Abstract
Wall teichoic acid (WTA) or related polyanionic cell wall glycopolymers are produced by most Gram-positive bacterial species and have been implicated in various cellular functions. WTA and the proton gradient across bacterial membranes are known to control the activity of autolysins but the molecular details of these interactions are poorly understood. We demonstrate that WTA contributes substantially to the proton-binding capacity of Staphylococcus aureus cell walls and controls autolysis largely via the major autolysin AtlA whose activity is known to decline at acidic pH values. Compounds that increase or decrease the activity of the respiratory chain, a main source of protons in the cell wall, modulated autolysis rates in WTA-producing cells but did not affect the augmented autolytic activity observed in a WTA-deficient mutant. We propose that WTA represents a cation-exchanger like mesh in the Gram-positive cell envelopes that is required for creating a locally acidified milieu to govern the pH-dependent activity of autolysins.
Introduction
The bacterial cell envelope governs vital processes including maintenance of cell shape, cell division, and protection against environmental challenges. In addition, it has a crucial role in the respiratory energy metabolism because the cytoplasmic membrane harbors the electron transport chain components, which generate a proton gradient across the membrane that is used to generate ATP or energize transport processes [1], [2].
The majority of Gram-positive bacteria contain polyanionic cell wall glycopolymers (CWGs) in their cell envelopes, which are covalently linked to either peptidoglycan (e.g. wall teichoic acid [WTA] or teichuronic acid) or membrane glycolipids (e.g. lipoteichoic acid [LTA] or succinylated lipoglycans) [3]. Several roles in the protection and maintenance of Gram-positive cell envelopes and in bacteria-host interaction have been assigned to CWGs after WTA or LTA-deficient mutants became available in Staphylococcus aureus and Bacillus subtilis [4]–[6]. Moreover, the cell envelope of B. subtilis cells has been shown to be protonated during respiratory metabolism [7], [8] and the polyanionic CWGs have been implicated in cation binding [9], [10]. However, it has remained unclear if the proton-binding capacity of WTA may impact on the pH-sensitive activity of cell wall-associated enzymes such as autolysins.
Peptidoglycan is built from long glycan strands that are cross-linked by peptide side chains. Peptidoglycan must be continuously synthesized to maintain cell integrity and viability and to allow for cell division. Peptidoglycan hydrolytic autolysins are critical for separating daughter cells after cell division [11], [12]. The maintenance of the peptidoglycan network requires a fine-tuned spatial and temporal control of autolysins to prevent suicidal cell death [13]. Yet, surprisingly little is known about the control of autolysins and the underlying regulatory principles are only superficially understood. Inactivation of AtlA, the major autolysin in S. aureus, or of the corresponding AtlE of Staphylococcus epidermidis is not lethal but the mutants form huge cell clusters as a result of incomplete cell separation [14]. While studying the role AtlA in S. aureus we observed that a WTA-deficient ΔtagO mutant (ΔtagO) [15] is much more susceptible to autolysis than the wild type when autolysins were artificially activated by washing the bacteria in buffer with low ionic strength [16]. We have recently demonstrated that WTA prevented AtlA binding to peptidoglycan in the bacterial side walls but not in the septum where WTA appeared to be less abundant or to have an altered structure. Similar observations have been made with the cell wall-binding LysM domain-containing autolysins LytF of B. subtilis [11] and Sle1 (or Aaa) and LytN of S. aureus [17]. Moreover, several studies have documented that dissipation of the membrane proton gradient leads to autolysin activation in Gram-positive bacteria [18], [19] but the molecular basis of this phenomenon has remained unclear.
Here, we show that WTA contributes substantially to the concentration of proton-binding sites in the cell wall and impacts on S. aureus autolysis largely via the major autolysin AtlA. Compounds that modulate the activity of the respiratory chain, a main source of protons in the cell wall, affected autolysis in the presence of WTA but not in its absence, which supports the notion that WTA contributes to the control of autolysin activity by governing the abundance of protons in the cell wall.
Results
The proton-binding capacity of WTA in the S. aureus cell wall
We hypothesized that the polyanionic properties of WTA may contribute to the binding of protons thereby affecting the local pH in the cell wall. In addition to WTA the cell envelopes of Gram-positive bacteria contain other negatively charged residues in LTA, peptidoglycan, phospholipid head groups, and surface-associated proteins. To assess if WTA contributes significantly to the overall density of negative charges in the cell envelope, the proton-binding capacities of S. aureus wild type and isogenic WTA-deficient tagO mutant (ΔtagO) were determined by Fourier Transform Infrared (FTIR) spectroscopy and a whole-cell titration approach.
Phosphate groups were abundant in wild type cell envelopes but below the FTIR detection limit in ΔtagO. Accordingly, the proton-binding sites in the mutant were 23% reduced compared to the wild type (Table 1), which confirms that WTA contributes substantially to the proton-binding capacity of the S. aureus cell envelope. ΔtagO complemented with a plasmid-encoded copy of tagO had the same proton-binding capacity as the wild type indicating that the difference between wild type and ΔtagO resulted from its inability to synthesize WTA. Since the abundance of protons in the cell envelope might affect the proton gradient across the cytoplasmic membrane the membrane potential of wild type, ΔtagO, and complemented mutant was measured by monitoring the distribution of the small lipophilic cation [3H]tetraphenylphosphonium bromide ([3H]TPP+). The three strains had similar membrane potentials around −120 mV (Tab. 1) indicating that WTA-dependent proton binding in the cell wall hardly impacts on the abundance of protons at the membrane surface.
Table 1. Proton-binding sites and phosphate groups in the cell envelope and membrane potential of S. aureus strains.
| Strain | Proton-binding site concentrations (µmol/mg bacteria) | Detection of phosphate group absorption bands by FTIR (cm−1) | Membrane potential (mV) |
| Wild type | 35.6 | 1. C-O-P-O-C stretch (1061 cm−1)a2. PO2 − antisymmetric stretch (1235 cm−1)a | −123 |
| ΔtagO | 27.5 | Bdb | −120 |
| ΔtagO complemented | 35.4 | Ndc | −122 |
, Assignment was made according to those reported previously in [42].
, Bd, below detection limit.
, Nd, not determined.
WTA affects autolysis via AtlA
The finding that WTA has a strong impact on the abundance of protons in the cell wall suggested that the activity of pH-sensitive cell-wall associated enzymes such as autolysins might be affected by the presence or absence of WTA. In line with this notion WTA has been shown to affect the activity of autolysins [20], [21]. Activity of AtlA, the major S. aureus autolysin, has its optimal activity at neutral pH and drops strongly at pH values below 6.5 [22] suggesting that its activity might be controlled by the presence of WTA.
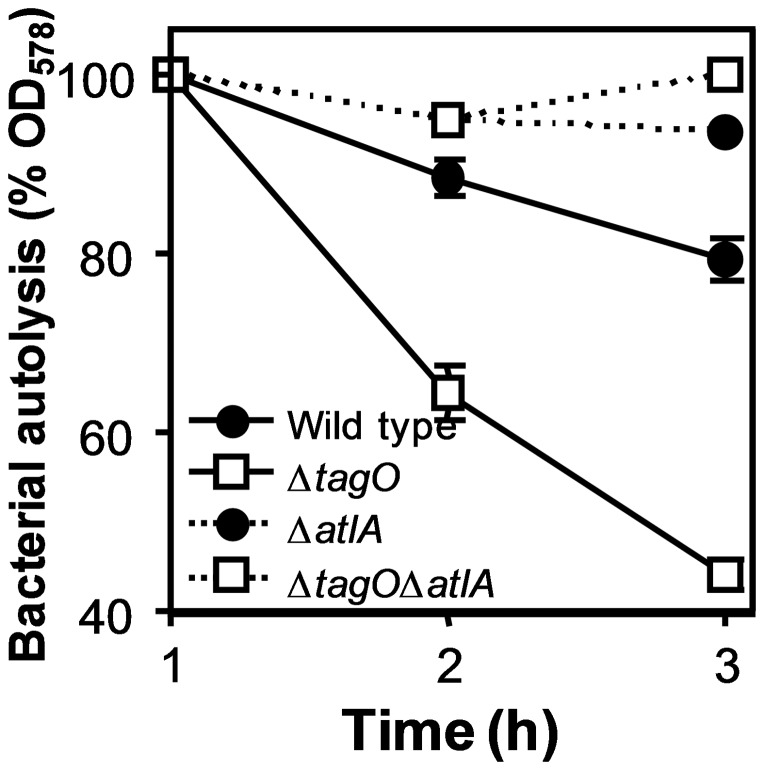
The autolytic activity of a WTA-deficient mutant ΔtagO followed a much faster kinetic than that of wild type or complemented ΔtagO in the absence of Triton X-100 (Fig. 1) or in its presence as described earlier [20]. In order to analyze if WTA deficiency impacts on autolysis via AtlA or via activation of one of the other autolysins atlA and tagO deletions were combined in strain ΔtagOΔatlA. This double mutant exhibited strongly reduced autolysis reaching a similar level as in the WTA-containing atlA-deficient strain indicating that WTA governs autolysis largely via AtlA.
Figure 1. Increased autolysis of S. aureus ΔtagO depends on the major autolysin AtlA.
AtlA-expressing or deficient strains are indicated by continuous or dotted lines, respectively. Means and SEM of 3 independent experiments are shown.
The respiratory chain affects autolysin activity in a WTA-dependent manner
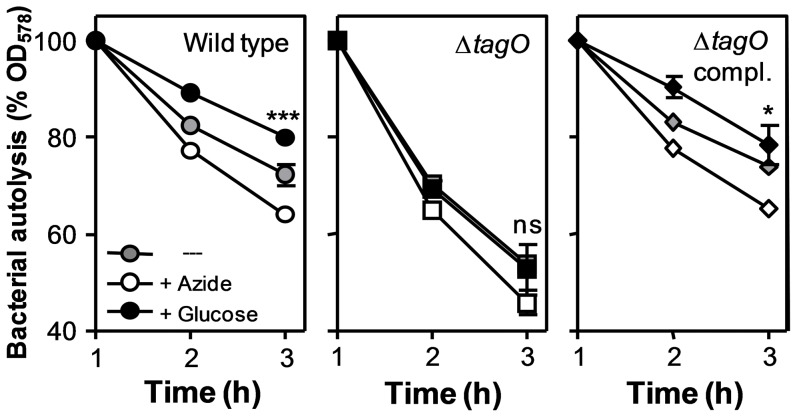
How the respiratory activity affects autolysis in S. aureus has remained elusive. We hypothesized that changes in the membrane proton gradient might lead to local pH changes that govern the pH-sensitive activity of AtlA in a WTA-dependent manner. In order to elucidate how the respiratory chain affects AtlA activity, autolysis was monitored in S. aureus cells whose proton gradient was dissipated by addition of the respiratory inhibitor azide with that of cells exposed to a high concentration of glucose (1%) to augment respiration.
In fact, azide led to an increase of autolysis while 1% glucose caused a further inhibition of autolysis of S. aureus wild type (Fig. 2), confirming that the respiratory capacity has a considerable impact on S. aureus autolysis. In contrast, the augmented autolysis rate of ΔtagO remained largely unchanged by the addition of azide or glucose indicating that respiration can only impact on autolysis when WTA is present. The complemented mutant behaved like the wild type (Fig. 2). These findings are in agreement with our hypothesis that AtlA activity is controlled by the local cell wall pH in dependence of the capacity of WTA to retain respiratory chain-derived protons.
Figure 2. Autolysis can be increased by addition of azide or inhibited by addition of glucose in WTA-containing but not in WTA-deficient S. aureus.
Means and SEM of 3 independent experiments are shown. Between-group differences were analyzed for significance by one-way ANOVA analysis. *, p<0.05; ***, p<0.001; ns, not significant.
Discussion
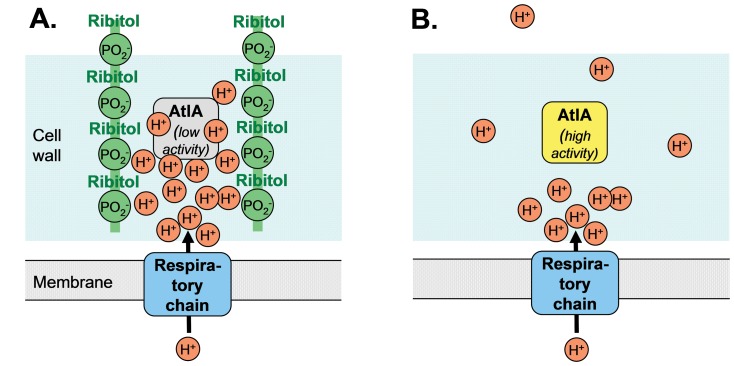
We provide evidence that the WTA polymers, which consist of a negatively charged backbone contribute substantially to the proton-binding capacity of the S. aureus cell wall. WTA is thought to act like a cation exchanger that retains protons in the cell wall (Fig. 3). It has been known for long that WTA and the membrane proton gradient can affect the autolytic activity of Gram-positive bacteria [18], [19] but it has remained mysterious if and how these phenomena are connected. It has recently been demonstrated that the presence of WTA reduces the affinity of AtlA and of other autolysins for S. aureus peptidoglycan and that AtlA binds preferentially to the cell division site where WTA appears to be less abundant or not yet fully matured [16], [17]. Accordingly, AtlA is evenly distributed on the surface of ΔtagO [16], which helps to understand the increased autolysin activity of ΔtagO compared to the parental strain. However, it does not explain why compounds that increase or decrease the membrane proton gradient also affect the activity of autolysins.
Figure 3. Model for the role of WTA in proton binding and control of autolysin activity.
(A) The negatively charged WTA phosphate groups retain protons in the cell wall, which creates an acidic environment keeping the activity of the major autolysin AtlA low. (B) In the absence of WTA protons are not retained, which avoids local acidification and leads to higher activity of AtlA.
Here, we demonstrate that S. aureus autolysis is sensitive to the activity of the respiratory chain in a WTA-dependent manner. A high membrane proton gradient probably causes local acidification (Fig. 3). Since AtlA, the major staphylococcal autolysin, exhibits reduced activity at pH values below 6.5 [22] autolysis may remain low during efficient respiratory growth in cell wall areas of high WTA abundance. Thus, WTA appears to control AtlA activity not only by preventing its binding but also by creating an acidic milieu that limits AtlA activity. The lower abundance of WTA in the septum [16] may help to recruit AtlA to the site of cell division and activate it by avoiding local acidification. It remains to be analyzed whether the two ways of how WTA controls autolysin activity are equally important for the control of autolysis in S. aureus or other Gram-positive bacteria. In addition to WTA additional mechanisms may contribute to proton gradient-dependent autolysin regulation in S. aureus. The membrane potential has recently been shown to govern the capacity of the LytSR two-component regulatory system to activate the cid and lrg operons encoding holins and anti-holins that also control autolysin activity [23].
WTA has many different functions including the targeting and control of peptidoglycan-biosynthetic proteins [24], [25], resistance to harmful molecules such as antimicrobial fatty acids [26], antimicrobial peptides [27], and lysozyme [28], interaction with inanimate surfaces to initiate biofilm formation [29], binding of cognate phages [30]–[32], and interaction with host receptors [33], [34]. Its additional role in governing proton binding and autolysin activity may explain its widespread occurrence and usually polyanionic nature. Non phosphate-containing but equally anionic cell wall glycopolymers such as the glucuronic acid-containing teichuronic acids found e.g. in bacilli [35], the succinylated CWGs of actinobacteria [36], or the pyruvylated CWGs of Bacillus anthracis [37] may have roles similar to those of the bona fide teichoic acids. Moreover, it is likely that the membrane-anchored LTA also contributes to the proton-scavenging capacities of Gram-positive bacteria [6]. Future studies are necessary to elucidate if our findings in S. aureus hold true for other Gram-positive bacteria and if WTA or WTA-biosynthetic enzymes do in fact represent attractive targets for new antibacterial strategies.
Materials and Methods
Bacteria, plasmids, and growth conditions
S. aureus strain SA113 and its derivatives ΔtagO and ΔatlA have been described recently [16], [33]. To construct a ΔatlAΔtagO double mutant the complemented ΔtagO was infected with phage Φ11 and the mutation was transduced to the ΔatlA strain. Colonies were screened for erythromycin and spectinomycin resistance with chloramphenicol sensitivity. The correct deletion of tagO in ΔatlA was confirmed by DNA sequencing. The WTA-deficient ΔtagO mutant was complemented using plasmid pRB474-SD-tagO, which was constructed by PCR-amplifying tagO from strain SA113 with optimized Shine-Dalgarno sequence and subcloning the amplicon into the pRB474 expression vector [38] to allow tagO expression from its constitutive veg promoter. All strains were grown in B medium (1% casein peptone, 0.5% yeast extract, 0.5% NaCl, 0.1% K2HPO4×3 H20, 0.1% glucose) containing appropriate antibiotics when necessary unless otherwise noted.
FTIR spectroscopy
FTIR spectra were recorded using a Bruker Vertex 80v FTIR spectrometer. The spectral resolution of the spectrometer was set to 2 cm-1 and 256 interferograms with an optical range of 4000 to 400 cm−1. To prepare S. aureus wild type and ΔtagO samples for FTIR measurement, 2 mg of freeze-dried bacterial sample were homogeneously mixed with 250 mg of dry potassium bromide (99.9%, Sigma-Aldrich) to prepare a KBr pellet. The samples were freeze-dried in order to remove water for FTIR measurements. Slight changes in structure duo to this procedure can, however, not be ruled out.
Acid-base titrations and modeling of titration data
Bacterial cells were grown until exponential phase and washed twice using ultrapure water. Cells were lyophilized and resuspended in 45 ml of 0.01 M NaNO3 background electrolyte at a concentration of 2 mg/ml for titration experiments. A 40 ml aliquot of the bacterial suspension was then transferred to a 150 ml Metrohm glass titration vessel. Bacterial suspensions were acidified to pH 3 and then covered with an air-tight lid fitted with an N2 gas line interface to prevent carbonate formation during the course of the titration and a pH electrode connected to an autotitrator (Metrohm Titrando 806) to monitor changes in H+ activity as a function of added titrant. The titrator settings were programmed as described previously [39], [40].
To account for the deprotonation of bacterial cell surface functional groups as a function of increasing solution pH the following dissociation mechanism is proposed: HL = L−+H+, where L− corresponds to a proton-binding functional group on the cell surface and H+∼H3O+ represents the hydronium ion species in solution. The apparent equilibrium constant for the proton dissociation mechanism described previously, Ka, can be defined as function of measured, [H+], and adjustable, [L−], parameters as follows: Ka = [H+].[L−]/[HL] where [H+] refers to the proton concentration calculated by the relationship [H+] = {H+} γH where, {H+} is the proton activity measured using the pH electrode, and γH is the proton activity coefficient for I = 0.01 M (KNO3). A multi-site Langmuir isotherm coupled to a linear programming optimization method (LPM) was used to calculate the concentration of functional groups on the cell surface and generate pKa (−log Ka) spectra where pKa is a measure of functional group acidity as described previously [39].
Membrane potentials
were determined with the small lipophilic cation [3H]TPP+ whose equilibrium across the cytoplasmic membrane is indicative of membrane potential as described previously [41]. Briefly, the test strains were grown in half-concentrated Muller-Hinton broth to an OD600 of 1, harvested, and then resuspended 1∶3 in fresh medium. [3H]TPP+ (25 Ci/mmol; Hartmann Analytic, Braunschweig, Germany) was added to a final concentration of 1 µCi/ml to cell suspensions and incubated for 10 min. Subsequently, aliquots were filtered onto 0.2-µm-pore-size cellulose acetate membranes (Schleicher & Schuell, Dassel, Germany) and washed twice with 50 mM phosphate buffer (pH 7.0). The filters were dried and radioactivity was measured using a Packard 1900CA liquid scintillation counter in the presence of Filtersafe (Zinsser Analytic, Frankfurt, Germany) scintillant.
Autolysis assays
Autolysis assays were performed essentially as described recently [16] with some modification. Briefly, cells were grown until exponential phase, washed twice with PBS at room temperature, and resuspended in prewarmed PBS at an optical density of 0.25 at 578 nm. Optical density was measured at hourly intervals for 3 h at 37°C. Cellular respiration was restored by addition of 1% glucose or inhibited using 50 mM sodium azide as indicated.
Acknowledgments
The authors would like to thank Marcus Nowak for the use of his FTIR equipment.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the German Research Foundation grants TRR34 to A.P. and F.G. and SFB766 to A.P., G.X., and F.G.; by the Bacteria Materials Interaction graduate programme of the University of Tübingen to A.P. and A.K.; and by a fellowship from the Humboldt Foundation to R.M. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb Perspect Biol. 2010;2:a000414. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Price CE, Driessen AJ. Biogenesis of membrane bound respiratory complexes in Escherichia coli. Biochim Biophys Acta. 2010;1803:748–766. doi: 10.1016/j.bbamcr.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 3.Weidenmaier C, Peschel A. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. NatRevMicrobiol. 2008;6:276–287. doi: 10.1038/nrmicro1861. [DOI] [PubMed] [Google Scholar]
- 4.Swoboda JG, Campbell J, Meredith TC, Walker S. Wall teichoic acid function, biosynthesis, and inhibition. Chembiochem. 2010;11:35–45. doi: 10.1002/cbic.200900557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia G, Kohler T, Peschel A. The wall teichoic acid and lipoteichoic acid polymers of Staphylococcus aureus. Int J Med Microbiol. 2009;300:148–154. doi: 10.1016/j.ijmm.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Reichmann NT, Grundling A. Location, synthesis and function of glycolipids and polyglycerolphosphate lipoteichoic acid in Gram-positive bacteria of the phylum Firmicutes. FEMS Microbiol Lett. 2011;319:97–105. doi: 10.1111/j.1574-6968.2011.02260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calamita HG, Ehringer WD, Koch AL, Doyle RJ. Evidence that the cell wall of Bacillus subtilis is protonated during respiration. Proc Natl Acad Sci U S A. 2001;98:15260–15263. doi: 10.1073/pnas.261483798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calamita HG, Doyle RJ. Regulation of autolysins in teichuronic acid-containing Bacillus subtilis cells. Mol Microbiol. 2002;44:601–606. doi: 10.1046/j.1365-2958.2002.02872.x. [DOI] [PubMed] [Google Scholar]
- 9.Heptinstall S, Archibald AR, Baddiley J. Teichoic acids and membrane function in bacteria. Nature. 1970;225:519–521. doi: 10.1038/225519a0. [DOI] [PubMed] [Google Scholar]
- 10.Schirner K, Marles-Wright J, Lewis RJ, Errington J. Distinct and essential morphogenic functions for wall- and lipo-teichoic acids in Bacillus subtilis. EMBO J. 2009;28:830–842. doi: 10.1038/emboj.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto H, Miyake Y, Hisaoka M, Kurosawa S, Sekiguchi J. The major and minor wall teichoic acids prevent the sidewall localization of vegetative DL-endopeptidase LytF in Bacillus subtilis. Mol Microbiol. 2008;70:297–310. doi: 10.1111/j.1365-2958.2008.06397.x. [DOI] [PubMed] [Google Scholar]
- 12.Zoll S, Patzold B, Schlag M, Gotz F, Kalbacher H, et al. Structural basis of cell wall cleavage by a staphylococcal autolysin. PLoS Pathog. 2010;6:e1000807. doi: 10.1371/journal.ppat.1000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rice KC, Bayles KW. Death's toolbox: examining the molecular components of bacterial programmed cell death. Mol Microbiol. 2003;50:729–738. doi: 10.1046/j.1365-2958.2003.t01-1-03720.x. [DOI] [PubMed] [Google Scholar]
- 14.Heilmann C, Hussein M, Peters G, G”tz F. Evidence for autolysin-mediated primary attachement of Staphylococcus epidermidis to a polysterene surface. MolMicrobiol. 1997;24:1013–1024. doi: 10.1046/j.1365-2958.1997.4101774.x. [DOI] [PubMed] [Google Scholar]
- 15.Weidenmaier C, Kokai-Kun JF, Kristian SA, Chanturiya T, Kalbacher H, et al. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat Med. 2004;10:243–245. doi: 10.1038/nm991. [DOI] [PubMed] [Google Scholar]
- 16.Schlag M, Biswas R, Krismer B, Kohler T, Zoll S, et al. Role of staphylococcal wall teichoic acid in targeting the major autolysin Atl. Mol Microbiol. 2010;75:864–873. doi: 10.1111/j.1365-2958.2009.07007.x. [DOI] [PubMed] [Google Scholar]
- 17.Frankel MB, Schneewind O. Determinants of Murein Hydrolase Targeting to Cross-wall of Staphylococcus aureus Peptidoglycan. J Biol Chem. 2012;287:10460–10471. doi: 10.1074/jbc.M111.336404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penyige A, Matko J, Deak E, Bodnar A, Barabas G. Depolarization of the membrane potential by beta-lactams as a signal to induce autolysis. Biochem Biophys Res Commun. 2002;290:1169–1175. doi: 10.1006/bbrc.2001.6317. [DOI] [PubMed] [Google Scholar]
- 19.Jolliffe LK, Doyle RJ, Streips UN. The energized membrane and cellular autolysis in Bacillus subtilis. Cell. 1981;25:753–763. doi: 10.1016/0092-8674(81)90183-5. [DOI] [PubMed] [Google Scholar]
- 20.Schlag M, Biswas R, Krismer B, Kohler T, Zoll S, et al. Role of staphylococcal wall teichoic acid in targeting the major autolysin Atl. Mol Microbiol. 2010;75:864–873. doi: 10.1111/j.1365-2958.2009.07007.x. [DOI] [PubMed] [Google Scholar]
- 21.Bierbaum G, Sahl HG. Induction of autolysis of staphylococci by the basic peptide antibiotic pep5 and nisin and their influence on the activity of autolytic enzymes. ArchMicrobiol. 1985;141:249–254. doi: 10.1007/BF00408067. [DOI] [PubMed] [Google Scholar]
- 22.Lutzner N, Patzold B, Zoll S, Stehle T, Kalbacher H. Development of a novel fluorescent substrate for Autolysin E, a bacterial type II amidase. Biochem Biophys Res Commun. 2009;380:554–558. doi: 10.1016/j.bbrc.2009.01.140. [DOI] [PubMed] [Google Scholar]
- 23.Patton TG, Yang SJ, Bayles KW. The role of proton motive force in expression of the Staphylococcus aureus cid and lrg operons. Mol Microbiol. 2006;59:1395–1404. doi: 10.1111/j.1365-2958.2006.05034.x. [DOI] [PubMed] [Google Scholar]
- 24.Atilano ML, Pereira PM, Yates J, Reed P, Veiga H, et al. Teichoic acids are temporal and spatial regulators of peptidoglycan cross-linking in Staphylococcus aureus. Proc Natl Acad Sci U S A. 2010;107:18991–18996. doi: 10.1073/pnas.1004304107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell J, Singh AK, Santa Maria JP, Jr, Kim Y, Brown S, et al. Synthetic lethal compound combinations reveal a fundamental connection between wall teichoic acid and peptidoglycan biosyntheses in Staphylococcus aureus. ACS Chem Biol. 2010;6:106–116. doi: 10.1021/cb100269f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohler T, Weidenmaier C, Peschel A. Wall teichoic acid protects Staphylococcus aureus against antimicrobial fatty acids from human skin. J Bacteriol. 2009;191:4482–4484. doi: 10.1128/JB.00221-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peschel A, Otto M, Jack RW, Kalbacher H, Jung G, et al. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins and other antimicrobial peptides. JBiolChem. 1999;274:8405–8410. doi: 10.1074/jbc.274.13.8405. [DOI] [PubMed] [Google Scholar]
- 28.Bera A, Biswas R, Herbert S, Kulauzovic E, Weidenmaier C, et al. Influence of wall teichoic acid on lysozyme resistance in Staphylococcus aureus. JBacteriol. 2007;189:280–283. doi: 10.1128/JB.01221-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gross M, Cramton S, G”tz F, Peschel A. Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. InfectImmun. 2001;69:3423–3426. doi: 10.1128/IAI.69.5.3423-3426.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eugster MR, Haug MC, Huwiler SG, Loessner MJ. The cell wall binding domain of Listeria bacteriophage endolysin PlyP35 recognizes terminal GlcNAc residues in cell wall teichoic acid. Mol Microbiol. 2011;81:1419–1432. doi: 10.1111/j.1365-2958.2011.07774.x. [DOI] [PubMed] [Google Scholar]
- 31.Xia G, Maier L, Sanchez-Carballo P, Li M, Otto M, et al. Glycosylation of wall teichoic acid in Staphylococcus aureus by TarM. J Biol Chem. 2010;285:13405–13415. doi: 10.1074/jbc.M109.096172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia G, Corrigan RM, Winstel V, Goerke C, Grundling A, et al. Wall Teichoic Acid-Dependent Adsorption of Staphylococcal Siphovirus and Myovirus. J Bacteriol. 2011;193:4006–4009. doi: 10.1128/JB.01412-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weidenmaier C, Kokai-Kun JF, Kristian SA, Chanturyia T, Kalbacher H, et al. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosokomial infections. NatMed. 2004;10:243–245. doi: 10.1038/nm991. [DOI] [PubMed] [Google Scholar]
- 34.Park KH, Kurokawa K, Zheng L, Jung DJ, Tateishi K, et al. Human serum mannose-binding lectin senses wall teichoic acid Glycopolymer of Staphylococcus aureus, which is restricted in infancy. J Biol Chem. 2010;285:27167–27175. doi: 10.1074/jbc.M110.141309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soldo B, Lazarevic V, Pagni M, Karamata D. Teichuronic acid operon of Bacillus subtilis 168. MolMicrobiol. 1999;31:795–805. doi: 10.1046/j.1365-2958.1999.01218.x. [DOI] [PubMed] [Google Scholar]
- 36.Delmas C, Gilleron M, Brando T, Vercellone A, Gheorghui M, et al. Comparative structural study of the mannosylated-lipoarabinomannans from Mycobacterium bovis BCG vaccine strains: characterization and localization of succinates. Glycobiology. 1997;7:811–817. doi: 10.1093/glycob/7.6.811. [DOI] [PubMed] [Google Scholar]
- 37.Choudhury B, Leoff C, Saile E, Wilkins P, Quinn CP, et al. The structure of the major cell wall polysaccharide of Bacillus anthracis is species-specific. JBiolChem. 2006;281:27932–27941. doi: 10.1074/jbc.M605768200. [DOI] [PubMed] [Google Scholar]
- 38.Brckner R. A series of shuttle vectors for Bacillus subtilis and Escherichia coli. Gene. 1992;122:187–192. doi: 10.1016/0378-1119(92)90048-t. [DOI] [PubMed] [Google Scholar]
- 39.Phoenix VR, Martinez RE, Konhauser KO, Ferris FG. Characterization and implications of the cell surface reactivity of Calothrix sp. strain KC97. Appl Environ Microbiol. 2002;68:4827–4834. doi: 10.1128/AEM.68.10.4827-4834.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez RE, Smith DS, Kulczycki E, Ferris FG. Determination of intrinsic bacterial surface acidity constants using a donnan shell model and a continuous pK(a) distribution method. J Colloid Interface Sci. 2002;253:130–139. doi: 10.1006/jcis.2002.8541. [DOI] [PubMed] [Google Scholar]
- 41.Raafat D, von Bargen K, Haas A, Sahl HG. Insights into the mode of action of chitosan as an antibacterial compound. Appl Environ Microbiol. 2008;74:3764–3773. doi: 10.1128/AEM.00453-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamm LK, Tatulian SA. Infrared spectroscopy of proteins and peptides in lipid bilayers. Q Rev Biophys. 1997;30:365–429. doi: 10.1017/s0033583597003375. [DOI] [PubMed] [Google Scholar]