Abstract
The matrix adhesion protein ameloblastin (AMBN) is one of the unique components of the mineralizing matrix of bones and teeth. Here we have focused on two cell types with AMBN expression to decipher AMBN function in developing dental, periodontal and bone tissues: mouse dental follicle cells (mDF) and periodontal ligament cells (mPDL). To test AMBN function, cell culture dishes for mDF and mPDL culture were exposed either to full length or C-terminal (AA 137–407) recombinant protein. Alternatively, cells were subjected to transient transfection using an AMBN-shRNA vector. Our cell culture studies documented that full-length AMBN-coated dishes promoted the attachment of mPDL and mDF cells as early as 1 hour post seeding. In order to identify potential intermediaries that might aid the effect of AMBN on adhesion, RhoA expression levels in AMBN coated and uncoated control dishes were assessed. These studies indicated that AMBN induced RhoA expression 4 hours post seeding, especially in mPDL cells. After four hours culture, the cell cycle inhibitor p27 was upregulated as well. In addition, exogenous AMBN and its C-terminal fragment reduced the proliferation of mDF and mPDL. Finally, transient transfection of mDF and mPDL cells with AMBN-shRNA vector resulted in a down-regulation of p27 in mPDL cells. Together, these data indicate that AMBN affects cell adhesion via RhoA and cell cycle progression through p27.
Keywords: extracellular matrix signaling, ameloblastin, integrin, RhoA, p27
Adhesion of cells to extracellular matrices is of fundamental importance for cellular functions, including cell differentiation, proliferation, and survival. Loss of adhesion to extracellular matrix, usually results in cell death (1,2), while exposure to extracellular matrices immediately places cells into a highly specific context of spatial and signaling instructions within an adhesion-dependent signaling scaffold (3,4). Many interactions between cells and their extracellular matrices are regulated by integrin cell surface receptors, which in turn affect intracellular signaling mediators, such as focal adhesion kinase, mitogen-activates protein kinases, and Rho-family GTPases (3,5). Through these signaling intermediaries, extracellular matrix signaling affects cell proliferation, cell death, cell shape, and gene expression (5). Control of cell cycle progression is one of the primary effects of extracellular matrix signaling, and this process is controlled by Cip/Kip family kinase inhibitors such as p27 (6). Both p27 and Rho-family GTPases are also involved in the regulation of actin dynamics and cell shape (7,8), and cytoplasmic p27 modulates actin dynamics by direct regulation of the RhoA pathway (9,10), suggesting that p27 interferes with RhoA activation and inhibits cell cycle progression.
One the extracellular matrix proteins that affects cells proliferation and adhesion is the AMBN matrix protein (11). AMBN inhibits ameloblast proliferation and is necessary for ameloblast adhesion (12). The AMBN protein sequence features a fibronectin interaction site (13) and heparin binding domains (14). In addition, there is a potential α2β1 integrin binding domain and a thrombospondin cell adhesion motif located in rodent ameloblastin proteins (12). Recent studies have indicated that AMBN regulates osteogenic differentiation through Src Kinase by affecting the interaction between CD63 and integrin β1 (15). Mutation of AMBN results in detachment of ameloblasts from the developing enamel matrix and a loss of cell polarity in AMBN null mice (16). Originally, AMBN was defined as an enamel specific protein (11). However, recent studies have demonstrated a broader expression pattern of AMBN in tissues including pulp (17), bone (18), Hertwig’s Epithelial Root Sheath (HERS) (19) and periodontal ligament (20).
The present study was prompted by the intriguing expression pattern of AMBN in HERS and periodontal ligament. In light of the high cell turnover in the periodontium and because of the importance of cell adhesion for periodontal ligament attachment to teeth and bones we asked the question how the extracellular matrix protein AMBN might affect cell proliferation and attachment in developing and mature cells of the periodontium, dental follicle and periodontal ligament. Studies were focused on Rho A and p27 as known regulators of attachment and proliferation (10). The present study provides mechanistic insights into the regulation of periodontal ligament proliferation and adhesion by the extracellular matrix protein AMBN.
Material and Methods
Cell culture
PDL and DF cells were isolated from mouse molars (Luan et al, 2002) and maintained in α-minimum essential medium (α-MEM, Gibco BRL, Gaithersburg, MD) supplemented with 10% fetal bovine serum (FBS, Atlanta Biological. Atlanta, GA), 100 U/ml penicillin, 100 µg/ml streptomycin and 25 ng/ml Amphotericin B (Gibco BRL) in a 5% CO2 atmosphere at 37 °C. The medium was changed twice a week.
AMBN protein expression and purification
The mouse AMBN coding region was amplified by PCR with a 5’ Nde1 site and a 3’ BamH1 site. The PCR products were inserted in the pET-28 expression vector (Novagen, Madison, WI) and subcloned into BE21 cells. The protein expression was induced with IPTG at a concentration of 1mg/ml at 32° C for 4 hours. Protein purification was performed used Ni-NTA agarose (Qiagen, Valencia, CA). PAGE electrophoresis and Western Blot are described elsewhere in this volume (21).
AMBN administration and manipulation
For AMBN administration, 35 mm untreated culture dishes (Corning, BD Scientific, Franklin Lakes, NJ) were coated with recombinant mouse AMBN (rmAMBN) at a concentration of 10 µg/ml at 4°C overnight, and blocked with 2% denatured BSA at room temperature for 1 hour. Alternatively, AMBN protein (1 µg/ml) was added into culture medium. For AMBN gene manipulation, AMBN shRNA and control shRNA plasmids (Santa Cruz Biotechnology, Santa Cruz, CA) were transfected into PDL cells according to manufacturer’s instructions. For blocking AMBN signaling pathways, neutralizing β1 integrin and CD63 (Santa Cruz) antibodies were applied to cell culture at a concentration of 10 mg/ml. The ERK inhibiter O126 (SIGMA, St Luis, MO) was used at 10 µM. The block was maintained throughout the entire experiment by keeping the inhibitor in the culture solution.
Cell attachment
Adhesion assays were performed using AMBN coated 35 mm culture dishes. After washing, 106 of PDL or DF cells were seeded into each dish and incubated at 37°C for 1 or 4 hours. Nonadherent cells were removed by washing with PBS and the remaining cells counted under a microscope.
Cell proliferation and MTT
Cells were cultured in rmAMBN-coated plates, or in the presence of AMBN protein in culture medium for 24 or 48 hours and thereafter trypsinized and counted. A second group of cells was incubated in MTT solution (2mg/ml of MTT in DMEM with 2% FBS) for 4 hours after initial culture. To quantify proliferative activity, the MTT stained cells were lysed in HCL/Isopropanol and the absorbance was detected at 570nm with background subtraction at 630nm (22).
Protein extraction and Western blot analysis
Cultured cells were collected and lysed using a lysis buffer containing 100mM Tris HCl pH 9.0, 200 mM KCl, 25 mM EGTA, 36 mM MgCl2, 2% deoxycholic acid and 10% DTT( v/v). Equal amounts of protein were subjected to SDS–polyacrylamide gel electrophoresis, and the separated proteins were transferred to a PVDF membrane (Immobilon P®, Millipore, Billerica, MA). The membrane was incubated with anti-RhoA or p27 primary antibodies at 1:1,000 dilution and kept at 4° C overnight (Abcam, Cambridge, MA). Immune complexes were detected with peroxidase-conjugated secondary antibody (Molecular Probes®, Carlsbad, CA) after 1 hour incubation at room temperature and enhanced by chemiluminescence reagents (Pierce Biotechnology, Rockford, IL). The amount of the protein expression was compared after normalization with β-actin as an internal calibrator in each lane.
Statistical analysis
Quantitative data were presented as means ± SD from three independent experiments and compared with one way ANOVA statistical analysis tests. The difference between groups was considered statistically significant at P<0.05.
Results
Effect of AMBN on cell adhesion
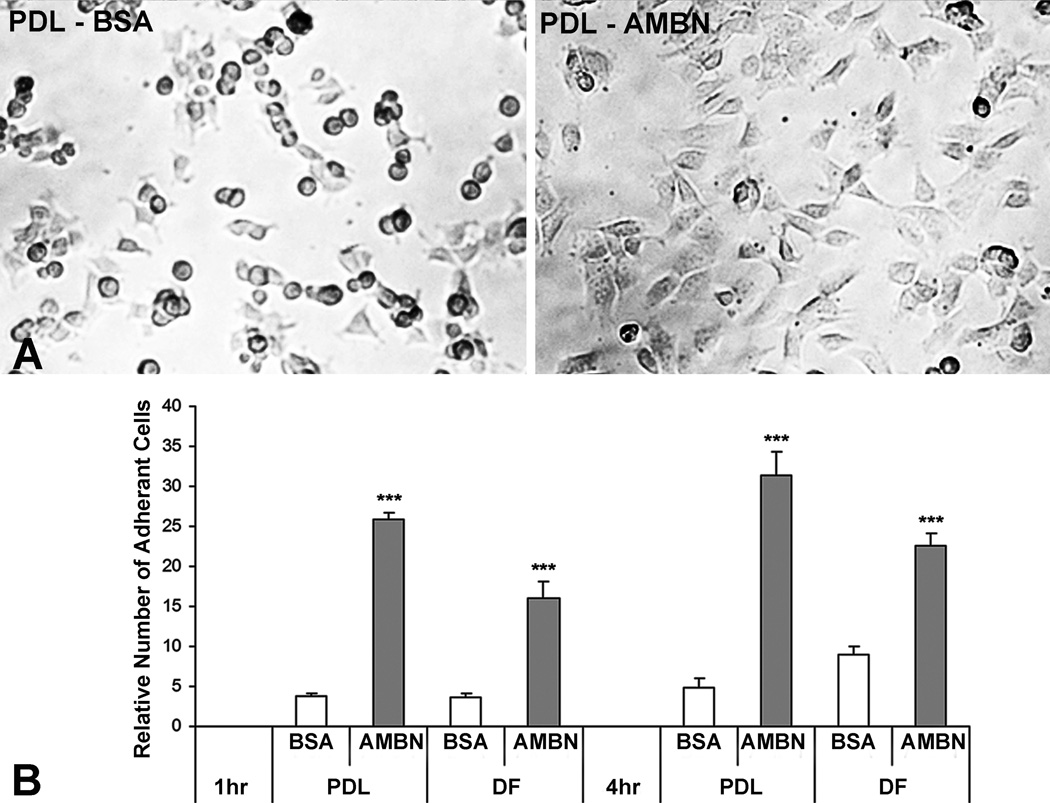
The AMBN protein is a secreted, extracellular matrix molecule and as such might either function as part of a protein matrix or as a soluble factor (11). To test the effect of AMBN on PDL and DF cells, AMBN protein was applied by coating untreated dishes or by adding AMBN directly into the culture medium. AMBN treatment significantly enhanced adhesion and spreading of both PDL and DF cells (Fig. 1). The PDL cells on the surface of AMBN-coated dishes were flat and formed cell processes while PDL cells on BSA-coated dishes were round and aggregated after 1 hour of culture (Fig. 1A). The number of attached PDL cells was five times higher and the number of attached DF cells was three times higher in the AMBN-treated group than that in BSA-treated group (Fig. 1B).
Figure 1. AMBN enhanced cell adhesion.
Mouse PDL and DF cells were cultured in a BSA- or AMBN-coated culture dished for 1 and 4 hours. (A) Phase contrast images of cell attachment and spreading. Note adherent and spreaded mPDL cells on the surface of AMBN-coated dishes compared to round and aggregated cells on BSA-coated dishes. (B) Relative number of attached PDL and DF cells. The number of adherent PDL cells in the BSA treatment group after 1 hour’s culture were set as one unit. Data are presented as mean ± SD of three different cultures. The effect of AMBN on cell adhesion was highly significant (p<0.001, ***)
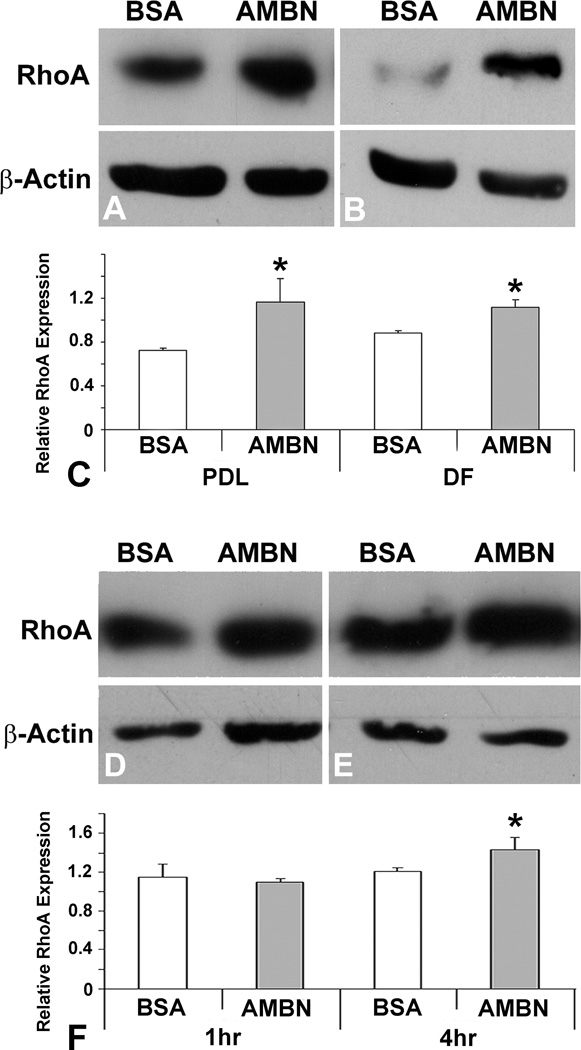
Previous studies have demonstrated that cell adhesion/spreading and RhoA formed a regulatory loop (23). To assess whether AMBN-mediated adhesion of PDL and DF cells stimulated RhoA activity, RhoA expression levels in response to AMBN treatment were examined using Western blot analysis. After four hours of cell seeding, there was a 1.6- fold increase in RhoA expression in AMBN-treated PDL cells and a 1.3 fold increase in DF cells compared to BSA-treated cells (Figs. 2A–C). To determine the time course of AMBN effect on RhoA expression during cell adhesion and spreading process, RhoA expression was analyzed using Western blotting after 1, 4 and 24 hours of cell seeding. There was no difference of RhoA expression between BSA- and AMBN-treated cells after 1 hour. The effect of AMBN was significantly increased after 4 hours, and maintained until 24 hours after treatment (Figs. 2D–G).
Figure 2. AMBN upregulated RhoA expression in mouse PDL and DF cells.
RhoA expression in PDL and DF cells cultured at different time points was analyzed by Western blotting. Western blot analysis compares BSA and AMBN on RhoA expression in PDL (A) and DF (B) cells 4 hour after cell seeding, illustrated by densitometry (C). The effect of AMBN in PDL cells was evaluated after 1 (D), 4 (E) and 24 (F) hour’s culture. Data are presented as mean ± SD of three different cultures. The star * represents p<0.05.
Effect of AMBN on cell proliferation
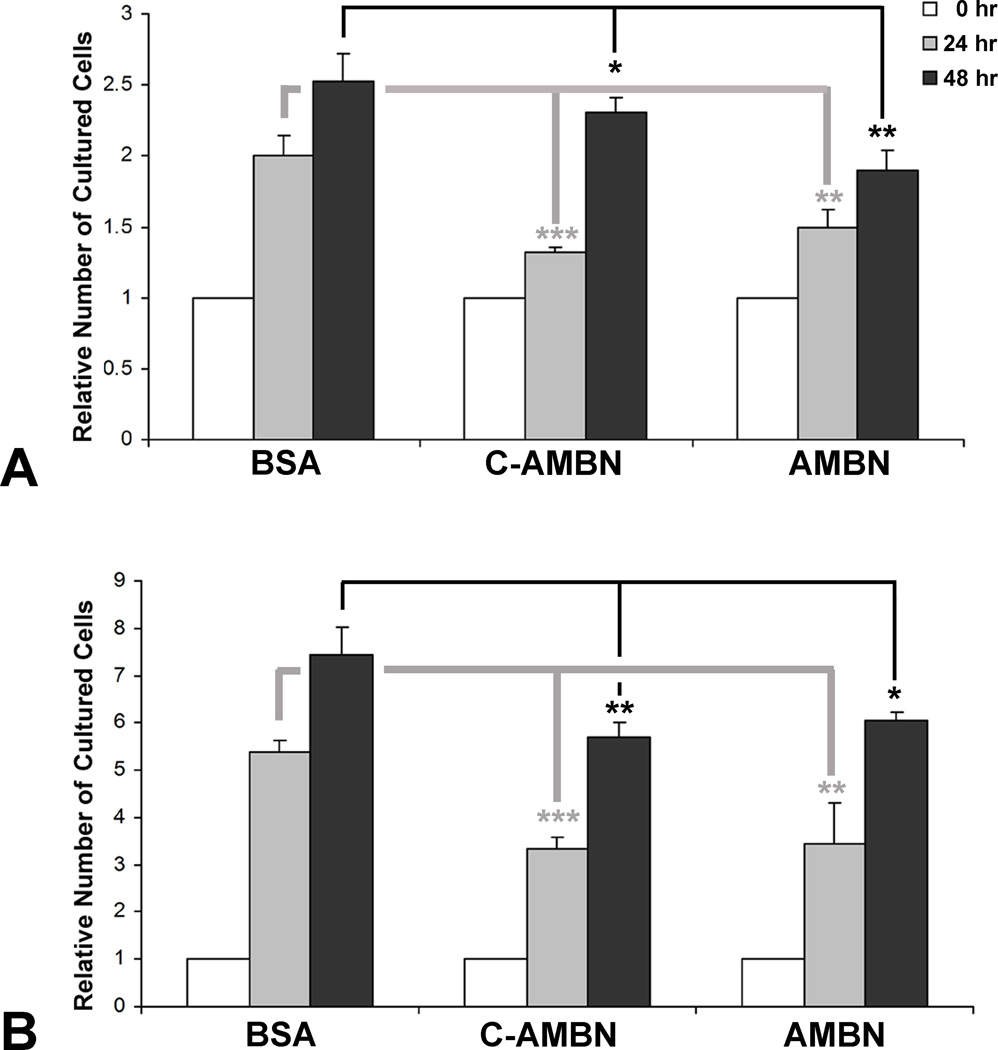
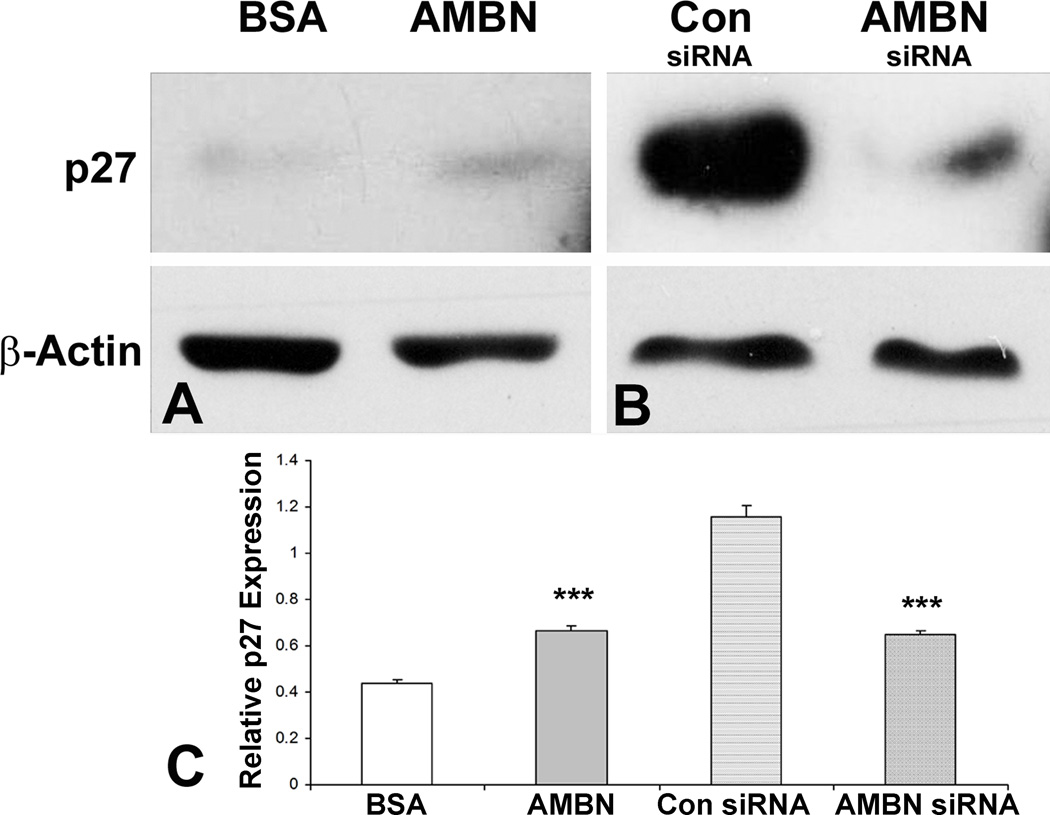
Cell adhesion involves changes not only in the cellular skeleton but also in cell function (24). AMBN treatment reduced both PDL and DF cell proliferation. The cell density in the presence of AMBN was lower than that in the presence of BSA (not shown). When compared to BSA control after 24 and 48 hours’ culture, full-length AMBN significantly decreased the number of PDL cells (25% and 24.7% respectively, Fig. 3A) and DF cells (36.4% and 18.9% respectively, Fig. 3B). The C-terminal fragment of AMBN showed similar effects (Figs. 3A and B). When added to PDL cell culture medium, mouse recombinant AMBN upregulated p27 expression by 1.5-fold after 24 hours culture (Figs. 4A and B). In contrast, knocking down endogenous AMBN by siAMBN significantly downregulated p27 expression (56.3%, Fig. 4C).
Figure 3. AMBN inhibited cell proliferation.
PDL and DF cells were cultured after adding BSA (1 µg/ml), the C-terminal AMBN fragment (C-AMBN, 1µg/ml) or AMBN (1µg/ml) for 24 and 48 hours and the number of cells was counted. The relative number of PDL (A) and DF (B) cells is presented using the original cell number (0 hour) as one unit.
Figure 4. AMBN modulated p27 expression.
AMBN function was manipulated by addition of exogenous AMBN protein (A) or knocking down the endogenous AMBN gene using AMBN siRNA (B). A scrambled siRNA (con siRNA) was used as a control (B). The effect of AMBN on p27 expression was analyzed using Western blot (A and B) and illustrated by densitometry (C).
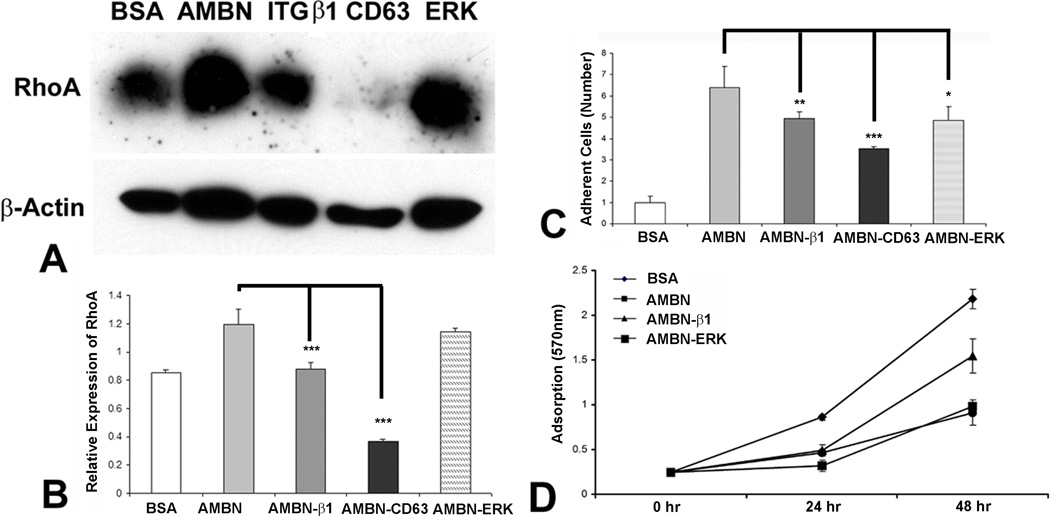
AMBN affects protein expression and cellular function through CD63, β1 integrin and ERK pathways
Rodent AMBN contains a DGEA cell adhesion domain, which interacts with α2β1 integrin (12). The interaction between integrin β1 and CD63 has been demonstrated to play a role in the AMBN signal pathway (15). We therefore hypothesized that AMBN may affects cell adhesion through integrin cell surface receptors. To determine whether β1 integrin and its interaction partner CD63 participates in AMBN-modulated cell adhesion and gene expression, we used an anti-β1 integrin or CD63 antibody (10µg/ml) to neutralize these receptors. In addition, we used the ERK1/2 inhibitor U0126 to inhibit the MER/ERK pathway. Our results indicated that all three inhibitors reduced the effect of AMBN 4 hours after cell seeding on RhoA expression (Figs 5A and B), cell adhesion (Fig. 5C) and cell proliferation (Fig. D).
Figure 5. Inhibition of AMBN function in protein expression, cell adhesion and proliferation by blocking CD63, integrin β1 and ERK pathways.
AMBN was compared to BSA, and AMBN function was selectively inhibited with CD63, β1 integrin antibodies (ITGβ1), or ERK inhibitor. (A) Western blot analysis of RhoA expression. (B) Densitometric evaluation of RhoA expression level 4 hours post seeding. (C) Relative number of adherent PDL cells following seeding for 4 hours. (D) MTT analysis of cell proliferation after 24 and 48 hour’s culture of mouse PDL cells. Cell proliferative activity is presented as OD value.
Discussion
Extracellular matrix proteins of the periodontium provide structural support and anchorage for cells and regulate intercellular communication (25). The present study focused on one the matrix molecules of the developing and mature periodontium, the extracellular matrix protein AMBN. In this study, the effect of AMBN on cell proliferation and attachment of dental follicle and periodontal ligament cells was determined and potential mechanisms by which AMBN regulates proliferation and attachment were explored. In order to mimic the extracellular matrix surface to which periodontal cells were exposed, culture dishes were coated with recombinant ameloblastin. Coating of culture dishes to test the effect of matrix molecules on cells is a common and well-established procedure to mimic extracellular matrix environments (26,27). Studies performed here demonstrated that AMBN affects cell adhesion via RhoA and cell cycle progression through p27.
In the present study, both soluble and immobilized AMBN enhanced PDL and DF cell adhesion. This effect may be in addition to endogenous levels of AMBN. Adhesion of cells to the ECM is crucial for craniofacial development (28). For example, loss of AMBN function resulted in the detachment of ameloblasts from dentin surface and caused enamel defects (13). The effect of AMBN on cell adhesion is not only found in ameloblasts (12) but also in mesenchymal PDL and DF cells, as demonstrated in the present study. One of the classic intracellular mediators of extracellular matrix induced cell adhesion is the small GTPase RhoA and RhoA levels gradually increase upon cell contact with surfaces, resulting in the formation of stress fibers and the maturation of focal adhesion (29). The present study indicated that AMBN significantly increased RhoA expression as cell attachment occurred during cell culture, suggesting that AMBN as an extracellular matrix molecule enhances periodontal cell attachment via RhoA.
Here it was demonstrated that recombinant mouse AMBN inhibited PDL and DF cell proliferation through modulating p27 expression. These studies are supported by earlier studies related to ameloblast proliferation indicating that recombinant AMBN inhibits proliferation and is required for maintaining the differentiation state of ameloblasts (16). However, recent studies demonstrated that AMBN shows growth-factor like activity (30) and promotes bone growth by enhancing proliferation of progenitor cells (31). Moreover, synthetic ameloblastin peptides did not enhance proliferation but stimulated differentiation of human periodontal ligament cells (32). AMBN protein structure and function studies from our group (see the related publication by Zhang et al. in this volume) and from other groups (15,33,34) provide an explanation for the difference in AMBN function related to adhesion and/or proliferation in various cell types. These studies indicated that the AMBN protein contains several cell adhesion domains and directly or indirectly interacts with integrins, CD63 and fibronectin, and activates the ERK pathway. Different fragments of AMBN protein may differentially interact with ECM proteins as well as cell surface receptors in different cell types, explaining the multitude of AMBN tissue-specific functions on a general level.
In summary, this study introduces AMBN as a typical extracellular matrix protein that is involved both in the regulation of proliferation and adhesion of periodontal cells. Here we show that AMBN greatly enhances the attachment of periodontal cells through the small GTPase RhoA, while AMBN reduces periodontal cell proliferation through the kinase inhibitor p27. These findings suggest that the extracellular matrix molecule AMBN is important for the attachment of developing and mature periodontal ligament cells and also reduces cell proliferation, which may be related to the control and maintenance of periodontal ligament cell differentiation.
Acknowledgement
Generous support for these studies by NIDCR grants DE18057 and DE19155 to XL is gratefully acknowledged.
References
- 1.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meredith JE, Jr, Fazeli B, Schwartz MA. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993;4:953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boudreau NJ, Jones PL. Extracellular matrix and integrin signalling: the shape of things to come. Biochem J. 1999;339:481–488. [PMC free article] [PubMed] [Google Scholar]
- 4.Gilmore AP. Anoikis. Cell Death Differ. 2005;12(Suppl 2):1473–1477. doi: 10.1038/sj.cdd.4401723. [DOI] [PubMed] [Google Scholar]
- 5.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 6.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 7.Nagahara H, Vocero-Akbani AM, Snyder EL, Ho A, Latham DG, Lissy NA, Becker-Hapak M, Ezhevsky SA, Dowdy SF. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat Med. 1998;4:1449–1452. doi: 10.1038/4042. [DOI] [PubMed] [Google Scholar]
- 8.McAllister SS, Becker-Hapak M, Pintucci G, Pagano M, Dowdy SF. Novel p27(kip1) C-terminal scatter domain mediates Rac-dependent cell migration independent of cell cycle arrest functions. Mol Cell Biol. 2003;23:216–228. doi: 10.1128/MCB.23.1.216-228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Besson A, Gurian-West M, Schmidt A, Hall A, Roberts JM. p27Kip1 modulates cell migration through the regulation of RhoA activation. Genes Dev. 2004;18:862–876. doi: 10.1101/gad.1185504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denicourt C, Dowdy SF. Cip/Kip proteins: more than just CDKs inhibitors. Genes Dev. 2004;18:851–855. doi: 10.1101/gad.1205304. [DOI] [PubMed] [Google Scholar]
- 11.Krebsbach PH, Lee SK, Matsuki Y, Kozak CA, Yamada KM, Yamada Y. Full-length sequence, localization, and chromosomal mapping of ameloblastin. A novel tooth-specific gene. J Biol Chem. 1996;271:4431–4435. doi: 10.1074/jbc.271.8.4431. [DOI] [PubMed] [Google Scholar]
- 12.Fukumoto S, Yamada A, Nonaka K, Yamada Y. Essential roles of ameloblastin in maintaining ameloblast differentiation and enamel formation. Cells Tissues Organs. 2005;181:189–195. doi: 10.1159/000091380. [DOI] [PubMed] [Google Scholar]
- 13.Beyeler M, Schild C, Lutz R, Chiquet M, Trueb B. Identification of a fibronectin interaction site in the extracellular matrix protein ameloblastin. Exp Cell Res. 2010;316:1202–1212. doi: 10.1016/j.yexcr.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Sonoda A, Iwamoto T, Nakamura T, Fukumoto E, Yoshizaki K, Yamada A, Arakaki M, Harada H, Nonaka K, Nakamura S, Yamada Y, Fukumoto Critical role of heparin binding domains of ameloblastin for dental epithelium cell adhesion and ameloblastoma proliferation. J Biol Chem. 2009;284:27176–27184. doi: 10.1074/jbc.M109.033464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iizuka S, Kudo Y, Yoshida M, Tsunematsu T, Yoshiko Y, Uchida T, Ogawa I, Miyauchi M, Takata T. Ameloblastin regulates osteogenic differentiation by inhibiting Src kinase via cross talk between integrin beta1 and CD63. Mol Cell Biol. 2011;31:783–792. doi: 10.1128/MCB.00912-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukumoto S, Kiba T, Hall B, Iehara N, Nakamura T, Longenecker G, Krebsbach PH, Nanci A, Kulkarni AB, Yamada Y. Ameloblastin is a cell adhesion molecule required for maintaining the differentiation state of ameloblasts. J Cell Biol. 2004;167:973–983. doi: 10.1083/jcb.200409077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fong CD, Cerny R, Hammarstrom L, Slaby I. Sequential expression of an amelin gene in mesenchymal and epithelial cells during odontogenesis in rats. Eur J Oral Sci. 1998;106(Suppl 1):324–330. doi: 10.1111/j.1600-0722.1998.tb02193.x. [DOI] [PubMed] [Google Scholar]
- 18.Spahr A, Lyngstadaas SP, Slaby I, Pezeshki G. Ameloblastin expression during craniofacial bone formation in rats. Eur J Oral Sci. 2006;114:504–511. doi: 10.1111/j.1600-0722.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- 19.Zeichner-David M, Oishi K, Su Z, Zakartchenko V, Chen LS, Arzate H, Bringas P., Jr Role of Hertwig's epithelial root sheath cells in tooth root development. Dev Dyn. 2003;228:651–663. doi: 10.1002/dvdy.10404. [DOI] [PubMed] [Google Scholar]
- 20.Nunez J, Sanz M, Hoz-Rodriguez L, Zeichner-David M, Arzate H. Human cementoblasts express enamel-associated molecules in vitro and in vivo. J Periodontal Res. 2010;45:809–814. doi: 10.1111/j.1600-0765.2010.01291.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Diekwisch TGH, Luan X. Structure and function of ameloblastin as an extracellular matrix protein: adhesion, calcium binding, and CD63-Interaction in human and mouse. doi: 10.1111/j.1600-0722.2011.00889.x. In this volume. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loveland BE, Johns TG, Mackay IR, Vaillant F, Wang ZX, Hertzog PJ. Validation of the MTT dye assay for enumeration of cells in proliferative and antiproliferative assays. Biochem Int. 1992;27:501–510. [PubMed] [Google Scholar]
- 23.Fukata M, Nakagawa M, Kuroda S, Kaibuchi K. Cell adhesion and Rho small GTPases. J Cell Sci. 1999;112:4491–4500. doi: 10.1242/jcs.112.24.4491. [DOI] [PubMed] [Google Scholar]
- 24.Buckley CD, Rainger GE, Bradfield PF, Nash GB, Simmons DL. Cell adhesion: more than just glue. Mol Membr Biol. 1998;15:167–176. doi: 10.3109/09687689709044318. [DOI] [PubMed] [Google Scholar]
- 25.Ten Cate AR. Oral histology: development, structure and function. 5th ed. Mosby; 1998. pp. 253–314. [Google Scholar]
- 26.Underwood PA, Bennett FA. The effect of extracellular matrix molecules on the in vitro behavior of bovine endothelial cells. Exp Cell Res. 1993;205:311–319. doi: 10.1006/excr.1993.1091. [DOI] [PubMed] [Google Scholar]
- 27.Flaim CJ, Teng D, Chien S, Bhatia SN. Combinatorial signaling microenvironments for studying stem cell fate. Stem Cells Dev. 2008;17:29–39. doi: 10.1089/scd.2007.0085. [DOI] [PubMed] [Google Scholar]
- 28.Kerrigan JJ, McGill JT, Davies JA, Andrews L, Sandy JR. The role of cell adhesion molecules in craniofacial development. J R Coll Surg Edinb. 1998;43:223–229. [PubMed] [Google Scholar]
- 29.Huveneers S, Truong H, Fassler R, Sonnenberg A, Danen EH. Binding of soluble fibronectin to integrin alpha5 beta1 - link to focal adhesion redistribution and contractile shape. J Cell Sci. 2008;121:2452–2462. doi: 10.1242/jcs.033001. [DOI] [PubMed] [Google Scholar]
- 30.Zeichner-David M, Chen LS, Hsu Z, Reyna J, Caton J, Bringas P. Amelogenin and ameloblastin show growth-factor like activity in periodontal ligament cells. Eur J Oral Sci. 2006;114(Suppl 1):244–253. doi: 10.1111/j.1600-0722.2006.00322.x. discussion 254–6, 381–2. [DOI] [PubMed] [Google Scholar]
- 31.Tamburstuen MV, Reppe S, Spahr A, Sabetrasekh R, Kvalheim G, Slaby I, Syversen U, Lyngstadaas SP, Reseland JE. Ameloblastin promotes bone growth by enhancing proliferation of progenitor cells and by stimulating immunoregulators. Eur J Oral Sci. 2011;118:451–459. doi: 10.1111/j.1600-0722.2010.00760.x. [DOI] [PubMed] [Google Scholar]
- 32.Kitagawa M, Kitagawa S, Nagasaki A, Miyauchi M, Uchida T, Takata T. Synthetic ameloblastin peptide stimulates differentiation of human periodontal ligament cells. Arch Oral Biol. 2011;56:374–379. doi: 10.1016/j.archoralbio.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Vymetal J, Slaby I, Spahr A, Vondrasek J, Lyngstadaas SP. Bioinformatic analysis and molecular modelling of human ameloblastin suggest a two-domain intrinsically unstructured calcium-binding protein. Eur J Oral Sci. 2008;116:124–134. doi: 10.1111/j.1600-0722.2008.00526.x. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Tannukit S, Zhu D, Snead ML, Paine ML. Enamel matrix protein interactions. J Bone Miner Res. 2005;20:1032–1040. doi: 10.1359/JBMR.050111. [DOI] [PubMed] [Google Scholar]