Abstract
Homologs of the transcriptional regulator PtxS are omnipresent in Pseudomonas, whereas PtxR homologues are exclusively found in human pathogenic Pseudomonas species. In all Pseudomonas sp., PtxS with 2-ketogluconate is the regulator of the gluconate degradation pathway and controls expression from its own promoter and also from the Pgad and Pkgu for the catabolic operons. There is evidence that PtxS and PtxR play a central role in the regulation of exotoxin A expression, a relevant primary virulence factor of Pseudomonas aeruginosa. We show using DNaseI-footprint analysis that in P. aeruginosa PtxR binds to the -35 region of the PtoxA promoter in front of the exotoxin A gene, whereas PtxS does not bind to this promoter. Bioinformatic and DNaseI-footprint analysis identified a PtxR binding site in the Pkgu and Pgad promoters that overlaps the -35 region, while the PtxS operator site is located 50 bp downstream from the PtxR site. In vitro, PtxS recognises PtxR with nanomolar affinity, but this interaction does not occur in the presence of 2-ketogluconate, the specific effector of PtxS. DNAaseI footprint assays of Pkgu and Pgad promoters with PtxS and PtxR showed a strong region of hyper-reactivity between both regulator binding sites, indicative of DNA distortion when both proteins are bound; however in the presence of 2-ketogluconate no protection was observed. We conclude that PtxS modulates PtxR activity in response to 2-ketogluconate by complex formation in solution in the case of the PtoxA promoter, or via the formation of a DNA loop as in the regulation of gluconate catabolic genes. Data suggest two different mechanisms of control exerted by the same regulator.
Introduction
The ubiquitous Gram-negative bacterium Pseudomonas aeruginosa is an opportunistic human pathogen which is a frequent cause of hospital-acquired infections including ventilator associated pneumonia, and catheter infections in immuno-compromised patients [1]. Furthermore, P. aeruginosa is an etiologic agent of ear infections [2] and causes infections in severely burned individuals [3] as well as in patients who suffer from cystic fibrosis [4]. The establishment of P. aeruginosa infection is accompanied by the synthesis of several extracellular and cell-associated virulence factors, amongst which is exotoxin A encoded by the toxA gene [5]. Similar to other extracellular virulence factors such as diphtheria-, cholera- and pertussis-toxin, exotoxin A is an ADP-ribosyl transferase that decorates host elongation factor-2, leading to the cessation of protein synthesis and eventually causes cell death [6].
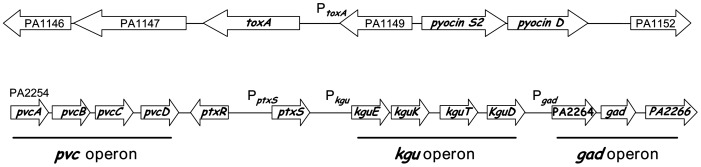
The regulation of the expression of toxA is complex and several gene products are involved in the process. Amongst other proteins the transcriptional regulators RegA, Vfr, Fur, PvdS, PtxR and PtxS have been suggested to play a role in the expression of toxA [7]–[19]. The most enigmatic regulators are PtxR and PtxS since so far no direct interaction of these proteins with the toxA promoter has been documented and the molecular mechanism by which PtxS governs toxA expression is unknown [8], [20]. PtxR is a LysR-type transcriptional regulator that is predicted to harbour a helix-turn-helix DNA binding domain in its N-terminal region and a potential effector recognition domain at its C-terminal extension. The ptxS gene, which in P. aeruginosa is located adjacent and transcribed divergently from the ptxR gene, encodes the regulator of the gluconate degradation pathway [20], Figure 1). PtxS is a member of the LacI family of transcriptional regulators and does not share any significant sequence similarities with PtxR (13% sequence identity in an alignment with 7 gaps), similar to other members of the LacI family PtxS has an N-terminal DNA-binding domain and a C-terminal effector binding domain. In several species of the genus Pseudomonas the role of PtxS in the control of the gluconate degradation pathway has been elucidated (21–24). PtxS was found to bind to a palindromic sequence (5′-TGAAACCGGTTTCA-3′) in the promoter region of the kgu and gad operons as well as to its own promoter [17], [24]. Proteins encoded by the kgu and gad operons are involved in the transport and conversion of 2-ketogluconate into 6-phosphogluconate, which is then funnelled into the Entner-Doudoroff pathway [22]–[27]. PtxS operates as a repressor that binds to the -10 region of the target promoters and occludes RNA polymerase access. PtxS recognizes 2-ketogluconate as an effector, and its binding causes the dissociation of this repressor from its DNA targets, which as a consequence allows transcriptional activation [24]. However, inspection of the toxA upstream region did not reveal any potential PtxS operator site; and the molecular mechanism of the regulatory impact of PtxS on toxA expression is unclear.
Figure 1. Genetic organization of the open reading frames which are under the control of PtxS and PtxR.
The physical organization of the region ptxS and ptxR genes was established by Hamood et al. [8].
This study was aimed at determining the molecular mechanisms by which PtxS and PtxR modulate toxA expression and gluconate catabolic gene expression in P. aeruginosa. We show that purified PtxR binds to the Pkgu and Pgad promoters (Table 1), and in contrast with an earlier report by Colmer and Hamood [20], we found that PtxR binds to PtoxA. PtxS binds to the P. aeruginosa Pkgu and Pgad promoters but not to the toxA promoter (Table 1). In solution PtxS and PtxR interact with nanomolar affinity, although this interaction does not occur in the presence of 2-ketogluconate. Furthermore, evidence is presented which indicates that the simultaneous binding of PtxR and PtxS to the Pgad and Pkgu promoter region provokes DNA loop formation and that in the presence of 2-ketogluconate the loop formation is abolished. Since PtxR binds the toxA promoter but PtxS does not, it appears that the role of PtxS in modulation of toxA is exerted through interaction of PtxS in solution with DNA bound PtxR. Our results indicate that the PtxS and PtxR pair of regulators uses different mechanisms to control expression of ketogluconate metabolism and expression of a virulence factor.
Table 1. Strains and plasmids used in this study.
| Genotype of relevant characteristics | References | |
| Strains | ||
| P. aeruginasa PAO1 | Serotype O5, wild type, Ap | [42] |
| P. aeruginasa ΔPtxS | ptxS::pCHESIΩ-Str | [20] |
| P. aeruginasa ΔPtxR | ptxR::pCHESIΩ-Tc | [20] |
| E. coli DH5αF’ | F’/hsdR17, recA1, gyrA | [39] |
| E. coli BL21 (DE3) | F-, ompI, hsdS B(r− Bm− B)gal, dam, met | [39] |
| Plasmids | ||
| pGEM-T | Cloning vector, Apr | Dominion |
| pMBL-T | Cloning vector, Apr | Dominion |
| Bgal::PtoxA | TcR, pMP220 bearing the promoter region of the toxA | This work |
| pMBL::PtxS | ptxS gene in pMBL vector, Apr | This work |
| pMBL::PtxR | ptxR gene in pMBL vector, Apr | This work |
| pET24b::PtxS | Derivative bearing the ptxS gene, Kmr | This work |
| pET24b::PtxR | Derivative bearing the ptxR gene, Kmr | This work |
| pGEM-T::PtoxA | pGEM-T containing the toxA promoter, Apr | This work |
| pGEM-T::Pkgu | pGEM-T containing the kgu promoter, Apr | This work |
| pGEM-T::Pgad | pGEM-T containing the gad promoter, Apr | This work |
Kmr, Strr, Tcr and Apr stand for resistance to kanamycin, streptomycin, tetracycline and ampicillin, respectively.
Results
PtxS Binds to Ketogluconate Operon Promoters and PtxR Binds to the Pkgu, Pgad and PtoxA Promoters, but not to the PptxS Promoter
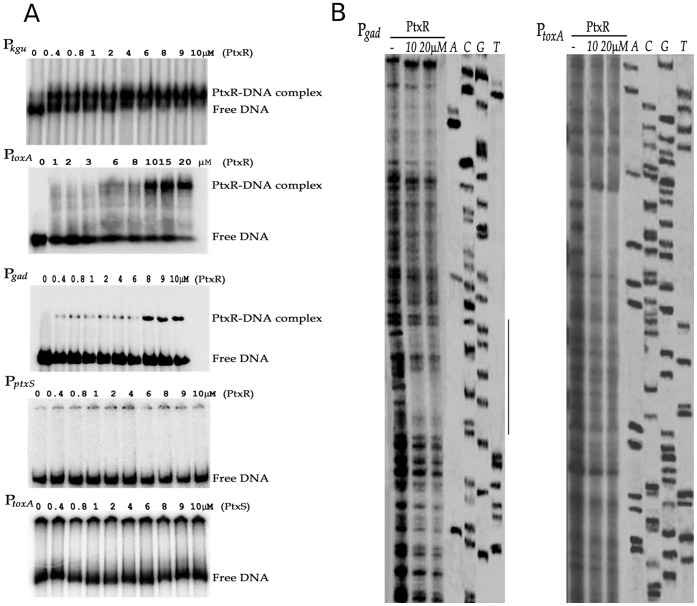
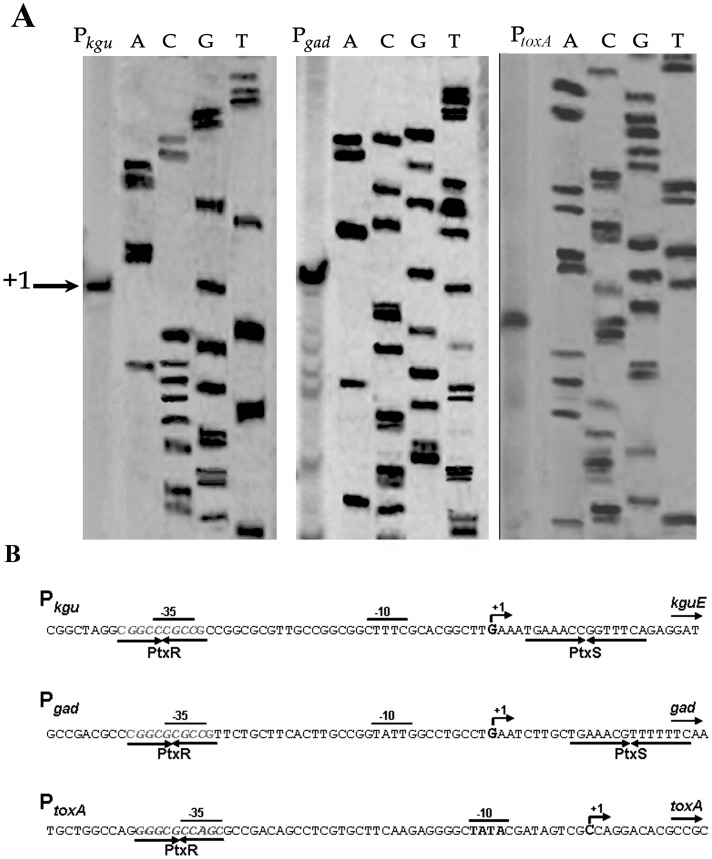
Since PtxR works in conjunction with PtxS to control expression of PtoxA and PtxS regulates the expression of the gad and kgu catabolic operons and its own synthesis [9], [17], [24], we first explored by EMSA (electrophoresis mobility shift assay) if PtxR and PtxS bind to promoters PptxS, Pkgu, Pgad and PtoxA. To this end PtxR and PtxS were produced as recombinant proteins and purified to homogeneity by affinity chromatography. As expected PtxS was able to retard DNA bearing the Pkgu, Pgad and PptxS promoters (Figure S1) but not the PtoxA promoter (Figure 2); surprisingly we found that PtxR binds to PtoxA, Pkgu and Pgad promoters but not to PptxS promoter (Figure 2). To define with precision the sites of interaction of PtxS and PtxR with their targets, we first determined by primer extension the +1 of all four promoters. A single main transcription start point was found and -10/−35 canonical sequences identified (Figure 3). To identify the PtxR and PtxS operator sites, DNAseI footprinting assays were then carried out with Pgad, Pkgu and PtoxA (Figure 2). In accordance to the work described by other authors PtxS recognized a palindromic sequence 5′-TGAAAN4TTTCA-3′ as its target in Pgad and Pkgu [17], [24]. With PtxR the footprint showed the protection of a distinct DNA fragment in the three assayed promoters with a palindromic sequence 5′-CGGCGCGCCCG-3′ that overlaps the -35 region of each promoter (Figure 3B). While our results confirm previous studies with PtxS, this is the first report showing that PtxR binds a specific DNA target sequence.
Figure 2. Interaction of PtxR with promoters Pkgu, PtoxA, Pgad and PptxS.
A) Electrophoretic mobility shift assays for the binding of PtxR to Pkgu , PtoxA , Pgad and PptxS and of PtxS to PtoxA. The size of the fragments used in EMSA were 289-, 248-, 495-, and 324-bp for the Pkgu, Pgad, PtoxA and PtxS respectively. Experiments were carried out with PtxR or PtxS concentrations in the range between 0.4 and 10 µM as described in Materials and Methods. Free DNA and DNA/protein complex are indicated. B) DNAseI footprinting assays of promoter P gad and PtoxA . Experiments were conducted as described in Materials and Methods. From left to right Lane 1: free DNA, lanes 2 and 3: DNA +10 or 20 µM PtxR, respectively, lanes 4 to 7: DNA sequencing ladder. The region protected by PtxR is indicated by a vertical line and the corresponding sequence is shown in Figure 3B.
Figure 3. Analysis of the Pkgu and Pgad promoters.
A) Determination of the transcription start point using primer extension analysis of the Pkgu (left), Pgad (central) and PtoxA (right) promoters. The sequencing ladder was used to estimate the size of the transcript. B) Sequences of the three promoters. The transcriptional start sites are indicated by arrows. The palindromic PtxS and PtxR binding sites, and the -10 and -35 binding sites for the RNA polymerase are shown.
PtxR Binds to its Target Operator
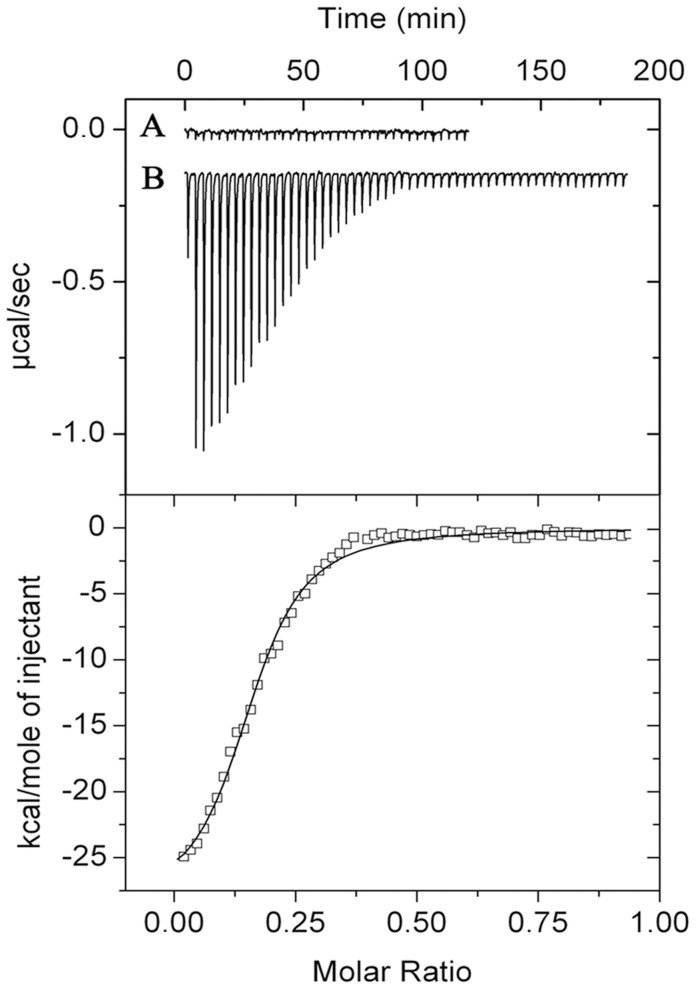
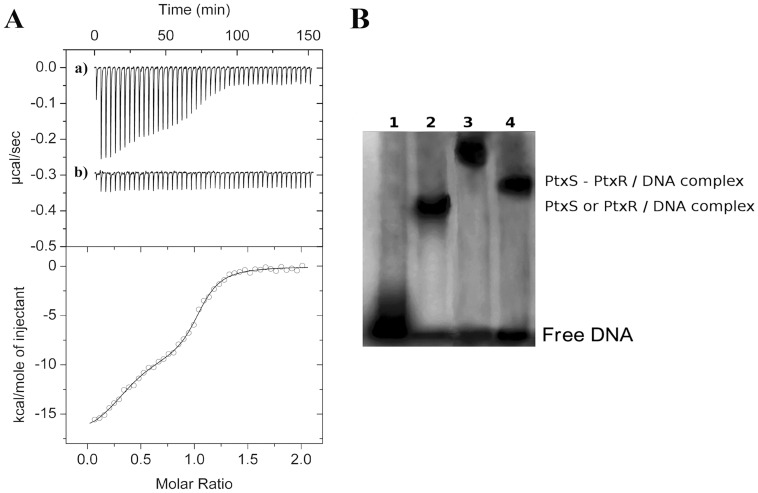
To further study the PtxR-DNA interaction, a 50-mer PtoxA DNA fragment was synthesised which contains the PtxR operator site at its centre and is flanked by additional promoter sequence to avoid potential context effects. The sequence spans the –8 to –58 positions of the promoter region. In order to investigate the potential interaction between PtxR and a duplexed form of this DNA, we carried out ITC assays (Figure 4). The results show that binding of PtxR to its target was driven by favourable enthalpy changes (ΔH = −27.3±0.4 kcal/mol) and counterbalanced by unfavourable entropy changes (TΔS = −17.6 kcal/mol). Binding was tight and a K D of 164±6 nM was determined. It should be noted that PtxR did not bind to the 50-mer duplex DNA containing the operator site of PtxS and it should be also noted that replacement of the central 5 nucleotides of the PtxR binding site in the PtoxA promoter prevents binding of PtxR to this mutant variant (Figure S2).
Figure 4. Microcalorimetric binding studies of PtxR to DNA.
In this series of experiments 5 µM of PtxR was titrated with 3.2 µl aliquots of a 50 µM solution containing the PtxR operator region of PtoxA. Upper panel: Raw titration data. (A) Titration of PtxR with buffer. (B) Titration of PtxR with DNA. Lower panel: Integrated and dilution corrected raw data. Data were fitted with the “One binding site model” of the MicroCal version of ORIGIN.
A three dimensional homology model of PtxR was built using the LysR family protein CrgA of Neisseria meningitidis (PDB: 3 hhg. [28]) as a template. The PtxR homology model revealed that residues K39, S40, E44, R47 and D52 in the HTH (helix-turn-helixcould be involved in interaction with the DNA (Figure S3). Site directed mutants were constructed in which these amino acids were replaced by alanine and the mutant proteins were purified to homogeneity and analysed by microcalorimetric titration assays with the 50-mer PtoxA DNA fragment. A complete absence of binding was observed for mutants K39A, S40A, E44A and D52A, indicating a crucial role of these amino acids for DNA-binding (Figure S2). Mutant R47A bound to DNA with an affinity reduced by a factor of 2 (K D of 240±11 nM).
PtxS Binds Tightly to PtxR While Free in Solution and While Bound to DNA
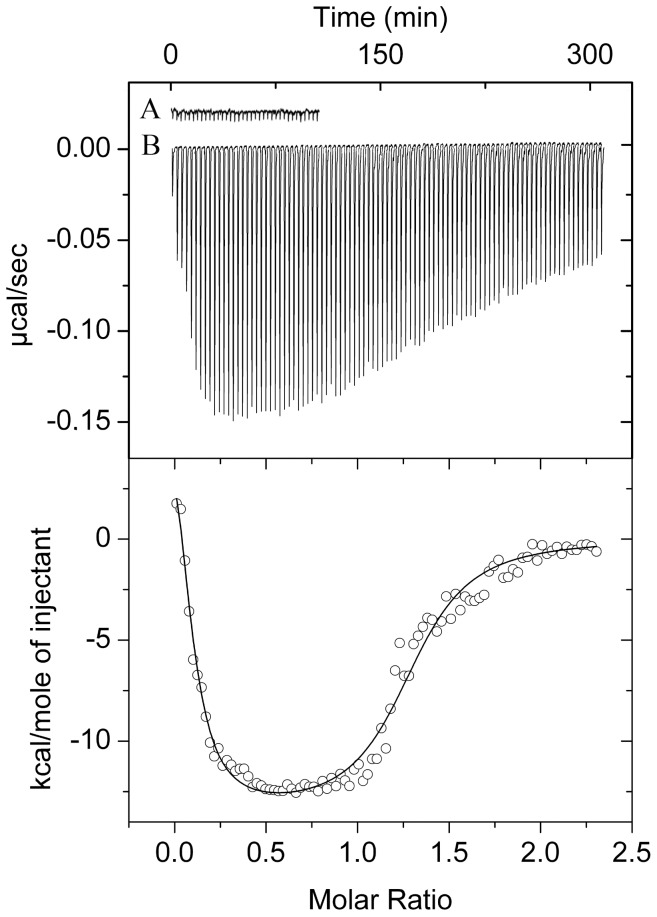
The EMSA pattern observed in Figure 2 suggests that PtxR binds to the PtoxA promoter, however, both PtxS and PtxR have binding sites on the Pkgu and Pgad promoters. This gave rise to a hypothesis in which the regulation of these promoters by PtxR and PtxS may not only be influenced by their ability to bind DNA but also due to potential interactions directly between the two regulators. To check this hypothesis, we carried out electrophoretic analysis with purified proteins by themselves or mixed. We observed that while PtxS and PtxR run as a single band, when the two proteins are mixed an extra band corresponding to the PtxS/PtxR heterodimer becomes visible (Figure S4). To further study this interaction, ITC (isothermal titration calorimetry) assays with PtxS and PtxR were carried out. The initial control experiment involved the injection of 50 µM PtxS into buffer, which gave rise to small and uniform peaks. Subsequently, PtxR (50 µM) was injected into a sample containing 50 µM PtxS, which lead to significant exothermic heat changes (Figure 5A) that saturated at a PtxS:PtxR ratio of approximately 1∶1, indicative of a direct PtxR/PtxS interaction. From the recorded thermogram we deduced the existence of two binding events; an analysis using the “Two binding site model” of the ORIGIN software produced a satisfactory fit (Figure 5A). A first high affinity event with a PtxS/PtxR stoichiometry of 0.33 was characterised by a K D = 12±3 nM and an enthalpy change (ΔH) of −17.8±0.9 kcal/mol. This was followed by an event of lower affinity (K D = 102±10 nM) and lower enthalpy change (ΔH = −9.1±0.5 kcal/mol). The stoichiometry of this second event was 0.69, which implied that the overall stoichiometry considering both events is 1.02 and support that PtxS and PtxR interact with a 1∶1 stoichiometry. The data are congruent with the binding of both proteins in a two-step process, although the exact nature of each step still needs to be determined.
Figure 5. Binding studies of PtxS to native and mutant PtxR.
(A) In this series of experiments 3 µM PtxR was titrated with 3.2 µl aliquots of 50 µM of PtxS in the absence (a), and in the presence (b) of 2-ketogluconate added at a final concentration of 1 mM. Lower panel: Integrated and dilution corrected raw data for the titration of PtxR with PtxS. Data were fitted with the “Two binding site model” of the MicroCal version of ORIGIN. (B) EMSA with target DNA in the absence (Lane 1) or in the presence of 10 µM PtxS (Lane 2), 10 µM PtxR (Lane 4) or 10 µM PtxS and PtxR (Lane 3).
In another series of experiments we determined if DNA-bound PtxR was recognized by PtxS. To this end, a 50-mer DNA fragment of PtoxA was used and a ten fold molar excess of this DNA fragment was mixed with homogeneous PtxR protein so that resulting final concentrations were 5 µM for PtxR and 50 µM for the DNA. Under these conditions all PtxR molecules were bound to DNA. This mixture was titrated with 50 µM PtxS (note: In a control experiment we previously established that PtxS does not bind to this 50-mer DNA fragment). As for the titration of both free proteins, the binding curve was biphasic (Figure 6). Data analysis revealed two events with approximate dissociation constants of 1.7±0.1 and 8.2±0.1 µM. This demonstrated that PtxS also binds to PtxR when it is previously bound to its target operator.
Figure 6. Microcalorimetric studies showing that DNA-bound PtxR is recognized by PtxS.
A mixture of 5 µM of PtxR was mixed with 50 µM of a 35-mer duplex DNA containing the PtxR site in PtoxA. Under these conditions PtxR is entirely saturated with DNA. Then the mixture was titrated with 3.2 µl aliquots of a 50 µM PtxS. A) Injection of PtxR in the buffer; B) injection of PtxS in the DNA/PtxR complex. Upper panel: Raw titration data. Lower panel: Integrated and dilution corrected raw data for the titration of the mixed PtxR/PtoxA with PtxS. Data were fitted with the “Two binding site model” of the MicroCal version of ORIGIN.
The possibility that PtxR binds simultaneously free and DNA-bound PtxS was also assessed using EMSA in which the electrophoretic mobility of free DNA was compared to that of the DNA in complex with each of the proteins or both proteins simultaneously. As shown in Figure 5B the mobility of DNA in complex with both PtxS and PtxR (lane 3) was reduced as compared to the complexes with each protein individually assay (lanes 2 and 4).
We have previously shown that PtxS interacts with 2-ketogluconate (2 KG) with high affinity [24], while PtxR did not interact with gluconate or 2-ketogluconate (Figure S5). Subsequent experiments were aimed at establishing whether 2-ketogluconate binding to PtxS impacts the interaction between both regulator proteins. To this end the microcalorimetric titration shown in Figure S5 was repeated with the only difference that 2-ketogluconate was added to both protein solutions at a final concentration of 1 mM. As shown in Figure S5 the titration resulted in small and uniform peaks which can be entirely attributed to dilution heats. This experiment demonstrates clearly that 2-ketogluconate binding to PtxS prevents a molecular interaction with PtxR.
Since PtxS interacts with free and DNA-bound PtxR, and PtxR binds to PtoxA, Pkgu and Pgad while PtxS has binding sites in only Pkgu and Pgad, different transcription scenarios are feasible in regards to the modulation of gene expression by PtxS and PtxR. We investigated these potential scenarios both in vitro and in vivo using Pgad and PtoxA .
Evidence that Transcriptional Control of the Pgad Promoter is Based on PtxR-PtxS Mediated DNA Loop Formation
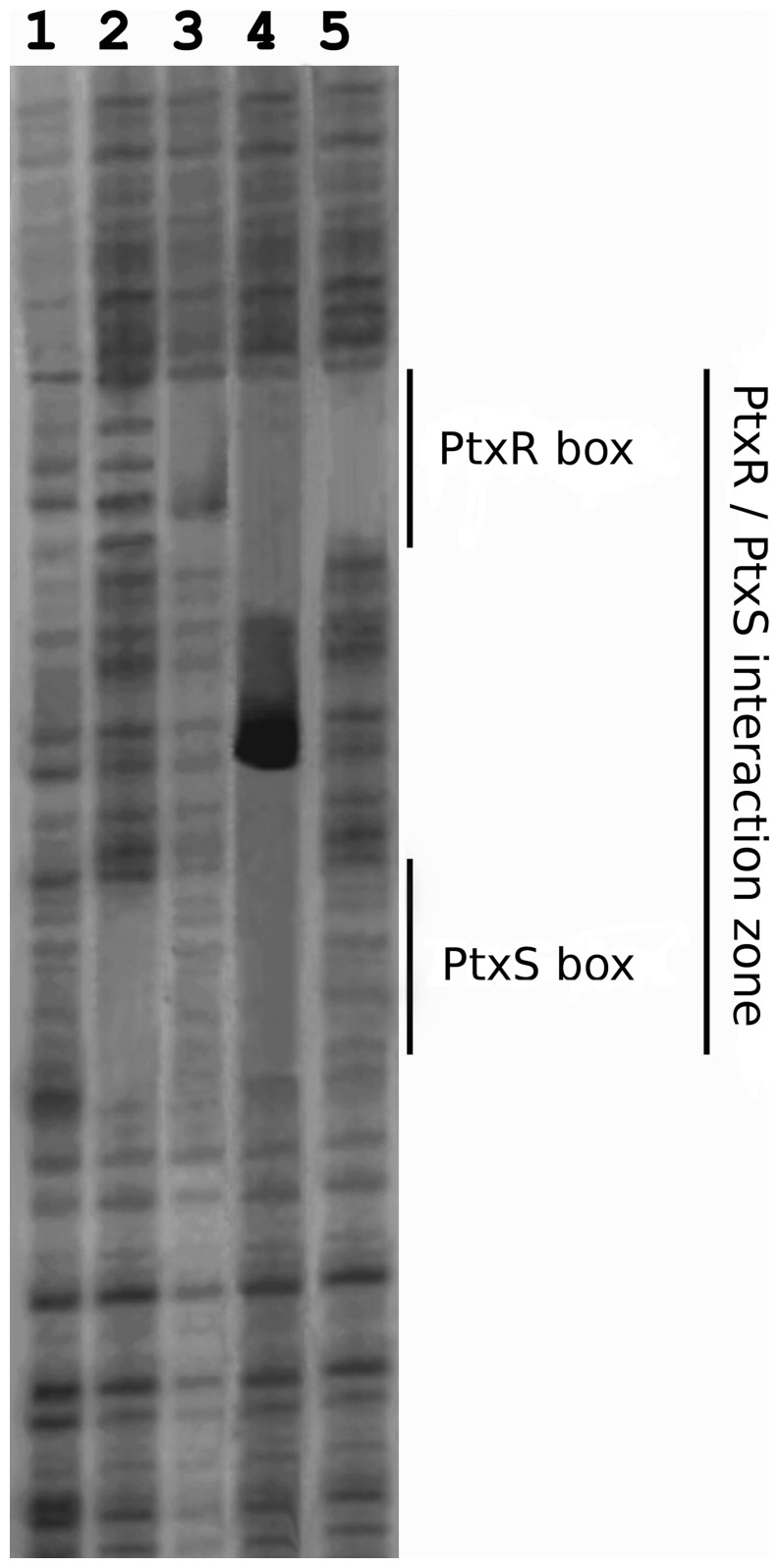
We have shown in this study that both PtxS and PtxR bind the Pgad promoter and that the specific sites for each regulator are relatively distanced from each other, and we have also demonstrated that both regulator proteins bind tightly to each other. Based on these finding, we hypothesized that the interaction of the two DNA-bound regulators induces the formation of a DNA loop structure. To this end, footprint assays were performed with the Pgad promoter in the absence of both regulators, with either PtxS or PtxR and with both regulator proteins.
Lane 1 in Figure 7 corresponds to the pattern of the Pgad promoter in the absence of protein, while lanes 2 and 3 correspond to the pattern obtained in the presence of PtxS and PtxR, respectively. The protection of DNA by bound PtxS (lane 2) and bound PtxR (lane 3) is apparent. When PtxS and PtxR were present with DNA, as is the case in lane 4, apart from the binding sites of both proteins a series of very strong bands appear which correspond to a DNAseI hyperreactivity region. This region of hyperreactivity is located between the binding sites of PtxR and PtxS and its precise location is indicated in Figure 3B. When the assay was repeated with PtxR and PtxS with 2-ketogluconate (lane 5), then the protection by PtxR was evident, while PtxS site was no protected as expected from the release of the protein from its DNA target site (lane 5). The data suggest the formation of a DNA loop in the presence of both regulator proteins in the absence of 2-ketogluconate. For PtoxA to which PtxR binds but PtxS does not, the footprint assay in the presence of PtxR with or without PtxS was similar though a bit larger shadow was seen when PtxS was present (not shown).
Figure 7. DNAseI footprinting assay of Pgad promoter with PtxS, PtxR or both proteins.
Conditions as in the legend for Figure 2. Lane 1, free DNA; Lane 2 DNA with 10 µM PtxS; Lane 3, DNA with 10 µM PtxR; lane 4 DNA with 10 µM PtxS +10 µM PtxR, lane 5 as lane 4 but with 1 mM 2-ketogluconate.
We used PtoxA::lacZ and Pkgu::lacZ to monitor expression of these promoters in vivo in the PAO1 background and isogenic ΔptxS and ΔptxR backgrounds. Expression from PtoxA or Pkgu in the parental background increased 3- to 4-fold in response to 2-ketogluconate. In the ΔptxR mutant expression in the absence of 2-ketogluconate was lower than in the parental strain and this level of expression did not increase with 2-ketogluconate. In ΔptxS expression was high regardless of 2-ketogluconate and close to the highest levels measured in the parental background with 2-ketogluconate. This suggests that PtxS interacts with PtxR and prevents PtxR activity as an activator of expression of toxA.
Discussion
Transcription regulation is the major method of gene expression control in prokaryotic cells and is modulated by proteins that interact with RNA polymerase, such as sigma factors and transcriptional regulators. The most common type of transcriptional regulators are made of two domains, one functioning as the sensor for signals, and the other a DNA binding domain. In some cases more than one protein is involved in the activation/repression of transcription in response to a signal as is the case with two-component regulatory systems. Recently some prokaryotic regulatory systems have been revealed to be more complex with an increasing number of reports of three proteins involved in regulation [28]–[34].
In E. coli about 50% of promoters are under the control of one specific regulator, while 50% of E. coli promoters are modulated by two or more transcriptional factors [34]. Promoters involved in the construction of cell structures, i.e., flagella, pili, and fimbrae, and complex cellular processes, i.e., virulence, biofilm formation, are often controlled by multiple environmental signals and different transcription factors operate to modulate this control. This seems to be the case for the control of the toxA gene of Pseudomonas aeruginosa that encodes the most toxic virulence factor of this microorganism [6], [8], [9]. For example, to transcribe this promoter RNA polymerase drives expression with either σ70 or the alternative PvdS sigma factor depending on iron conditions; in addition the global regulator Vfr modulates expression from PtoxA. Ferrell et al. [11] showed that Vfr regulates toxA by influencing the level of expression of ptxR but one of the most enigmatic features was that the expression of toxA is modulated by PtxR and PtxS but no binding to DNA was reported. In this study we confirmed that in a ΔptxR background, expression of toxA is almost three-fold lower than in the parental strain, an observation which confirms the positive role of PtxR. We show in this study that PtxR binds PtoxA and that PtxS and PtxR, two one-component regulators belonging to different families, interact with each other to modulate expression of toxA and the catabolism of gluconate.
We have previously reported that PtxS binds to identical sites at PptxS, Pkgu and Pgad [24]. Using EMSA assays we have now shown that PtxR recognized the promoters Pkgu , Pgad and PtoxA (Figure 2), albeit with different binding affinity. These different affinities could be due to local differences in DNA structure and some minor sequence differences as has been observed for other repressors, e.g. the TtgV repressor binds more tightly to the PttgD promoter than PttgG because of local differences in DNA sequence and the bending angle of the DNA, which influences the level of transcription [35]. In this study we also show using footprint assays and ITC, that PtxR binds to the toxA promoter, and that the operator site corresponds to a short palindrome whose sequence is 5′- CGCCGCCGCG -3′, this motif was found to overlap the -35 site of RNA polymerase.
There is evidence that PtxS negatively regulates ptxR expression [20], and we confirmed in this work that PtxR enhances the transcription of toxA. Beta-galactosidase measurements showed that the specific effector molecule of PtxS, 2-ketogluconate, induces toxA expression in vivo (Table 2). ITC data provide evidence that 2-ketogluconate is recognized by PtxS but not by the regulator PtxR. The increase in toxA promoter activity in the parental strain in the presence of 2-ketogluconate is thus mediated by the binding of this effector to PtxS and by the activating role which PtxR has on the PtoxA promoter. A major conclusion of this work resides thus in the demonstration of the link between carbon metabolism and the expression of the virulence factor gene toxA; however, exactly how these results fit within the infection/virulence process cannot be derived from only these data and a series of in vivo assays with tissue cultures and animals are currently being designed to answer these questions.
Table 2. Expression of PtoxA in the wild-type, ptxS and ptxR deficient backgrounds.
| Strain | PtoxA:’lacZ | Pkgu::’lacZ | ||
| Without ketogluconate | + ketogluconate | Without ketogluconate | + ketogluconate | |
| wt | 415±40 | 1545±90 | 300±50 | 850±100 |
| ΔptxS | 1630±40 | 1390±50 | 875±50 | 800±100 |
| ΔptxR | 175±60 | 210±25 | 230±5 | 250±50 |
The promoter region of toxA or kgu was cloned into pMP220 (TcR) and the resulting plasmid electroporated into the indicated strains. Cells were grown on M9 minimal medium with citrate (15 mM) and overnight cultures were diluted 50-fold in the same medium in the absence or in the presence of 2-ketogluconate (5 mM). β-galactosidase activity was determined in cells in the exponential phase of growth after 3 hours of incubation. Data are the average of 3 independent assays each performed in duplicate.
PtxS Uses Two Repressor Mechanisms Depending on the Promoter it Regulates
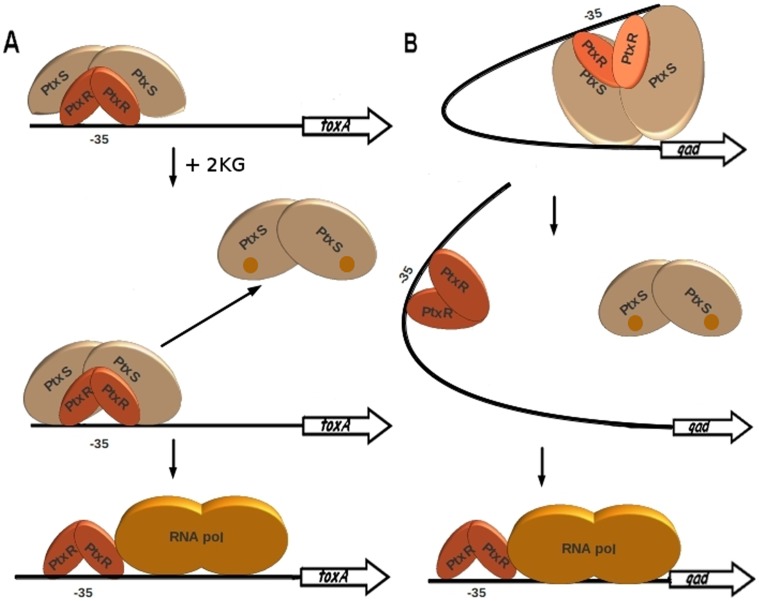
The PtxR operator in Pkgu and Pgad overlaps the -35 region and the PtxS site is found around 50 bp downstream. In analogy to these promoters PtoxA also has a PtxR operator site overlapping the -35 region but our data showed that PtxS does not bind to this promoter. ITC data demonstrated that PtxS forms a tight complex with PtxR, either in its free form or when bound to DNA. The molecular mechanism of PtxS mediated regulation of PtoxA expression is therefore likely based on the formation of a protein complex at the -35 region. The binding of PtxS in solution to DNA-bound PtxR prevents the activation effect of PtxR on transcription. Binding of 2-ketogluconate to PtxS causes the latter protein to dissociate (Figure 8A). The activator role of bound PtxR might reside in the recruitment of the RNA polymerase and to allow transcription. Therefore, the role of PtxS is likely to be that of interfering with this process by blocking PtxR-RNA polymerase interaction or simply by steric hindrance.
Figure 8. Schematic diagrams of the PtoxA and Pgad regulation models.
Left.- The PtxR dimer binds the -35 region of PtoxA and PtxS in solution inhibits other interactions; with 2-ketogluconate (2 KG) PtxS is released and PtxR can recruit RNA polymerase to promote transcription. Right.- PtxS and PtxR bound to their operator sites interact and induce DNA bending, when 2-ketogluconate is present the PtxS repressor is released and PtxR recruits RNA polymerase to facilitate transcription from the catabolic promoter.
In contrast to PtoxA, PtxR and PtxS bind to both Pkgu and Pgad. Given that both proteins also interact with each other we conducted experiments to verify whether DNA bound regulators also interact with each other when bound to DNA. Since the operator sites are spaced by around 50 bp, an interaction between these regulators would introduce a DNA loop. Data reported in this work are entirely consistent with this hypothesis. DNAseI footprint assays showed a site of hyperreactivity in an area between both operators, that is taken as evidence of DNA distorsion probably via DNA loop formation. The loop would prevent RNA polymerase access to the promoter, while in the presence of 2-ketogluconate the PtxS protein is released and recruitment of RNA polymerase allow transcription of ketogluconate genes. The results of this study have led to define the molecular mechanism by which the concerted action of PtxS and PtxR modulates toxA expression.
Materials and Methods
Bacterial Strains and Plasmids used in this Study
The genotype or the relevant characteristics of the bacterial strains and plasmids used in this study are listed in Table 1. Bacterial strains were grown in LB medium or in modified M9 minimal medium with 5 mM citrate or 2-ketogluconate as the sole C-source [36]. When required, antibiotics were added to the culture medium to reach a final concentration of 25 µg/ml kanamycin, 50 µg/ml ampicillin and 10 µg/ml tetracycline. Escherichia coli strain DH5α was used for plasmid construction and E. coli BL21 (DE3) was used for protein production.
Expression and Purification of His-tagged PtxS and PtxR Proteins in Escherichia coli
To produce polyhistidine-tagged proteins, the ptxS and ptxR genes were cloned into plasmid pET24b(+). For this primers PtxSPAO1.f and PtxSPAO1.r or primers PtxRPAO1.f and PtxRPAO1.r (Table S1) were used to amplify the ptxS and ptxR genes, respectively, from chromosomal DNA of P. aeruginosa PAO1. The amplicons that contained restriction sites for NheI and XhoI were digested and then cloned into the pMBL vector to yield pMBL::PtxS and pMBL::pPtxR (Table 1). The NheI/XhoI fragments were subsequently excised from these plasmids and cloned into NheI-XhoI-digested pET24b(+) to produce pET24b::PtxS and pET24b::PtxR, which allow the expression of PtxS and PtxR recombinant proteins containing a C-terminal hexahistidine tag. Escherichia coli BL21 (DE3) harbouring the pET24b derivatives was grown in 2 litre Erlenmeyer flasks containing 250 ml of LB supplemented with 25 µg/ml kanamycin. Cultures were incubated at 30°C with shaking until a turbidity at 660 nm of 0.6 was reached, then 1 mM isopropyl-β-D-thiogalactopyranoside was added to induce the expression of the ptxS and ptxR genes. The cultures were then incubated at 18°C overnight and cells were harvested by centrifugation (30 min at 20,000×g), and stored at −80°C until used for protein purification.
For protein purification, cells were suspended in 25 ml of buffer A (50 mM Tris-HCl pH 7.9; 300 mM NaCl; 1 mM DTT; 10 mM imidazol) supplemented with a tablet of complete™ EDTA-free protease inhibitor. Cells were lysed by three passes through a French Press at a pressure of 1000 p.s.i. The cell suspension was then centrifuged at 20,000×g for 1 hour. The pellet was discarded and the supernatant was filtered and loaded onto a 5 ml His-Trap chelating column (GE Healthcare, St. Gibes, UK) previously equilibrated with buffer A.
The proteins were eluted with a 10 to 500 mM gradient of imidazol in buffer A, the protein concentration was determined as described [37], and protein purity was verified by SDS-PAGE (sodium dodecyl sulphate-polyacrylamide gel electrophoresis). The apparently homogenous protein was dialyzed overnight against buffer B (50 mM HEPES pH 7.9, 300 mM NaCl, 1 mM DDT, and 10% [v/v] glycerol), adjusted to 11 mg/ml and stored at −80°C.
Site-directed Mutagenesis
PtxR mutants were generated by amplification of the ptxR gene in plasmid pET24b::PtxR using pfu turbo DNA polymerase (Stratagene) and 39 mer overlapping primers (Table S1) that incorporated appropriate mismatches to introduce the desired mutation(s) [37]. The nature of each mutant allele was confirmed by DNA sequencing. The PtxR mutant proteins were produced in E. coli BL21 (DE3) transformed with the appropriate plasmid. A standard purification following the protocol described above yielded 8 to 10 mg of homogeneous protein per L of culture.
Isothermal Titration Calorimetry
Microcalorimetric experiments were carried out at 20°C using a VP-microcalorimeter (Microcal, Amherst, MA). PtxS and PtxR proteins and DNA were dialyzed against 50 mM HEPES buffer, pH 7.9; 300 mM NaCl; 1 mM dithiothreitol; 10% (v/v) glycerol. For DNA binding studies, oligonucleotides corresponding to both strands of the PtxR binding site at the toxA promoter (5′-GATATCGGCTGCTGGCCAGGGGCG CCAGCGCCGACAGCCTCGTGCTTCAA-3′) were synthesized. Annealing was carried out by mixing 200 µM of each of the complementary oligonucleotides in 50 mM phosphate buffer pH 7.0, 0.5 mM EDTA, 2.5 M NaCl. The mixture was incubated at 90°C for 30 min and then the samples were allowed to cool to room temperature. Typically, reverse titrations (DNA into protein) involved the injection of aliquots of 15–50 µM DNA into a solution of 5 µM of PtxS or PtxR proteins [38]. All data were corrected using the heat changes arising from injection of the ligand into buffer. The titration data were analyzed using the “one-binding site model” of the MicroCal version of ORIGIN. The parameters ΔH (reaction enthalpy), K A (binding constant, K A = 1/K D), and n (reaction stoichiometry) were determined from the curve fit. The change in free energy (ΔG) and in entropy (ΔS) was calculated from the values of K A and ΔH with the equation:
where R is the universal molar gas constant and T is the absolute temperature.
Transcriptional Fusions to ‘lacZ
To obtain a transcriptional fusion of the promoter of the toxA and kgu genes to the ‘lacZ reporter, the corresponding region was amplified using P. aeruginosa strain PAO1 chromosomal DNA as template and primers incorporating PstI and BglII restriction sites. Upon amplification, the DNA fragments were cloned into the pGEM-T plasmid. Clones were sequenced to verify the absence of mutations (Table 1). The PstI-BglII fragment was subsequently excised from the pGEM-T derivative and cloned into the pMP220 promoter probe vector using the same restriction sites. Resulting plasmids were transformed into wild-type P. aeruginosa PAO1 and its ptxS and ptxR isogenic mutants.
β-Galactosidase Assays
P. aeruginosa PAO1 and its isogenic ptxS or ptxR mutants were grown in minimal medium with citrate as the sole C-source in the presence or absence of 5 mM of 2-ketogluconate. Overnight cultures were diluted to a turbidity of 0.01 in the same minimal medium. Growth was continued at 37°C and after 3 hours aliquots were taken and ß-galactosidase activity was determined with o-nitrophenyl-ß-D-galactoside as a substrate in permeabilized whole cells as described by Miller [39]. At least three independent assays were performed, and activity was expressed in Miller Units.
RNA Extraction and Primer Extension
P. aeruginosa PAO1 cells were grown on M9 minimal medium supplemented with 5 mM 2-ketogluconate. RNA was extracted using the TRI reagent protocol (Ambion) and its integrity was assessed by agarose gel electrophoresis. RNA concentration was determined spectrophotometrically at 260 nm. Primer extension reactions were performed as described by Marqués et al. [40] with the set of primers indicated in Table S1.
Electrophoretic Mobility Shift Assays
The Pkgu, PptxS, Pgad and PtoxA promoter regions of P. aeruginosa PAO1 were amplified by PCR using pGEM-T:Pkgu, pGEM-T:PptxS, pGEM-T:Pgad, and pGEM-T:PtoxA respectively, as templates and the set of primer pairs indicated in Table S1. Amplified fragments were isolated from agarose gels and end-labelled with [γ-32P] deoxy-ATP using T4 polynucleotide kinase. A 10 µl sample containing approximately 2 nM of labelled DNA (1.5×104 cpm) was incubated with increasing concentrations of purified PtxS or PtxR for 1 h at 30°C in 10 µl of binding buffer (50 mM Tris-HCl pH 7.5; 10 mM NaCl, 0.5 M magnesium acetate, 0.1 mM EDTA; 1 mM DTT, 5% [vol/vol] glycerol) containing 20 µg/ml of polyd(IC) and 200 µg/ml bovine serum albumin. DNA-protein complexes were resolved by electrophoresis in 4% (wt/vol) nondenaturing polyacrylamide gels in 1×TBE using a BioRad electrophoresis apparatus as described previously [41]–[43].
DNaseI Footprinting
DNA fragments containing Pgad and PtoxA of P. aeruginosa PAO1 were amplified as outlined above. DNA was labelled with [γ-32P] deoxy-ATP and 10 µl samples containing 2 nM of probe were mixed with different amounts of PtxR (10 and 20 µM) in binding buffer for the formation of the DNA-PtxR complex. Samples were incubated at 30°C for 1 h, which was followed by the addition of DNase I (0.4 U; Roche Biochemicals). After incubation for 30 min, the reaction was stopped by adding 2 µl of 500 mM EDTA. DNA was extracted with phenol-chloroform, ethanol precipitated and dissolved in 10 µl of sequence loading buffer. After incubation at 95°C for 5 min, DNA was loaded onto a 6.5% (wt/vol) DNA sequencing gel [37]. Appropriate sequencing reactions were loaded onto the gels along with the footprinting samples and used as a size ladder for identification of the sequences of protected sites.
Supporting Information
Interaction of PtxS with promoters P kgu , P toxA , P gad and P ptxS . Electrophoretic mobility shift assays for the binding of PtxS to Pkgu , Pgad and PptxS. Experiments were carried out with PtxS concentrations in the range between 0.4 to 10 µM. Free DNA and DNA/protein complex are indicated. The size of the fragments used in this assay is given in the Legend for Figure 2.
(TIF)
Amino acids involved in DNA binding. A) The PtxR homology model was built based on the 3D structure of the CrgA protein of Neisseria that presents 40% identity to PtxR. B) amino acids which are potentially involved in DNA binding are highlighted. In the zoom of the recognition helix of the HTH motif. Amino acids which were mutated to alanine are shown in the ball-and-stick form.
(TIF)
Lack of interaction of PtxR with a mutant variant of P toxA promoter or of a mutant PtxR (D52A) with wild-type toxA promoter. A) The microcalorimetric titration with the 50-mer toxA nucleotide exhibiting 5 nucleotide changes (5-GATATCGGCTGCTGGCCAGGCCGACAGCCTCGTGCTTCAA-3′) was carried out as described in the legend for Figure 4 in this article. B) The EMSA assay of the wild-type PtoxA promoter with increasing concentrations of PtxRD52A was carried out as described in Figure 2A.
(TIF)
Native gel electrophoresis of PtxS, PtxR and PtxS/PtxR samples. Native gel polyacrylamide electrophoresis was prepared as described by Fenner et al. (43). Homogenous 10 µM samples of PtxS (lane 1), PtxR (lane 2), PtxS+PtxR (lane 3) and PtxS/PtxR with 1 mM 2-ketogluconate (lane 4) were solved for 1 h at 120 V. Gels were stained with Coomassie Brilliant Blue staining solution (1 g of Serva Blue R-250 into 1 L of water/methanol/acetic acid (50∶40:10)). Data were confirmed by western-blot using an anti-His tag antibody.
(TIF)
Microcalorimetric analysis of the interaction of PtxR and PtxS with 2-ketogluconate. Upper panel: A) Titration of 120 µM PtxR with 3.2 µl aliquots of 500 µM 2-ketogluconate. B) Titration of 120 µM de PtxS with 3.2 µl aliquots 500 µM 2-ketogluconate. Lower panel: Integrated and dilution corrected peak areas of raw data shown in B. Data were fitted with the “One binding site model” of the MicroCal version of ORIGIN.
(TIF)
Sequences of primers used in this study.
(DOC)
Acknowledgments
We thank M.M. Fandila for secretarial assistance and B. Pakuts for improving the English in this manuscript. We thank Prof. Abdul. N. Hamood and Prof. Udo Blaesi for providing us with P. aeruginosa mutants.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by the European Research Area Network (ERANET) programme Pathogenomics within the ADHERS BIO2008-04419-E/project. We also received Fondos Europeos para El Desarrollo (FEDER) grants BIO-2006-05668, BIO2010-17227 and CVI-3010. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chastre J, Fagon JY. Ventilator-associated pneumonia. J Bacteriol. 2002;192:4357–4366. [Google Scholar]
- 2.Dohar JE, Hebda PA, Veeh R, Awad M, Costerton JW, et al. Mucosal biofilm formation on middle-ear mucosa in a nonhuman primate model of chronic suppurative otitis media. Laryngoscope. 2005;8:1469–1472. doi: 10.1097/01.mlg.0000172036.82897.d4. [DOI] [PubMed] [Google Scholar]
- 3.Montie TC, Doyle-Huntzinger D, Craven RC, Holder IA. Loss of virulence associated with absence of flagellum in an isogenic mutant of Pseudomonas aeruginosa in the burned-mouse model. Infect Immun. 1982;3:1296–1298. doi: 10.1128/iai.38.3.1296-1298.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hassett DJ, Korfhagen TR, Irvin RT, Schurr MJ, Sauer K, et al. Pseudomonas aeruginosa biofilm infections in Cystic Fibrosis: insights into pathogenic processes and treatment strategies. Expert Opin. Ther Targets. 2010;14:117–130. doi: 10.1517/14728220903454988. [DOI] [PubMed] [Google Scholar]
- 5.Iglewski BH, Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin. Proc Natl Acad Sci USA. 1975;6:2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamood AN, Colmer-Hamood JA, Carty NL. Regulation of Pseudomonas aeruginosa Exotoxin A synthesis. In Pseudomonas: Virulence and Gene Regulation.Vol. 2. Edited by J.L. Ramos. Kluwer. Academic/Plenum publishers, New York. pp 389–423. 2004.
- 7.Frank DW, Iglewski BH. Kinetics of toxA and regA mRNA accumulation in Pseudomonas aeruginosa. J Bacteriol. 1988;10:4477–4483. doi: 10.1128/jb.170.10.4477-4483.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamood AN, Colmer JA, Ochsner UA, Vasil ML. Isolation and characterization of a Pseudomonas aeruginosa gene, ptxR, which positively regulates exotoxin A production. Mol Microbiol. 1996;1:97–110. doi: 10.1046/j.1365-2958.1996.6251337.x. [DOI] [PubMed] [Google Scholar]
- 9.Colmer JA, Hamood AN. Characterization of ptxS, a Pseudomonas aeruginosa gene which interferes with the effect of the exotoxin A positive regulatory gene, ptxR. Mol Gen Genet. 1998;3:250–259. doi: 10.1007/s004380050729. [DOI] [PubMed] [Google Scholar]
- 10.Gaines JM, Carty NL, Tiburzi F, Davinic M, Visca P, et al. Regulation of the Pseudomonas aeruginosa toxA, regA and ptxR genes by the iron-starvation sigma factor PvdS under reduced levels of oxygen. Microbiology. 2007;153:4219–4233. doi: 10.1099/mic.0.2007/011338-0. [DOI] [PubMed] [Google Scholar]
- 11.Ferrell E, Carty NL, Colmer-Hamood JA, Hamood AN, West SE. Regulation of Pseudomonas aeruginosa ptxR by Vfr. Microbiology. 2008;154:431–439. doi: 10.1099/mic.0.2007/011577-0. [DOI] [PubMed] [Google Scholar]
- 12.Davinic M, Carty NL, Colmer-Hamood JA, San Francisco M, Hamood AN. Role of Vfr in regulating exotoxin A production by Pseudomonas aeruginosa. Microbiology. 2009;155:2265–2273. doi: 10.1099/mic.0.028373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barton HA, Johnson Z, Cox CD, Vasil AI, Vasil ML. Ferric uptake regulator mutants of Pseudomonas aeruginosa with distinct alterations in the iron-dependent repression of exotoxin A and siderophores in aerobic and microaerobic environments. Mol Microbiol. 1996;5:1001–1017. doi: 10.1046/j.1365-2958.1996.381426.x. [DOI] [PubMed] [Google Scholar]
- 14.Hedstrom RC, Funk CR, Kaper JB, Pavlovskis OR, Galloway DR. Cloning of a gene involved in regulation of exotoxin A expression in Pseudomonas aeruginosa. . Infect Immun. 1986;51:37–42. doi: 10.1128/iai.51.1.37-42.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ochsner UA, Vasil AI, Vasil ML. Role of the ferric uptake regulator of Pseudomonas aeruginosa in the regulation of siderophores and exotoxin A expression: purification and activity on iron-regulated promoters. J Bacteriol. 1995;177:7194–7201. doi: 10.1128/jb.177.24.7194-7201.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ochsner UA, Johnson Z, Lamont IL, Cunliffe HE, Vasil ML. Exotoxin A production in Pseudomonas aeruginosa requires the iron-regulated pvdS gene encoding an alternative sigma factor. Mol Microbiol. 1996;21:1019–1028. doi: 10.1046/j.1365-2958.1996.481425.x. [DOI] [PubMed] [Google Scholar]
- 17.Swanson BL, Hamood AN. Autoregulation of the Pseudomonasaeruginosa protein PtxS occurs through a specific operator site within the ptxS upstream region. J Bacteriol. 2000;182:4366–4371. doi: 10.1128/jb.182.15.4366-4371.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Storey DG, Frank DW, Farinha MA, Kropinski AM, Iglewski BH. Multiple promoters control the regulation of the Pseudomonas aeruginosa regA gene. Mol Microbiol. 1990;4:499–503. doi: 10.1111/j.1365-2958.1990.tb00616.x. [DOI] [PubMed] [Google Scholar]
- 19.West SE, Kaye SA, Hamood AN, Iglewski BH. Characterization of Pseudomonas aeruginosa mutants that are deficient in exotoxin A synthesis and are altered in expression of regA, a positive regulator of exotoxin A. Infect Immun. 1994;62:897–903. doi: 10.1128/iai.62.3.897-903.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colmer JA, Hamood AN. Molecular analysis of the Pseudomonas aeruginosa regulatory genes ptxR and ptxS. Can J Microbiol. 2001;47:820–838. [PubMed] [Google Scholar]
- 21.Del Castillo T, Duque E, Ramos JL. A set of activators and repressors control peripheral glucose pathways in Pseudomonas putida to yield a common central intermediate. J Bacteriol. 2008;190:2331–2339. doi: 10.1128/JB.01726-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Del Castillo T, Ramos JL. Simultaneous catabolite repression between glucose and toluene metabolism in Pseudomonas putida is channeled through different signaling pathways. J Bacteriol. 2007;189:6602–6610. doi: 10.1128/JB.00679-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del Castillo T, Ramos JL, Rodríguez-Herva JJ, Führer T, Sauer U, et al. Convergent peripheral pathways catalyze initial glucose catabolism in Pseudomonas putida: Genomic and flux analysis. J Bacteriol. 2007;189:5142–5152. doi: 10.1128/JB.00203-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daddaoua A, Krell T, Alfonso C, Morel B, Ramos JL. Compartmentalized glucose metabolism in Pseudomonas putida is controlled by the PtxS repressor. J Bacteriol. 2010;192:4357–4366. doi: 10.1128/JB.00520-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Entner N, Doudoroff M. Glucose and gluconic acid oxidation of Pseudomonas saccharophila. J Biol Chem. 1952;196:853–862. [PubMed] [Google Scholar]
- 26.Führer T, Fischer E, Sauer U. Experimental identification and quantification of glucose metabolism in seven bacterial species. J Bacteriol. 2005;187:1581–1590. doi: 10.1128/JB.187.5.1581-1590.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conway T. The Entner-Doudoroff pathway: history, physiology and molecular biology.FEMS Microbiol Rev. 1992;9:1–27. doi: 10.1111/j.1574-6968.1992.tb05822.x. [DOI] [PubMed] [Google Scholar]
- 28.Sainsbury S, Lane LA, Ren J, Gilbert RJ, Saunders NJ, Robinson CV, et al. The structure of CrgA from Neisseria meningitidis reveals a new octameric assembly state for LysR transcriptional regulators. Nucleic Acids Res. 2009;37:4545–4558. doi: 10.1093/nar/gkp445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galperin MY. Diversity of structure and function of response regulator output domains. Curr Opin Microbiol. 2010;13:150–159. doi: 10.1016/j.mib.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buelow DR, Raivio TL. Three (and more) component regulatory systems-auxiliary regulators of bacterial histidin kinase. Mol Microbiol. 2010;75:547–566. doi: 10.1111/j.1365-2958.2009.06982.x. [DOI] [PubMed] [Google Scholar]
- 31.Krell T, Lacal J, Muñoz-Martínez F, Reyes-Darias JA, et al. Diversity at its best: bacterial taxis. Env Microbiol. 2011;13:1115–1124. doi: 10.1111/j.1462-2920.2010.02383.x. [DOI] [PubMed] [Google Scholar]
- 32.Scharf BE. Summary of useful methods for two-component system research. Curr Opin Microbiol. 2010;13:246–252. doi: 10.1016/j.mib.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Thomason PA, Traynor D, Cavet G, Chang WT, Harwood AJ, et al. An intersection of the cAMP/PKA and two-component signal transduction systems in Dictyostelium. EMBO J. 1998;17:2838–2845. doi: 10.1093/emboj/17.10.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishihama A. Prokaryotic genome regulation: multifactor promoters, multitarget regulators and hierarchic networks. FEMS Microbiol Rev. 2010;34:628–645. doi: 10.1111/j.1574-6976.2010.00227.x. [DOI] [PubMed] [Google Scholar]
- 35.Fillet S, Vélez M, Lu D, Zhang X, Gallegos MT, Ramos JL. TtgV represses two different promoters by recognizing different sequences. J Bacteriol. 2009;191:1901–1909. doi: 10.1128/JB.01504-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abril MA, Michán C, Timmis KN, Ramos JL. Regulator and enzyme specificities of the TOL plasmid-encoded upper pathway for degradation of aromatic hydrocarbons and expansion of the substrate range of the pathway. J Bacteriol. 1989;171:6782–6790. doi: 10.1128/jb.171.12.6782-6790.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daniels C, Daddaoua A, Lu D, Zhang X, Ramos JL. Domain cross-talk during effector binding to the multidrug binding TtgR regulator. J BiolChem. 2010;285:21372–21381. doi: 10.1074/jbc.M110.113282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krell T. Microcalorimetry: A response to challenges in modern biotechnology. Microb Biotechnol. 2008;1:126–136. doi: 10.1111/j.1751-7915.2007.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller JH. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y. 1972.
- 40.Marqués S, Ramos JL, Timmis KN. Analysis of the mRNA structure of the Pseudomonas putida TOL meta fission pathway operon around the transcription initiation point, the xylTE and the xylFJ regions. Biochim Biophys Acta. 1993;1216:227–236. doi: 10.1016/0167-4781(93)90149-8. [DOI] [PubMed] [Google Scholar]
- 41.Sasse J, Gallagher SR. Staining proteins in gels. Curr Protoc Immunol. 2004;8:89. doi: 10.1002/0471142735.im0809s58. [DOI] [PubMed] [Google Scholar]
- 42.Hancock RE, Carey AM. Outer membrane of Pseudomonas aeruginosa: heat-2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979;140:902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fenner C, Traut RR, Mason DT, Wikman-Coffelt J. Quantification of Coomassie Blue stained proteins in polyacrylamide gels based on analyses of eluted dye. Anal Biochem. 1975;63:595–602. doi: 10.1016/0003-2697(75)90386-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Interaction of PtxS with promoters P kgu , P toxA , P gad and P ptxS . Electrophoretic mobility shift assays for the binding of PtxS to Pkgu , Pgad and PptxS. Experiments were carried out with PtxS concentrations in the range between 0.4 to 10 µM. Free DNA and DNA/protein complex are indicated. The size of the fragments used in this assay is given in the Legend for Figure 2.
(TIF)
Amino acids involved in DNA binding. A) The PtxR homology model was built based on the 3D structure of the CrgA protein of Neisseria that presents 40% identity to PtxR. B) amino acids which are potentially involved in DNA binding are highlighted. In the zoom of the recognition helix of the HTH motif. Amino acids which were mutated to alanine are shown in the ball-and-stick form.
(TIF)
Lack of interaction of PtxR with a mutant variant of P toxA promoter or of a mutant PtxR (D52A) with wild-type toxA promoter. A) The microcalorimetric titration with the 50-mer toxA nucleotide exhibiting 5 nucleotide changes (5-GATATCGGCTGCTGGCCAGGCCGACAGCCTCGTGCTTCAA-3′) was carried out as described in the legend for Figure 4 in this article. B) The EMSA assay of the wild-type PtoxA promoter with increasing concentrations of PtxRD52A was carried out as described in Figure 2A.
(TIF)
Native gel electrophoresis of PtxS, PtxR and PtxS/PtxR samples. Native gel polyacrylamide electrophoresis was prepared as described by Fenner et al. (43). Homogenous 10 µM samples of PtxS (lane 1), PtxR (lane 2), PtxS+PtxR (lane 3) and PtxS/PtxR with 1 mM 2-ketogluconate (lane 4) were solved for 1 h at 120 V. Gels were stained with Coomassie Brilliant Blue staining solution (1 g of Serva Blue R-250 into 1 L of water/methanol/acetic acid (50∶40:10)). Data were confirmed by western-blot using an anti-His tag antibody.
(TIF)
Microcalorimetric analysis of the interaction of PtxR and PtxS with 2-ketogluconate. Upper panel: A) Titration of 120 µM PtxR with 3.2 µl aliquots of 500 µM 2-ketogluconate. B) Titration of 120 µM de PtxS with 3.2 µl aliquots 500 µM 2-ketogluconate. Lower panel: Integrated and dilution corrected peak areas of raw data shown in B. Data were fitted with the “One binding site model” of the MicroCal version of ORIGIN.
(TIF)
Sequences of primers used in this study.
(DOC)