Introduction
Pathological Q waves may appear on the 12-lead electrocardiogram (ECG) during acute ST-segment elevation myocardial infarction (STEMI) and signify loss of electrical excitation. Anterior wall STEMI often results in loss of R waves in the precordial leads and subsequent appearance of pathological Q waves. Not infrequently, very early loss of R waves may be visible in patients presenting with anterior STEMI and occasionally, these R waves reappear in the first months following the index event. Despite reports in the literature [1–9], the prognostic significance of R-wave reappearance (RWR) in the setting of primary percutaneous coronary intervention (pPCI) remains uncertain. In this report we present two patients with anterior STEMI who underwent uncomplicated pPCI. The follow-up ECGs were indicative of the amount of myocardial recovery after the acute event as shown by cardiovascular magnetic resonance (CMR) imaging.
Case report
Case 1
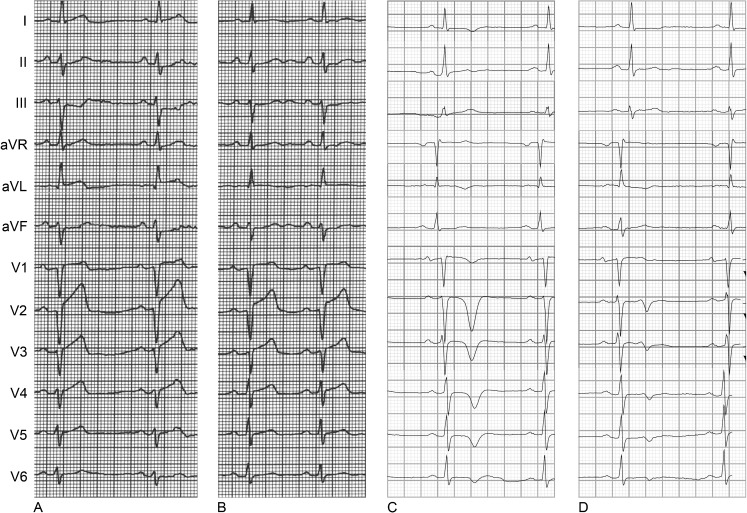
A 75-year-old man with no prior history of cardiac disease was admitted to our coronary care unit because of acute chest pain with radiation to the left arm accompanied by vegetative symptoms for 3 h. Smoking was the patient’s only risk factor for coronary artery disease. The ECG on admission showed ST-segment elevation in leads aVL and V1–V4 with loss of R waves in leads V1–V3 indicating anterior wall STEMI (Fig. 1a). Emergency coronary angiography revealed an occlusion of the left anterior descending (LAD) artery immediately distal to the origin of the first diagonal branch. Uncomplicated PCI with stenting resulted in restoration of TIMI 3-graded flow approximately 4 h after symptom onset. Peak CK-MB level was 148 μg/l. Post-procedural ECG showed >50% ST-segment resolution but persistent loss of R waves in leads V1–V3 (Fig. 1b).
Fig. 1.
The 12-lead ECG recorded a before primary PCI, b after primary PCI, c after 1 month and d after 2 months (patient 1)
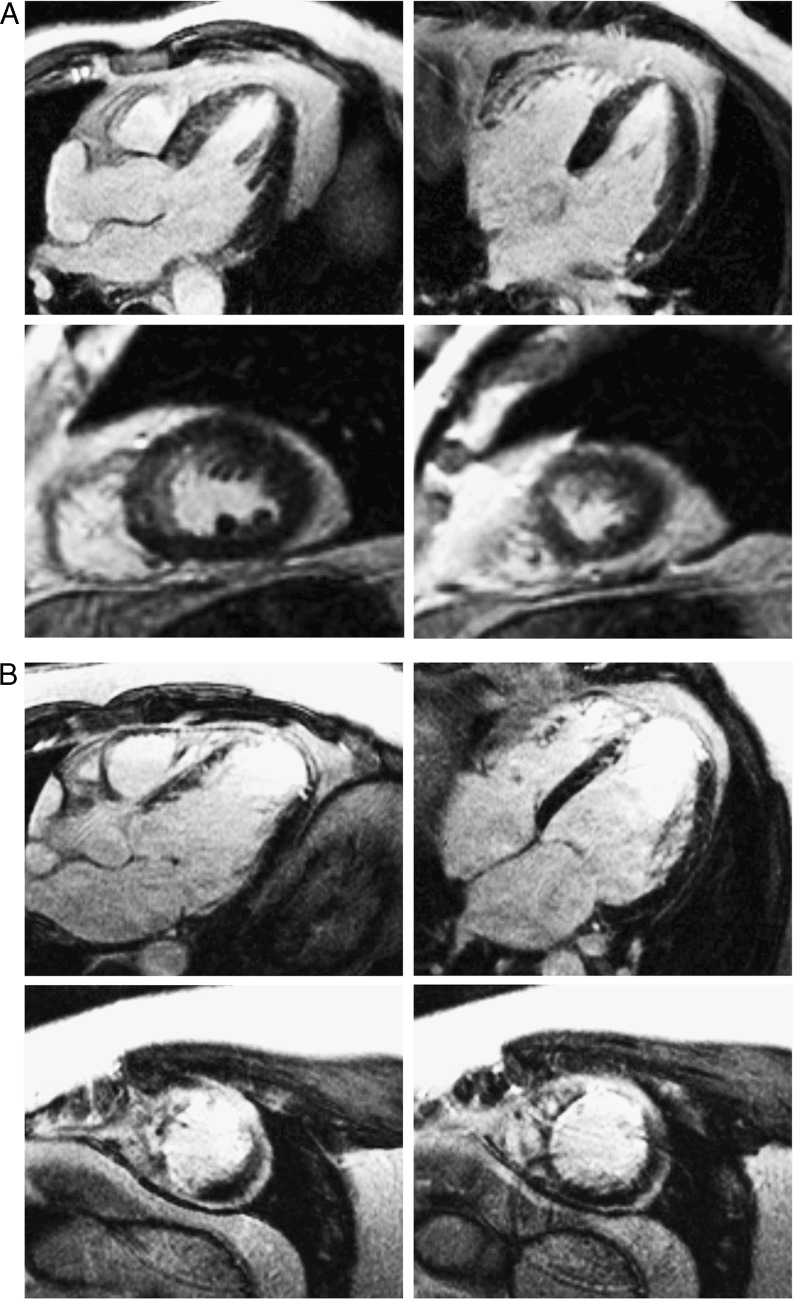
Nevertheless, at the outpatient clinic, 1 month after the MI, the first follow-up ECG showed RWR, most evident in leads V2–V3 (Fig. 1c). At 3 months after the index event, the second follow-up ECG showed persistent RWR in V1–V3 with higher R-wave voltage amplitude in V1–V2 as compared with the previously recorded ECGs (Fig. 1d). CMR at 10 months after the index event showed only limited perfusion defects (Fig. 3a). As a result, we did not observe regional wall motion abnormalities and the left ventricular ejection fraction (LVEF) was 62%.
Fig. 3.
Cardiovascular magnetic resonance imaging in patient 1 (a) and patient 2 (b). a CMR shows limited perfusion defects. b CMR in shows transmural necrosis of the anteroseptum
Case 2
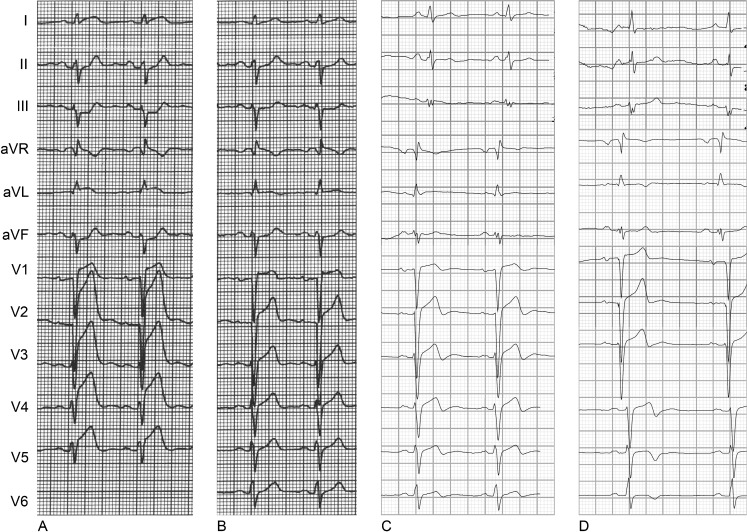
A 49-year-old man with no prior history of cardiac disease was admitted to our coronary care unit because of typical acute chest pain lasting 3.5 h. The patient’s risk factors for coronary artery disease were a positive family history and hypertension. The ECG on admission showed ST-segment elevation in leads aVR, aVL and V1–V5 with early loss of R waves in leads V1 and V2 indicating anterior wall STEMI (Fig. 2a). Emergency coronary angiography showed an LAD occlusion immediately distal to the origin of the first diagonal branch. Uncomplicated PCI with stenting resulted in restoration of TIMI-3 graded flow at 4.5 h after symptom onset. Peak CK-MB was 480 μg/l. Post-procedural ECG showed >50% ST-segment resolution and R-wave loss in leads V1–V2 (Fig. 2b).
Fig. 2.
The 12-lead ECG recorded a before primary PCI, b after primary PCI, c after 1 week and d after 4 months (patient 2)
The follow-up ECGs recorded at 1 week and 4 months after the index event showed persistent R-wave loss (Fig. 2c, d). CMR showed transmural necrosis of the anteroseptum (Fig. 3b) and LVEF was 45%.
Discussion
This case report describes two anterior wall STEMI patients with early R-wave loss in the precordial leads. Both patients had a proximal LAD occlusion, and uncomplicated PCI with stenting was performed within 5 h after symptom onset. Follow-up ECGs in case 1 showed complete RWR, while follow-up ECGs in case 2 showed persistent loss of R waves. In both cases CMR, performed within 1 year after the index event, showed that the occurrence of RWR was indicative of the amount of myocardial recovery.
RWR is not an uncommon phenomenon in anterior STEMI patients undergoing successful reperfusion therapy. The incidence rates reported in the literature are 39% and 77% during 6 and 6–60 month follow-up periods, respectively [5, 7].
The appearance of pathological Q waves in the chronic phase of STEMI has been classically associated with myocardial necrosis, whereas Q waves in the early stages of STEMI may result from a transient loss of electrical function due to intense ischaemia [10]. The exact mechanism of RWR has not been clarified, but the time course may give insight into the mechanism involved in this phenomenon. After the acute phase of STEMI, the infarcted myocardium may gradually shrink as part of the remodelling process. If myocardial fibres are then able to cover the infarcted area, this may result in RWR [11]. However, this process may take several months to years while the RWR in case 1 occurred within 1 month. Bateman et al. [1] explained early RWR by the existence of an ‘electrically silent’ area in context of myocardial stunning with transient loss of potential in cellular membrane. Finally, the exact position of precordial electrodes could vary during recording of the ECGs influencing R-wave voltage. This seems of minor influence in case 1 as all follow-up ECGs consistently showed complete RWR.
The prognostic significance of RWR remains unclear. Several studies [2, 4, 6, 7, 9] reported that patients with RWR had a smaller infarct size and better left ventricular function whereas Iwasaki et al. [5] showed no such association. The CMR in case 1 showed a smaller infarct size and peri-infarct border zone as compared with case 2. The peri-infarct border zone is postulated to represent a mix of viable and scarred myocardium. This heterogeneous region may act as a substrate for ventricular arrhythmic events which is an indicator of prognosis [12]. In this case, the occurrence of RWR corresponded with a better myocardial recovery revealed by CMR and therefore may provide important prognostic information. By reporting these cases, we would like to create awareness of this phenomenon and encourage future research to enlighten its significance.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Bateman TM, Czer LS, Gray RJ, et al. Transient pathologic Q waves during acute ischemic events: an electrocardiographic correlate of stunned but viable myocardium. Am Heart J. 1983;106(6):1421–1426. doi: 10.1016/0002-8703(83)90056-X. [DOI] [PubMed] [Google Scholar]
- 2.Coll S, Betriu A, de Flores T, et al. Significance of Q-wave regression after transmural acute myocardial infarction. Am J Cardiol. 1988;61(10):739–742. doi: 10.1016/0002-9149(88)91058-2. [DOI] [PubMed] [Google Scholar]
- 3.Haiat R, Chiche P. Transient abnormal Q waves in the course of ischemic heart disease. Chest. 1974;65(2):140–144. doi: 10.1378/chest.65.2.140. [DOI] [PubMed] [Google Scholar]
- 4.Ishikawa K, Shimizu M, Ohno M, et al. Clinical significance of abnormal Q wave disappearance in acute transmural myocardial infarction. Jpn Circ J. 1991;55(3):213–220. doi: 10.1253/jcj.55.213. [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki K, Kusachi S, Hina K, et al. Q-wave regression unrelated to patency of infarct-related artery or left ventricular ejection fraction or volume after anterior wall acute myocardial infarction treated with or without reperfusion therapy. Am J Cardiol. 1995;76(1):14–20. doi: 10.1016/S0002-9149(99)80793-0. [DOI] [PubMed] [Google Scholar]
- 6.Jaarsma W, Visser CA, van Eenige MJ, et al. Left ventricular wall motion with and without Q-wave disappearance after acute myocardial infarction. Am J Cardiol. 1987;59(6):516–518. doi: 10.1016/0002-9149(87)91159-3. [DOI] [PubMed] [Google Scholar]
- 7.Nagase K, Tamura A, Mikuriya Y, et al. Significance of Q-wave regression after anterior wall acute myocardial infarction. Eur Heart J. 1998;19(5):742–746. doi: 10.1053/euhj.1997.0850. [DOI] [PubMed] [Google Scholar]
- 8.Wasserman AG, Bren GB, Ross AM, et al. Prognostic implications of diagnostic Q waves after myocardial infarction. Circulation. 1982;65(7):1451–1455. doi: 10.1161/01.CIR.65.7.1451. [DOI] [PubMed] [Google Scholar]
- 9.Yasuda M, Iida H, Itagane H, et al. Significance of Q wave disappearance in the chronic phase following transmural acute myocardial infarction. Jpn Circ J. 1990;54(12):1517–1524. doi: 10.1253/jcj.54.12_1517. [DOI] [PubMed] [Google Scholar]
- 10.Atar S, Birnbaum Y. Ischemia-induced ST-segment elevation: classification, prognosis, and therapy. J Electrocardiol. 2005;38(4 Suppl):1–7. doi: 10.1016/j.jelectrocard.2005.06.098. [DOI] [PubMed] [Google Scholar]
- 11.Kalbfleisch JM, Shadaksharappa KS, Conrad LL, et al. Disappearance of the Q-deflection following myocardial infarction. Am Heart J. 1968;76(2):193–198. doi: 10.1016/0002-8703(68)90194-4. [DOI] [PubMed] [Google Scholar]
- 12.Yan AT, Shayne AJ, Brown KA, et al. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation. 2006;114(1):32–39. doi: 10.1161/CIRCULATIONAHA.106.613414. [DOI] [PubMed] [Google Scholar]