Abstract
pBSSB1 is a 27 kb non-bacteriophage-related linear plasmid first found in Salmonella enterica serovar Typhi (S. Typhi), but the mechanism underlying the replication of pBSSB1 is currently unknown. Previous reports showed that the factor for inversion stimulation (Fis) encoded by fis can affect the replication, transcription and other processes through binding DNA. Here, a fis deletion mutant of S. Typhi (Δfis) was prepared through the homologous recombination mediated by suicide plasmid and the loss of pBSSB1 in Δfis was observed surprisingly by pulsed field gel electrophoresis (PFGE). Subsequently, the loss of pBSSB1 was verified by PCR and Southern blot. In addition, the motility of Δfis was deficient and the flagellin of Δfis could not be detected by 2-dimensional polyacrylamide gel electrophoresis. All these results show that Fis is essential for the stability of pBSSB1 and affects the motility of S. Typhi.
Introduction
The factor for inversion stimulation (Fis) encoded by fis is a small DNA-bending nucleotide-associated protein which plays a role in the transcriptional regulation of a number of genes in diverse bacterial species [1]. Fis was found initially as a co-factor of the site-specific recombination system. It was reported that Fis of E. coli is composed of two similar subunits and each subunit consists of 98 amino acids. In the structure of Fis, there is a typical α-helix-turn-α-helix (helix-turn-helix, HTH) domain which could bind to the major groove of the DNA double helix [2]. Fis has wide regulatory roles, such as regulating the bacterial growth, virulence, and flagellum [3]. In addition, Fis can change the structure of bacterial nucleic acid and affect the replication, transcription and other processes through binding the DNA [4].
Plasmid is an extra chromosomal, self replicating genetic element which in many cases is circular. In 1979, the first linear plasmid of prokaryote was found in Streptomyces rochei which could produce antibiotics [5]. So far linear plasmids have been found in about a dozen of Streptomyces, and the molecular size of these linear plasmids is between 12–640 kb [6]. Subsequently, another kind of linear plasmids were also found in Borrelia [7]. In 2007, a linear plasmid named pBSSB1 was reported to be present in Salmonella enterica serovar Typhi (S. Typhi) z66-positive strain by Baker et al [8]. pBSSB1, which is about 27kb-sized, is the first non-bacteriophage-related linear plasmid found in Enterobacteriaceae, and it mediates the unidirectional flagellar phase variation of S. Typhi z66-positive strain [8], [9]. However, the mechanism underlying the replication of pBSSB1 is currently unknown.
The protein DnaA, which recognizes the origin of replication oriC, is essential for the DNA replication of Bacteria. With the help of DnaC protein, DnaB, a helicase can bind to oriC region to open the DNA double helix so as to initiate replication. Results of in vitro research show that Fis competes with DnaA protein for the origin of replication, oriC to affect DNA replication in E. coli [10], [11]. Besides, Fis can bind to the transcription start site of dnaA operon to suppress its expression [12]. dnaA operon is composed of dnaA, dnaN and recF, coding for the DnaA protein which can recognize the origin of replication oriC, β subunit of DNA polymerase III which is responsible for the extension of the newly-replicated DNA chains and the RecF protein which is involved in the recombination and the repair of DNA, respectively [12], [13]. In light of these functions of Fis, we hypothesized that Fis may play a very important role in the replication of pBSSB1.
In this study, we prepared a fis deletion mutant of S. Typhi (Δfis) through the homologous recombination mediated by suicide plasmid, and performed pulsed field gel electrophoresis (PFGE) to investigate the genome structure of Δfis. It was surprisingly found that the 27 kb linear plasmid has disappeared in Δfis. The loss of linear plasmid in Δfis was verified by PCR and Southern blot. Moreover, only the complementary strain Δfis(pBADfis) can host this linear plasmid while the Δfis and Δfis(pBAD) cannot. These results suggest that fis is essential for the stability of plasmid pBSSB1. Since it was previously reported that the gene fljB z66 located on pBSSB1 encodes the flagellum and is responsible for the motility of S. Typhi z66-positive strain [8], the motility and flagellin of Δfis was examined by semi-solid agar plates and two-dimensional polyacrylamide gel electrophoresis respectively. The results show that the motility of Δfis was deficient and the flagellin of Δfis could not be detected.
Materials and Methods
Bacterial Strains and Plasmids
S. Typhi GIFU10007, a z66-positive wild-type strain was used in this study. Mutants and plasmids used in this work are listed in Table 1.
Table 1. Strains and plasmids used in the present study.
| Strain or plasmid | Relevant characteristics | Reference or source |
| Strains | ||
| S. Typhi GIFU10007 | wild-type strain; z66+ | 14 |
| SY372λpir | E. coli host strain of suicide plasmid | Laboratory collection |
| Δfis | GIFU10007(Δfis); z66- | This work |
| Δfis (pBAD) | Δfis containing pBAD empety vector | This work |
| Δfis (pBADfis) | Δfis containing pBADfis recombinant plasmid | This work |
| S. Typhi GIFU10007-1 | GIFU10007 containing pBSSB2; Kanar | This work |
| Δfis(pBADfis)(pBSSB2) | Δfis(pBADfis) containing pBSSB2; Kanar | This work |
| Plasmids | ||
| pGMB151 | suicide plasmid; sacB; Ampr | 14 |
| pGMBfis | pGMB151 containing fis | This work |
| pBAD/gIII | Expression vector; Ampr | 15 |
| pBADfis | pBAD containing fis | This work |
| pET-28a-c(+) | Kanar | Laboratory collection |
| pKD46 | Red helper plasmid; Ampr | Laboratory collection |
| pBSSB2 | pBSSB1 containing a kanamycin resistance gene | This work |
Construction of the fis Deletion Mutant of S. Typhi
Primers used in this study are listed in Table 2. To generate the Δfis, primer pairs F1A/B and F2A/B were used to amplify the fragments F1 (499-bp) and F2 (314-bp) located upstream and downstream of the gene fis, respectively. A BamHI site was added to the 5′-termini of primers F1A and F2B, and a SalI site was added to the 5′-termini of primers F1B and F2A. Two fragments F1 and F2 were amplified from S. Typhi GIFU10007 and digested with Sal I and ligated with DNA Ligation Kit Ver.2 (TaKaRa) to form the homologous fragment, in which 159-bp of the gene fis was absent. The fragment was then inserted into the BamH I site of the suicide plasmid pGMB151, which carries a sucrose-sensitivity gene sacB. The suicide plasmid carrying the deletion of fis gene was transferred into wild-type strain by electroporation as previously described [14], [15]. The mutant strain was selected by PCR with primers F1A and F2B. Finally, the selected candidate of the fis deletion mutant was confirmed by sequencing analysis and designated as Δfis.
Table 2. Primers used in this study.
| Primers | Sequence(5′-) | Purpose |
| F1A(BamH I) | AGGGATCCGGCAGTTAAGCAGAAAGT | fis mutant construction |
| F1B(Sal I) | CTGTCGACGTTACCTGATCCTGAGAGTT | |
| F2A(Sal I) | TATGTCGACTGCTGCTCTGATGATGG | |
| F2B(BamH I) | TCAGGATCCCACCATACCGTCGAAAT | |
| P-fis-A(Nco I) | TACCATGGATACGCTATTGAGGACGC | Complementary expressionof fis in Δfis |
| P-fis-B(Sal I) | CTGTCGACTTAGTTCATGCCGTATT | |
| Pa(23173) | TAACAGATAGCCACACACAGT | Confirmation of the presenceor absence of pBSSB1 by PCR |
| Pb(23750) | TCAAGGAAGACTGAGATTTGT | |
| P-Kana-A | AATTGATAAAGGAAAGTGGTTCCGTTATAAAAATGGCTTATTCGATATAAGGTCTGACGCTCAGTGGA | Insertion of kanamycin cassettewithin pBSSB1 |
| P-Kana-B | TAGTGGCTCAAAGAGTATTAGAAATTGACAGAGAAAAGAAAGCAGAATGATTTCGGCCTATTGGTTAA | |
| ORF1-A | GAGAAGATGCCCGTAA | Confirmation of the insertionof kanamycin cassette withinpBSSB1 by PCR |
| Kana-B | ATGGCTCATAACACCC |
Pulsed-field Gel Electrophoresis (PFGE)
A single colony of S. Typhi wild strain and mutant strain Δfis was inoculated into 4 ml LB, and cultured overnight with shaking (250 r/min) at 37°C. Bacteria were collected by centrifugation (4000 r/min, 10 min, 4°C) and washed three times with buffer PIV (10 mmol/L Tris, 1 mol/L NaCl, pH7.6). The pelleted bacteria were resuspended in 1 ml buffer PIV and then mixed with 2% low melting agarose gel to make the cell plugs for PFGE. The cell plugs were digested with the fresh lysis buffer (6 mmol/L Tris, 0.1 molL EDTA, 1 mol/L NaCl, 0.5% Brij-58, 0.2% sodium deoxycholate, 0.5% SDS, RNaseA 20 µg/ml, lysozyme enzyme 1 mg/ml) overnight at 37°C. After being washed with buffer ES (0.5 mol/L EDTA, 1% SDS), the cell plugs were digested with buffer ESP (including protease K 100 µg/ml of the ES) overnight at 50°C to digest bacterial protein, and finally washed with TE buffer. The DNA of bacteria in the cell plugs was separated on 1.0% agarose gels by electrophoresis with a CHEF Mapper system (Bio-Rad, USA) in the 0.5×TBE buffer. The electrophoresis was performed at 6 V/cm and 14°C. The pulse time increased from 1 to 20 s during 18 h run. DNA-PFGE marker (Bio-Rad, USA) was used as the size marker.
Verification of the Absence of Linear Plasmid pBSSB1 in Δfis by PCR and Southern-blot
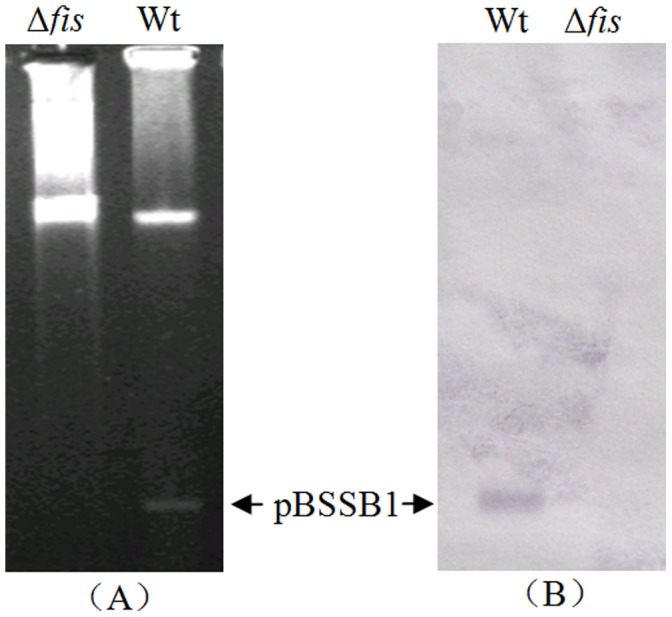
To verify the absence of linear plasmid pBSSB1 in Δfis, a pair of primers Pa(23173) and Pb(23750) (Table 2), which was designed according to the sequence of pBSSB1 reported previously [8], was used to amplify the corresponding fragments in order to investigate whether the linear plasmid pBSSB1 was absent in the mutant strain Δfis of S. Typhi. In addition, the separated DNA fragments from PFGE was transferred onto the nitrocellulose membrane and subjected to Southern-blot with the biotin-labeled DNA fragments as the probe.
Complementary Expression of fis in Δfis
Primers P-fis-A and P-fis-B (Table 2), specific to upstream and downstream regions of the gene fis were used to amplify a promoterless fis gene with pfu DNA polymerase (Fermentas). An Nco I site and a Sal I site were added to the 5′-termini of primers P-fis-A and P-fis-B, respectively. An approximately 297 bp amplicon was inserted into the Nco I and Sal I sites of the expression vector pBAD/gIII (Invitrogen) to form the recombinant plasmid (pBADfis). The positive plasmid pBADfis was verified by digestion with Nco I and Sal I and sequence analysis. The Δfis was transformed with pBADfis and designated as Δfis(pBADfis). As a control, the Δfis was also transformed with the empty vector pBAD/gIII and designated as Δfis(pBAD). Expression of fis in Δfis(pBADfis) was induced by L-arabinose (0.2% wt/vol).
Insertion of Kanamycin Cassette within pBSSB1
A kanamycin resistance gene was inserted within pBSSB1 between ORF001 and ORF002 using the lambda Red recombinase (one-step method) as described by Datsenko and Wanner [16]. First, the Red helper plasmid pKD46 was isolated and transformed into wild type S. Typhi GIFU10007 by electroporation (2.5 kV, 600 ohms, 25 µF; Bio-Rad Gene Pulser). Then, the kanamycin resistance gene was amplified with the primers P-Kana-A and P-Kana-B (Table 2) using plasmid pET-28a-c(+) DNA as a template. PCR-amplified DNA was precipitated and re-suspended in 10 µl of nuclease-free water. Re-suspended DNA was transformed by electroporation into S. Typhi GIFU10007 cells containing pKD46 plasmid as described above. Transformed cells were recovered for 1 h at 37°C in 800 µl of SOC, and then plated onto LB medium supplemented with 25 µg/ml kanamycin. Finally, the insertion of kanamycin cassette into pBSSB1 was confirmed by PCR with the primers ORF1-A and Kana-B (Table 2). The pBSSB1 plasmid with the kanamycin resistance gene insert was designated as pBSSB2, and S. Typhi GIFU10007 harboring pBSSB2 was designated as S. Typhi GIFU10007-1.
Transformation of Δfis, Δfis(pBAD) and Δfis(pBADfis) with pBSSB2
The pBSSB2 plasmid was isolated from S. Typhi GIFU10007-1 using an alkaline lysis method originally described by Kado and Liu [17]. The quality and quantity of the extracted pBSSB2 DNA were tested by electrophoresis and an ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, USA) respectively. Then, Δfis(pBADfis), Δfis(pBAD) and Δfis was transformed with the pBSSB2 DNA by electroporation (2.5 kV, 600 ohms, 25 µF; Bio-Rad Gene Pulser). Transformed cells were screened on the LB medium supplemented with 25 µg/ml kanamycin. Transformation of Δfis or Δfis(pBAD) or Δfis(pBADfis) with pBSSB2 was finally confirmed by PCR with primers Pa(23173) and Pb(23750) (Table 2).
Motility Assay
Bacteria were cultured overnight at 37°C in LB broth. Each 4 µl of culture was inoculated into the centre of a 0.3% semisolid LB agar plate cntaining L-arabinose (0.2% wt/vol). The plates were incubated at 37°C for 10 hours and motility was assessed qualitatively by examining the diameter of circular swimming which was formed by the growing motile bacterial cells.
Two-dimensional Polyacrylamide Gel Electrophoresis and Mass Spectrometry Analysis
Bacterial proteins were extracted from wild-type and fis mutant strain strains. The proteins were firstly separated by isoelectrofocusing electrophoresis and then subjected to SDS-PAGE electrophoresis (Bio-rad). After staining with Coomassie Brilliant Blue G-250, differential expression of bacterial proteins between the wild-type and fis mutant strains were detected and analyzed by Mass spectrometry.
Results
The Loss of Linear Plasmid pBSSB1 in Δfis of S. Typhi
The DNA of bacteria in the agarose, which was not digested by restriction enzymes, was separated by PFGE. As shown in Figure 1(A), two DNA bands were stained and the upper band may be the chromosomal DNA while the lower band is the linear plasmid pBSSB1 DNA. This result indicates that the linear plasmid pBSSB1 was lost in the Δfis of S. Typhi. To better verify the loss of the linear pBSSB1 plasmid, we used biotin labeled DNA fragment which was amplified by a pair of specific primers designed according to the sequence of pBSSB1 plasmid as probes to hybridize the DNA bands from PFGE by Southern blot (figure 1(B)). In addition, a specific DNA fragment of pBSSB1 can not detected by PCR with primers designed according to the pBSSB1 DNA sequence (data not shown). Moreover, the pBSSB2 plasmid DNA can only be transformed into the complementary strain Δfis(pBADfis) while the Δfis and Δfis(pBAD) cannot host the plasmid pBSSB2 DNA. These results show that Fis is essential for the stability of this linear plasmid in S. Typhi.
Figure 1. Identification of linear plasmid pBSSB1 by PFGE (A) and Southern-blot (B).
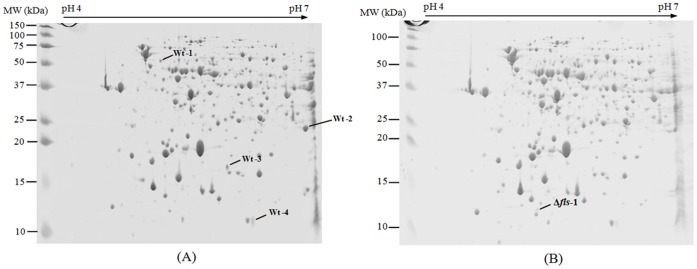
Fis Affects the Motility of Δfis
As shown in Figure 2, the motility of Δfis was greatly decreased in comparison with the wild-type parental strain. However, motility was restored, to some extent in the complemented strains Δfis(pBADfis) and Δfis(pBADfis)(pBSSB2). In addition, the bacterial proteins of Δfis of S. Typhi were compared with those of wild-type strain by two-dimensional polyacrylamide gel electrophoresis and mass spectrometry analysis. As shown in Figure 3, five bacterial proteins (Wt-1, Wt-2, Wt-3, Wt-4 and Δfis-1), whose expression was obviously different between the mutant strain Δfis and wild-type strain, were found by two-dimensional electrophoresis analysis. Among these bacterial proteins, the flagellin (Wt-1) encoded by fljB z66 gene was confirmed to be absent in the mutant strain Δfis by mass spectrometry analysis. These results demonstrate that Fis may affect the bacterial motility due to the loss of linear plasmid pBSSB1 in Δfis of S. Typhi GIFU10007.
Figure 2. Effect of Fis on the motility of S. Typhi GIFU10007.
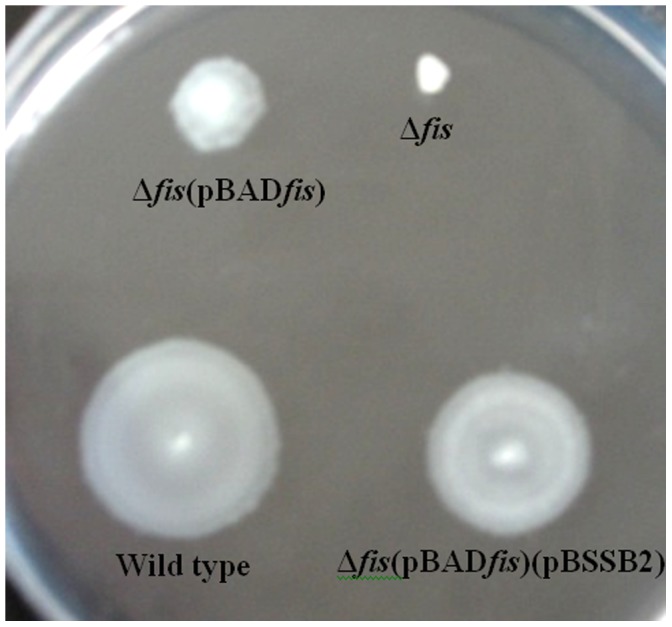
Figure 3. Two-dimensional polyacrylamide gel electrophoresis profile of wild-type strain (A) and Δfis (B).
Discussion
Although linear plasmids are relatively common in bacterial species such as Streptomyces and Borrelia, pBSSB1 is the first non-bacteriophage-related linear plasmid to be described in the Enterobacteriaceae that contains no detectable homology sequence of bacteriophage [8]. However, little is known about the replication of this linear plasmid. Fis is a very important small nucleotide-associated protein which plays a role in affecting the bacterial chromosome structure and the initiation of DNA replication [12]. In this study, the fact that pBSSB1 disappeared in the mutant strain Δfis is a significant observation. This means that Fis is essential for the stability of the linear plasmid pBSSB1. There are reports to show that either the formation of multicopy plasmid dimers or the associated reduction in copy number leads to the instability of the plasmid [18], [19]. The global regulator Fis is essential for the stable maintenance of plasmid ColE1 through binding to cer of ColE1in a sequence-specific manner [20]. ColE1-like plasmids are less stable in fis mutant hosts and it is conceivable that instability caused by the mutation is due to altered Fis binding site [21]. Therefore, Fis may influence the stability of pBSSB1 plasmid by affecting a specific gene. However, there was no obvious differential expression of genes contributing to DNA replication found by the microarray and proteomic analysis between the wild-type and Δfis (data not shown).
Changes in GC skew, which was reported to be associated with the origin of replication on plasmids and bacterial chromosomes, were previously used to predict the internal origin of bi-directional linear replication [22], [23]. It was reported that the change in GC skew ((G-C)/(G+C)) is present in the middle of pBSSB1 and this region may be the origin of the replication [8]. Therefore, it is speculated that the replication of pBSSB1 is initiated from the middle and prolonged bi-directionally and Fis may be essential for the initiation of replication of this plasmid. In addition, it has been suggested that pBSSB1 may possess terminal protein (Tp) covalently bound to the 5′ end of the DNA, which is very similar to linear plasmids from Streptomyces [8]. Many linear plasmids are replicated bi-directionally from an internal origin, which leaves single-stranded gaps of 250-300 nt at the 3′ ends, and these gaps are proposed to be patched by Tp-primed DNA synthesis [24], [25]. Therefore, Fis may also affect the replication of pBSSB1 through the regulation on the Tp of this plasmid. All these hypotheses need further experiments to clarify.
Previous studies showed that S. Typhi z66 positive strain is a biphasic Salmonella serovar, harbouring the fliC gene in the chromosome and fljB z66 gene in the pBSSB1 plasmid [8], [14]. FljAz66, which is encoded by fljA z66 gene located downstream of fljB z66 gene, can inhibit the expression of fliC similar to most biphasic Salmonella [9], [15]. In this study, the loss of plasmid pBSSB1 in Δfis may relieve the repression of FljAz66 on the expression of fliC. Previous study showed that the expression of the flagellar genes selected from the early (flhD), middle (fliA) and late (fliC) stages of flagellar biosynthesis was strongly repressed in the fis mutant [3]. In the present study, we also found that many genes contributing to flagellar biosynthesis and motility were strongly down regulated in Δfis by microarray analysis of the differential expression between the wild-type and Δfis (data not shown). Therefore, Fis might affect the bacterial motility due to the loss of pBSSB1 plasmid and the decreased expression of fliC in Δfis of S. Typhi.
In summary, this study is the first to demonstrate that Fis is essential for the stability of pBSSB1 and affects the motility of S. Typhi.
Acknowledgments
We thank T. Ezaki (Gifu University) for bacterial strains and continuous support.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by National Natural Science Foundation of China (31000076) and Natural Science Foundation for colleges and universities in Jiangsu Province (10KJB310001). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Browning DF, Grainger DC, Busby SJ. Effects of nucleoid-associated proteins on bacterial chromosome structure and gene expression. Curr Opin Microbiol. 2010;13:773–780. doi: 10.1016/j.mib.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Yuan HS, Finkel SE, Feng JA, Kaczor-Grzeskowiak M, Johnson RC, et al. The molecular structure of wild-type and a mutant Fis protein: Relationship between mutational changes and recombinational enhancer function or DNA binding. Proc Natl Acad Sci U S A. 1991;88:9558–9562. doi: 10.1073/pnas.88.21.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly A, Goldberg MD, Carroll RK, Danino V, Hinton JC, et al. A global role for Fis in the transcriptional control of metabolism and type III secretion in Salmonella enterica serovar Typhimurium. Microbiology. 2004;150:2037–2053. doi: 10.1099/mic.0.27209-0. [DOI] [PubMed] [Google Scholar]
- 4.Cameron AD, Stoebel DM, Dorman CJ. DNA supercoiling is differentially regulated by environmental factors and FIS in Escherichia coli and Salmonella enterica. Mol Microbiol. 2011;80:85–101. doi: 10.1111/j.1365-2958.2011.07560.x. [DOI] [PubMed] [Google Scholar]
- 5.Hayakawa T, Otake N, Yonehara H, Tanaka T, Sakaguchi K. Isolation and characterization of plasmids from Streptomyces. J Antibiot. 1979;32:1348–1350. doi: 10.7164/antibiotics.32.1348. [DOI] [PubMed] [Google Scholar]
- 6.Zhong L, Cheng Q, Tian X, Zhao L, Qin Z. Characterization of the replication, transfer, and plasmid/lytic phage cycle of the Streptomyces plasmid-phage pZL12. J Bacteriol. 2010;192:3747–3754. doi: 10.1128/JB.00123-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plasterk RH, Simon MI, Barbour AG. Transposition of structural genes to an expression sequence on a linear plasmid causes antigenic variation in the bacterium Borrelia hermsii. Nature. 1985;318:257–263. doi: 10.1038/318257a0. [DOI] [PubMed] [Google Scholar]
- 8.Baker S, Hardy J, Sanderson KE, Quail M, Goodhead I, et al. A novel linear plasmid mediates flagellar variation in Salmonella Typhi. PLoS Pathog. 2007;3:e59. doi: 10.1371/journal.ppat.0030059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker S, Holt K, Whitehead S, Goodhead I, Perkins T, et al. A linear plasmid truncation induces unidirectional flagellar phase change in H: z66 positive Salmonella Typhi. Mol Microbiol. 2007;66:1207–1218. doi: 10.1111/j.1365-2958.2007.05995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryan VT, Grimwade JE, Camara JE, Crooke E, Leonard AC. Escherichia coli prereplication complex assembly is regulated by dynamic interplay among Fis, IHF and DnaA. Mol Microbiol. 2004;51:1347–1359. doi: 10.1046/j.1365-2958.2003.03906.x. [DOI] [PubMed] [Google Scholar]
- 11.Gille H, Egan JB, Roth A, Messer W. The FIS protein binds and bends the origin of chromosomal DNA replication, oriC, of Escherichia coli. Nucleic Acids Res. 1991;19:4167–4172. doi: 10.1093/nar/19.15.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wold S, Crooke E, Skarstad K. The Escherichia coli Fis protein prevents initiation of DNA replication from oriC in vitro. Nucleic Acids Res. 1996;24:3527–3532. doi: 10.1093/nar/24.18.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Froelich JM, Phuong TK, Zyskind JW. Fis Binding in the dnaA operon promoter region. J Bacteriol. 1996;178:6006–6012. doi: 10.1128/jb.178.20.6006-6012.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang X, Phung LV, Dejsirilert S, Tishyadhigama P, li Y, et al. Cloning and Characterization of the gene encoding the z66 antigen of Salmonella enterica serovar Typhi. FEMS Microbiol Lett. 2004;234:239–246. doi: 10.1016/j.femsle.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 15.Zou X, Huang X, Xu S, Zhou L, Sheng X, et al. Identification of a fljA gene on a linear plasmid as a repressor gene of fliC in Salmonella enterica serovar Typhi. Microbiol Immunol. 2009;53:191–197. doi: 10.1111/j.1348-0421.2009.00106.x. [DOI] [PubMed] [Google Scholar]
- 16.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kado CI, Liu ST. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981;145:1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Summers DK, Sherratt DJ. Multimerization of high copy number plasmids causes instability: ColE1 encodes a determinant essential for plasmid monomerization and stability. Cell. 1984;36:1097–1103. doi: 10.1016/0092-8674(84)90060-6. [DOI] [PubMed] [Google Scholar]
- 19.Summers DK, Beton CW, Withers HL. Multicopy plasmid instability: the dimer catastrophe hypothesis. Mol Microbiol. 1993;8:1031–1038. doi: 10.1111/j.1365-2958.1993.tb01648.x. [DOI] [PubMed] [Google Scholar]
- 20.Blaby IK, Summers DK. The role of FIS in the Rcd checkpoint and stable maintenance of plasmid ColE1. Microbiology. 2009;155:2676–2682. doi: 10.1099/mic.0.029777-0. [DOI] [PubMed] [Google Scholar]
- 21.Balding C, Blaby I, Summers D. A mutational analysis of the ColE1-encoded cell cycle regulator Rcd confirms its role in plasmid stability. Plasmid. 2006;56:68–73. doi: 10.1016/j.plasmid.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Lobry JR, Louarn JM. Polarisation of prokaryotic chromosomes. Curr Opin Microbiol. 2003;6:101–108. doi: 10.1016/s1369-5274(03)00024-9. [DOI] [PubMed] [Google Scholar]
- 23.Picardeau M, Lobry JR, Hinnebusch BJ. Analyzing DNA strand compositional asymmetry to identify candidate replication origins of Borrelia burgdorferi linear and circular plasmids. Genome Res. 2000;10:1594–1604. doi: 10.1101/gr.124000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang CC, Huang CH, Li CY, Tsay YG, Lee SC, et al. The terminal proteins of linear Streptomyces chromosomes and plasmids a novel class of replication priming proteins. Mol Microbiol. 2002;43:297–305. [PubMed] [Google Scholar]
- 25.Tsai HH, Huang CH, Tessmer I, Erie DA, Chen CW. Linear Streptomyces plasmids form superhelical circles through interactions between their terminal proteins. Nucleic Acids Res. 2011;39:2165–2174. doi: 10.1093/nar/gkq1204. [DOI] [PMC free article] [PubMed] [Google Scholar]