The mice received 100 µl of 2.5% TNBS solution in 50% ethanol by intra-colonic instillation using a 3.5 F catheter, which was inserted 4 cm into the colon under mild ketamine/xylazine anesthesia. Mice received MC-12 25 mg/kg or vehicle ip once a day for two days. Body weight was monitored daily and mice were euthanized on the third day when blood and colon tissues were collected and processed as described above. Cross sections of colon tissue were stained with H&E, and the histological score was determined as in the DSS model.
ELISA
The serum levels of TNF-α, IFN-γ, IL-1β, IL-6 and IL-10 were measured using the Milliplex Map kit (EMD Millipore, Billerica, MA) that following the manufacturer’s instructions. Briefly, 25 µl frozen serum were incubated with 25 µl magnetic beads and 25 µl assay buffer overnight at 4°C. After washing with a Hand-Held Mag Plate Washer (Affymetrics, Santa Clara, CA), the beads were incubated at room temperature with 25 µl biotinylated secondary antibody and 25 µl streptavidin-phycoerythrin for 1 hr each. Fluorescence intensity was determined using the Bio-Plex 200 System (Bio-Rad, Hercules, CA) with a low PMT calibration. All samples were run in duplicate and analyzed on the same day.
Myeloperoxidase Activity
MPO activity was measured using a commercial kit and following the instructions of the manufacturer (Invitrogen, Eugene, OR). Briefly, a portion of colon tissue was homogenized in PBS, centrifuged at 10,000×g for 15 min and 50 µl of the supernatant from each sample were added into a 96-well microplate. Then we added 50 µl of 2X APF working solution to all samples and standard wells and incubated the plate at room temperature for 30 min. We stopped the reaction by adding 10 µl of 10X chlorination inhibitor. We measured the fluorescence intensity using a Multiplate Reader (Molecular Devices, Sunnyvale, CA) with excitation at 485 nm and emission at 530 nm [41].
Cytosolic Phospholipase A2 (cPLA2) Activity
We determined cPLA2 activity using the cPLA2 assay kit and following the instructions of the manufacturer (Cayman Chemical, Ann Arbor, MI). Briefly, a portion of colon tissue was homogenized in cold PBS and centrifuged at 10,000×g for 15 min and 10 µl of supernatant from each sample and 5 µl assay buffer were added into the wells of a 96-well microplate. We initiated the reaction by adding 200 µl substrate solution to all wells and incubating for 5 min at room temperature. The fluorescence intensity was measured using a Multiplate Reader (Molecular Devices, Sunnyvale, CA) with excitation at 485 nm and emission at 530 nm [33].
PGE2 Competitive Enzyme Immunoassay (EIA)
PGE2 level in colon tissue and PGE2 metabolites in serum were extracted according to manufacturer’s protocol and measured using the PGE2 EIA Kit (#514010, Cayman Chemical, Ann Arbor, MI) and PGE metabolite EIA kit (#514531, Cayman Chemical, Ann Arbor, MI), respectively. Concentrations (pg/ml) were estimated from the absorbance of the calculated standard curve. Results were calculated using the Cayman Chemical computer spreadsheet.
Immunohistochemistry
Immunohistochemical staining for phospho-NF-κB (activated form of NF-κB) and cyclooxygenase-2 (COX-2) was performed on colon tissue samples. Briefly, paraffin-embedded sections (4 µm thick) were deparaffinized, rehydrated, and microwave-heated for 15 min in 0.01 mol/L citric buffer (pH 6.0) for antigen retrieval. Then, 3% hydrogen peroxide was applied to block endogenous peroxidase activity. After 30 min of blocking with normal serum (Invitrogen, Carlsbad, CA), the primary rabbit anti-phospho-NF-κB p65 ser276 antibody (henceforth p-p65, Cell Signaling, Danvers, MA) or rabbit anti-COX-2 polyclonal antibody (Cayman Chemical, Ann Arbor, MI) or the corresponding control isotype IgG were applied and incubated overnight at 4°C. Slides were washed thrice with PBS, each for 5 min. The biotinylated secondary antibody and the streptavidin-biotin complex were applied, each for a 60 min incubation at room temperature. After rinsing with PBS, the slides were immersed for 10 min in 3,3′-diaminobenzidine (Sigma, St. Louis, MO) solution (0.4 mg/mL, with 0.003% hydrogen peroxide), monitored under the microscope and the reaction was terminated with distilled water. Slides were then counterstained with hematoxylin, dehydrated, and coverslipped. Five fields per section were photographed and the percentage of positive cells in colonic epithelium was determined [42]. The intensity of cytoplasmic staining of COX-2 was assessed semi-quantitatively as previously described [43]: negative: no staining or <10% positive cells; 1+: weak staining or 10–25% positive cells; 2+: moderate staining or 25–50% positive cells; 3+: strong staining or >50% positive cells.
Electrophoretic Mobility Shift Assay (EMSA)
After the indicated treatment, cell nuclear fractions were isolated from 3×106 cells as previously described [44]. The NF-κB activity was determined using the LightShift chemiluminescent EMSA kit (Thermo Fisher Scientific, Rockford, IL) and following the manufacturer’s instructions. Briefly, the nuclear extracts were incubated with biotin-labeled DNA probes (5′-AGTTGAGGGGACTTTCCCAGGC-3′) at 37°C for 20 min, then loaded onto the polyacrylamide gel and transferred to a nylon membrane. The membrane was exposed to UV-light for 10 min for cross-linking of the transferred DNA, incubated with stabilized streptavidin-horseradish peroxide conjugate in blocking buffer for 15 min and covered with substrate working solution, followed by exposure to X-ray film.
Real-time Quantitative PCR
About 100 mg of colon tissue were placed in 1 ml of cold Trizol (Invitrogen Life Technologies, Carlsbad, CA), homogenized immediately with a rotor power homogenizer, and RNA was extracted following the manufacturer’s instructions. Total RNA was retrotranscribed with M-MLV reverse transcriptase (Invitrogen Life Technologies, Carlsbad, CA) using random primers. Real-time quantitative PCR was performed in a CFD-3200 Opticon detector (BioRad, Hercules, CA) using QuantiTect SYBR Green PCR Kit (Qiagen, Valencia CA). The PCR cycling conditions were: 40 cycles of 60 seconds at 94°C, 30 seconds at 51.4°C and 30 seconds at 72°C. PCR primers (forward and reverse primers) were designed based on published sequences: TNF-α: AGGCTGCCCCGACTACGT and GACTTTCTCCTGGTATGAGATAGCAAA; IFN-γ: CAGCAACAGCAAGGCGAAA and CTGGACCTGTGG GTTGTTGAC; IL-1β: TCGCTCAGGGTCACAAGAAA and CATCAGAGGCAAGGAGGAAAAC; IL-6: ACAAGTCGGAGGCTTAATTACACAT and ATGTGTAATTAAGCCTCCGACTTGT; IL-10: ATGCTGCCT GCTCTTACTGACTG and TTGCCATTGCACAACTCTTTTC; β-actin: AGATTACTGCTCTGGCTCCTA and CAAAGAAAGGGTGTAAAACG. Relative expression levels of mRNA were normalized to β-actin.
Statistics
Data were expressed as mean ± SEM and analyzed by comparing means with One-Way ANOVA using the SPSS program (Version 11.5.0). An additional post hoc test for multiple comparisons was performed. p<0.05 denotes statistically significant differences. The comparison between groups for COX-2 IHC staining was performed using Pearson’s χ2 method.
Supporting Information
Human colon tissue sections stained immunohistochemically with an anti-p-p65 antibody. A: Normal colon crypt epithelial cells show minimal to no staining for p-p65. B: Colonic mucosa with colitis showing markedly increased p-p65 staining, almost exclusively nuclear, in both epithelial and interstitial cells. C. The results of the “ % positive cells for p-p65” in 15 normal and 21 colitis samples stained with p-p65. Values are mean ± SEM. The difference between two groups is statistical highly significant; p<0.0001.
(TIF)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by National Institutes of Health grants: R01CA139454 and R01CA09242308. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Vatn MH. Natural history and complications of IBD. Curr Gastroenterol Rep. 2009;11:481–487. doi: 10.1007/s11894-009-0073-8. [DOI] [PubMed] [Google Scholar]
- 2.Mayer L. Evolving paradigms in the pathogenesis of IBD. J Gastroenterol. 2010;45:9–16. doi: 10.1007/s00535-009-0138-3. [DOI] [PubMed] [Google Scholar]
- 3.Szigethy E, McLafferty L, Goyal A. Inflammatory bowel disease. Child Adolesc Psychiatr Clin N Am 19: 301–318, ix. 2010. [DOI] [PubMed]
- 4.Devlin SM, Panaccione R. Evolving inflammatory bowel disease treatment paradigms: top-down versus step-up. Gastroenterol Clin North Am. 2009;38:577–594. doi: 10.1016/j.gtc.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Benchimol EI, Seow CH, Steinhart AH, Griffiths AM. Traditional corticosteroids for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008. CD006792. [DOI] [PMC free article] [PubMed]
- 6.de Silva S, Devlin S, Panaccione R. Optimizing the safety of biologic therapy for IBD. Nat Rev Gastroenterol Hepatol. 2010;7:93–101. doi: 10.1038/nrgastro.2009.221. [DOI] [PubMed] [Google Scholar]
- 7.Campbell KJ, Perkins ND. Regulation of NF-kappaB function. Biochem Soc Symp. 2006. pp. 165–180. [DOI] [PubMed]
- 8.Zhang Z, Huang L, Zhao W, Rigas B. Annexin 1 induced by anti-inflammatory drugs binds to NF-kappaB and inhibits its activation: anticancer effects in vitro and in vivo. Cancer Res. 2010;70:2379–2388. doi: 10.1158/0008-5472.CAN-09-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perretti M, D’Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9:62–70. doi: 10.1038/nri2470. [DOI] [PubMed] [Google Scholar]
- 10.Perretti M, Gavins FN. Annexin 1: an endogenous anti-inflammatory protein. News Physiol Sci. 2003;18:60–64. doi: 10.1152/nips.01424.2002. [DOI] [PubMed] [Google Scholar]
- 11.Wirtz S, Neurath MF. Mouse models of inflammatory bowel disease. Adv Drug Deliv Rev. 2007;59:1073–1083. doi: 10.1016/j.addr.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Kawada M, Arihiro A, Mizoguchi E. Insights from advances in research of chemically induced experimental models of human inflammatory bowel disease. World J Gastroenterol. 2007;13:5581–5593. doi: 10.3748/wjg.v13.i42.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roncucci L, Mora E, Mariani F, Bursi S, Pezzi A, et al. Myeloperoxidase-positive cell infiltration in colorectal carcinogenesis as indicator of colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2008;17:2291–2297. doi: 10.1158/1055-9965.EPI-08-0224. [DOI] [PubMed] [Google Scholar]
- 14.Perez-Chacon G, Astudillo AM, Balgoma D, Balboa MA, Balsinde J. Control of free arachidonic acid levels by phospholipases A2 and lysophospholipid acyltransferases. Biochim Biophys Acta. 2009;1791:1103–1113. doi: 10.1016/j.bbalip.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Haeggstrom JZ, Rinaldo-Matthis A, Wheelock CE, Wetterholm A. Advances in eicosanoid research, novel therapeutic implications. Biochem Biophys Res Commun. 2010;396:135–139. doi: 10.1016/j.bbrc.2010.03.140. [DOI] [PubMed] [Google Scholar]
- 16.Gustafson-Svard C, Lilja I, Sjodahl R, Tagesson C. Cytosolic phospholipase A2 and cyclooxygenase-2 mediate release and metabolism of arachidonic acid in tumor necrosis factor-alpha-primed cultured intestinal epithelial cells (INT 407). Scand J Gastroenterol. 1995;30:1000–1007. doi: 10.3109/00365529509096345. [DOI] [PubMed] [Google Scholar]
- 17.Fantini MC, Pallone F. Cytokines: from gut inflammation to colorectal cancer. Curr Drug Targets. 2008;9:375–380. doi: 10.2174/138945008784221206. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 2008;14:4280–4288. doi: 10.3748/wjg.14.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 20.Danese S, Mantovani A. Inflammatory bowel disease and intestinal cancer: a paradigm of the Yin-Yang interplay between inflammation and cancer. Oncogene. 2010;29:3313–3323. doi: 10.1038/onc.2010.109. [DOI] [PubMed] [Google Scholar]
- 21.Atreya I, Atreya R, Neurath MF. NF-kappaB in inflammatory bowel disease. J Intern Med. 2008;263:591–596. doi: 10.1111/j.1365-2796.2008.01953.x. [DOI] [PubMed] [Google Scholar]
- 22.Karrasch T, Jobin C. NF-kappaB and the intestine: friend or foe? Inflamm Bowel Dis. 2008;14:114–124. doi: 10.1002/ibd.20243. [DOI] [PubMed] [Google Scholar]
- 23.Andresen L, Jorgensen VL, Perner A, Hansen A, Eugen-Olsen J, et al. Activation of nuclear factor kappaB in colonic mucosa from patients with collagenous and ulcerative colitis. Gut. 2005;54:503–509. doi: 10.1136/gut.2003.034165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takada Y, Ray N, Ikeda E, Kawaguchi T, Kuwahara M, et al. Fos proteins suppress dextran sulfate sodium-induced colitis through inhibition of NF-kappaB. J Immunol. 2010;184:1014–1021. doi: 10.4049/jimmunol.0901196. [DOI] [PubMed] [Google Scholar]
- 25.Herfarth H, Brand K, Rath HC, Rogler G, Scholmerich J, et al. Nuclear factor-kappa B activity and intestinal inflammation in dextran sulphate sodium (DSS)-induced colitis in mice is suppressed by gliotoxin. Clin Exp Immunol. 2000;120:59–65. doi: 10.1046/j.1365-2249.2000.01184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marrero JA, Matkowskyj KA, Yung K, Hecht G, Benya RV. Dextran sulfate sodium-induced murine colitis activates NF-kappaB and increases galanin-1 receptor expression. Am J Physiol Gastrointest Liver Physiol. 2000;278:G797–804. doi: 10.1152/ajpgi.2000.278.5.G797. [DOI] [PubMed] [Google Scholar]
- 27.Bhattacharyya S, Dudeja PK, Tobacman JK. ROS, Hsp27, and IKKbeta mediate dextran sodium sulfate (DSS) activation of IkappaBa, NFkappaB, and IL-8. Inflamm Bowel Dis. 2009;15:673–683. doi: 10.1002/ibd.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hendel J, Nielsen OH. Expression of cyclooxygenase-2 mRNA in active inflammatory bowel disease. Am J Gastroenterol. 1997;92:1170–1173. [PubMed] [Google Scholar]
- 29.Paiotti AP, Miszputen SJ, Oshima CT, de Oliveira Costa H, Ribeiro DA, et al. Effect of COX-2 inhibitor after TNBS-induced colitis in Wistar rats. J Mol Histol. 2009;40:317–324. doi: 10.1007/s10735-009-9243-0. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka K, Suemasu S, Ishihara T, Tasaka Y, Arai Y, et al. Inhibition of both COX-1 and COX-2 and resulting decrease in the level of prostaglandins E2 is responsible for non-steroidal anti-inflammatory drug (NSAID)-dependent exacerbation of colitis. Eur J Pharmacol. 2009;603:120–132. doi: 10.1016/j.ejphar.2008.11.058. [DOI] [PubMed] [Google Scholar]
- 31.Kefalakes H, Stylianides TJ, Amanakis G, Kolios G. Exacerbation of inflammatory bowel diseases associated with the use of nonsteroidal anti-inflammatory drugs: myth or reality? Eur J Clin Pharmacol. 2009;65:963–970. doi: 10.1007/s00228-009-0719-3. [DOI] [PubMed] [Google Scholar]
- 32.Barros KV, Xavier RA, Abreu GG, Martinez CA, Ribeiro ML, et al. Soybean and fish oil mixture increases IL-10, protects against DNA damage and decreases colonic inflammation in rats with dextran sulfate sodium (DSS) colitis. Lipids Health Dis. 2010;9:68. doi: 10.1186/1476-511X-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SW, Rhee HJ, Ko J, Kim YJ, Kim HG, et al. Inhibition of cytosolic phospholipase A2 by annexin I. Specific interaction model and mapping of the interaction site. J Biol Chem. 2001;276:15712–15719. doi: 10.1074/jbc.M009905200. [DOI] [PubMed] [Google Scholar]
- 34.Lim LH, Pervaiz S. Annexin 1: the new face of an old molecule. Faseb J. 2007;21:968–975. doi: 10.1096/fj.06-7464rev. [DOI] [PubMed] [Google Scholar]
- 35.Peterson JW, Dickey WD, Saini SS, Gourley W, Klimpel GR, et al. Phospholipase A2 activating protein and idiopathic inflammatory bowel disease. Gut. 1996;39:698–704. doi: 10.1136/gut.39.5.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogler G, Brand K, Vogl D, Page S, Hofmeister R, et al. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115:357–369. doi: 10.1016/s0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- 37.Lee KM, Kang BS, Lee HL, Son SJ, Hwang SH, et al. Spinal NF-kB activation induces COX-2 upregulation and contributes to inflammatory pain hypersensitivity. Eur J Neurosci. 2004;19:3375–3381. doi: 10.1111/j.0953-816X.2004.03441.x. [DOI] [PubMed] [Google Scholar]
- 38.Newton R, Kuitert LM, Bergmann M, Adcock IM, Barnes PJ. Evidence for involvement of NF-kappaB in the transcriptional control of COX-2 gene expression by IL-1beta. Biochem Biophys Res Commun. 1997;237:28–32. doi: 10.1006/bbrc.1997.7064. [DOI] [PubMed] [Google Scholar]
- 39.Clapper ML, Cooper HS, Chang WC. Dextran sulfate sodium-induced colitis-associated neoplasia: a promising model for the development of chemopreventive interventions. Acta Pharmacol Sin. 2007;28:1450–1459. doi: 10.1111/j.1745-7254.2007.00695.x. [DOI] [PubMed] [Google Scholar]
- 40.Dieleman LA, Palmen MJ, Akol H, Bloemena E, Pena AS, et al. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moreels TG, Nieuwendijk RJ, De Man JG, De Winter BY, Herman AG, et al. Concurrent infection with Schistosoma mansoni attenuates inflammation induced changes in colonic morphology, cytokine levels, and smooth muscle contractility of trinitrobenzene sulphonic acid induced colitis in rats. Gut. 2004;53:99–107. doi: 10.1136/gut.53.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin YZ, Yao SY, Veach RA, Torgerson TR, Hawiger J. Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J Biol Chem. 1995;270:14255–14258. doi: 10.1074/jbc.270.24.14255. [DOI] [PubMed] [Google Scholar]
- 43.Hori Y, Oda Y, Kiyoshima K, Yamada Y, Nakashima Y, et al. Oxidative stress and DNA hypermethylation status in renal cell carcinoma arising in patients on dialysis. J Pathol. 2007;212:218–226. doi: 10.1002/path.2176. [DOI] [PubMed] [Google Scholar]
- 44.Williams JL, Ji P, Ouyang N, Liu X, Rigas B. NO-donating aspirin inhibits the activation of NF-kappaB in human cancer cell lines and Min mice. Carcinogenesis. 2008;29:390–397. doi: 10.1093/carcin/bgm275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Human colon tissue sections stained immunohistochemically with an anti-p-p65 antibody. A: Normal colon crypt epithelial cells show minimal to no staining for p-p65. B: Colonic mucosa with colitis showing markedly increased p-p65 staining, almost exclusively nuclear, in both epithelial and interstitial cells. C. The results of the “ % positive cells for p-p65” in 15 normal and 21 colitis samples stained with p-p65. Values are mean ± SEM. The difference between two groups is statistical highly significant; p<0.0001.
(TIF)