Abstract
Please cite this paper as: Hall et al. (2012) Avian influenza in shorebirds: experimental infection of ruddy turnstones (Arenaria interpres) with avian influenza virus. Influenza and Other Respiratory Viruses DOI: 10.1111/j.1750‐2659.2012.00358.x.
Background Low pathogenic avian influenza viruses (LPAIV) have been reported in shorebirds, especially at Delaware Bay, USA, during spring migration. However, data on patterns of virus excretion, minimal infectious doses, and clinical outcome are lacking. The ruddy turnstone (Arenaria interpres) is the shorebird species with the highest prevalence of influenza virus at Delaware Bay.
Objectives The primary objective of this study was to experimentally assess the patterns of influenza virus excretion, minimal infectious doses, and clinical outcome in ruddy turnstones.
Methods We experimentally challenged ruddy turnstones using a common LPAIV shorebird isolate, an LPAIV waterfowl isolate, or a highly pathogenic H5N1 avian influenza virus. Cloacal and oral swabs and sera were analyzed from each bird.
Results Most ruddy turnstones had pre‐existing antibodies to avian influenza virus, and many were infected at the time of capture. The infectious doses for each challenge virus were similar (103·6–104·16 EID50), regardless of exposure history. All infected birds excreted similar amounts of virus and showed no clinical signs of disease or mortality. Influenza A‐specific antibodies remained detectable for at least 2 months after inoculation.
Conclusions These results provide a reference for interpretation of surveillance data, modeling, and predicting the risks of avian influenza transmission and movement in these important hosts.
Keywords: Avian, infection, influenza, pathogenesis, shorebird, turnstone
Introduction
The role of shorebirds, particularly sandpipers and allied birds (Order Charadriiformes; Family Scolopacidae), in the epidemiology of avian influenza virus (AIV) is unclear. Although numerous AIVs have been isolated from these taxa, consistent positive results have been reported only from ruddy turnstones (subfamily Arenariinae, Arenaria interpres) and sympatric shorebirds at Delaware Bay, USA. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10
Olsen et al. 5 determined that worldwide AIV prevalence from shorebird surveillance is 0·8%, and Ip et al. 11 found a prevalence of 0·04% in Alaskan Scolopacidae. At Delaware Bay, the prevalence in spring shorebirds has ranged from 4·4% to 14·2%. 3 , 8 This contrasts with the prevalence of AIV found in waterfowl that typically peaks during autumnal southern migrations, when large numbers of young, immunologically naïve birds congregate. 12 AIVs isolated from shorebirds also exhibit more annual diversity than those isolated from waterfowl. 3 Thus, AIV infection in shorebirds is different from other reservoirs and the causes and implications of these differences are unknown.
Our understanding of avian influenza in shorebirds is largely limited to data acquired in field studies. With the exception of a study with highly pathogenic avian influenza (HPAIV) in dunlin (Calidris alpina), no information is available on pathogenesis, viral shedding patterns, minimum infectious dose, immune response, or clinical outcome of AIV infection in shorebirds. In this study, we experimentally challenged ruddy turnstones with AIV isolates to examine the course and outcome of AIV infection. This knowledge is essential to understanding the disease dynamics in this important AIV reservoir.
Materials and methods
Ruddy turnstone acquisition and husbandry
Ruddy turnstones were captured at Delaware Bay in May 2009 (n = 40) and 2010 (n = 40). The birds were transported to the National Wildlife Health Center (NWHC), Madison, WI, where they were housed in HEPA‐filtered isolator cages (2–3 birds/cage) within a BSL3 facility. Birds were provided water and food ad libitum.
Experimental design and AIV inoculation
On arrival at the NWHC, oral and cloacal swabs and serum were collected from all birds. Swabs were tested for AIV infection, and sera were tested for AIV antibodies. On the basis of these pre‐inoculation data, birds were divided into cohorts and allowed to acclimatize for 5 (2009) or 13 (2010) days. The purpose of the longer 2010 acclimation period was to allow any pre‐existing viral infections to clear. The low pathogenic avian influenza virus (LPAIV) isolates used to challenge the birds were A/shorebird/DE/42/2006 (H7N3) and A/northernpintail/California/44242‐758/2006 (H5N2). The HPAIV isolate used was A/whooper swan/Mongolia/244/05 (H5N1). In 2009, a LPAIV H7N3 isolate was chosen because it was originally isolated from Charadriiforms. Therefore, serological and virus negative birds were preferentially assigned to be challenged with this virus. The remaining seropositive birds were assigned to either the H7N3 or the HPAIV (H5N1) trials. In 2010, seronegative birds were assigned to be challenged with the H7N3 shorebird isolate (Table 1). We used LPAIV H5N2 to compare this waterfowl isolate with the H7N3 isolate. Uninoculated birds, four in 2009 and two in 2010, served as negative controls for each trial.
Table 1.
Influenza virus isolates, inocula titers, and experimental design used to challenge ruddy turnstones
| Bird ID | First challenge | Second challenge | Bird ID | First challenge | Second challenge |
|---|---|---|---|---|---|
| Virus (Dose)* | Virus (Dose)** | Virus (Dose) | Virus (Dose)** | ||
| 1 | Control (−)*** | Control (−)*** | 5† | Control (−)†† | H5N1 (6·25)*** |
| 2 | Control (−)*** | Control (−)*** | 70† | Control (−)*** | H5N1 (6·25)*** |
| 3 | H7N3 (1·25)†† | H7N3 (3·75)†† | 16 | H5N2 (2)*** | H5N1 (5·25)*** |
| 55 | H7N3 (1·25)†† | H7N3 (3·75)†† | 20 | H5N2 (2)*** | H5N1 (5·25)†† |
| 10 | H7N3 (1·25)†† | H7N3 (3·75)†† | 51 | H5N2 (2)*** | H5N1 (5·25)*** |
| 8 | H7N3 (1·25)*** | H5N1 (4·25)†† | 7 | H5N2 (2)*** | H5N1 (6·25)*** |
| 6 | H7N3 (1·25)*** | H5N1 (4·25)*** | 9 | H5N2 (2)*** | H5N1 (6·25)*** |
| 29 | H7N3 (1·25)†† | Held Long Term (−)*** | 11 | H5N2 (2)*** | H5N1 (6·25)*** |
| 13 | H7N3 (3·25)†† | H7N3 (3·75)†† | 67 | H5N2 (4)*** | Control (−)*** |
| 14 | H7N3 (3·25)†† | H7N3 (3·75)†† | 79 | H5N2 (4)*** | Control (−)*** |
| 12 | H7N3 (3·25)*** | H7N3 (3·75)*** | 66 | H5N2 (4)*** | ††† |
| 68 | H7N3 (3·25)†† | H5N1 (4·25)†† | 59 | H5N2 (4)†† | H7N3 (3·75)†† |
| 63 | H7N3 (3·25)*** | H5N1 (4·25)*** | 52 | H5N2 (4)*** | H5N1 (5·25)*** |
| 61 | H7N3 (3·25)†† | Held Long Term (−)*** | 54 | H5N2 (4)*** | H5N1 (5·25)*** |
| 15 | H7N3 (5·25)†† | H7N3 (3·75)*** | 4† | H5N2 (6)*** | H7N3 (3·75)*** |
| 64 | H7N3 (5·25)*** | H5N1 (4·25)*** | 69 | H5N2 (6)*** | H7N3 (3·75)*** |
| 30 | H7N3 (5·25)*** | H5N1 (4·25)*** | 57† | H5N2 (6)*** | H7N3 (4·75)*** |
| 65 | H7N3 (5·25)*** | H5N1 (5·25)*** | 58 | H5N2 (6)*** | H7N3 (4·75)*** |
| 18 | H7N3 (5·25)†† | Held Long Term (−)*** | 60† | H5N2 (6)†† | H7N3 (4·75)†† |
| 19 | H7N3 (5·25)†† | Held Long Term (−)*** | 80 | H5N2 (6)*** | H7N3 (4·75)*** |
Avian influenza virus isolates used to inoculate ruddy turnstones: A/shorebird/DE/42/2006 (H7N3); A/northernpintail/California/44242‐758/2006 (H5N2); A/whooperswan/Mongolia/244/05 (H5N1).
*Viral titers confirmed in embryonating egg culture and expressed as Log10 EID50.
**Inocula used in second round of experimental infections.
***Serologically positive (IDEXX ELISA) to avian influenza virus at time of inoculation.
†These birds were naturally infected with avian influenza virus at time of arrival at the NWHC as determined by RT‐PCR analysis of cloacal swabs.
††Serologically negative (IDEXX ELISA) to avian influenza virus at time of inoculation.
†††Bird 66 euthanized because of injury.
Viral inocula were passaged and titered in embryonating eggs. Six birds/cohort were inoculated intranasally (10 μl) and intrachoanally (90 μl) with one of three doses of LPAIV or HPAIV. The titers of the highest dose of each inoculum were calculated by the method of Reed and Muench 13 in 10‐day‐old embryonating chicken eggs (37·5°C, 50% humidity) at the time of inoculation. The initial doses administered in the 2009 trial were based on the infectious dose (101·7 EID50/100 μl, HPAIV H5N1), determined in dunlin, another shorebird species. 14
Birds that excreted no detectable viral RNA orally or cloacally and failed to seroconvert were assumed to not have been infected. Thus, they were available for subsequent challenge with an isolate to which they had not been previously exposed. Four birds that were initially seronegative but seroconverted after H7N3 inoculation were maintained for a total of 63 days post‐inoculation (DPI). Blood samples were periodically taken and tested for the presence of AIV antibodies using blocking enzyme‐linked immunosorbent assays (bELISA).
Sampling
Blood samples (200 μl) were collected by jugular venipuncture on arrival at the NWHC, prior to inoculation (DPI 0), DPI 7, and DPI 14 (2009) or DPI 15 (2010). Sera were separated by centrifugation in serum separator tubes (Becton Dickinson, Franklin Lakes, NJ, USA) and stored at −20°C. Birds were weighed and monitored daily to ascertain health status. Cloacal and oropharyngeal swabs were obtained daily using Dacron®‐tipped applicators, placed in cryovials containing viral transport medium and stored at −80°C until analysis.
Serology
Blocking enzyme‐linked immunosorbent assays were performed using the IDEXX multispecies ELISA according to the manufacturer’s directions (IDEXX Laboratories, Westbrook, ME, USA). This assay utilizes influenza A nucleoprotein as the coating antigen. Hemagglutination inhibition (HI) assays were based on Palmer et al. 15 using the virus isolates employed for the infection trials as well as A/ruddy turnstone/DE/70/2010(H6N4), a common virus subtype in turnstones at Delaware Bay in 2010. Chicken and horse red blood cells were used for HI assays.
Virus neutralization assays
Pre‐ and post‐infection sera from selected birds were examined for neutralizing activity to the H6N4 isolate, an isolate circulating in shorebirds during 2010. Sera were heat‐treated at 56°C for 30 minutes and diluted to 1:10 or 1:30 with PBS. A 25 μl sample of each sera dilution was combined with 25 μl virus dilution (100 EID50) and incubated for 30 minutes at room temperature. Serum/virus mixtures were brought to 0·6 ml with PBS, and 0·2 ml was injected into each of three embryonating chicken eggs and incubated for 48 hours at 35°C. Hemagglutinating activity of allantoic fluid was tested from each egg. Sera from the four birds housed long term were tested for neutralizing activity to the H7N3 virus using the same methods, except that 1:5, 1:10, 1:30, and 1:100 serum dilutions were used.
Virus isolation
Virus isolation was performed on cloacal swab samples taken upon arrival at the NWHC in embryonating chicken egg culture, as described above. Influenza subtypes were determined from virus isolates by sequence analysis of the viral RNA. 16 The presence of viable virus in all swabs from experimentally infected birds with RT‐PCR C t values was also confirmed by virus isolation in embryonating egg culture.
RNA extraction and real‐time RT‐PCR
Viral RNA was extracted from cloacal and oropharyngeal swabs by using the MagMAX™ 96 AI/ND Viral RNA Isolation Kit (Ambion, Austin, TX, USA) following the manufacturer’s procedures. Real‐time RT‐PCR was performed using procedures, primers, and probes for the detection of influenza virus RNA. 17 , 18 RT‐PCR assays were performed using Qiagen OneStep® RT‐PCR kit (Valencia, CA, USA).
Results
Pre‐inoculation virological and serological status
Based on RT‐PCR analyses of cloacal swabs taken on arrival at the NWHC, 11/40 birds in 2009 and 5/40 in 2010 were excreting influenza viral RNA. From these RT‐PCR swab samples, four AIVs were isolated in 2009 [H10N7 (3); H10N1 (1)], and another four were isolated in 2010 (all H6N4). Regardless of virological status, all birds were in apparent good health, maintained or gained weight in captivity, and showed no overt signs of illness throughout the study.
Most of the birds had pre‐existing antibodies to AIV: 28/40 (70%) in 2009 and 26/40 (65%) in 2010. HI analyses against the virus isolates used for inoculation showed no inhibition, implying no recent exposure to those viruses (data not shown).
2009 AIV experimental challenge
In both 2009 challenge trials, regardless of inoculation dose (H7N3; 101·75, 100·75, 100·1 EID50/100 μl or H5N1; 103·5, 102·5, 101·5 EID50/100 μl), none of the ruddy turnstones showed overt signs of disease or excreted detectable viral RNA orally or cloacally, and only those birds naturally infected prior to inoculation developed antibodies to AIV. All birds remained healthy for 14 DPI, based on daily observations and increase in body mass (data not shown). We therefore concluded the doses were below the minimum threshold required to infect ruddy turnstones.
2010 AIV experimental challenge
In 2010, we increased the inoculation doses. Table 2 shows the proportion of birds infected and excreting viral RNA at each virus dose. The birds were sampled for 14 DPI and housed an additional 14 days, at which time they all remained in good health. To better define the infectious dose, we performed a second round of infection with the H7N3 isolate using birds that had remained seronegative or had been inoculated with the H5N2 isolate (Table 1). The remaining turnstones were inoculated with three doses of HPAIV (H5N1) (Table 2). Based on logistic regression analyses, 19 we estimated the 50% infectious dose (ID50) for each isolate. The ID50 in turnstones for the H7N3 shorebird isolate was 103·61 EID50/100 μl (95% CI 3·18–4·05); the H5N2 waterfowl isolate was 104·01 EID50/100 μl (95% CI 3·24–4·78); and H5N1 was 104·16 EID50/100 μl (95% CI 3·39–4·93). It must be kept in mind that the ID50 calculation of the H5N1 isolate is only an approximation due to the fact that none of the doses administered resulted in <50% of the inoculated birds becoming infected.
Table 2.
Proportion of ruddy turnstones infected with avian influenza virus at each dose administered
| Isolate | Dose* (Log10 EID50) | No. infected**/No. inoculated |
|---|---|---|
| A/northernpintail/California/44242‐758/2006 (H5N2) | 6·0 | 6/6 |
| 4·0 | 3/6 | |
| 2·0 | 0/6 | |
| A/shorebird/DE/42/2006 (H7N3) | 5·25 | 5/6 |
| 4·75*** | 4/4 | |
| 3·75*** | 8/10 | |
| 3·25 | 1/6 | |
| 2·75*** | 0/6 | |
| 1·25 | 0/6 | |
| A/whooperswan/Mongolia/244/2005 (H5N1) | 6·25*** | 6/6 |
| 5·25*** | 4/6 | |
| 4·25*** | 4/5 |
*Viral titers confirmed in embryonating egg culture using method of Reed and Muench. 13
**Birds were considered infected on the basis of oral/cloacal excretion of viral RNA and seroconversion.
***Results from second round of inoculations.
Viable H7N3, H5N2, and H5N1 viruses were isolated from birds infected with each of the viruses. All infected birds predominantly excreted virus RNA orally (3, 4, 5); and few RT‐PCR positive cloacal swabs (C t range 30·74–40·33) were detected (data not shown). The relative amounts of viral RNA excreted orally were similar for each virus isolate including H5N1 (Figure 1). The duration of virus excretion typically lasted 5–7 DPI, though several birds inoculated with the H5N1 and H5N2 isolates excreted detectable viral RNA orally up to 10 DPI.
Table 3.
RT‐PCR analysis of oral swabs from ruddy turnstones experimentally inoculated with influenza isolate A/shorebird/DE/42/2006 (H7N3)
| Bird ID | Dose* | DPI 0 | DPI 1 | DPI 2 | DPI 3 | DPI 4 | DPI 5 | DPI 6 | DPI 7 | DPI 9 |
|---|---|---|---|---|---|---|---|---|---|---|
| 61 | 3·25 | –** | – | 32·5c | 38·49c | – | – | – | – | – |
| 68*** | 3·25 | – | – | – | – | – | – | – | – | – |
| 63 | 3·25 | – | – | – | – | – | – | – | – | – |
| 12 | 3·25 | – | – | – | – | – | – | – | – | – |
| 13 | 3·25 | – | – | – | – | – | – | – | – | – |
| 14 | 3·25 | – | – | – | – | – | – | – | – | – |
| 3 | 3·75 | – | 27·77† | 34·53† | – | – | – | – | – | – |
| 10 | 3·75 | – | – | – | – | – | – | – | – | – |
| 55 | 3·75 | – | – | – | – | – | – | – | – | – |
| 15 | 3·75 | – | 29·24 | 33·87 | 39·95 | – | – | – | – | – |
| 13 | 3·75 | – | 39·43 | 32·3 | – | – | – | – | – | – |
| 14 | 3·75 | – | 29·68† | 32·91 | 34·08 | 38·06† | 32† | 35 | 39·43 | – |
| 68 | 3·75 | – | – | – | 35·77 | – | – | – | – | – |
| 59 | 3·75 | – | 31·51† | 30·43† | 31·33† | 29·48† | 28·07† | 27·59† | 32·86† | 39·48† |
| 4 | 3·75 | – | 33·83† | 33·45† | 40·62 | – | – | – | – | – |
| 69 | 3·75 | – | 35·92† | 38·08† | –† | 38·01 | – | – | – | – |
| 60 | 4·75 | – | 30·7† | 34·21 | – | 39·97 | 40·14 | – | – | – |
| 80 | 4·75 | – | 37·31 | – | 40·34 | – | – | – | – | – |
| 57 | 4·75 | – | – | 39·25 | 37·05 | – | – | – | – | – |
| 58 | 4·75 | – | 32·82 | 32·99† | 35·72 | 31·98† | 28·52† | 39·24 | – | – |
| 15*** | 5·25 | – | – | – | – | – | – | – | – | – |
| 18 | 5·25 | – | 29·19†c | 31·93c | 34·34c | 38·85c | 40·25c | – | – | – |
| 64 | 5·25 | – | 30·71†c | 36·82c | 39·31c | – | – | – | – | – |
| 19 | 5·25 | – | 29·67†c | 32·71†c | 35·28c | 38·25†c | – | – | – | – |
| 17 | 5·25 | – | 28·48†c | 28·58c | 29·95†c | 34·01c | 39·98c | – | – | – |
| 65 | 5·25 | – | 30·87†c | 25·9†c | 27·37†c | 27·94†c | 27·14†c | 33·07†c | 34·29c | 37·16†c |
DPI, days post‐inoculation.
*Inocula doses expressed as Log10 EID50/100 μl. No bird inoculated with lower viral titers became infected based on viral excretion and seroconversion except bird 29 that seroconverted but did not shed viral RNA (not shown). This bird was housed long term to determine the stability of antibody response.
**C t values are from RT‐PCR analyses using avian influenza H7 specific primers and probe. Samples with no C t values are shown with –. cIndicates that RT‐PCR positive cloacal swabs were detected on the DPI shown.
***These birds were uninfected and remained seronegative from the first challenge round and were reused in a second challenge with this virus isolate.
†Presence of viable virus confirmed by virus isolation in embryonating egg culture.
Table 4.
RT‐PCR analysis of oral swabs from ruddy turnstones experimentally inoculated with influenza isolate A/northern pintail/California/44242‐758/2006 (H5N2)
| Bird ID | Dose* | DPI 0 | DPI 1 | DPI 2 | DPI 3 | DPI 4 | DPI 5 | DPI 6 | DPI 7 | DPI 9 |
|---|---|---|---|---|---|---|---|---|---|---|
| 5 | Control | –** | – | – | – | – | – | – | – | – |
| 70 | Control | – | – | – | – | – | – | – | – | – |
| 7 | 2 | – | – | – | – | – | – | – | – | – |
| 9 | 2 | – | 38·09*** | – | – | – | – | – | – | – |
| 11 | 2 | – | – | – | – | – | – | – | – | – |
| 16 | 2 | – | – | – | – | – | – | – | – | – |
| 20 | 2 | – | 39·51*** | – | – | –c | – | –c | – | – |
| 51 | 2 | – | 38·23*** | – | – | – | – | – | – | – |
| 52 | 4 | – | – | – | – | – | – | – | – | – |
| 59 | 4 | – | – | – | – | – | – | – | – | – |
| 53 | 4 | – | – | 37·6 | 37·85† | 32·46† | – | –c | – | – |
| 54 | 4 | – | – | – | – | – | – | – | – | – |
| 67 | 4 | – | 30·1† | 30·08† | 34·18c | – | – | – | – | – |
| 66 | 4 | – | – | 33·85 | 33·99 | – | 37·82 | – | – | – |
| 57 | 6 | – | – | – | – | 39·79 | 37·56 | – | – | – |
| 58 | 6 | – | 34·95 | 37·21 | 36·26 | 34·64 | 38·24 | – | – | – |
| 60 | 6 | – | 33·85 | 35·2 | 38·63 | – | – | – | – | – |
| 62 | 6 | – | 37·11 | 35·4 | 32·55 | – | – | 38·93 | – | 39·7 |
| 4 | 6 | – | 30·89c | 32·1 | 35·54 | – | – | – | – | – |
| 69 | 6 | – | 30·8c | 36·64 | – | – | – | – | – | – |
DPI, days post‐inoculation.
*Inocula doses expressed as Log10 EID50/100 μl. No bird inoculated with lower viral titers became infected based on viral excretion and seroconversion (not shown).
**C t values are from RT‐PCR analyses using avian influenza H5 specific primers and probe. Samples with no C t values are shown with –. cIndicates that RT‐PCR positive cloacal swabs were detected on the DPI shown.
***The presence of viral RNA detected on DPI1 only was assumed to be residual inoculum.
†Presence of viable virus confirmed by virus isolation in embryonating egg culture.
Table 5.
RT‐PCR analysis of oral swabs from ruddy turnstones experimentally inoculated with highly pathogenic avian influenza isolate A/whooperswan/Mongolia/244/05 (H5N1)
| Bird ID | Dose* | DPI 0 | DPI 1 | DPI 2 | DPI 3 | DPI 4 | DPI 5 | DPI 6 | DPI 7 | DPI 8 | DPI10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 67 | Control | –** | – | – | – | – | – | – | – | – | – |
| 79 | Control | – | – | – | – | – | – | – | – | – | – |
| 64 | 4·25 | – | 26·9*** | 33·46 | 36·38 | – | 38·75 | –c | – | – | – |
| 12 | 4·25 | – | 31·26 | 28·01*** | 34·55 | 33·37 | 27·83*** | 31·49 | 37·13 | – | 38·21 |
| 63 | 4·25 | – | – | – | 30·31*** | 31·13 | 32·51***c | 33·81 | 36·59 | 34·79 | 38·09 |
| 8 | 4·25 | – | – | – | – | 38·66*** | – | – | – | 39·89 | – |
| 6 | 4·25 | – | 29·97 | 30·05 | 33·29c | 33·56c | 29·28c | 28·81 | 31·86*** | 34·84 | – |
| 30 | 4·25 | – | ‐ | 37·36 | – | – | – | – | – | – | – |
| 54 | 5·25 | – | 37·28 | 34·12 | – | 39·49 | – | – | – | – | – |
| 65 | 5·25 | – | – | – | – | 37·44 | – | – | – | – | – |
| 52 | 5·25 | – | 31·75 | 37·04 | – | – | – | – | – | – | – |
| 51 | 5·25 | – | 36·75 | 36·61 | 38·89 | – | – | 39·55 | – | – | – |
| 20 | 5·25 | – | 32·77 | 32·78 | 36·54 | – | – | – | – | – | – |
| 16 | 5·25 | – | 27·2*** | 31·26 | 37·15 | 38·77*** | – | – | – | – | – |
| 11 | 6·25 | – | 30·49 | 32·01 | 35·84 | 39·79 | 37·56 | – | – | – | – |
| 9 | 6·25 | – | 27·08*** | 35·63 | 36·3 | 38·74 | – | – | – | – | – |
| 7 | 6·25 | – | 34·51 | 35·22 | 37·93 | – | – | – | – | – | – |
| 70 | 6·25 | – | 27*** | 30·42 | 33·61 | 33·16 | 33·88 | 27·27 | – | – | – |
| 5 | 6·25 | – | 37·27 | – | 37·28 | – | – | – | – | – | – |
DPI, days post‐inoculation.
*Inocula doses expressed as Log10 EID50/100 μl. No bird inoculated with lower viral titers became infected based on viral excretion and seroconversion (not shown).
**C t values are from RT‐PCR analyses using avian influenza H5 specific primers and probe. Samples with no C t values are shown with –. cIndicates that RT‐PCR positive cloacal swabs were detected on the DPI shown.
***Presence of viable virus confirmed by virus isolation in embryonating egg culture.
Figure 1.
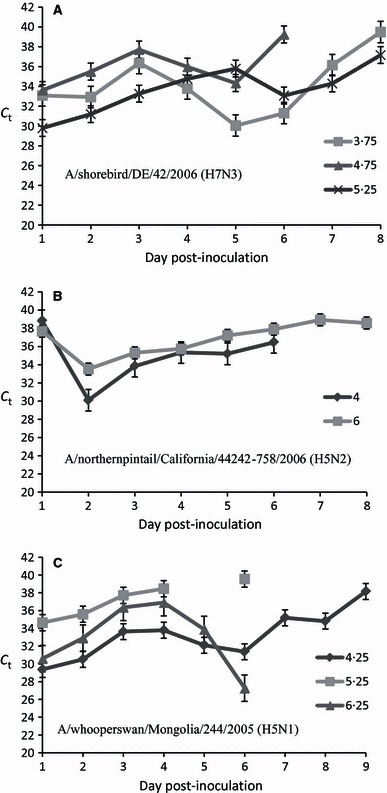
Oral excretion of avian influenza virus RNA from experimentally inoculated ruddy turnstones. Cycle threshold (C t) values determined by H5 or H7 hemagglutinin‐specific RT‐PCR analyses for 8 or 9 days post‐inoculation (DPI). Each panel represents the mean C t values of infected birds inoculated with (A) low pathogenic avian influenza virus A/shorebird/DE/42/2006 (H7N3), (B) A/northernpintail/California/44242‐758/2006 (H5N2), or (C) highly pathogenic avian influenza virus A/whooperswan/Mongolia/244/2005 (H5N1). Error bars represent the standard deviation at each time point. The titers of virus are expressed as log10 EID50/100 μl.
The turnstones that were initially serologically naïve and that became infected after virus challenge, all developed detectable influenza antibodies by 7 DPI. HI analyses of 14 DPI sera using the inocula strains as antigens showed that only one bird developed detectable HI activity and that was to H5N1 in chicken erythrocytes. Using horse erythrocytes, six of the birds challenged with H5N1 revealed agglutinating inhibition with titers ranging from 40 to 640 (data not shown). However, not all birds that were infected and excreting HPAIV developed detectable HI antibody (1:40), and only one bird challenged with H7N3 and one with H5N2 developed HI activity. The reasons for the failure to detect HI activity in turnstones are not known and illustrate the difficulties and differences in applying standard methods to new species.
The four birds that seroconverted after inoculation with the H7N3 shorebird isolate and maintained for 63 DPI showed a slight trend toward seronegativity by bELISA; one bird (#61) was borderline seronegative with a sample/negative control (S/N) ratio of 0·50 at 63 DPI (Figure 2). Despite becoming seropositive after infection with AIV, none of the birds’ sera neutralized H7N3 virus at any dilution (1:10 or greater) or time point tested (14–63 DPI). In fact, using standard virus neutralization assays, we failed to detect any neutralizing activity in any bird that we examined (data not shown), again illustrating the need for additional research on immune responses in these birds.
Figure 2.
Stability of serum antibodies in ruddy turnstones after inoculation with low pathogenic avian influenza virus A/shorebird/DE/42/2006 (H7N3). Birds were maintained for the number of days post‐inoculation (DPI) indicated, and sample/negative control (S/N) ratios were determined in four birds by using IDEXX MultiSpecies ELISA. S/N ratios <0.50 are considered seropositive.
Regardless of virus inoculum or dose, none of the ruddy turnstones developed overt signs of disease, showed any ill effects, or died, even after inoculation with H5N1. In fact, based on their maintenance and gain of body mass, the birds remained healthy for the duration of this study.
Discussion
Working with ruddy turnstones from Delaware Bay was complicated. Wild birds are often a challenge to house and maintain, especially in biocontainment facilities. We developed the techniques and expertise to house and maintain these birds for relatively long periods of time, sufficient to perform several rounds of experimental infections. Upon capture and transport to the NWHC, some birds were actively infected with AIV, and many more were serologically positive. Fortunately, none of the birds arrived infected with the AIV subtypes with which they were later inoculated or showed evidence of prior exposure to those subtypes. However, all of the experimental data must be viewed in the context that many of the birds had histories with influenza that may have affected the outcomes of these AIV experimental infections.
Regardless of whether an LPAIV or HPAIV isolate was used as inoculum, whether the isolate was from a waterfowl (H5N2) or shorebird (H7N3) species, or whether the birds were previously exposed to AIV, the infectious doses were essentially equivalent, and all birds remained healthy. The consistency of these results lends confidence that they are accurate measurements of AIV infection in this species. The relative amounts of virus excreted by birds infected with different isolates were also similar and were predominantly detected in oral swabs. This contrasts with field sampling data, where cloacal shedding is typical. The reason for this difference is unknown but may be a result of the route of experimental inoculation used for this study, that is, intranasal/intrachoanal, as opposed to an oral/fecal transmission cycle in natural settings. Most field studies of AIV in shorebirds have been preferentially based on cloacal and/or fecal sampling 20 ; thus, the reported virus prevalence in these birds may be an underestimate. Alternatively, the conditions in which these birds were maintained, with unfamiliar food and altered behavior, may have contributed to skewed patterns of excretion. Clearly, additional studies are needed to examine this issue.
These experimental infection results in turnstones contrast with those in another shorebird species. 14 The ID50 of H5N1 in dunlins was 101·7 EID50/100 μl, whereas in turnstones, the infectious dose was 104·16 EID50/100 μl, and no infected bird died or showed any ill effects. Why the two species react differently is unknown, but different hosts react differently to AIV infection. For example, wood ducks (Aix sponsa) proved highly susceptible to HPAIV infection, with 100% mortality, 21 whereas northern pintails (Anas acuta), became infected but did not exhibit any mortality. 22 Another difference may be that the dunlin study used naïve juvenile birds and this study used adult turnstones with varied histories of influenza exposure.
All of the turnstones experimentally infected with H5N1 were AIV seropositive: some had exposure to AIV prior to capture; some had been inoculated with H5N2, and others with H7N3. Fereidouni et al. 23 showed that previous infection with AIV heterosubtypes provides varying degrees of protection against H5N1 infection and mortality. However, we found no differences in infection rates between any of the previously exposed birds, whether they had been exposed to H5, H7, or to an undetermined hemagglutinin subtype. The lack of virus neutralizing activity, despite the stability of antibody levels, indicates no prevention of subsequent infection with other subtypes. Clearly, the immune responses of shorebirds differ from those of poultry and waterfowl, which are better understood. Developing adequate, accurate methods to define these responses in ruddy turnstones and other species of shorebirds is crucial to determining their roles in transporting and transmitting AIV in nature.
Avian influenza ecology at Delaware Bay is unique. High virus and serological prevalence in ruddy turnstones at a time of year when AIV activity in waterfowl is typically low raises many questions about the processes and disease dynamics occurring at this location. Ruddy turnstones are the species with the highest prevalence of influenza at Delaware Bay, and this study reports the results of experimental infection studies in ruddy turnstones in an age class and time of year most relevant to the ecology of AIV. In adult ruddy turnstones, the infectious dose, the amount, and the duration of virus excretion were remarkably similar, regardless of AIV inocula. This study provides a basis for future experimental work to assess and plan surveillance studies, develop risk analyses, and evaluate the epidemiological impacts of shorebirds as hosts of AIV.
Acknowledgements
Critical components of this study required the technical assistance of David Walker at St. Jude Children’s Research Hospital, Robin E. Russell of the NWHC, and the exemplary work of the animal care staff at the NWHC. This work has been funded, in whole or in part, by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract Nos. HHSN266200700005C and HHSN266200700007C, and by the American Lebanese Syrian Associated Charities. Use of trade or product names does not imply endorsement by the United States government.
References
- 1. Kawaoka Y, Chambers TM, Sladen WL, Webster RG. Is the gene pool of influenza viruses in shorebirds and gulls different from that in wild ducks? Virology 1988; 163:247–250. [DOI] [PubMed] [Google Scholar]
- 2. Fouchier RAM, Olsen B, Bestebroer TM et al. Influenza A virus surveillance in wild birds in Northern Europe in 1999 and 2000. Avian Dis 2003; 47:857–860. [DOI] [PubMed] [Google Scholar]
- 3. Krauss S, Walker D, Pryor SP et al. Influenza A viruses of migrating wild aquatic birds in North America. Vector Borne Zoonotic Dis 2004; 4:177–189. [DOI] [PubMed] [Google Scholar]
- 4. Krauss S, Obert CA, Franks J et al. Influenza in migratory birds and evidence of limited intercontinental virus exchange. PLoS Pathog 2007; 3:1684–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Olsen B, Munster VJ, Wallensten A, Waldenström J, Osterhaus ADME, Fouchier RAM. Global patterns of influenza A virus in wild birds. Science 2006; 312:384–388. [DOI] [PubMed] [Google Scholar]
- 6. Alexander DJ. Summary of avian influenza activity in Europe, Asia, Africa, and Australasia, 2002–2006. Avian Dis 2007; 51:161–166. [DOI] [PubMed] [Google Scholar]
- 7. Munster VJ, Baas C, Lexmond P et al. Spatial, temporal, and species variation in prevalence of influenza A viruses in wild migratory birds. PLoS Pathog 2007; 3:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hanson BA, Luttrell MP, Goekjian VH et al. Is the occurrence of avian influenza virus in Charadriiformes species and location dependent? J Wildl Dis 2008; 44:351–361. [DOI] [PubMed] [Google Scholar]
- 9. Winker K, Spackman E, Swayne DE. Rarity of influenza A virus in Spring shorebirds, Southern Alaska. Emerg Infect Dis 2008; 14:1314–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iverson SA, Takekawa JY, Schwarzbach S et al. Low prevalence of avian influenza virus in shorebirds on the Pacific coast of North America. Waterbirds 2008; 31:602–610. [Google Scholar]
- 11. Ip HS, Flint PL, Franson JC et al. Prevalence of influenza A viruses in wild migratory birds in Alaska: patterns of variation in detection at a crossroads of intercontinental flyways. Virol J 2008; 5:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev 1992; 56:152–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reed LJ, Muench H. A simple method for estimating fifty per cent endpoints. Am J Hyg 1938; 27:493–497. [Google Scholar]
- 14. Hall JS, Franson JC, Gill RE Jr et al. Experimental challenge and pathology of highly pathogenic avian influenza virus H5N1 in dunlin (Calidris alpina), an intercontinental migrant shorebird species. Influenza Other Respi Viruses 2011; 5:365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Palmer DF, Coleman MT, Dowdle WR, Schild GC. Advanced Laboratory Techniques for Influenza Diagnosis. Atlanta, GA: United States Department of Health, Education and Welfare, 1975. [Google Scholar]
- 16. Hoffmann E, Stech J, Guan Y, et al. Universal primer set for the full‐length amplification of all influenza A viruses. Arch Virol 2001; 146:2275–2289. [DOI] [PubMed] [Google Scholar]
- 17. Spackman E, Senne DA, Myers TJ. Development of a real‐time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J Clin Microbiol 2002; 40:3256–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spackman E, Ip H, Suarez DL, Slemons RD, Stallknecht DE. Analytical validation of a real‐time reverse transcription polymerase chain reaction test for Pan‐American lineage H7 subtype avian influenza viruses. J Vet Diagn Invest 2008; 20:612–616. [DOI] [PubMed] [Google Scholar]
- 19. Development Core Team . R A Language and Environment for Statistical Computing. R Vienna, Austria: Foundation for Statistical Computing, 2011; ISBN 3‐900051‐07‐0, http://www.R‐project.org/. [Google Scholar]
- 20. Krauss S, Stallknecht DE, Negovetich NJ, Niles LJ, Webby RJ, Webster RG. Coincident ruddy turnstone migration and horseshoe crab spawning creates an ecological ‘hot spot’ for influenza viruses. Proc Biol Sci 2010; 277:3373–3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brown JD, Stallknecht DE, Beck JR, Suarez DL, Swayne DE. Susceptibility of North American ducks and gulls to H5N1 highly pathogenic avian influenza viruses. Emerg Infect Dis 2006; 12:1663–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brown JD, Stallknecht DE, Valeika S, Swayne DE. Susceptibility of wood ducks to H5N1 highly pathogenic avian influenza virus. J Wildl Dis 2007; 43:660–667. [DOI] [PubMed] [Google Scholar]
- 23. Fereidouni SR, Starick E, Beer M. Highly pathogenic avian influenza virus infection of mallards with homo‐ and heterosubtypic immunity induced by low pathogenic avian influenza viruses. PLoS ONE 2009; 4:e6706. [DOI] [PMC free article] [PubMed] [Google Scholar]


