Abstract
Rationale: Asthma is characterized by disordered airway physiology as a consequence of increased airway smooth muscle contractility. The underlying cause of this hypercontractility is poorly understood.
Objectives: We sought to investigate whether the burden of oxidative stress in airway smooth muscle in asthma is heightened and mediated by an intrinsic abnormality promoting hypercontractility.
Methods: We examined the oxidative stress burden of airway smooth muscle in bronchial biopsies and primary cells from subjects with asthma and healthy controls. We determined the expression of targets implicated in the control of oxidative stress in airway smooth muscle and their role in contractility.
Measurements and Main Results: We found that the oxidative stress burden in the airway smooth muscle in individuals with asthma is heightened and related to the degree of airflow obstruction and airway hyperresponsiveness. This was independent of the asthmatic environment as in vitro primary airway smooth muscle from individuals with asthma compared with healthy controls demonstrated increased oxidative stress–induced DNA damage together with an increased production of reactive oxygen species. Genome-wide microarray of primary airway smooth muscle identified increased messenger RNA expression in asthma of NADPH oxidase (NOX) subtype 4. This NOX4 overexpression in asthma was supported by quantitative polymerase chain reaction, confirmed at the protein level. Airway smooth muscle from individuals with asthma exhibited increased agonist-induced contraction. This was abrogated by NOX4 small interfering RNA knockdown and the pharmacological inhibitors diphenyleneiodonium and apocynin.
Conclusions: Our findings support a critical role for NOX4 overexpression in asthma in the promotion of oxidative stress and consequent airway smooth muscle hypercontractility. This implicates NOX4 as a potential novel target for asthma therapy.
Keywords: asthma, airway smooth muscle, airway hyperresponsiveness, NOX4, SOD2
At a Glance Commentary
Scientific Knowledge on the Subject
Asthma is characterized by variable airflow obstruction and airway hyperresponsiveness as a consequence of increased airway smooth muscle contractility. The underlying cause of this hypercontractility is poorly understood.
What This Study Adds to the Field
We identified an increased burden of oxidative stress associated with up-regulated nicotinamide adenine dinucleotide phosphate oxidase (NOX) 4 expression in the airway smooth muscle in asthma. Airway smooth muscle from individuals with asthma is intrinsically hypercontractile. Pharmacological inhibition and small interfering RNA knockdown of NOX4 normalizes this hypercontractility, suggesting that NOX4 may be a novel therapeutic target for asthma.
Asthma affects over 300 million people worldwide. Importantly, asthma control is suboptimal in about half of sufferers, and 10% are refractory to current antiinflammatory and bronchodilator therapies (1, 2). There is therefore an urgent need for new therapies. Asthma is characterized by variable airflow obstruction and airway hyperresponsiveness as a consequence of increased airway smooth muscle contractility (1, 2). This heightened airway smooth muscle (ASM) contraction is considered to be largely due to the local asthmatic milieu with infiltration by inflammatory cells, in particular eosinophils and T cells, into the submucosa (2–4), to mast cells in the ASM bundle (4, 5), and to up-regulation of Th2 cytokines (6, 7).
Interestingly, there is an increasing body of evidence that ASM is fundamentally altered in asthma compared with healthy controls, suggesting that abnormalities in these structural cells may also play a critical role in the development of the abnormal physiology in asthma and may contribute to the persistent airway inflammation. ASMs from individuals with asthma exhibit increased synthetic capacity (8, 9), mitochondrial biogenesis (10), altered calcium homeostasis (10, 11), and in some reports (10–12), but not all (13, 14), increased proliferation. Critically, there is emerging evidence that ASM from individuals with asthma is hypercontractile as demonstrated by an increased velocity of contraction in response to electrical field stimulation at the single-cell level (15) and in cell populations using gel contraction assays (16).
The asthma phenotype is dependent upon dynamic interactions between the host and its environment. Exposure to environmental stimuli and the consequent inflammatory response is likely to be responsible for the increased oxidative stress observed within the asthmatic airway, as evidenced by increased 8-isoprostane in sputum and breath condensate (17). Whether the ASM in asthma is under an increased oxidative stress burden and whether this affects ASM function is uncertain. We hypothesized that the burden of oxidative stress in ASM in asthma is heightened, in part mediated by a fundamental abnormality in the asthmatic ASM, which in turn promotes ASM hypercontractility.
Some of the results of these studies have been previously reported in the form of an abstract (18).
Methods
Detailed methods are available in the online supplement.
Subjects and Cells
Subjects with asthma (n = 51) and healthy control subjects (n = 28) were recruited from Leicester, UK. Asthma severity was defined by Global Initiative for Asthma (GINA) treatment steps (mild–moderate GINA 1–3, severe GINA 4–5) (2). Primary ASM was isolated from bronchial biopsies and cells were used in experiments between passages 2 and 6. The study was approved by the Leicestershire Ethics Committee, and patients gave their written informed consent.
Immunohistochemistry and Western Blotting
Two-micrometer sections from glycomethacrylate-embedded bronchial biopsies were stained using an 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) monoclonal antibody (Abcam, Cambridge, UK), or isotype control (Dako, Ely, UK) and assessed using a semiquantitative score from no to very high staining (0–5). SDS-PAGE and Western blotting were prepared using ASM lysates (19) with a nicotinamide adenine dinucleotide phosphate oxidase (NOX) 4 rabbit polyclonal antibody (Abcam).
ASM DNA Damage and Intracellular ROS
DNA damage was assessed using the human 8-oxoguanine glycosylase 1 (hOGG1)-modified comet assay (hOGG1 comet) (20). Intracellular reactive oxygen species (ROS) was assessed by incubating the ASM with 10 μM 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate for 30 minutes (21), washed, and incubated with PBS or 10 mM H2O2, and fluorescence was read after 1 h.
Flow Cytometry and Immunofluorescence
ASMs were stained with rabbit polyclonal anti-NOX4 (Abcam, Cambridge, UK), anti–NOX 1–3 and 5 (Insight Biotechnology, Wembley, UK) indirectly labeled with fluorescein isothiocyanate (FITC; Dako), or α-smooth muscle actin-FITC direct conjugate (Sigma Gillingham, Dorset, UK), or bradykinin B2 receptor indirectly labeled with FITC (BD, Oxford, UK) or isotype controls (Dako) and assessed by flow cytometry (BD FACScan; BD), as previously described (22). ASMs were stained for immunofluorescence with rabbit polyclonal NOX1–5 or anti-superoxide dismutase (SOD)2 (Abcam) or isotype controls, indirectly labeled with FITC, and counterstained with 4′,6′-diamidino-2 phenylindole (Sigma).
Gene Array and Analysis
ASM RNA expression was examined using the Human Genome U133A probe array (Affymetrix, Santa Clara, CA) and analyzed to determine genes that demonstrated significant differential expression between health and disease by more than twofold up- or down-regulated following 1,000 permutations or were present in five or more individuals with asthma/healthy control subjects versus one or less healthy control subjects/individuals with asthma.
Real-Time Reverse Transcription–Polymerase Chain Reaction
Real-time reverse transcription–polymerase chain reaction was performed (SuperScript Vilo cDNA synthesis kit, Express SYBR GreenER qPCR Supermix Universal; Invitrogen, Paisley, UK). Relative quantification was done using the comparative 2−ΔΔCt method and expressed as fold change as previously described (23). The internal normalizer gene was 18S RNA amplified with 18S forward primer (h18SRNA.891F:GTTGGTTTTCGGAACTGAGG) and 18S reverse primer (h18SRNA.1090R:GCATCGTTTATGGTCGGAAC); amplification of NOX4 was with primers forward (hNox4.598F:TGGCTGCCCATCTGGTGAATG) and reverse (hNox4.878R:GCATCGTTTATGGTCGGAAC).
Assessment of ASM Contraction by Collagen Gel Analysis
ASM cells were harvested post-treatment with either NOX4 inhibitor diphenyleneiodonium (1 or 5 μM for 6 hours; Sigma), apocynin (10 μM for 30 min; Acros Organics, Fischer Scientific, Loughborough, UK), SOD mimetic manganese (III) tetrakis (4-benzoic acid) porphyrin chloride (10, 50, or 100 μg/ml for 6 h; Enzo Life Sciences, Exeter, UK), or post-transfection (nucleofection; Lonza AG, Cologne, Germany) with two NOX4 or SOD2 small interfering RNAs (siRNAs) with a negative control (Stealth RNAi; Invitrogen), and their contractile properties were assessed using collagen gel contraction (22). The mean (SEM) % viability of cells recovered from the gels following collagenase digestion was 87 (3)%. Transfection efficiency was greater than 90%, and knockdown efficiency for NOX4 was 80–90% and for SOD2 90–95%.
Statistical Analysis
Statistical analysis was performed using PRISM Version 4 (GraphPad, La Jolla, CA). Parametric data were presented as mean (SEM) and nonparametric data as median (interquartile range). Analysis between groups was by t tests or Mann-Whitney tests and across groups by ANOVA with appropriate post hoc pairwise comparisons. P < 0.05 was considered significant.
Results
We sought to determine the degree of oxidative stress in the ASM bundle in asthma. We investigated expression by the ASM bundles in bronchial biopsies from subjects with mild to moderate or severe asthma compared with healthy control subjects of 8-oxodG, a biomarker of oxidative damage, by immunohistochemistry. These subjects were extensively phenotyped and their clinical characteristics are as shown in Table 1. We found that in asthma, independent of disease severity, there was a marked increased nuclear 8-oxodG staining (Figures 1A–1E). Critically, this was inversely correlated with FEV1/FVC (%) (r = −0.38; P = 0.02; n = 37) (Figure 1F) and airway hyperresponsiveness (r = −0.43; P = 0.025; n = 27), suggesting that there is an association between the burden of oxidative stress and the features of disordered airway physiology that characterize asthma.
TABLE 1.
CLINICAL CHARACTERISTICS OF INDIVIDUALS WITH ASTHMA AND HEALTHY CONTROLS
| Normal | Mild–Moderate Asthma (GINA 1–3) | Severe Asthma (GINA 4–5) | |
| Number | 9 | 18 | 10 |
| Age* | 42 (5) | 49 (4) | 53 (3) |
| Male/female | 5/4 | 7/11 | 4/6 |
| Never/current/ex-smokers | 8/1/0 | 14/4/0 | 7/3/0 |
| Atopy, n (%) | 4 (44) | 12 (67) | 7 (70) |
| PC20FEV1, mg/ml† | >16 | 0.46 (0.17–1.3)‡ | 0.78 (0.26–2.3)‡ |
| FEV1, % predicted* | 94 (3) | 82 (7) | 77 (7)‡ |
| Pre-BD FEV1/FVC, %* | 82 (2) | 72 (2)‡ | 69 (5)‡ |
| BD response, %* | 0 (0) | 11 (4)‡ | 11 (3)‡ |
| Sputum cell counts | |||
| TCC* | 0.9 (0.1) | 2.4 (0.5) | 4.7 (1.1)‡ |
| Eosinophil, %§ | 0.4 (0.8) | 1.0 (5.2) | 4.6 (13.3)‡ |
| Neutrophil, %* | 56 (12) | 48 (7) | 62 (8) |
| Macrophage, %* | 37 (12) | 39 (7) | 26 (6)‡ |
| Lymphocyte, %* | 2.0 (1.5) | 1.2 (0.3) | 0.8 (0.4) |
| Epithelial cells, %* | 5 (4) | 6 (1) | 2 (1) |
| 8-oxodG semiquantitative score | |||
| Epithelium§ | 4.5 (1.25) | 5 (0.5) | 5 (0.75) |
| Lamina propria§ | 4 (1) | 5 (0.75) | 5 (0.75) |
| Airway smooth muscle§ | 2 (2.25) | 4.5 (1.75)‡ | 4.5 (2)‡ |
Definition of abbreviations: BD = bronchodilator; GINA = Global Initiative for Asthma; 8-oxodG = 8-oxo-7,8-dihydro-2′-deoxyguanosine; TCC = total cell count.
Mean (SEM).
Geometric mean (95% confidence interval).
P < 0.05 compared to control.
Median (interquartile range).
Figure 1.
Representative photomicrographs of bronchial biopsies illustrating 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) immunostaining in (A) an isotype control, (B) a subject with severe asthma, (C) the airway smooth muscle (ASM) bundle of a healthy control, and (D) the ASM bundle in a subject with severe asthma. Scale bars, 100 μm (A and B) and 25 μm (C and D). (E) Semiquantitative intensity score of 8-oxodG in ASM cells in subjects with asthma compared with healthy subjects. Data are shown as mean ± SEM; n = 37; comparison across groups Kruskal-Wallis P = 0.022 and between groups post hoc Dunn's pairwise comparison P values as shown. (F) 8-oxodG staining intensity was correlated negatively with airflow obstruction (FEV1/FVC %) (Spearman's rank correlation coefficient).
We then considered whether this exaggerated oxidative stress burden was increased in ASM independent of the local in vivo environment. We cultured primary ASM isolated by microdissection from bronchial biopsies from individuals with asthma and healthy controls (see Table E1 in the online supplement) and assessed the levels of oxidatively damaged DNA using hOGG1-modified single-cell gel electrophoresis, or “comet” assay, before and after exposure to hydrogen peroxide (100 μM). Background levels of DNA damage consisting of single-strand breaks and alkali-labile and 8-oxodG sites in primary ASM cells from individuals with asthma were significantly elevated compared with levels from healthy control subjects (mean difference [95% confidence interval {CI}] in tail moment 2.2 [1.8 to 2.5]; P < 0.001) (Figure 2A). Analysis by percentage tail DNA revealed similar findings (data not shown). Following hydrogen peroxide (10 mM) exposure, there was an increase in the production of ROS, which was more marked in asthmatic ASM compared with that from healthy control subjects (mean fold difference [95% CI] 8.1 [3.0 to 19.1]; P = 0.029) (Figure 2B). DNA damage following hydrogen peroxide challenge was comparable in health and disease (Figure 2A), suggesting that the maximal response is similar, but sensitivity to oxidative stress is heightened in asthmatic ASM. These observations support the view that there are intrinsic differences in the function of ASM derived from individuals with asthma and suggest that these abnormalities can be present in the absence of the asthmatic environment, although this does not exclude the possibility that these effects may be further augmented by the proinflammatory milieu and heightened oxidative stress burden in the asthmatic airway.
Figure 2.
(A) Detection of DNA damage induced by hydrogen peroxide in airway smooth muscle (ASM) cells using the human 8-oxoguanine glycosylase 1-modified alkaline comet assay. Reactive oxygen species (ROS) promotes formation of 8-oxo-7,8-dihydroguanine in DNA. Human 8-oxoguanine glycosylase 1 is an 8-oxoguanine DNA glycosylase that recognizes and removes the damaged DNA caused by ROS. Tail moment is defined as the product of the tail length and the total DNA in the tail. It incorporates the smallest detectable size of migrating DNA (comet tail length) and the fragments (represented by the intensity of DNA in the tail). Data are shown as mean ± SEM (n = 9). (B) Detection of intracellular ROS, induced by hydrogen peroxide in ASM cells after 1 hour using 2′,7′-dichlorofluorescin diacetate, which detects hydrogen peroxide, peroxyl radicals, and peroxynitrite anions (mean ± SEM; n = 8). (C) Intracellular flow cytometric data shown as a scatter plot (mean ± SEM). (D) Representative flow cytometric analysis of NOX4 cell surface expression and intracellular NOX4 expression. Gray line corresponds to IgG isotype control, black line NOX4+. (E) Quantitative reverse transcription–polymerase chain reaction analysis of NOX4 mRNA in normal and asthmatic ASM cells (mean ± SEM; n = 23). (F) NOX4 mRNA expression correlated negatively with airflow obstruction. Comparisons were made across groups by Kruskal-Wallis test with post hoc Dunn's pairwise comparison for nonparametric data and by paired and unpaired t tests as appropriate for parametric data. Correlations were assessed by the Spearman rank test.
To determine the potential mechanisms in asthma driving the increased sensitivity to oxidative stress in ASM, we chose to examine messenger RNA (mRNA) expression using genome-wide microarrays of unstimulated ASM from six individuals with asthma and six healthy control subjects. We identified 17 transcripts that were significantly up-regulated and 20 down-regulated in asthma versus healthy controls (more than twofold, with a false discovery rate of 29% following 1,000 permutations). Additionally, 23 transcripts were present in five or more individuals with asthma versus one or fewer healthy control subjects and 11 transcripts in five or more healthy control subjects versus one or fewer individuals with asthma (Tables E2a–E2c).
From the gene array data, we selected genes that are involved in the generation and detoxification of ROS, namely NOX4, a subtype of the ROS-generating nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, and SOD2, which were up- and down-regulated in asthma respectively. The other NOX isoforms, 1–3 and 5, were not identified by gene array in ASM from healthy subjects or those with asthma. Abnormalities in NOX4 and SOD2 expression have the potential to act in concert with an increase in oxidative stress via ROS generation through the up-regulation of NOX4 and decrease ROS removal through the down-regulation of SOD2. NOX4 has been implicated in the pathogenesis of pulmonary fibrosis, and its expression is increased in interstitial lung disease in humans (24, 25) and in murine models (25). We confirmed that NOX4 protein and mRNA expression is increased in asthma by flow cytometry (7.7 [1.4]% vs. 21.4 [5.4]%; mean difference [95% CI] 13.7 [0.2 to 27.3]; P = 0.024) (Figures 2C and 2D) and quantitative real-time reverse transcription–polymerase chain reaction (Figure 2E; P = 0.002). The amount of mRNA expression was related to the FEV1/FVC (%) (r = −0.41; P = 0.046; n = 23) (Figure 2F). Interestingly, this correlation was remarkably similar to the relationship between airflow obstruction and the in vivo burden of oxidative stress. In contrast, we were unable to confirm differential SOD1 or SOD2 expression by ASM from individuals with asthma and healthy subjects at the mRNA or protein level (Figures E1A–E1D). Similarly, we did not identify differences in the expression of NOX1–3 or 5 by ASM between subjects with asthma or healthy controls using flow cytometry and immunofluorescence (data not shown). Taken together, these data suggest that the intrinsic differences in oxidative stress burden in ASM from individuals with asthma and healthy controls may be due to the differential NOX4 expression.
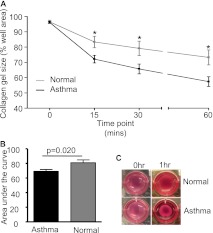
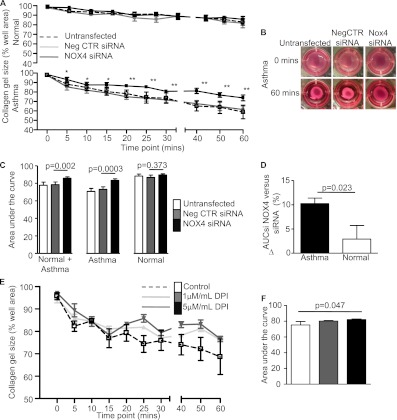
Disordered airway physiology is the hallmark of asthma with an exaggerated response to smooth muscle spasmogens. Here we confirm, using a gel contraction assay, that ASM agonist-induced contraction is increased in asthma compared with healthy control subjects (mean difference in area under the curve contraction [95% CI] 11.85 [2.1 to 21.6]; P = 0.02) (Figures 3A–3C). Transforming growth factor (TGF)-β has been implicated in increasing ASM contractility, in part via up-regulation of α-smooth muscle actin expression (22, 25). However, neither basal TGF-β release nor α-smooth muscle actin expression was different between ASM from individuals with asthma and that from healthy control subjects (Figures E2A–E2C). Bradykinin B2 receptor was also not different between ASM from individuals with asthma and that from control subjects (mean difference in median fluorescence intensity [95% CI] −24 [−174 to 126], P = 0.71; n = 8). We therefore considered that NOX4 overexpression in asthma may predispose the asthmatic ASM to heightened agonist-induced contraction. We found that the agonist-induced ASM contraction of the healthy control donors and those with asthma combined was inhibited by anti-NOX4 siRNA (mean difference [95% CI] −7.3 [−11.2 to −3.5]; P = 0.002). Interestingly, this effect was predominately due to the attenuation of the contractile response of the donors with asthma. The hypercontractility seen in the individuals with asthma was abrogated by an anti-NOX4 siRNA (mean difference [95% CI] −10.25 [−13.6 to −7.3]; P = 0.0003) (Figures 4A–4C). The reduction in the contractile response mediated by NOX4 siRNA was significantly greater in ASM from individuals with asthma than in that from healthy control subjects (mean difference [95% CI] 7.3 [1.3 to 13.4]; P = 0.023) (Figure 4D) and was replicated by a second NOX4-specific siRNA (mean difference [95% CI] 8.6 [2.0 to 15.2]; P = 0.017). Likewise, the agonist-induced contraction of asthmatic ASM was attenuated by the NOX inhibitor apocynin (mean difference in area under the curve contraction [95% CI] 9.1 [0.6 to 17.8]; P = 0.042; n = 4) and in a concentration-dependent manner by diphenyleneiodonium (Figures 4E and 4F).
Figure 3.
(A) Percentage contraction of collagen gels impregnated with airway smooth muscle from donors with asthma (n = 19) versus healthy control donors (n = 8) over 1 hour following stimulation with 1 nM bradykinin, (B) area under the curve gel contraction (mean ± SEM), and (C) representative gel photographs taken at 0 hour and 1 hour time points. The comparison was made by unpaired t test. *P < 0.05.
Figure 4.
(A) Percentage contraction of collagen gels, following stimulation with 1 nM bradykinin, impregnated with airway smooth muscle from donors with asthma versus healthy control donors untransfected or transfected with negative control small interfering RNA (siRNA) or NOX4 siRNA, (B) representative gel photographs taken at 0 hour and 1 hour time points, and (C) area under the curve (AUC) gel contraction (mean ± SEM, n = 6 asthma, n = 4 healthy control). Between group comparisons were made by paired t tests, *P < 0.05, **P < 0.01. (D) Comparison of the difference in AUC between negative control siRNA and NOX4 siRNA in asthma versus healthy control. (E) Percentage contraction of collagen gels impregnated with airway smooth muscle from donors with asthma versus healthy control donors following stimulation with 1 nM bradykinin in the presence and absence of diphenyleneiodonium (1–5 μg/ml) (n = 4). (F) AUC gel contraction (mean ± SEM, n = 4). Across-group comparisons were made by linear regression.
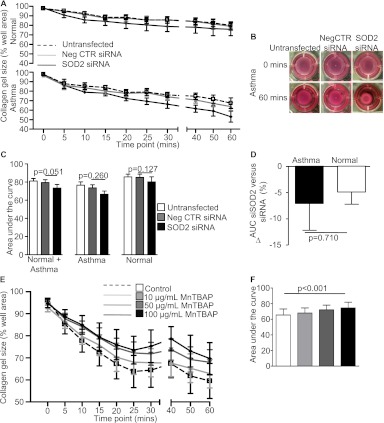
We were unable to demonstrate differential SOD2 expression between ASM from individuals with asthma and that from healthy control subjects; however, we considered whether perturbing SOD2 may affect ASM contraction. Indeed, an anti-SOD2 siRNA increased the contractility of both asthmatic and healthy control ASM donors combined to a degree similar to anti-NOX4, but did not reach statistical significance (mean difference [95% CI] 6.0 [−1.6 to 11.2]; P = 0.051) (Figures 5A–5C). Importantly, in contrast to anti-NOX4, the effect of anti-SOD2 was similar in subjects with asthma (7.2 [−8.6 to 23]; P = 0.26) and healthy controls (4.9 [−2.5 to 12.3]; P = 0.13) (Figures 5A–5C). The increase in the contractile response induced by SOD2 siRNA was not significantly different between ASM from individuals with asthma and that from healthy controls (mean difference [95% CI] 2.2 [−11.5 to 15.9]; P = 0.71) (Figure 5D) and was replicated by a second SOD2-specific siRNA (mean difference [95% CI] 3.5 [−4.3 to 11.3]; P = 0.31). Similarly, the SOD mimetic manganese (III) tetrakis (4-benzoic acid) porphyrin chloride attenuated agonist-induced contractions of asthmatic ASM in a concentration-dependent manner (Figures 5E and 5F). This suggests that changes in SOD2 expression and function may affect ASM contraction, but that this does not represent an intrinsic abnormality of asthma. Importantly, neither anti-NOX4 nor anti-SOD2 siRNA significantly affected the ASM α-smooth muscle actin expression (Figures E3A–E3D), suggesting that their effects upon ASM contraction are independent of the contractile protein expression.
Figure 5.
(A) Percentage contraction of collagen gels, following stimulation with 1 nM bradykinin, impregnated with airway smooth muscle from donors with asthma versus healthy control donors untransfected or transfected with negative control small interfering RNA (siRNA) or superoxide dismutase 2 siRNA, (B) representative gel photographs taken at 0 hour and 1 hour time points, and (C) AUC gel contraction (mean ± SEM, n = 8). Between-group comparisons were made by paired t tests. (D) Comparison of the difference in AUC between negative control siRNA and superoxide dismutase 2 siRNA in asthma versus healthy control. (E) Percentage contraction of collagen gels impregnated with airway smooth muscle from asthmatic versus healthy control donors following stimulation with 1 nM bradykinin in the presence and absence of manganese (III) tetrakis (4-benzoic acid) porphyrin chloride (10–100 mg/ml) (n = 6). (F) AUC gel contraction (mean ± SEM, n = 6). Across-group comparisons were made by linear regression.
Discussion
This is the first report to identify that the oxidative stress burden in asthmatic ASM in vivo is increased and in vitro primary ASM exhibited increased ROS generation, resulting in oxidative stress and increased background levels of oxidatively damaged DNA. Critically, we have identified that NOX4 expression is intrinsically up-regulated in asthma. Both the degree of oxidative damage in vivo and the NOX4 expression in vitro were similarly related to the degree of airflow obstruction. Most importantly, this agonist-induced ASM hypercontractility in asthma was eliminated by inhibition of NOX4. Indeed, the agonist-induced contraction of ASM from individuals with asthma was normalized by NOX4 siRNA, strongly supporting a critical role for NOX4 in the asthma paradigm.
Current evidence supports the view that the burden of oxidative stress is up-regulated in the airway of individuals with asthma (17). An elevated oxidative stress burden may be a consequence of the inflammatory milieu in asthma or due to abnormalities in the dynamic and complex processes involved in the generation and detoxification of ROS. Here we found that the oxidative stress burden in asthmatic ASM was increased in vivo and in vitro, suggesting that an abnormality in the handling of ROS persists in primary culture. Indeed, we found using genome-wide transcriptome arrays and confirmed by quantitative polymerase chain reaction and at the protein level by flow cytometry that expression of NOX4 was intrinsically up-regulated in ASM derived from subjects with asthma. The expression of the other NOX isoforms 1–3 and 5 were not different between asthma and health, and therefore our results do not support a role for a nonspecific NOX effect, but rather suggest a specific role for NOX4. Although we demonstrated differential expression by gene array of the mitochondrial enzyme SOD2 in ASM from individuals with asthma and healthy controls, we were unable to validate this observation by quantitative polymerase chain reaction or protein expression. Therefore, our findings support the view that NOX4 rather than mitochondrial enzymes may be more important in driving the intrinsic abnormalities in asthmatic ASM. This increased expression of NOX4 in asthma has the potential to increase the generation of ROS and provides a possible explanation for the heightened burden of oxidative stress observed in vivo. Whether this intrinsic difference between asthma and health has clinical implications is therefore critically important.
The cardinal feature of asthma is disordered airway physiology, which is largely a consequence of increased ASM contraction (1, 2). We found that the burden of oxidative stress observed in the ASM bundle in vivo and the expression of NOX4 in primary ASM cells were related to the degree of airflow obstruction, suggesting that oxidative stress driven by increased NOX4 expression may contribute to impaired lung function in asthma. We confirmed and extended the earlier fundamental observation that ASM from individuals with asthma exhibit increased agonist-induced contraction compared with healthy control subjects (15, 16). This was not a consequence of differences in expression of α-smooth muscle actin or bradykinin B2 receptor by ASM in disease versus health. Importantly, we found that both NOX4 siRNA and pharmacological inhibition attenuated this exaggerated contractile response in asthmatic ASM. The importance of oxidative stress upon agonist-induced contraction was also demonstrated by the increased contraction in response to SOD2 siRNA and reduced contraction in the presence of the SOD2 mimetic. However, critically, these effects were similar between ASM from individuals with asthma and that from healthy control subjects, supporting the view that this mechanism is not differentially regulated in health and disease. Indeed, the intrinsic difference observed in the agonist-induced contraction between asthmatic and normal ASM was normalized by NOX4 knockdown, implicating the intrinsic up-regulation of NOX4 as critical in the development of ASM hypercontractility in asthma.
One potential criticism of our study is that in the absence of an animal model, we are unable to demonstrate that manipulation of NOX4 expression in vivo modulates ASM contraction. Thus, whether NOX4 is necessary or sufficient to induce ASM hypercontractility in asthma remains unknown. We used the gel contraction assay as a model of ASM contraction. Gel contraction occurs over a longer time period than agonist-induced bronchoconstriction in vivo and reflects the need for the force of contraction of the ASM within the gel to be sufficient to cause gel contraction. However, this method has been validated as a measure of ASM contraction in previous studies (16, 22), and we are therefore confident that it reflects in vivo differences. Intriguingly, it is also uncertain whether this NOX4 up-regulation in asthmatic ASM represents the primary abnormality in asthma or a secondary phenomenon. A previous study has reported abnormal mitochondrial biogenesis in ASM from individuals with asthma (10). This led to increased oxygen consumption and altered intracellular calcium homeostasis. NOX4 is up-regulated in hypoxic conditions, acting as a hypoxia sensor (26). The consequent increased production of ROS may provide a positive feedback loop enhancing mitochondrial biogenesis. Therefore, it is likely that these two observations are intimately related, with one potentially augmenting the other. Nevertheless, that NOX4 may be a secondary event rather than the primary abnormality in the pathogenesis of asthma does not detract from its potential clinical relevance, as strategies aimed at normalizing NOX4 expression in asthma may have important clinical outcomes.
In conclusion, we have demonstrated that the oxidative stress burden in ASM from individuals with asthma is heightened together with an up-regulation of NOX4. ASM from individuals with asthma exhibited increased agonist-induced contraction, which was abrogated by siRNA knockdown or pharmacological inhibition, implicating NOX4 as a novel target for asthma therapy. Pharmacological inhibitors of NOX4 are already in early development for pulmonary fibrosis (27). We therefore suggest that this approach may present an important novel target for asthma, which could be tested in clinical trials in the near future.
Supplementary Material
Acknowledgments
The authors thank all the research volunteers who participated in the study, and also the following people for their valuable assistance throughout the study: M. Bafadhel, D. Desai, B. Hargadon, M. Pantoli, M. Karbaschi, A. Gavrila, J. Balfour, and G. Stewart.
Footnotes
Supported by a Wellcome Trust Senior Clinical Fellowship and AirPROM (FP7 270,194). The research was performed in laboratories partly funded by the European Regional Development Fund (ERDF 05,567). The Wellcome Trust, the European Commission, and the European Regional Development Fund had no involvement in the design of the study, data collection, analysis and interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript.
Author Contributions: A.S., F.H., E.G., R.S., and C.D. contributed to the study design, experiments, data collection, and interpretation; M.C. and R.A.J.C. were involved in the design of the study, supervision, and interpretation; and C.E.B. was involved in the study design, volunteer recruitment, data collection, supervision, data interpretation, and data analysis and had full access to the data and is responsible for the integrity of the data and final decision to submit. All authors contributed to the writing of the manuscript and have approved the final version for submission.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201107-1281OC on November 22, 2011
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bousquet J, Mantzouranis E, Cruz AA, Aït-Khaled N, Baena-Cagnani CE, Bleecker ER, Brightling CE, Burney P, Bush A, Busse WW, et al. Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization Consultation on Severe Asthma. J Allergy Clin Immunol 2010;126:926–938 [DOI] [PubMed] [Google Scholar]
- 2. Global strategy for asthma management and prevention [Internet]; c2005 [updated 2009; accessed October 2011]. Available from: www.ginasthma.com.
- 3.Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol 2008;8:218–230 [DOI] [PubMed] [Google Scholar]
- 4.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med 2002;346:1699–1705 [DOI] [PubMed] [Google Scholar]
- 5.Berger P, Girodet PO, Begueret H, Ousova O, Perng DW, Marthan R, Walls AF, Tunon de Lara JM. Tryptase-stimulated human airway smooth muscle cells induce cytokine synthesis and mast cell chemotaxis. FASEB J 2003;17:2139–2141 [DOI] [PubMed] [Google Scholar]
- 6.Panina-Bordignon P, Papi A, Mariani M, Di Lucia P, Casoni G, Bellettato C, Buonsanti C, Miotto D, Mapp C, Villa A, et al. The C–C chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J Clin Invest 2001;107:1357–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brightling CE, Symon FA, Birring SS, Bradding P, Pavord ID, Wardlaw AJ. TH2 cytokine expression in bronchoalveolar lavage fluid T lymphocytes and bronchial submucosa is a feature of asthma and eosinophilic bronchitis. J Allergy Clin Immunol 2002;110:899–905 [DOI] [PubMed] [Google Scholar]
- 8.Brightling CE, Ammit AJ, Kaur D, Black JL, Wardlaw AJ, Hughes JM, Bradding P. The CXCL10/CXCR3 axis mediates human lung mast cell migration to asthmatic airway smooth muscle. Am J Respir Crit Care Med 2005;171:1103–1108 [DOI] [PubMed] [Google Scholar]
- 9.Chan V, Burgess JK, Ratoff JC, O'connor BJ, Greenough A, Lee TH, Hirst SJ. Extracellular matrix regulates enhanced eotaxin expression in asthmatic airway smooth muscle cells. Am J Respir Crit Care Med 2006;174:379–385 [DOI] [PubMed] [Google Scholar]
- 10.Trian T, Benard G, Begueret H, Rossignol R, Girodet PO, Ghosh D, Ousova O, Vernejoux JM, Marthan R, Tunon-de-Lara JM, et al. Bronchial smooth muscle remodeling involves calcium-dependent enhanced mitochondrial biogenesis in asthma. J Exp Med 2007;204:3173–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahn K, Hirst SJ, Ying S, Holt MR, Lavender P, Ojo OO, Siew L, Simcock DE, McVicker CG, Kanabar V, et al. Diminished sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) expression contributes to airway remodelling in bronchial asthma. Proc Natl Acad Sci USA 2009;106:10775–10780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roth M, Johnson PR, Borger P, Bihl MP, Rüdiger JJ, King GG, Ge Q, Hostettler K, Burgess JK, Black JL, et al. Dysfunctional interaction of C/EBPalpha and the glucocorticoid receptor in asthmatic bronchial smooth-muscle cells. N Engl J Med 2004;351:560–574 [DOI] [PubMed] [Google Scholar]
- 13.Kaur D, Hollins F, Saunders R, Woodman L, Sutcliffe A, Cruse G, Bradding P, Brightling C. Airway smooth muscle proliferation and survival is not modulated by mast cells. Clin Exp Allergy 2010;40:279–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward JE, Harris T, Bamford T, Mast A, Pain MC, Robertson C, Smallwood D, Tran T, Wilson J, Stewart AG. Proliferation is not increased in airway myofibroblasts isolated from asthmatics. Eur Respir J 2008;32:362–371 [DOI] [PubMed] [Google Scholar]
- 15.Ma X, Wang Y, Stephens NL. Serum deprivation induces a unique hypercontractile phenotype of cultured smooth muscle cells. Am J Physiol 1998;274:1206–1214 [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto H, Moir LM, Oliver BG, Burgess JK, Roth M, Black JL, McParland BE. Comparison of gel contraction mediated by airway smooth muscle cells from patients with and without asthma. Thorax 2007;62:848–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montuschi P, Barnes PJ, Ciabattoni G. Measurement of 8-isoprostane in exhaled breath condensate. Methods Mol Biol 2010;594:73–84 [DOI] [PubMed] [Google Scholar]
- 18.Hollins F, Sutcliffe A, Gomez E, Doe C, Saunders R, Challiss J, Brightling C. Novel mechanisms of airway smooth muscle contraction and relaxation: are these therapeutically targetable? [abstract]. American Thoracic Society Meeting 2011;183:A1264 [Google Scholar]
- 19.Gomez E, Powell ML, Bevington A, Herbert TP. A decrease in cellular energy status stimulates PERK-dependent eIF2alpha phosphorylation and regulates protein synthesis in pancreatic beta-cells. Biochem J 2008;410:485–493 [DOI] [PubMed] [Google Scholar]
- 20.Cooke MS, Duarte TL, Cooper D, Chen J, Nandagopal S, Evans MD. Combination of azathioprine and UVA irradiation is a major source of cellular 8-oxo-7,8-dihydro-2’-deoxyguanosine. DNA Repair (Amst) 2008;7:1982–1989 [DOI] [PubMed] [Google Scholar]
- 21.Touyz RM, Schiffrin EL. Ang II–stimulated superoxide production is mediated via phospholipase D in human vascular smooth muscle cells. Hypertension 1999;34:976–982 [DOI] [PubMed] [Google Scholar]
- 22.Woodman L, Siddiqui S, Cruse G, Sutcliffe A, Saunders R, Kaur D, Bradding P, Brightling C. Mast cells promote airway smooth muscle cell differentiation via autocrine up-regulation of TGF-beta 1. J Immunol 2008;181:5001–5007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manea A, Tanase LI, Raicu M, Simionescu M. JAK/STAT signaling pathway regulates Nox1 and Nox4-based NADPH oxidase in human aortic smooth muscle cells. Aterioscler Thromb Vasc Biol 2010; 30:105–112 [DOI] [PubMed] [Google Scholar]
- 24.Amara N, Goven D, Prost F, Muloway R, Crestani B, Boczkowski J. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts. Thorax 2010;65:733–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med 2009;15:1077–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rathore R, Zheng YM, Niu CF, Liu QH, Korde A, Ho YS, Wang YX. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCepsilon signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med 2008;45:1223–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laleu B, Gaggini F, Orchard M, Fioraso-Cartier L, Cagnon L, Houngninou-Molango S, Gradia A, Duboux G, Merlot C, Heitz F, et al. First in class, potent, and orally bioavailable NADPH oxidase isoform 4 (Nox4) inhibitors for the treatment of idiopathic pulmonary fibrosis. J Med Chem 2010;53:7715–7730 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.