Neurons in the central nervous system are characterized by complex axonal arborization patterns. For example, the principal neurons of the cerebral cortex, the pyramidal cells, send axonal projections locally and distally to both cortical and subcortical targets (1), to ultimately communicate with other neurons through thousands of synaptic terminals. The axon normally is considered a very reliable transmission line in which active and stable propagation of signals occurs. The currency of neuronal communication, the action potential (AP), is initiated at or near the soma (Fig. 1). Then, through active processes involving voltage-gated ion channels, the AP propagates down the axon throughout the branched structure of its arbor. However, the necessary branching of axons, which is required for efficient divergent output, puts an undue strain on the transmission line (see ref. 2 for a comprehensive review). Further, the safety factor for transmission of APs can be dynamically reduced when electrical conditions in the vicinity of the axon are not perfect, and propagation failures will occur.
Figure 1.
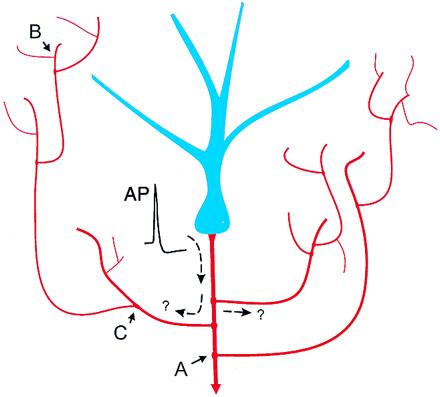
Output signals from neurons must follow a circuitous and sometimes insecure path. The soma and dendrites (blue) of neurons receive synaptic input, integrate the resulting electrical signals, and generate action potential (AP) output. APs then actively propagate down the axon (red) and its branches to reach multiple synaptic terminals and thereby to communicate with other neurons. Axons can be highly branched and have complicated bifurcations with membrane inhomogeneities such as swellings and incompatibilities between mother and daughter diameters. Reliability of propagation will be altered at each of these inhomogeneities (see text). Will the initiated AP faithfully propagate to all its branches? This will depend on both the active electrical properties of the axon and its geometry. Either complete or selective block at different daughter branches may occur, and regulation of propagation has interesting implications for routing of efferent information. A failure to propagate past point A would have widespread effect—subcortical output (descending axon with arrowhead indicates a subcortically projecting cell) would be totally blocked, as would information flow to the branch that extends up to the upper right of the cell. By contrast, failure at point C would influence a smaller local region on the left side of the cell, whereas failure at B would influence only a few synaptic terminals in a very localized region.
In this issue of PNAS, Cox et al. (3) provide evidence that, at least for neocortical pyramidal neurons, propagation is extremely reliable. In a technical tour de force, using the high spatial and temporal resolution afforded by the method of two-photon scanning laser scanning microscopy, they were able to detect signs of electrical activity in individual submicron neuronal structures, including axons, branch points, and axonal swellings. The latter, from which most measurements were made, were likely to be synaptic terminals. Supragranular pyramidal neurons of rat sensorimotor cortex were recorded by somatic recordings with patch-clamp electrodes containing a fluorescent dye. The dye, a calcium indicator, would diffuse into the axonal structures over a period of several minutes and would indicate axonal activity through changes in fluorescence caused by Ca2+ entry through voltage-gated channels. This method allowed Cox and colleagues to monitor electrical activity through a large extent of the axonal arbor. Indeed, they were able to resolve activity-dependent signals from second to fourth order branches as far as 500 μm from the soma. The central finding was that APs initiated in the soma always propagated to the observed swellings, regardless of the distance into the axonal tree. In other words, if a Ca2+ signal was observed in response to a single AP, it was also observed with every subsequent AP, even when applied in rapid succession.
Two other tests provide further confidence in this result. In the first, neurons were treated with current injections designed to condition the neuronal membrane potential to very negative levels, far beyond the normal resting level of −60 mV. This type of conditioning will reduce excitability by deinactivating voltage-gated K+ channels and has been shown to produce failures of intercellular communication in hippocampal neurons (4). However, Cox et al. found that such negative conditioning steps had no effect on propagation of electrical signals in neocortical axons. In a second test, they applied the neuromodulator adenosine, which mainly produces depression of neural activity through activation of K+ channels and inhibition of Ca2+ channels. They found that adenosine did reduce Ca2+ entry but did not alter reliability of AP invasion into terminals. Thus, the margin of error for propagation is quite high in the studied axons.
The idea that AP propagation in branched axonal structures may not be completely reliable was first systematically studied by Barron and Matthews (5) in the spinal cord. They recorded electrical signals at two different locations that, based on response latency and frequency following, seemed to represent activity of a single axon. When they applied repetitive electrical stimuli, they found that signals recorded at one point along the axon intermittently would vanish at the second location. More recent studies have used a variety of vertebrate and invertebrate preparations to examine the mechanisms and patterns of branch point failure (2). Studies in invertebrates (6–8) or the vertebrate neuromuscular junction (9) in particular have been very informative, because it is often possible to directly and simultaneously record from multiple axon branches before and after the bifurcations and/or from the postsynaptic target. From these studies it is clear that use-dependent changes can degrade propagation reliability through mechanisms such as ion accumulation and inactivation of voltage-dependent ion channels.
Why is propagation so reliable in neocortical axons (3, 10) in light of the findings of reduced reliability in other preparations? At this point we can only speculate, given that the detailed electrical properties of axons in the cortex are poorly understood. A brief discussion of geometric considerations may be useful, however. In order for there to be successful transmission of the electrical signal from one structure to another, there must be a match between the impedance of the input and output structures. APs propagate through the structure of the axon by an active process. Local regions of depolarization lead to openings of voltage-gated Na+ channels and Na+ entry. The depolarizing current then spreads to adjacent, not-yet-activated portions of the membrane to result in a propagating wave (11). In regions where there is an overabundance of membrane, for example, when an axon dilates or branches (Fig. 2), there may be insufficient current produced in the active portion of the membrane to initiate a regenerative response in the adjacent section, in which case propagation will fail. The so-called 3/2 power law developed by Goldstein and Rall (12) describes an ideal relationship between the geometry of mother and daughter branches with otherwise identical properties. When the branches are appropriately sized, the impedance is perfectly matched (Fig. 2A) so that AP propagation proceeds through the branchpoint with high reliability and no discontinuity in conduction. By contrast, when the daughter branches have relatively large diameters (Fig. 2B), as can occur in neocortical pyramidal neurons (13), then propagation slows as it enters the branchpoint and reliability decreases. Similarly, en passant synapses, which are swellings in the axon, also present a significant electrical load (impedance mismatch, Fig. 2C) on the arriving AP. Thus, it is all the more striking that neocortical axons can perform reliably under such unfavorable conditions. One possible explanation for high reliability might be that axonal structures are endowed with inhomogeneous distributions of ion channels that compensate for the increased electrical load. For example, a higher density of Na+ channels at swellings as compared with the mother axon would lower the threshold for AP entry into the branch or terminal and improve propagation.
Figure 2.
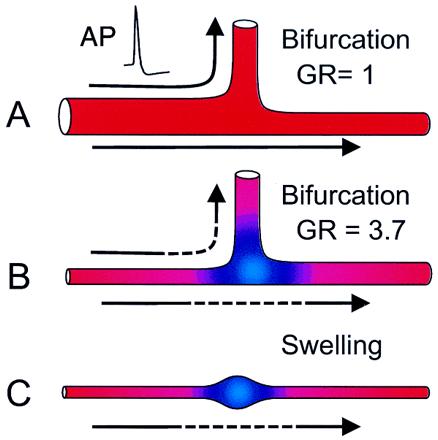
AP propagation through axonal structures. Two different bifurcations and a swelling are shown. Direction flow is from soma via the mother branch on the left toward the daughter branches on the top and right. (A) In the first example, propagation is completely reliable because the ratio of the daughter and mother branches is optimal for impedance matching [Goldstein–Rall (GR) ratio = 1]. (B) In the second case, the large diameters of the daughter branches place a significant load on the mother branch (GR ratio = 3.7) so that propagation is slowed (blue color and dashed arrows) and may fail to enter one or both of the daughter branches. (C) In the final case, propagation is similarly slowed as it enters a swelling and may then fail to propagate into the terminal and beyond.
Although the current results (3) are clear, the issue of branch point failure in the central nervous system may not be fully resolved for the finest terminal axonal branches until methods are developed to record from these structures. Meanwhile, the theoretical possibility of dynamic changes in axon propagation within axonal arbor is intriguing, as it provides an additional layer of computing power to the neuron (14). Control of propagation within the axonal tree could be very powerful in routing information (Fig. 1), and modest changes in ion channel activity, such as those produced by synaptic input, would have profound effects on information flow. In commenting on the paucity of synaptic connections onto axons, Koch (15) notes, “It is anybody's guess why the nervous system did not avail itself of the opportunity to precisely (in space and time) filter or gate action potentials.” Given the results of the present study, it appears that the neuronal soma and dendrites, with their plethora of intrinsic electrical machinery and wide range of synaptic receptors, are responsible for doing the majority of computation required for proper input/output coding.
Footnotes
See companion article on page 9724.
References
- 1.Feldman M L. In: Cellular Components of the Cerebral Cortex. Peters A C, Jones E G, editors. New York: Plenum; 1984. pp. 123–189. [Google Scholar]
- 2.Swadlow H A, Kocsis J D, Waxman S G. Annu Rev Biophys Bioeng. 1980;9:143–179. doi: 10.1146/annurev.bb.09.060180.001043. [DOI] [PubMed] [Google Scholar]
- 3.Cox C L, Denk W, Tank D, Svoboda K. Proc Natl Acad Sci USA. 2000;97:9724–9728. doi: 10.1073/pnas.170278697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Debanne D, Guérineau N C, Gähwiler B H, Thompson S M. Nature (London) 1997;389:286–289. doi: 10.1038/38502. [DOI] [PubMed] [Google Scholar]
- 5.Barron D H, Matthews B H. J Physiol (London) 1935;85:73–103. doi: 10.1113/jphysiol.1935.sp003303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fatt P, Katz B. J Exp Biol. 1953;30:433–439. [Google Scholar]
- 7.Grossman Y, Parnas I, Spira M E. J Physiol (London) 1979;295:283–305. doi: 10.1113/jphysiol.1979.sp012969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith D O. J Physiol (London) 1980;301:243–259. doi: 10.1113/jphysiol.1980.sp013202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krnjevic K, Miledi R. J Physiol (London) 1959;149:1–22. doi: 10.1113/jphysiol.1959.sp006321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen C, Stevens C F. Proc Natl Acad Sci USA. 1994;91:10380–10383. doi: 10.1073/pnas.91.22.10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodgkin A L, Huxley A F. J Physiol (London) 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein S S, Rall W. Biophys J. 1974;14:731–757. doi: 10.1016/S0006-3495(74)85947-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deschênes M, Landry P. Brain Res. 1980;191:538–544. doi: 10.1016/0006-8993(80)91302-5. [DOI] [PubMed] [Google Scholar]
- 14.Chung S H, Raymond S A, Lettvin J Y. Brain Behav Evol. 1970;3:72–101. doi: 10.1159/000125464. [DOI] [PubMed] [Google Scholar]
- 15.Koch C. Biophysics of Computation: Information Processing in Single Neurons. New York: Oxford Univ. Press; 1999. [Google Scholar]


