Abstract
Background
Research on efficacious treatments for apathy in Alzheimer’s disease has been hindered by a lack of consensus diagnosis, difficulties in measurement, and studies with small sample sizes.
Methods
In designing the Apathy in Dementia Methylphenidate Trial (ADMET), a trial to evaluate the efficacy and safety of methylphenidate for the treatment of apathy in Alzheimer’s disease, we encountered the following issues: defining and measuring apathy, distinguishing apathy and depression, determining an appropriate test treatment, selecting relevant secondary outcomes, recruiting participants, and deciding on a suitable method for treatment unmasking. ADMET is a 6-week randomized, double-masked, placebo-controlled multicenter clinical trial with two parallel treatment groups assigned in a 1:1 ratio with randomization stratified by clinical center. The recruitment goal is 60 randomized participants over 2 years. The primary outcomes are change in apathy severity as measured by the Apathy Evaluation Scale and the Alzheimer Disease Cooperative Study-Clinical Global Impression of Change.
Conclusion
The design decisions made for ADMET are important elements to be considered in trials assessing the safety and efficacy of medications for clinically significant apathy in Alzheimer’s disease.
Keywords: Alzheimer dementia, methylphenidate, apathy, randomized trial
1. Introduction
The number of people living with Alzheimer’s disease (AD) world-wide is expected to exceed 80 million by 2040 (1). Neuropsychiatric symptoms affect most people with AD at some point during the illness (2–5). Apathy is one of the most common neuropsychiatric symptoms with prevalence estimates ranging from 36% to 70% of people with AD (2,6) and is associated with increased care needs and caregiver burden and distress (7,8), increased risk of institutionalization (9), and higher costs of care (10). Given the prevalence and consequences of apathy in AD dementia, identifying methods of managing apathetic symptoms is an important public health priority.
The Apathy in Dementia Methylphenidate trial (ADMET) is an investigator-initiated clinical trial funded by an American Recovery and Reinvestment Act award from the National Institute on Aging (NIA) issued in September of 2009.
2. Issues in designing a trial to test treatments for apathy in people with dementia
2.1 Defining and measuring apathy
DSM-IV (TR) (11) supplies no formal definition or diagnostic criteria for apathy although it is cited as a symptom of several disorders. Apathy is particularly common in AD (12), fronto-temporal dementias (13), Parkinson’s disease (14), and after stroke (15). Given these heterogenous contexts, it was important for ADMET to incorporate a definition of apathy that included both mood and cognitive symptoms. Existing definitions included those by Marin (16): “diminished motivation not attributable to diminished level of consciousness, cognitive impairment, or emotional distress”, a definition that seems to exclude dementia, and Stuss (17): “absence of responsiveness to stimuli as demonstrated by a lack of self-initiated action”, a perhaps easier definition to apply to demented AD patients. However, a consensus definition or diagnostic criteria for apathy in dementia did not exist.
We used the Neuropsychiatric Inventory (NPI) (18) apathy subscale to specify inclusion criterion in the study. We defined clinically significant apathy as apathy that 1) occurs daily (i.e., “very frequently” NPI rating), or 2) occurs at least once a week (“often” or “frequently” NPI rating) with evident severity (“moderate” or “marked” NPI rating). Our definition limited study participants to those with apathy based impairment of daily life at a clinically significant severity level for whom pharmacological treatment is appropriate.
After ADMET had begun recruitment, a task force proposed diagnostic criteria for apathy in neurodegenerative diseases (19) that show substantial overlap with our NPI inclusion criteria. A significantly increased apathy score on the NPI has been reported for individuals in a clinical practice setting who fulfilled the proposed diagnostic criteria for apathy (20).
We considered several scales for longitudinal assessment of apathy severity, including the NPI apathy subscale to be consistent with the entry criteria, or the Dementia Apathy Interview and Rating (DAIR) (21), scale which had been developed specifically for measuring apathy in dementia. We chose to use the Apathy Evaluation Scale (AES) because of its widespread use in measuring apathy (22) and previous use as a primary outcome measure in a trial of treatment of apathy in AD (23).
The AES is an 18-item scale designed to measure apathy as a neuropsychiatric symptom, defined as “reduced motivation which is not due to emotional distress, intellectual impairment or loss of consciousness” (24). In ADMET, study personnel administer the AES informant version to participants’ study partners. The informant version has good internal consistency (Cronbach’s α = 0.94) and test-retest (Pearson’s r = 0.94) reliability (25).
ADMET will also use a clinician rating of target behavior change similar to the approach used in the Depression in Alzheimer’s Disease Study-2 (DIADS-2) (26) and the Citalopram for Agitation in Alzheimer’s Disease (CitAD) trial (27). We modified the ADCS-CGIC by adding apathy-specific interview questions and probes based on Marin’s core features of apathy: lack of initiative, lack of interest and emotional blunting (16).
2.2 Distinguishing between apathy and depression
Although depressive symptoms overlap those of apathy (28–30) they are distinct (16,31,32) with respect to the patient’s emotional and affective experience. Depression is characterized by dysphoria (i.e., sadness and distress) while apathy presents with emotional indifference. The locus of suffering and distress tends to be different. In depression, the patient is the primary sufferer; while in apathy, the family and caregivers are commonly distressed, but not the patient. Further, apathy is also associated with lack of insight (32). Marin, et al (33) found that people with AD frequently had elevated apathy scores unassociated with elevated depression; while individuals with major depressive disorder had elevation of both depression and apathy scores.
We believed that depression and apathy would respond differently to treatment and therefore chose to exclude individuals meeting DSM-IV criteria for Major Depressive Episode at enrollment. We do, however, include apathetic individuals currently taking antidepressant medication but not meeting DSM-IV depression criteria
2.3 Recruiting participants
We began recruitment for ADMET in May, 2010. Initially recruitment was slow; we enrolled only four participants between May 2010 and the end of July 2010. We quickly identified two recruitment barriers.
Focusing on the individual was not a fruitful approach. With most major neuropsychiatric symptoms in AD, the individual appears overtly to be in distress. In contrast, with apathy the individual does not complain and appears not to experience distress; rather the caregiver experiences increased distress. As a result, we focused recruitment efforts on caregivers.
Referring clinicians often did not appreciate apathy as neuropsychiatric symptom due to its ‘silent’ quality. We consequently found it necessary to enhance local educational efforts towards an increased appreciation of apathy symptoms. Identifying and overcoming these barriers resulted in markedly improved recruitment; since the beginning of August 2010, we have been recruiting a mean of four participants per month. ADMET is currently exceeding recruitment goals with completion of enrollment expected by October 2011.
2.4 Determining an appropriate test treatment
The evidence base for choosing a pharmacologic treatment of apathy is limited, and we considered several classes of known neuroactive medications. We chose not to test cholinesterase inhibitors even though they may be modestly effective in reducing apathy (34–36). Many potential participants would already be using a cholinesterase inhibitor. Discontinuation of medication was not in the patients’ best interests and limiting the study population to individuals not currently taking a cholinesterase inhibitor would limit generalizability. To minimize the potentially confounding effect of changes in cholinesterase inhibitor treatment on our primary apathy outcomes, we required that these patients be on a stable dose of these medications for one month prior to randomization.
We rejected antipsychotics as treatment for apathy in AD given the lack of evidence from controlled trials suggesting treatment effect (37–40) and concerns about increased mortality risk resulting in an FDA “black box” warning (41–43).
There is no evidence to support the use of antidepressants for the treatment of apathy in AD (44) and, in fact, selective serotonin reuptake inhibitors (SSRIs) may increase apathy (45,46). Thus we chose not to consider antidepressants as treatment for apathy in the ADMET study.
The best treatment alternative for symptoms of apathy appears to be a dopamine enhancing agent. Activity in the dopaminergic mesolimbic brain reward system may correlate with motivated behavior in both healthy and neurologically impaired populations (47). Dysfunction in the brain reward system correlates with apathy symptoms in people with AD (48). We considered the psychostimulants dextroamphetamine, amantadine, and bupropion, but data on the use of these drugs for apathy is limited (49–51). Preliminary data on the on the efficacy and safety of methylphenidate for the treatment of apathy in AD in a double-blind, randomized, placebo-controlled crossover trial (13 participants) showed significantly greater improvement in apathy (as measured by the AES) in the methylphenidate phase compared with the placebo phase (see Figure 1), although a significantly greater proportion of participants also experienced at least one adverse event in the methylphenidate phase (23). Given this preliminary data and other data suggesting that methylphenidate causes fewer side-effects than other psychostimulants in the elderly (52), we chose methylphenidate (IR Ritalin®, target dose 10 mg BID) as the active comparator for ADMET.
Figure 1. Change scores for methylphenidate versus placebo in 13 patients.
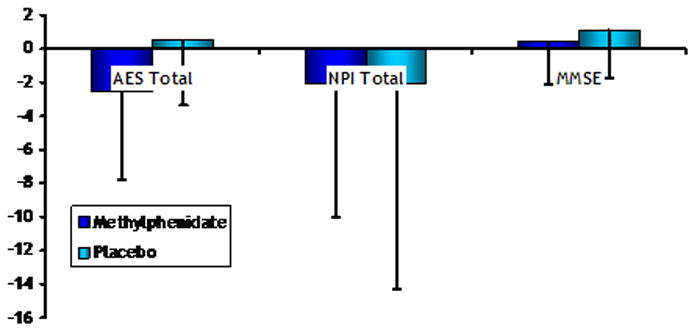
Data from preliminary, randomized, cross-over study of 13 patients on cholinesterase inhibitors. The shaded bars are the mean change (end of treatment minus baseline) such that negative change values indicate improvement, and the lines indicate the standard deviation of the change.
2.5 Including other relevant secondary outcomes
Little is known about the effects of methylphenidate on cognitive functioning in AD. Galynker et al. (53) reported approximately 1.8 point increase in Mini Mental State Examination (MMSE) (54) scores associated with open-label treatment of AD patients with 10–20 mg daily of methylphenidate and preliminary data showed little difference in MMSE scores between treatment groups (see Figure 1) (23). We chose the MMSE as a brief measure of global cognitive function given its widespread use for assessment of efficacy and toxicity in neuropsychiatric trials (55).
Because the dopaminergic system also plays a key role in attention, inattention often coexists with apathy. Baseline attention may predict response to methylphenidate treatment of apathy which may in turn improve attention (23). We chose to measure attention with Wechsler Memory Scale – Revised (WMS-R) digit span tests (56) which has been reported to effectively measure attention, concentration, sequencing, number facility and auditory short-term memory (57–59) in dementia populations and to be a sensitive measure of drug response in methylphenidate trials (60).
Important secondary safety outcomes include incident delusions and hallucinations as measured by the NPI, weight loss, changes in blood pressure, incident abnormal electrocardiograms, other expected adverse events associated with methylphenidate, and all serious adverse events.
2.6 Determining when to unmask patients, study partners and study staff
Decisions regarding unmasking require striking a balance between facilitating clinical care and maintaining masking and allocation concealment. Unmasking each study participant at closeout might improve recruitment and facilitate clinical care, but could also sabotage masking and allocation concealment of other participants. We elected to inform patients and their study partners of the treatment assignment immediately after completing data collection at the close out visit but we do not simultaneously unmask clinical center personnel. Sealed envelopes with the treatment assignments and the study monitor’s contact information are given to participants and partners with instructions to discuss the study with their primary care physicians. Clinical center personnel remain masked until study end to prevent selection and information biases. Emergency unmasking before the week 6 visit is allowed in emergency situations only.
3. Design of Apathy in Dementia Methylphenidate trial
3.1 Study organization
ADMET has three recruiting clinical centers located in Baltimore, Charleston and Toronto, and two resource centers (chair’s office and coordinating center). The ADMET Steering Committee (SC) is the primary decision-making body; and is comprised of one voting member from each center.
The ADMET Data Safety and Monitoring Committee (DSMC) includes three voting members who are appointed by the NIA and have expertise in biostatistics, psychiatry and neurology. They are independent of ADMET conduct and review accumulating, unmasked data on the safety and efficacy of methylphenidate compared with placebo. The DSMC makes recommendations to the SC and the NIA. NIA and study leadership participate in DSMC meetings to address trial conduct, including protocol implementation and data analysis.
3.2 Eligibility and consent
Study participants, who are recruited from outpatient facilities, nursing homes, and community outreach, have possible or probable Alzheimer’s disease as defined by National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) (61) criteria with Mini-Mental State Examination (MMSE) (54) scores of 10 or higher. Participants also have “clinically significant apathy” and do not meet criteria for major depression. A study partner, who accompanies the participant to study visits, is a friend, family member or caregiver, spends several hours a week with the participant, and supervises the participant’s care. Patients were excluded from participation if they had either a comorbid condition or were taking concomitant medications that made it unsafe to use methylphenidate. The detailed list of eligibility criteria for ADMET is given in Table 1.
Table 1.
Data collection schedule
| Assessment visits and contacts (weeks from baseline)* | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | |
| Procedures | |||||||
| Review of consent | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Review inclusion/exclusion | ✓ | . | ✓ | . | ✓ | . | ✓ |
| Collect demographic information | ✓ | ||||||
| Review history | ✓ | . | ✓ | . | ✓ | . | ✓ |
| Perform ongoing medical monitoring | ✓ | . | ✓ | . | ✓ | . | ✓ |
| Review of medication use | . | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Dispense of study drug | ✓ | . | ✓ | ✓. | ✓ | . | . |
| Patient assessments | |||||||
| AES | ✓ | . | ✓ | . | ✓ | . | ✓ |
| ADCS-CGI | ✓ | . | ✓ | . | ✓ | . | ✓ |
| Digit span | ✓ | . | ✓ | . | ✓ | . | ✓ |
| MMSE | ✓ | . | ✓ | . | ✓ | . | ✓ |
| NPI | ✓ | ✓ | |||||
| Safety measures | |||||||
| Review of adverse events | . | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Blood collection for electrolyte panel | ✓ | . | ✓ | . | ✓ | . | ✓ |
| Review ECG | ✓ | . | ✓ | . | . | . | ✓ |
| Study partner intervention | |||||||
| Psychosocial intervention | ✓ | . | ✓ | . | ✓ | . | ✓ |
AES, Apathy Evaluation Scale, ADCS-CGI, Alzheimer’s Disease Cooperative Study - Clinical Global Impression; NPI, Neuropsychiatric Inventory; MMSE, Mini-mental State Examination
In-person clinic visits occur at baseline and weeks 2, 4 and 6 following baseline. Telephone contacts occur at weeks 1, 3, and 5following baseline.
Consent is obtained from the participant if he or she is capable of providing consent. If not, then consent is obtained from an authorized legal representative and the participant is asked to provide assent following the guidelines proposed by the Alzheimer’s Association (62) and procedures approved by each clinical center’s Institutional Review Board (IRB) or Research Ethics Board (REB) in accordance with local law. Consent is also obtained from the study partners who accompany the participants on study visits, if required by the local ethics board. The ADMET protocol was reviewed and approved by the IRB or REB at each clinical center, chair’s office, and coordinating center.
3.3 Randomization and masking
The coordinating center created the treatment assignment schedule using a documented, auditable SAS program (SAS/STAT® software, Version 9.1 of the SAS System for Windows; Copyright © 2000–2004 SAS Institute Inc., Cary NC, USA). The randomization scheme was stratified by clinical center with permuted length blocks. Participants are randomized in a 1:1 ratio to receive methylphenidate or matching placebo. Clinical centers request and receive treatment assignments via the online ADMET data system following confirmation of eligibility. Participants, study partners, and clinical center personnel are all masked to treatment assignment.
3.4 Study treatment protocol
The dose, treatment administration, and treatment duration of methylphenidate were chosen based on strategies of previous trials in the elderly depressed (63,64), open label administration guidelines in demented populations (52), and primarily because of the preliminary study of methylphenidate for apathy that provided data on the safety and efficacy of 10 mg BID methylphenidate (23).
Participants begin treatment by taking one study drug capsule twice a day (corresponding to 10 mg/day in the methylphenidate group) for three days. The study drug is administered by a caregiver or taken by the patient under the supervision of a caregiver. Study staff telephone the patient and/or study partner on day three to discuss tolerability and if acceptable, increase the dose to the target of two capsules twice a day (20 mg/day). Study physicians may recommend no dose increase if there are side-effects at the lower dose and may lower the dose if necessary for safety concerns.
All study partners and participants (when possible) receive the ADMET standardized psychosocial intervention, a 20 – 30 minute counseling session at study visits, educational materials, and 24-hour availability of study staff for crisis management assistance. During the psychosocial intervention a study clinician provides referrals for caregivers if they are in need of counseling or psychiatric assessment. This psychosocial intervention maintains compliance with the existing standard of care (65,66).
3.5 Data collection
Scheduled in-person study visits and telephone contacts occur regardless of adherence to assigned treatment to allow analyses to be performed according to the intention-to-treat philosophy. In-person visits are scheduled at baseline and weeks 2, 4 and 6 following baseline with telephone contacts scheduled at weeks 1, 3, and 5 (Table 2). Study personnel may conduct unscheduled visits or telephone contacts to provide medical monitoring, encourage compliance, or offer counseling.
Table 2.
Design Summary
| Apathy in dementia methylphenidate trial (ADMET) |
Objectives
|
Type of trial
|
Setting
|
Primary outcome measures
|
Other outcomes
|
Study population
|
Power calculations
|
Treatment groups
|
Stratification of randomization
|
Masking
|
Inclusion criteria
|
Exclusion criteria
|
Duration of follow-up
|
Data collection schedule
|
Study personnel enter data into a web-based data entry system. Data are stored in redundant databases on a password protected web server and archived daily by the coordinating center. The data entry system performs error and consistency checks during data entry. The coordinating center also conducts audits of clinical center data during the trial.
3.6 Definition of comparisons, analyses and power calculations
The primary outcome measures are the AES (30) and the ADCS-CGIC scale (67). Secondary outcome measures include the NPI apathy scores (18), Digit span forward and backward tasks (68), MMSE (54) and adverse events.
Definition of primary comparisons
We will base the primary assessment of efficacy on the intention-to-treat comparison of the difference in the change in AES scores from baseline to week 6 and the comparison at week 6 of the ratings for the apathy domain of the ADCS-CGIC. We hypothesize that the methylphenidate group will have a larger reduction in AES scores (corresponding to more improvement in apathy symptoms) than the placebo group by week 6. We also hypothesize that the proportion of patients with scores indicating worsening of apathy at week 6 (compared with baseline) on the ADCS-CGIC will be lower on methylphenidate than placebo.
Analysis of AES
The primary comparison will be the difference in the change in AES from baseline to week 6 using a t-test. We will also conduct longitudinal analyses of AES scores to compare treatment groups over time using a linear mixed effects model with random intercept and slope for each participant using all available data for that participant. Additionally, we will perform sensitivity analyses to assess the potential impact of missing data by imputing the missing outcomes using the method of multiple imputation (69,70).
Analysis of ADCS-CGIC
Proportional odds logistic regression will be used to compare the ADCS-CGIC ratings (ranging from 1 to 7) at week 6 between the groups to capitalize on the ordinal structure of the outcome.
Analysis of secondary outcomes
We will conduct analyses on change in the NPI domain ratings from baseline to week 6 using t-tests with transformations of the outcome variable if necessary. Other secondary outcomes to be examined longitudinally by treatment group include global cognition (MMSE), digit span and rates of adverse events. Time-to serious adverse events will also be compared using survival methods.
Power calculations
We conducted power calculations for the two primary hypotheses using information from our preliminary study (48). With 60 participants (1:1 allocation ratio), the power to detect a difference of 3.3 in the change from baseline to week 6 on the AES is at least 80% assuming a two-sided type I error of 5%. Significant attrition is not expected since the trial is only 6 weeks long so we did not adjust the sample size for potential losses to follow-up.
We calculated power for the ADCS-CGIC outcome by using standard two sample power estimations for comparing two proportions and assumed that 20% to 30% of participants assigned to placebo would show moderate or marked improvement. With 60 participants, the study will have greater than 80% power to detect a difference of 35% between the proportion of participants who improve (or worsen) in the methylphenidate group compared with the placebo group. The binary power calculation can be taken as a lower bound of the power for the proportional odds analysis described above.
3.7 Adverse event monitoring
Adverse events are collected by systematic, close-ended questions on known or expected side effects of methylphenidate, open-ended questions about unexpected side effects, and review of results of electrolyte panels and electrocardiograms (ECG). We also measure weight at each visit and have defined weight loss of 7% or more as an adverse event. Serious adverse events are collected at the time of event regardless of presumed association with methylphenidate and reported to IRBs and regulatory agencies as appropriate.
3.8 Quality assurance
Study personnel met for in-person training on all study assessments and passed a test for knowledge of study protocol and procedures prior to data collection. The study handbook and policy and procedures memoranda contain detailed information about standardized methods for performing study procedures. The coordinating center continually monitors performance of the clinical centers and presents performance information to the SC and DSMC; performance data include (but are not limited to) enrollment, completed and missed visits, losses to follow-up, protocol deviations, and data edit queries. The coordinating center performs site visits to all clinical centers to review facilities, staffing, study documentation, consent forms, IRB submissions and approvals, forms and data management and study drug accounting.
4. Discussion
There has been only limited study of treatment of apathy in dementia. Our preliminary data suggested that methylphenidate may be efficacious for treating apathy, and we will test this possibility in ADMET, the first parallel group, multicenter, randomized controlled trial of a medication intervention for apathy in AD. Given the current state of knowledge, we designed ADMET as a phase II trial, with the objective to show “proof-of-concept” rather than definitively testing our hypothesis. Definitive testing requires a subsequent trial if ADMET demonstrates acceptable risk-benefit profile of methylphenidate treatment on apathy in AD.
ADMET’s design involved decisions on several design issues, including developing an operational definition of apathy, selection of a promising intervention, and review and choice of an instrument to accurately measure change in apathy. We will carry the lessons learned to a possible phase III study. We discovered that excluding patients on antidepressants hindered recruitment as well as limiting generalizability of results, and the SC revised the protocol to allow stable treatment of both SSRIs and serotonin and norepinephrine reuptake inhibitors (SNRIs). Early in the trial, we expected apathy to have higher prevalence in those with more cognitive impairment but have found apathy to also be prevalent in patients with higher levels of functioning and have adjusted our enrollment criteria accordingly. In a larger trial, we would like to collect more complete data on cognition and its relationship to both apathy and treatment with methylphenidate.
Trials for potential treatment of apathy symptoms in patients with AD are challenging to design and conduct. Research has been complicated by a lack of consensus diagnosis and difficulties in measurement. Our tribulations may be able to serve as a guide for future trials.
Acknowledgments
Grant funding: National Institute on Aging, R01 AG033032-01
Sources of support
Grant funding:
National Institute on Aging and National Institute of Mental Health, R01 AG033032-01
Steering Committee voting members (responsibilities: study design and conduct)
Resource center representatives:
Jacobo Mintzer, MD, MBA (chair)
Roberta Scherer, PhD (vice chair)
Clinical center directors:
Krista Lanctôt, PhD
Jacobo Mintzer, MD, MBA
Paul Rosenberg, MD
Research group
Resource centers (responsibility: study administration)
Chair’s office, Medical University of South Carolina, Charleston:
Jacobo Mintzer, MD, MBA, study chair
David Bachman, MD, medical monitor
Coordinating center, Johns Hopkins Bloomberg School of Public Health, Baltimore:
Roberta Scherer, PhD, director
Lea Drye, PhD, deputy director
Anne Shanklin Casper, MA, CCRP, lead coordinator
Gabrielle Jenkins
David Shade, JD
Matthew Toepfner
Vijay Vaidya, MSc, MPH
Project Office, National Institute on Aging, Bethesda:
Laurie Ryan, PhD, project officer
Clinical centers (responsibility: data collection)
Johns Hopkins Bayview and Johns Hopkins School of Medicine, Baltimore:
Paul Rosenberg, MD, director
Alyssa Bergey Carol Gogel, RN
Christopher Marano, MD
Esther Oh
Julia Pedroso, RN, MA, lead coordinator
Gwenn Smith
Lynn Smith
Martin Steinberg, MD
Medical University of South Carolina, Charleston:
Jacobo Mintzer, MD, MBA, director
Elizabeth Safrit
Jane Ann Sweeny
Marilyn Stuckey
Amanda Watts, lead coordinator
Jan Watts
Arthur Williams
Sunnybrook Health Sciences Centre, Toronto
Krista Lanctôt, PhD, director
Sandra Black
Jennifer Bray
Jaclyn Cappell, lead coordinator
Sarah Chau
Nathan Herrmann, MD
Joanne Lawrence
Abby Li
Ken Shulman, MD
Gayla Tennen
Study pharmacy
Sunnybrook Health Sciences Centre, Department of Pharmacy, Toronto
John Iazzetta, PharmD
Data Safety and Monitoring Committee voting members (responsibility: review of accumulating data on safety and efficacy)
Stephan Arndt, PhD, chair
Kristine Yaffe, MD
Jeffrey Cummings, MD
References
- 1.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366 (9503):2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinberg M, Shao H, Zandi P, et al. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2008;23 (2):170–177. doi: 10.1002/gps.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyketsos CG, Steinberg M, Tschanz JT, et al. Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry. 2000;157 (5):708–714. doi: 10.1176/appi.ajp.157.5.708. [DOI] [PubMed] [Google Scholar]
- 4.Mega MS, Cummings JL, Fiorello T, et al. The spectrum of behavioral changes in Alzheimer’s disease. Neurology. 1996;46 (1):130–135. doi: 10.1212/wnl.46.1.130. [DOI] [PubMed] [Google Scholar]
- 5.Lyketsos CG, Lopez O, Jones B, et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288 (12):1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 6.Tatsch MF, Bottino CM, Azevedo D, et al. Neuropsychiatric symptoms in Alzheimer disease and cognitively impaired, nondemented elderly from a community-based sample in Brazil: prevalence and relationship with dementia severity. Am J Geriatr Psychiatry. 2006;14 (5):438–445. doi: 10.1097/01.JGP.0000218218.47279.db. [DOI] [PubMed] [Google Scholar]
- 7.Freels S, Cohen D, Eisdorfer C, et al. Functional status and clinical findings in patients with Alzheimer’s disease. J Gerontol. 1992;47 (6):M177–M182. doi: 10.1093/geronj/47.6.m177. [DOI] [PubMed] [Google Scholar]
- 8.Kaufer DI, Cummings JL, Christine D, et al. Assessing the impact of neuropsychiatric symptoms in Alzheimer’s disease: the Neuropsychiatric Inventory Caregiver Distress Scale. J Am Geriatr Soc. 1998;46 (2):210–215. doi: 10.1111/j.1532-5415.1998.tb02542.x. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee S, Murray J, Foley B, et al. Predictors of institutionalisation in people with dementia. J Neurol Neurosurg Psychiatry. 2003;74 (9):1315–1316. doi: 10.1136/jnnp.74.9.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrmann N, Lanctot KL, Sambrook R, et al. The contribution of neuropsychiatric symptoms to the cost of dementia care. Int J Geriatr Psychiatry. 2006;21 (10):972–976. doi: 10.1002/gps.1594. [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 12.Aalten P, Verhey FR, Boziki M, et al. Neuropsychiatric syndromes in dementia. Results from the European Alzheimer Disease Consortium: part I. Dement Geriatr Cogn Disord. 2007;24 (6):457–463. doi: 10.1159/000110738. [DOI] [PubMed] [Google Scholar]
- 13.Fatemi Y, Boeve BF, Duffy J, et al. Neuropsychiatric aspects of primary progressive aphasia. J Neuropsychiatry Clin Neurosci. 2011;23 (2):168–172. doi: 10.1176/appi.neuropsych.23.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Starkstein SE, Brockman S. Apathy and Parkinson’s disease. Curr Treat Options Neurol. 2011;13 (3):267–273. doi: 10.1007/s11940-011-0118-9. [DOI] [PubMed] [Google Scholar]
- 15.Jorge RE, Starkstein SE, Robinson RG. Apathy following stroke. Can J Psychiatry. 2010;55 (6):350–354. doi: 10.1177/070674371005500603. [DOI] [PubMed] [Google Scholar]
- 16.Marin RS. Differential diagnosis and classification of apathy. Am J Psychiatry. 1990;147 (1):22–30. doi: 10.1176/ajp.147.1.22. [DOI] [PubMed] [Google Scholar]
- 17.Stuss DT, van Reekum R, Murphy KJ. Differentiation of states and causes of apathy. In: Borod J, editor. The Neuropsychology of Emotion. New York: Oxford University Press; 2000. pp. 340–363. [Google Scholar]
- 18.Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44 (12):2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 19.Robert P, Onyike CU, Leentjens AF, et al. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24 (2):98–104. doi: 10.1016/j.eurpsy.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Mulin E, Leone E, Dujardin K, et al. Diagnostic criteria for apathy in clinical practice. Int J Geriatr Psychiatry. 2011;26 (2):158–165. doi: 10.1002/gps.2508. [DOI] [PubMed] [Google Scholar]
- 21.Strauss ME, Sperry SD. An informant-based assessment of apathy in Alzheimer disease. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15 (3):176–183. [PubMed] [Google Scholar]
- 22.Clarke DE, Ko JY, Kuhl EA, et al. Are the available apathy measures reliable and valid? A review of the psychometric evidence. J Psychosom Res. 2011;70 (1):73–97. doi: 10.1016/j.jpsychores.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrmann N, Rothenburg LS, Black SE, et al. Methylphenidate for the treatment of apathy in Alzheimer disease: prediction of response using dextroamphetamine challenge. J Clin Psychopharmacol. 2008;28 (3):296–301. doi: 10.1097/JCP.0b013e318172b479. [DOI] [PubMed] [Google Scholar]
- 24.Marin RS. Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci. 1991;3 (3):243–254. doi: 10.1176/jnp.3.3.243. [DOI] [PubMed] [Google Scholar]
- 25.Marin RS, Wilkosz PA. Disorders of diminished motivation. J Head Trauma Rehabil. 2005;20(4):377–388. doi: 10.1097/00001199-200507000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Martin BK, Frangakis CE, Rosenberg PB, et al. Design of Depression in Alzheimer’s Disease Study-2. Am J Geriatr Psychiatry. 2006;14 (11):920–930. doi: 10.1097/01.JGP.0000240977.71305.ee. [DOI] [PubMed] [Google Scholar]
- 27.Drye LT, Ismail Z, Porsteinsson AP, et al. Citalopram for agitation in Alzheimer’s disease (CitAD): design and methods. Alzheimers Dement. 2011 doi: 10.1016/j.jalz.2011.01.007. Ref Type: In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onyike CU, Sheppard JM, Tschanz JT, et al. Epidemiology of apathy in older adults: the Cache County Study. Am J Geriatr Psychiatry. 2007;15 (5):365–375. doi: 10.1097/01.JGP.0000235689.42910.0d. [DOI] [PubMed] [Google Scholar]
- 29.Starkstein SE, Fedoroff JP, Price TR, et al. Apathy following cerebrovascular lesions. Stroke. 1993;24 (11):1625–1630. doi: 10.1161/01.str.24.11.1625. [DOI] [PubMed] [Google Scholar]
- 30.Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991;38 (2):143–162. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- 31.Levy ML, Cummings JL, Fairbanks LA, et al. Apathy is not depression. J Neuropsychiatry Clin Neurosci. 1998;10 (3):314–319. doi: 10.1176/jnp.10.3.314. [DOI] [PubMed] [Google Scholar]
- 32.Starkstein SE, Petracca G, Chemerinski E, et al. Syndromic validity of apathy in Alzheimer’s disease. Am J Psychiatry. 2001;158 (6):872–877. doi: 10.1176/appi.ajp.158.6.872. [DOI] [PubMed] [Google Scholar]
- 33.Marin RS, Firinciogullari S, Biedrzycki RC. Group differences in the relationship between apathy and depression. J Nerv Ment Dis. 1994;182 (4):235–239. doi: 10.1097/00005053-199404000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Cummings JL, Mackell J, Kaufer D. Behavioral effects of current Alzheimer’s disease treatments: a descriptive review. Alzheimers Dement. 2008;4 (1):49–60. doi: 10.1016/j.jalz.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 35.Feldman H, Gauthier S, Hecker J, et al. A 24-week, randomized, double-blind study of donepezil in moderate to severe Alzheimer’s disease. Neurology. 2001;57 (4):613–620. doi: 10.1212/wnl.57.4.613. [DOI] [PubMed] [Google Scholar]
- 36.Gauthier S, Feldman H, Hecker J, et al. Efficacy of donepezil on behavioral symptoms in patients with moderate to severe Alzheimer’s disease. Int Psychogeriatr. 2002;14 (4):389–404. doi: 10.1017/s104161020200858x. [DOI] [PubMed] [Google Scholar]
- 37.Rocca P, Marino F, Montemagni C, et al. Risperidone, olanzapine and quetiapine in the treatment of behavioral and psychological symptoms in patients with Alzheimer’s disease: preliminary findings from a naturalistic, retrospective study. Psychiatry Clin Neurosci. 2007;61 (6):622–629. doi: 10.1111/j.1440-1819.2007.01729.x. [DOI] [PubMed] [Google Scholar]
- 38.Paleacu D, Barak Y, Mirecky I, et al. Quetiapine treatment for behavioural and psychological symptoms of dementia in Alzheimer’s disease patients: a 6-week, double-blind, placebo-controlled study. Int J Geriatr Psychiatry. 2008;23 (4):393–400. doi: 10.1002/gps.1892. [DOI] [PubMed] [Google Scholar]
- 39.Street JS, Clark WS, Kadam DL, et al. Long-term efficacy of olanzapine in the control of psychotic and behavioral symptoms in nursing home patients with Alzheimer’s dementia. Int J Geriatr Psychiatry. 2001;16 (Suppl 1):S62–S70. doi: 10.1002/1099-1166(200112)16:1+<::aid-gps569>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 40.Ballard C, Waite J. The effectiveness of atypical antipsychotics for the treatment of aggression and psychosis in Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD003476. doi: 10.1002/14651858.CD003476.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294 (15):1934–1943. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- 42.U.S. Food and Drug Administration. Deaths with Antipsychotics in Elderly Patinets with Behavioral Disturbances. FDA Public Health Advisory, Centre for Drug Evaluation and Research; 2005. Ref Type: Generic. [Google Scholar]
- 43.Ballard C, Hanney ML, Theodoulou M, et al. The dementia antipsychotic withdrawal trial (DART-AD): long-term follow-up of a randomised placebo-controlled trial. Lancet Neurol. 2009;8 (2):151–157. doi: 10.1016/S1474-4422(08)70295-3. [DOI] [PubMed] [Google Scholar]
- 44.Lyketsos CG, DelCampo L, Steinberg M, et al. Treating depression in Alzheimer disease: efficacy and safety of sertraline therapy, and the benefits of depression reduction: the DIADS. Arch Gen Psychiatry. 2003;60 (7):737–746. doi: 10.1001/archpsyc.60.7.737. [DOI] [PubMed] [Google Scholar]
- 45.Barnhart WJ, Makela EH, Latocha MJ. SSRI-induced apathy syndrome: a clinical review. J Psychiatr Pract. 2004;10 (3):196–199. doi: 10.1097/00131746-200405000-00010. [DOI] [PubMed] [Google Scholar]
- 46.Wongpakaran N, van RR, Wongpakaran T, et al. Selective serotonin reuptake inhibitor use associates with apathy among depressed elderly: a case-control study. Ann Gen Psychiatry. 2007;6:7. doi: 10.1186/1744-859X-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bressan RA, Crippa JA. The role of dopamine in reward and pleasure behaviour--review of data from preclinical research. Acta Psychiatr Scand Suppl. 2005;(427):14–21. doi: 10.1111/j.1600-0447.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- 48.Lanctot KL, Moosa S, Herrmann N, et al. A SPECT study of apathy in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;24 (1):65–72. doi: 10.1159/000103633. [DOI] [PubMed] [Google Scholar]
- 49.Roccaforte WH, Burke WJ. Use of psychostimulants for the elderly. Hosp Community Psychiatry. 1990;41 (12):1330–1333. doi: 10.1176/ps.41.12.1330. [DOI] [PubMed] [Google Scholar]
- 50.van Reekum R, Bayley M, Garner S, et al. N of 1 study: amantadine for the amotivational syndrome in a patient with traumatic brain injury. Brain Inj. 1995;9 (1):49–53. doi: 10.3109/02699059509004571. [DOI] [PubMed] [Google Scholar]
- 51.Corcoran C, Wong ML, O’Keane V. Bupropion in the management of apathy. J Psychopharmacol. 2004;18 (1):133–135. doi: 10.1177/0269881104043284. [DOI] [PubMed] [Google Scholar]
- 52.Maletta G, Mattox KM, Dysken M. Guidelines for prescribing psychoactive drugs. Geriatrics. 2000;55(3):65–6. 79. Update 2000. [PubMed] [Google Scholar]
- 53.Galynker I, Ieronimo C, Miner C, et al. Methylphenidate treatment of negative symptoms in patients with dementia. J Neuropsychiatry Clin Neurosci. 1997;9 (2):231–239. doi: 10.1176/jnp.9.2.231. [DOI] [PubMed] [Google Scholar]
- 54.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:196–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 55.Storga D, Vrecko K, Birkmayer JG, et al. Monoaminergic neurotransmitters, their precursors and metabolites in brains of Alzheimer patients. Neurosci Lett. 1996;203 (1):29–32. doi: 10.1016/0304-3940(95)12256-7. [DOI] [PubMed] [Google Scholar]
- 56.Wechsler D. Wechsler Memory Scale - revised manual. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 57.Hale JB, Hoeppner JB, Fiorello CA. Analyzing digit span components for assessment of attention processes. Journal of Psychoeducational Assessment. 2002;20:128–143. [Google Scholar]
- 58.Larrabee GJ, Curtiss G. Construct validity of various verbal and visual memory tests. J Clin Exp Neuropsychol. 1995;17 (4):536–547. doi: 10.1080/01688639508405144. [DOI] [PubMed] [Google Scholar]
- 59.Vanderploeg RD, Schinka JA, Retzlaff P. Relationships between measures of auditory verbal learning and executive functioning. J Clin Exp Neuropsychol. 1994;16 (2):243–252. doi: 10.1080/01688639408402635. [DOI] [PubMed] [Google Scholar]
- 60.Kaelin DL, Cifu DX, Matthies B. Methylphenidate effect on attention deficit in the acutely brain-injured adult. Arch Phys Med Rehabil. 1996;77 (1):6–9. doi: 10.1016/s0003-9993(96)90211-7. [DOI] [PubMed] [Google Scholar]
- 61.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34 (7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 62.Research consent for cognitively impaired adults: recommendations for institutional review boards and investigators. Alzheimer Dis Assoc Disord. 2004;18 (3):171–175. doi: 10.1097/01.wad.0000137520.23370.56. [DOI] [PubMed] [Google Scholar]
- 63.Lazarus LW, Moberg PJ, Langsley PR, et al. Methylphenidate and nortriptyline in the treatment of poststroke depression: a retrospective comparison. Arch Phys Med Rehabil. 1994;75 (4):403–406. doi: 10.1016/0003-9993(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 64.Wallace AE, Kofoed LL, West AN. Double-blind, placebo-controlled trial of methylphenidate in older, depressed, medically ill patients. Am J Psychiatry. 1995;152 (6):929–931. doi: 10.1176/ajp.152.6.929. [DOI] [PubMed] [Google Scholar]
- 65.Hogan DB, Bailey P, Carswell A, et al. Management of mild to moderate Alzheimer’s disease and dementia. Alzheimers Dement. 2007;3 (4):355–384. doi: 10.1016/j.jalz.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 66.Herrmann N, Gauthier S, Lysy PG. Clinical practice guidelines for severe Alzheimer’s disease. Alzheimers Dement. 2007;3 (4):385–397. doi: 10.1016/j.jalz.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 67.Schneider LS, Olin JT, Doody RS, et al. Validity and reliability of the Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11 (Suppl 2):S22–S32. doi: 10.1097/00002093-199700112-00004. [DOI] [PubMed] [Google Scholar]
- 68.Wheeler D. Wechsler Adult Intelligence Scale - Revised, Manual. New York: Psychological Corporation; 1981. [Google Scholar]
- 69.Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc. 1996;91:473–489. [Google Scholar]
- 70.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons; 1996. [Google Scholar]


