Abstract
Objective
To assess whether there is an association between statin use and the occurrence of polymyalgia rheumatic (PMR) in the spontaneous reporting database of the World Health Organisation (WHO).
Methods
We conducted a case/non-case study based on individual case safety reports (ICSR) in the WHO global ICSR database (VigiBase). Case reports containing the adverse event term polymyalgia rheumatica (WHOART or MedDRA Preferred Term) were defined as cases. Non-cases were all case reports containing other adverse event terms. Each case was matched to five non-cases by age, gender, and time of reporting. Case reports regarding a statin as suspected or concomitant drug were identified using the Anatomical Therapeutic Chemical (ATC) classification. Multivariate logistic regression was used to calculate reporting odds ratios (RORs) with 95% confidence intervals (CI).
Results
We identified 327 reports of PMR as cases and 1635 reports of other ADRs as non-cases. Among cases, statins were more frequently reported as suspected agent (29.4%) compared to non-cases (2.9%). After adjustment for several covariates, statins were significantly associated with reports of PMR (ROR 14.21; 95% CI 9.89–20.85).
Conclusion
The results of this study lends support to previous anecdotal case reports in the literature suggesting that the use of a statin may be associated with the occurrence of PMR. Further studies are needed to study the strength of the association in more detail and to elucidate the underlying mechanism.
Introduction
The 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, or statins, effectively lower cholesterol levels and significantly reduce the risk of cardiovascular events [1].
Recently, several studies have shown that these agents have anti-inflammatory and immunomodulatory properties which may eventually lead to immune dysregulation [2], [3]. Hence, statins might facilitate the development of autoimmunity, eventually resulting in autoimmune diseases. Previously, we observed in a population-based study that statins were associated with an increased risk of developing RA [4]. Furthermore, cases of statin-associated lupus-like syndrome, dermatomyositis, and vasculitis have been reported [5]–[11] Moreover, in a study comprising data from spontaneous case reports we have found an association between statin use and the occurrence of a lupus-like syndrome [12].
Three case reports suggested that statin use can trigger the development of polymyalgia rheumatica (PMR) [9]–[11]. PMR is an inflammatory rheumatic disease predominantly seen in the elderly and characterised by muscle pain and morning stiffness in the neck, shoulders, and/or pelvic girdle [13], [14]. The association between statin use and PMR has not yet been studied in depth. Therefore, we evaluated the association between statin use and the occurrence of PMR, using a case/non-case approach, in Vigibase the database of the WHO Uppsala Monitoring Centre containing individual case safety reports (ICSRs) of adverse drug reactions (ADRs).
Methods
Study Population
The association between the use of statins and PMR was evaluated using the database of the World Health Organisation Uppsala Monitoring Centre (WHO UMC), Sweden. The database (VigiBase) contains the global ICSRs of suspected adverse reactions to pharmaceutical products submitted through National Pharmacovigilance Centres by 90 countries around the world. When we extracted the data for the present study, the database contained more than 4.6 million ICSRs of suspected ADRs [15]. At the national level, ADRs are reported by health-care professionals, pharmaceutical companies, and in some countries by patients. Details about suspected ADRs such as age, gender, reporting date, country, nature of the ADR, suspected drugs, concomitantly used drugs, and interacting drugs are available in the VigiBase. ADRs are coded according to WHO Adverse Reaction Terminology (WHO-ART) or the Medical Dictionary for Regulatory Activities (MedDRA) [15]. The reported drugs are encoded using the WHO Drug Dictionary Enhanced, which includes the WHO Anatomical Therapeutic Chemical (ATC) classification [16]. Information in these reports is not homogenous, at least with regard to origin, completeness of documentation or the likelihood that the suspected drugs caused the adverse events [15].
Design
A case/non-case approach was used to evaluate the association between the use of statins and PMR. In VigiBase, cases were identified as all ICSRs of ADRs containing the WHO-ART or MedDRA preferred term ‘polymyalgia rheumatica’ [15]. Reports were only included when data on gender and age were available. Since we were interested in incident cases of PMR, ADR-reports with the preferred term ‘polymyalgia rheumatica aggravated’ were excluded from the study. Each case was matched to five non-cases by age, gender, and calendar year of reporting. Non-cases were reports concerning all other adverse reactions.
Definition of Exposure
Exposure to statins was defined as the reporting of statins as a suspected or concomitant drug for an ADR. The ATC codes for statins were C10AA (HMG-CoA reductase inhibitors), C10BA (HMG-CoA reductase inhibitors in combination with other lipid modifying agents), and C10BX (HMG-CoA reductase inhibitors and other combinations) [16].
Covariates
Concomitant medication such as drugs with immunomodulatory activity, i.e., use of anti-arrhythmic drugs, antihypertensives, antidiabetic agents, non-steroidal anti-inflammatory drugs (NSAIDs), antidepressants, anti-epileptics, and proton pump inhibitors were considered as covariates [17].
Statistical Analysis
Characteristics of the cases and non-cases were analysed using T-test, Chi-square test and Fisher’s exact test as appropriate. Means and standard deviations (SD) or percentages were obtained for continuous and categorical variables, respectively. The association between reporting of statins and PMR was assessed using logistic regression analysis and expressed as Reporting Odds Ratios (ROR) accompanied with 95% confidence intervals (CI). The ROR provides a ratio of the odds of exposure in reports of cases and non-cases. The ROR was calculated by dividing the numerator (the number of cases with statins as the suspected drug divided by the number of cases with another suspected drug) by the denominator (the number of non-cases with statins as the suspected drug divided by the number of non-cases with another suspected drug). Covariates that acted as confounders were included in the model if each of them induced a change of crude β estimates of the exposure-outcome association of at least 10% [18]. All tests were two-sided with a rejection of the null hypothesis at a p-value of less than 0.05. All the analyses were conducted using SPSS 16.0 statistical software (SPSS Inc. Chicago, Illinois, USA).
Sensitivity Analysis
We carried out five sensitivity analyses: 1) since reporters may not always be aware of statin-associated PMR we expanded the definition of statin use by including reports where statins were classified as concomitant (i.e. unsuspected) drug for an ADR.
To explore the influence of potential misclassification of patients with an ADR-report of PMR, we conducted four additional sensitivity analyses: 2) including only ADR-reports reported by physicians, 3) including only ADR-reports of patients older than 50 years [19], [20], 4) defining cases of PMR as an ADR with the single WHO-ART or MedDRA adverse reaction term PMR in which statins were reported as suspected drug, and 5) defining cases of PMR as only ADR of WHO-ART adverse reaction term PMR in which statins were reported as suspected or concomitant drug. In the latter two analyses, ADRs with two or more WHO-ART adverse reaction terms were excluded from the analysis.
Results
Baseline Characteristics
In VigiBase, we identified 327 reports of PMR (cases) that were matched with 1,635 reports of other ADRs (non-cases). The distribution of baseline characteristics for cases of PMR and non-cases are shown in Table 1. Characteristics were similar between cases and non-cases except for anti-depressant and anti-arrhythmic drugs that were more frequently reported in non-cases than in patients with PMR. In 104 of 327 cases of PMR, statins were reported as suspected or concomitant drug. Of these 104 cases, 96 reported statins as suspected drug whereas eight reported statins as concomitant drug. The distribution of statins reported as suspected drug in cases and non-cases are presented in figure 1.
Table 1. Baseline characteristics of the Polymyalgia Rheumatica study population.
| Characteristics | Cases | Non-cases | p-value |
| (n = 327) | (n = 1,635) | ||
| Mean Age (SD), y | 67.7 (11.0) | 67.7 (11.0) | NA* |
| Age categories | |||
| <50, % (n) | 6.1 (20) | 6.1 (100) | NA* |
| ≥50, % (n) | 93.9 (307) | 93.9 (1,535) | NA* |
| Sex | |||
| Male, % (n) | 37.9 (124) | 37.9 (620) | NA* |
| Female, % (n) | 62.1 (203) | 62.1 (1,015) | NA* |
| Statins | |||
| Suspected, % (n) | 29.4 (96) | 2.9 (47) | <0.001 |
| Suspected or Concomitant, % (n) | 31.8 (104) | 7.9 (129) | 0.08 |
| Comedication | |||
| Anti-arrhythmic drugs, % (n) | 2.8 (9) | 5.7 (93) | 0.03 |
| Antihypertensives, % (n) | 26.0 (85) | 21.7 (354) | 0.09 |
| Antidiabetics, % (n) | 4.3 (14) | 5.3 (86) | 0.46 |
| Non-statins Lipid modifyingagents, % (n) | 0.9 (3) | 0.7 (12) | 0.73 |
| NSAIDs†, % (n) | 2.1 (7) | 1.5 (24) | 0.34 |
| Corticosteroids, % (n) | 2.5 (8) | 1.5 (25) | 0.24 |
| DMARDs‡, % (n) | 1.2 (4) | 1.8 (30) | 0.64 |
| Antidepressants, % (n) | 1.2 (4) | 4.5 (74) | 0.003 |
| Antiepileptics, % (n) | 0.3 (1) | 1.5 (25) | 0.10 |
| Acid inhibitors, % (n) | 4.6 (15) | 3.6 (59) | 0.40 |
NA indicate not applicable because cases and non-cases were matched by age and gender.
NSAIDs: Non-steroidal anti-inflammatory drugs.
DMARDs: Disease-modifying anti-rheumatic drugs.
Figure 1. Exposure to statins in cases and non-cases.
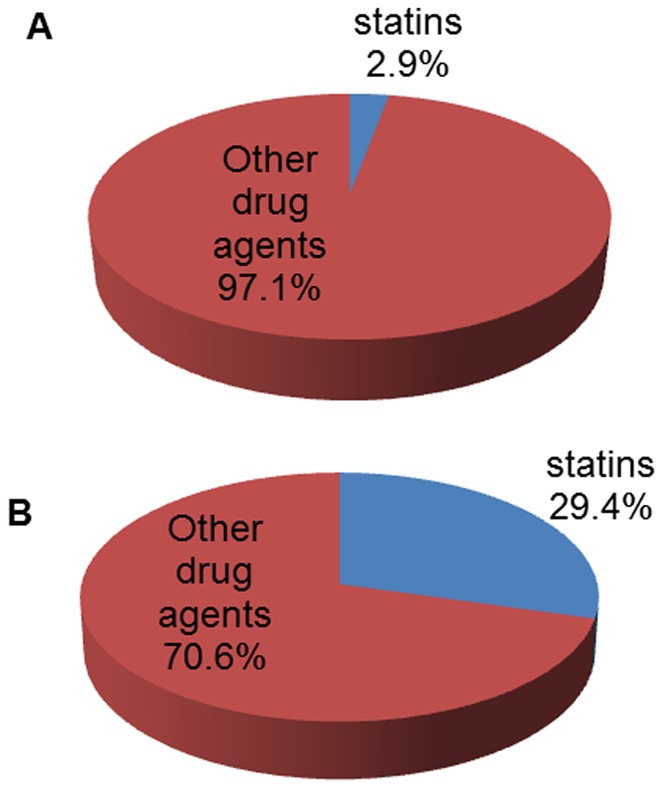
1A. Exposure to drugs in reports of other adverse drug reactions (non-cases). 1B. Exposure to drugs in reports of polymyalgia rheumatica (cases).
The characteristics of 96 cases of PMR with reported statins as suspected drug are presented in table 2. Most of these cases were reported by physicians in the late ‘90s and mainly originated from the United States, Germany, and Great Britain. Simvastatin and atorvastatin were more often reported as the suspected drug than other types of statins. The time to onset of PMR after starting statins ranged from one day to 5.7 years (mean, 11.9 months; median, 3.7 months). In 26 of 96 ADR-reports statin withdrawal was recorded. Of these 26 reported cases, eight cases reported that PMR abated while eight cases reported no effect. In six cases, a rechallenge with statins was reported resulting in the recurrence of PMR.
Table 2. Detailed information on the 96 case reports with statins as suspected drug.
| Characteristic | No. suspected statins | Characteristic | No. suspected statins |
| (n = 96) | (n = 96) | ||
| Year of reporting | Time to onset (days) | ||
| 1990–1999 | 59 | 1–90 | 35 |
| 2000–2006 | 37 | 91–365 | 17 |
| Type of statin | >365 | 23 | |
| Simvastatin | 35 | Not recorded | 21 |
| Pravastatin | 8 | Outcome | |
| Lovastatin | 10 | Recovered | 15 |
| Atorvastatin | 26 | Not (or not yet) recovered | 23 |
| Fluvastatin | 6 | Recovered with sequelae | 3 |
| Cerivastatin | 6 | Not recorded | 55 |
| Rosuvastatin | 5 | Dechallenge (Action) | |
| Country of origin | Drug withdrawn | 26 | |
| Australia | 9 | Dose not changed | 12 |
| Canada | 4 | Not recorded | 58 |
| Finland | 2 | Dechallenge (Result) | |
| France | 2 | Reaction abated | 8 |
| Germany | 18 | No effect observed | 8 |
| Great Brittan | 15 | Not applicable | 12 |
| Ireland | 1 | Not recorded | 68 |
| New Zealand | 3 | Rechallenge (Action) | |
| Norway | 1 | Rechallenge | 6 |
| Sweden | 3 | No rechallenge | 20 |
| Switzerland | 1 | Not recorded | 70 |
| The Netherlands | 3 | Rechallenge (Result) | |
| United States | 34 | Reaction recurred | 6 |
| Reporter | Not applicable | 20 | |
| General Practitioner | 31 | Not recorded | 70 |
| Physician | 8 | Causality | |
| Specialist | 5 | Probable | 3 |
| Hospital | 2 | Possible | 21 |
| Manufacturer | 2 | Not assessed | 10 |
| Consumer | 2 | Not recorded | 62 |
| Other* | 2 | ||
| Not reported | 44 |
“Other” includes consumer reports and various types of reports from other health professionals then physicians.
Association between Reporting of Statins and PMR
The association between the use of statins and PMR is shown in Table 3. Overall, statins were more often reported in patients with PMR in comparison with patients who had experienced other ADRs (adjusted ROR 14.21 [95% CI 9.89–20.85]; P = 0.001). The results were consistent when we conducted five sensitivity analyses; 1) by expanding the exposure definition to statins as suspected or concomitant drug for an ADR, the adjusted ROR for the occurrence of PMR was 6.03 (95% CI 4.40–8.25; P = 0.001), 2) including only ADR-reports reported by physicians: (adjusted ROR 14.70 [95% CI 7.07–30.65]; P = 0.001), 3) including only ADR-reports of patients older than 50 years: (adjusted ROR 14.01 [95% CI 6.98–24.83]; P = 0.001), 4) including only ADR-reports of PMR without other ADRs, in which statins were reported as suspected drug: (adjusted ROR 13.90 [95% CI 8.65–22.35]; P = 0.001), or 5) including only ADR-reports of PMR without other ADRs and in addition where statins were reported as suspected or concomitant drug: (adjusted ROR 5.75 [95% CI 3.74–8.83]; P = 0.001).
Table 3. Association between the use of statins and polymyalgia rheumatica (PMR).
| Characteristics | Cases (%) | Non-cases (%) | ROR Crude (95% CI) | ROR adjusted (95% CI)|| | p value¶ |
| n = 327 | n = 1635 | ||||
| Suspected statins | 96 (29.4) | 47 (2.9) | 14.43 (9.89–21.05) | 14.21 (9.69–20.85) | <0.001 |
| Sensitivity analysis 1* | n = 327 | n = 1635 | |||
| Suspected or concomitant statins | 104 (31.8) | 129 (7.9) | 5.66 (4.20–7.63) | 6.03 (4.40–8.25) | <0.001 |
| Sensitivity analysis 2† | n = 76 | n = 848 | |||
| Suspected statins | 18 (23.7) | 19 (2.2) | 14.15 (6.97–28.75) | 14.70 (7.07–30.65) | <0.001 |
| Sensitivity analysis 3‡ | n = 307 | n = 1535 | |||
| Suspected statins | 88 (28.6) | 43 (2.8) | 14.22 (7.15–26.92) | 14.01 (6.98–24.83) | <0.001 |
| Sensitivity analysis 4§ | n = 157 | n = 1635 | |||
| Suspected statins | 44 (28.0) | 47 (2.9) | 14.18 (8.93–22.52) | 13.90 (8.65–22.35) | <0.001 |
| Sensitivity analysis 5§ | n = 157 | n = 1635 | |||
| Suspected or concomitant statins | 46 (29.3) | 129 (7.9) | 5.55 (3.72–8.29) | 5.75 (3.74–8.83) | <0.001 |
cases of PMR were defined as all ADR-reports of PMR.
only ADR-reports reported by physicians.
only ADR-reports of patients older than 50 years.
cases of PMR were defined as only a report with the preferred term “PMR”.
adjusted for age, gender, reporting year, the use of anti-arrhythmic drugs, antihypertensives, anti-depressants, and anti-epileptics.
p values are for ROR adjusted.
Discussion
In the present study, we observed an association between statin use and reporting of PMR in VigiBase. In six reports the recurrence had been recorded of PMR after reexposure to statins, which strengthened the suspicion regarding the drug’s causal involvement in these patients. In the absence of information on the treatment of the PMR, in our study it could not be determined whether clinical improvement was related to statin discontinuation or to treatment with corticosteroids. In two previously published cases [9], [11], however, information on treatment regimes has been provided. The observation that clinical improvement had occurred within a month after discontinuation of the statin and without the administration of corticosteroids, was highly suggestive of a causal role of the statin in these two patients.
In our study, cases were predominantly older than 50 years and female (67%) which is in line with published studies reporting incidence rates of non-drug associated PMR [21], [22].
Several limitations of our study need to be addressed. First, data were obtained from a spontaneous reporting system without additional clinical assessment or qualitative verifications by the authors. For instance, in many ADR-reports no information was available about the withdrawal of statins, the course of PMR and the response to steroid therapy.
Second, there is gross but variable underreporting and it is likely that only a fraction of the actual adverse events that occurred (perhaps less than 10%) has been reported [23], [24]. ADRs to relatively new drugs, severe ADRs, and ADRs which are not listed in the summary of product characteristics tend to be more often reported [23]. To control for possible time trends of reporting, we matched non-cases for the calendar year of reporting.
Third, we cannot exclude the possibility of unmeasured and/or inadequately measured residual confounding. Furthermore, in VigiBase confounders are difficult to determine because they were not always recorded in the reports. Unfortunately, in our patients no data were available on vitamin D deficiency, which may be an important risk factor for statin-associated muscle complaints [25]. Recently, a case report [26], case series [27], and two cross sectional studies have found an association between vitamin D insufficiency and statin-induced myalgia [28], [29]. Importantly, vitamin D has been shown to modulate the immune response [30]. For instance in humans, high doses vitamin D therapy results in the inhibition of T helper (Th) 1 and Th17 cells and the promotion of Th2 and regulatory T cells [31].
Fourth, reporters may not be aware of the possible association between PMR and the use of statins use, and therefore it may not have been reported, or the statin may have been regarded as a concomitant drug only. In a sensitivity analysis including all reports of statins, suspected or not, the association between statin use and PMR was still observed, although somewhat attenuated.
Fifth, clinical details about the patients with PMR were scarce and we were not able to recognise possible diagnostic misclassification [32]. The reports in Vigibase of PMR were submitted by medical specialists, GPs, manufactures or patients. Information on clinical features, such as elevation of the inflammatory markers (erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP)), response to steroids and disease course, were often not recorded. However, when we only included case reports from physicians, the association between statin use and the occurrence of PMR was still present.
Since PMR occurs almost exclusively in patients aged 50 years and older [19], [20], we performed a sensitivity analysis in which we excluded patients who were younger than 50 years from the study. We still found an association between statin use and the occurrence of PMR.
The symptoms of PMR are very characteristic, although other conditions may mimic PMR [13], [14]. Statins are an established cause of muscular injury and specifically the inclusion of cases of myositis, non-specific myalgias, and/or myopathy may have occurred, the more so since laboratory findings, i.e., serum CK were often not recorded. On the other hand, patients with statin-associated myopathy may have normal serum CK levels which make it sometimes difficult to distinguish PMR from myopathic syndromes [33].
As yet, the possible pathophysiology underlying statin-associated PMR is uncertain.
Recently, it has been postulated that in statin-associated necrotising myopathy, statins may induce neo-antigens as a result of muscle damage which are subsequently presented to the immune system [34], [35]. A similar mechanism may be operative in statin-associated PMR.
We believe that the use of a case/non-case approach in a study with ICSRs of ADRs is, notwithstanding the limitations of our data, an appropriate approach in pharmacovigilance and drug safety research [36]. To our knowledge, this is the first study to assess the association between statin use and the occurrence of PMR in a large spontaneous reporting database. Our findings are consistent in various sensitivity analyses.
We postulate that the use of statins may be associated with an increased occurrence of PMR. Our study presents a pharmacovigilance signal and supports previous anecdotal case reports. We think that further research towards confirming and explaining the association between statin use and PMR is warranted.
Acknowledgments
The authors wish to acknowledge the help of Riny Janssen in providing background data for the study and Kristina Star in commenting on an earlier version of the manuscript. In addition, we thank the National Pharmacovigilance Centres for their contribution to the WHO-ADR database. The opinions and conclusions in this study are not necessarily those of the various National Centres or of the World Health Organisation.
Footnotes
Competing Interests: The Department of Pharmacoepidemiology and Clinical Pharmacology, Utrecht Institute for Pharmaceutical Sciences, employing authors HJI de Jong, SRF Saldi, OH Klungel, PC Souverein and RHB Meyboom has received unrestricted funding for pharmacoepidemiological research from GlaxoSmithKline, Novo Nordisk, private–public funded Top Institute Pharma (www.tipharma.nl and includes co-funding from universities, government and industry) and the Dutch Medicines Evaluation Board. This does not alter the authors’ adherence to all the PLoS ONE policies on sharing data and materials.
Funding: Funding for this study was obtained from the National Institute for Public Health and the Environment (project S340040). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, et al. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 2.Arnaud C, Braunersreuther V, Mach F. Toward immunomodulatory and anti-inflammatory properties of statins. Trends Cardiovasc Med. 2005;15:202–206. doi: 10.1016/j.tcm.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: A prospective study of the JUPITER trial. Lancet. 2009;373:1175–1182. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- 4.De Jong HJ, Klungel OH, van Dijk L, Vandebriel RJ, Leufkens HG, et al. Use of statins is associated with an increased risk of rheumatoid arthritis. Ann Rheum Dis. 2012;71:648–654. doi: 10.1136/ard.2011.155622. [DOI] [PubMed] [Google Scholar]
- 5.Noel B. Lupus erythematosus and other autoimmune diseases related to statin therapy: A systematic review. Journal of the European Academy of Dermatology and Venereology. 2007;21:17–24. doi: 10.1111/j.1468-3083.2006.01838.x. [DOI] [PubMed] [Google Scholar]
- 6.Golomb BA, Evans MA. Statin adverse effects: A review of the literature and evidence for a mitochondrial mechanism. Am J Cardiovasc Drugs. 2008;8:373–418. doi: 10.2165/0129784-200808060-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haroon M, Devlin J. A case of ANCA-associated systemic vasculitis induced by atorvastatin. Clin Rheumatol. 2008. pp. S75–77. [DOI] [PubMed]
- 8.Sen D, Rosenstein E, Kramer N. ANCA-positive vasculitis associated with simvastatin/ezetimibe: Expanding the spectrum of statin-induced autoimmunity? International Journal of Rheumatic Diseases. 2010;13:e29–e31. doi: 10.1111/j.1756-185X.2010.01481.x. [DOI] [PubMed] [Google Scholar]
- 9.Goëb V, Guillemant N, Vittecoq O, Le Loët X. Cerivastatin-induced polymyalgia rheumatica-like illness. Clin Rheumatol. 2004;23:179. doi: 10.1007/s10067-003-0772-8. [DOI] [PubMed] [Google Scholar]
- 10.Kay J, Finn D, Stone J. Case records of the massachusetts general hospital. case 4–2006. A 79-year-old woman with myalgias, fatigue, and shortness of breath. N Engl J Med. 2006;354:623–630. doi: 10.1056/NEJMcpc059040. [DOI] [PubMed] [Google Scholar]
- 11.Rudski L, Rabinovitch MA, Danoff D. Systemic immune reactions to HMG-CoA reductase inhibitors. report of 4 cases and review of the literature. Medicine. 1998;77:378–383. doi: 10.1097/00005792-199811000-00002. [DOI] [PubMed] [Google Scholar]
- 12.De Jong HJ, Cohen Tervaert JW, Saldi SR, Vandebriel RJ, Souverein PC, et al. Association between statin use and lupus-like syndrome using spontaneous reports. Semin Arthritis Rheum. 2011;41:373–81. doi: 10.1016/j.semarthrit.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Samanta A, Kendall J. A fresh look at polymyalgia rheumatica. Rheumatology (Oxford) 2002;41:1455–1456. doi: 10.1093/rheumatology/41.12.1455. [DOI] [PubMed] [Google Scholar]
- 14.Salvarani C, Cantini F, Boiardi L, Hunder GG. Polymyalgia rheumatica and giant-cell arteritis. N Engl J Med. 2002;347:261–271. doi: 10.1056/NEJMra011913. [DOI] [PubMed] [Google Scholar]
- 15.Lindquist M. Vigibase, the WHO global ICSR database system: Basic facts. Drug Inf J. 2008;42:408–419. [Google Scholar]
- 16.World Health Organisation (WHO) Collaborating Centre for Drug Statistics Methodology - Nordic Council on Medicines. Guidelines for ATC classification and DDD assigment. Oslo, Norway. 1999.
- 17.Dedeoglu F. Drug-induced autoimmunity. Curr Opin Rheumatol. 2009;21:547–551. doi: 10.1097/BOR.0b013e32832f13db. [DOI] [PubMed] [Google Scholar]
- 18.Greenland S, Robins JM. Confounding and misclassification. Am J Epidemiol. 1985;122:495–506. doi: 10.1093/oxfordjournals.aje.a114131. [DOI] [PubMed] [Google Scholar]
- 19.Chuang TY, Hunder GG, Ilstrup DM, Kurland LT. Polymyalgia rheumatica: A 10-year epidemiologic and clinical study. Ann Intern Med. 1982;97:672–680. doi: 10.7326/0003-4819-97-5-672. [DOI] [PubMed] [Google Scholar]
- 20.Healey LA. Long-term follow-up of polymyalgia rheumatica: Evidence for synovitis. Semin Arthritis Rheum. 1984;13:322–328. doi: 10.1016/0049-0172(84)90012-x. [DOI] [PubMed] [Google Scholar]
- 21.Smeeth L, Cook C, Hall AJ. Incidence of diagnosed polymyalgia rheumatica and temporal arteritis in the united kingdom, 1990–2001. Ann Rheum Dis. 2006;65:1093–1098. doi: 10.1136/ard.2005.046912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salvarani C, Gabriel SE, O’Fallon WM, Hunder GG. Epidemiology of polymyalgia rheumatica in olmsted county, minnesota, 1970–1991. Arthritis Rheum. 1995;38:369–373. doi: 10.1002/art.1780380311. [DOI] [PubMed] [Google Scholar]
- 23.Lumley CE, Walker SR, Hall GC, Stanton N, Grob PR. The under-reporting of adverse drug reactions in general practice. Pharmaceut Med. 1986;1:205–212. [Google Scholar]
- 24.Martin RM, Kapoor KV, Wilton LV, Mann RD. Underreporting of suspected adverse drug reactions to newly marketed ("black triangle") drugs in general practice: Observational study. BMJ. 1998;317:119–120. doi: 10.1136/bmj.317.7151.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta A, Thompson PD. The relationship of vitamin D deficiency to statin myopathy. Atherosclerosis. 2011;215:23–29. doi: 10.1016/j.atherosclerosis.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 26.Bell DS. Resolution of statin-induced myalgias by correcting vitamin D deficiency. South Med J. 2010;103:690–692. doi: 10.1097/SMJ.0b013e3181e21088. [DOI] [PubMed] [Google Scholar]
- 27.Lee P, Greenfield JR, Campbell LV. Vitamin D insufficiency–a novel mechanism of statin-induced myalgia? Clin Endocrinol (Oxf) 2009;71:154–155. doi: 10.1111/j.1365-2265.2008.03448.x. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed W, Khan N, Glueck CJ, Pandey S, Wang P, et al. Low serum 25 (OH) vitamin D levels (<32 ng/mL) are associated with reversible myositis-myalgia in statin-treated patients. Transl Res. 2009;153:11–16. doi: 10.1016/j.trsl.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Duell PB, Connor WE. Abstract 3701: Vitamin D deficiency is associated with myalgias in hyperlipidemic subjects taking statins. Circulation. 2008;118:S470. [Google Scholar]
- 30.Peelen E, Knippenberg S, Muris AH, Thewissen M, Smolders J, et al. Effects of vitamin D on the peripheral adaptive immune system: A review. Autoimmun Rev. 2011;10:733–43. doi: 10.1016/j.autrev.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Smolders J, Peelen E, Thewissen M, Cohen Tervaert JW, Menheere P, et al. Safety and T cell modulating effects of high dose vitamin D3 supplementation in multiple sclerosis. PLoS One. 2010;5:e15235. doi: 10.1371/journal.pone.0015235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dasgupta B, Borg FA, Hassan N, Barraclough K, Bourke B, et al. BSR and BHPR guidelines for the management of polymyalgia rheumatica. Rheumatology (Oxford) 2010;49:186–190. doi: 10.1093/rheumatology/kep303a. [DOI] [PubMed] [Google Scholar]
- 33.Phillips P, Haas R, Bannykh S, Hathaway S, Gray N, et al. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med. 2002;137:581–585. doi: 10.7326/0003-4819-137-7-200210010-00009. [DOI] [PubMed] [Google Scholar]
- 34.Grable-Esposito P, Katzberg HD, Greenberg SA, Srinivasan J, Katz J, et al. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve. 2010;41:185–190. doi: 10.1002/mus.21486. [DOI] [PubMed] [Google Scholar]
- 35.Christopher-Stine L, Casciola-Rosen LA, Hong G, Chung T, Corse AM, et al. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum. 2010;62:2757–2766. doi: 10.1002/art.27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson AM, Thabane L, Holbrook A. Application of data mining techniques in pharmacovigilance. Br J Clin Pharmacol. 2004;57:127–134. doi: 10.1046/j.1365-2125.2003.01968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


