Abstract
Experimental evidence points to the importance of mitochondrial transport defects in contributing to major neurodegenerative diseases, such as Parkinson’s disease (PD). Studies of mitochondrial transport along single axons are difficult with traditional dissociated culture systems and the fragility of the midbrain dopaminergic cultures precludes their survival in previously developed microfluidic devices with an enclosed architecture. Using soft lithography, we generated a microdevice from polydimethylsiloxane (PDMS) for the purpose of studying the transport of mitochondria along single dopaminergic axons. The device comprises of two large open culture chambers connected by a parallel array of microchannels that achieves fluidic separation of axons from the soma and allows the tracking of mitochondrial movement along oriented axons. Dopaminergic neurons from midbrain cultures were successfully cultured within the microdevices for up to 4 weeks and extended their axons across the microchannels. Axonal mitochondria within the microchannels were labeled by transduction with a mitochondrial-targeted DsRed2 lentiviral vector or with the mitochondria-specific dye, Mitotracker Deep Red and were visually tracked with conventional confocal microscopy. The methodology and device that we have described here will allow further study of the role of mitochondrial transport defects play in major neurodegenerative diseases.
Keywords: Parkinson’s disease, Axonal Transport, Soft Lithography
1. Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder affecting more than 20 million people worldwide(Mayeux 2003; Kim-Han, Antenor-Dorsey et al. 2011). The major clinical symptoms of this disorder arise from the loss of dopaminergic (DA) neurons. It has been suggested that it is the loss of the axonal projections to the striatum (60–70%) compared to the loss of DA cell bodies (~30%) (Cheng, Ulane et al. 2010), which initiates the disorder. Support for this notion comes from recent studies showing that genes whose mutations are responsible for familial forms of PD, such as Parkin and Pink1, encode for proteins that regulate mitochondrial trafficking and impairment (Yu, Sun et al. 2011). Moreover, the PD-mimetic, 1-methyl-4-phenylpyridinium (MPP+) (Blum, Torch et al. 2001) rapidly leads to abnormal trafficking of DA axonal mitochondria and loss of mitochondrial function (Kim-Han, Antenor-Dorsey et al. 2011). As described, however, the latter studies are currently hampered by the lack of a simple, standardized device to rapidly separate axons from cell bodies.
To study axon transport, investigators have used modified or unmodified versions of compartmented Campenot chambers (Campenot 1977; Ivins, Bui et al. 1998) to isolate axons from the cell body. These devices seal a piece of Teflon to a petri dish using silicone grease and the Teflon acts as a divider to create two or more compartments; three compartments in the original Campenot and two compartments in Ivin’s modified Campenot. Previously, we have used Ivin’s modified compartment chamber, which consists of a semicircular Teflon piece walled off at one end by a glass cover slip supported by a layer of collagen/matrigel and sealed to a Petri dish using silicone vacuum grease. Neurons are seeded on one side of the glass cover slip and extend axons through the collagen to the axonal side of the chamber. Although effective at isolating the axons from the neurons, one downside of this design and the original Campenot chamber is that the isolated axons are randomly oriented making it difficult to distinguish between anterograde and retrograde transport. A major advance in axon trafficking came from microfluidic devices designed by the Jeon lab that are fabricated using soft lithographic techniques. These devices orient the axons by forcing them to grow through a parallel array of micro-channels that connect somal and axonal compartments (Taylor, Rhee et al. 2006). While previous reports showed that dorsal root ganglion (Kim, Karthikeyan et al. 2009) and hippocampal neurons (Taylor, Blurton-Jones et al. 2005) can be successfully cultured in these devices, we have found that they are not suitable for culturing midbrain DA cultures, as the majority of DA neurons do not survive within the enclosed somal compartment, and those that remain fail to extend axons into the axonal compartment (Kim-Han, Antenor-Dorsey et al. 2011).
To overcome the limitations of previous compartmented devices and create a culture platform for investigating the role of mitochondrial transport in axonal degeneration during PD, we designed a microdevice with a large “open” compartment versus the enclosed compartments devised by Taylor et al., (2005). This “open” chamber is directly adjacent to an array of parallel microchannels that will allow us to 1) culture midbrain DA neurons 2) isolate DA axons 3) orient the axons to allow determination of retrograde and anterograde directions and 4) visualize these axons by live cell imaging so that we can record the movement of axonal mitochondria over time. The advantage of miniaturization is that we can place two devices on a single 35mm petri dish thus allowing us to run more than one condition (e.g. control vs. experimental) on the same dish at the same time. Using these devices and a line of transgenic mice whose DA neurons express GFP (DA/GFP) (Kim-Han, Antenor-Dorsey et al. 2011), we can easily study the effects that PD-mimetic have on mitochondrial transport and other transport processes.
2. Materials and Methods
2.1. Microfluidic Device
The master wafer for the microdevice was fabricated using standard photo-and soft lithographic techniques as described previously (Taylor, Rhee et al. 2006). A CAD file of the design was drawn in AutoCAD (Autodesk CA) and used to generate a high-resolution transparency mask (CAD/Art services, OR). A two-step photolithographic technique was used to generate the silicon wafer. First, the 5 µm tall micro-channels were made by spin-coating a negative photoresist SU8-2 (Microchem USA) onto a silicon wafer. The transparency printed with a negative pattern of the micro-channels was placed over the wafer and exposed to UV so that photoresist within the exposed area was crosslinked. Uncrosslinked photoresist on the wafer was then dissolved and washed away using SU8 developer (Microchem USA). After baking, the wafer with the micro-channels was then used to generate the somal and axonal compartments by a similar spin-coating process using SU8 2050 (Microchem USA). The transparency mask of the compartments were placed over the wafer, exposed to UV, developed and then baked to generate the final wafer.
2.2. Replica molding
Polydimethylsiloxane (PDMS) was used to create individual microdevices using the master wafer. Briefly, Sylgard 184 (Dow Corning, USA) was mixed in a 10:1 (base:curing agent) ratio and poured over the master wafer. After removing the bubbles using a vacuum desiccator, the PDMS was baked overnight at 60°C. Each device was then cut out from the PDMS mold and a 6 mm diameter circular hole punch was used to create the axonal and somal compartments. The microdevice and a custom cut 35 mm dish with a glass cover slip bottom were treated in a plasma cleaner (Harrick Plasma USA) for two minutes to generate a tight seal between microdevice and the glass. The finished microdevices were then sterilized by UV exposure for 30 minutes and coated with poly-D-lysine (PDL) diluted in sterile water at 0.2 mg/mL overnight prior to cell plating.
2.3. Cell Culture
DA/GFP cultures from Tg(TH-EGFP)DJ76GSAT transgenic mice (Jackson Laboratories, Bar Harbor Maine) were prepared as previously described (Kim-Han, Antenor-Dorsey et al. 2011). Briefly, DA/GFP males were mated with wild type females. The brains were isolated from embryos on embryonic day 14 (E14) and were screened for GFP fluorescence prior to dissection of the midbrain. Isolated neurons (70,000 cells per somal compartment of the device) were plated in DMEM/F-12, 5% fetal bovine serum (FBS) supplemented with B27 (Invitrogen) and penicillin/streptomycin (Sigma). The axonal compartments were supplemented with 300 ng/mL of Netrin I (R&D Systems) to direct axonal outgrowth. Mitochondrial movement was then assessed on day in vitro (DIV) 13 or 14.
2.4. Optical Imaging
Two techniques were used to label mitochondria. First, a mitochondrial-targeted DsRed2 was generated by inserting a mitochondrial targeting sequence from COX IV (12 amino acids) in front of the DsRed2 sequence (Clontech). MitoDsRed2 was then subcloned into a FUGW lentiviral expression vector. The lentivirus was generated using HEK293T cells (Araki, Sasaki et al. 2004). The DA/GFP cells were transduced with lentivirus at DIV2 for 6 hours and then imaged at DIV 13 or 14. Alternatively, mitochondria were labeled with 50 nM of Mitotracker Deep Red (MTR; Invitrogen) on DIV12 and then imaged on either DIV 13 or 14. Images were taken using a Zeiss LSM510 Meta NLO Multiphoton System (Carl Zeiss) on Axiovert 200M inverted microscope with 40x water objective [C-Apochromat 40x/1.2W Corr.1.2 numerical aperture, collar correction (0.14–0.18)] equipped with a 5% CO2/37°C environmental chamber. Filters used for visualizing a given fluorescent marker included 488 nm argon laser and 505 long pass emission filter (GFP), 543 nm HeNe laser and 560 long pass emission filter (MitoDsRed2) and a 633 nm HeNe laser and 650–710 band pass emissions filter (MitoTracker Deep Red FM). A total of sixty images of mitochondrial movement at 5 second intervals were recorded to create time-lapse images and generate kymographs for quantifying mitochondrial movement.
2.5 Image analysis
Kymographs generated using ImageJ/Multiple Kymograph (NIH, Bethesda, MD) were analyzed as described previously (Kim-Han, Antenor-Dorsey et al. 2011). Briefly, diagonal lines were drawn for each moving particle on a kymograph. The angle and length of the lines were then used to calculate the direction and speed of the moving mitochondria. Only mitochondria that moved more than 5 µm in length for at least 15 seconds were counted.
2.6 Modeling toxin diffusion
Transport of MPP+ into the somal compartment of a Jeon microfluidic device (approximated by a rectangular block that is 7 mm in length, 1.5 mm in width, and 100 µm in height) was modeled as a convection-diffusion problem using COMSOL multiphysics software version 4.0a (COMSOL Inc, Burlington MA). The concentration of MPP+ within the compartment was initially set to zero and the concentration of the MPP+ at the entrance of the compartment was set to 2 µM. The model assumed diffusion through water at 37°C and a diffusion coefficient of 1 µm/ms.
3. Results and Discussion
3.1. “Open compartment” microdevice shows improved viability and axon growth for midbrain DA/GFP neurons
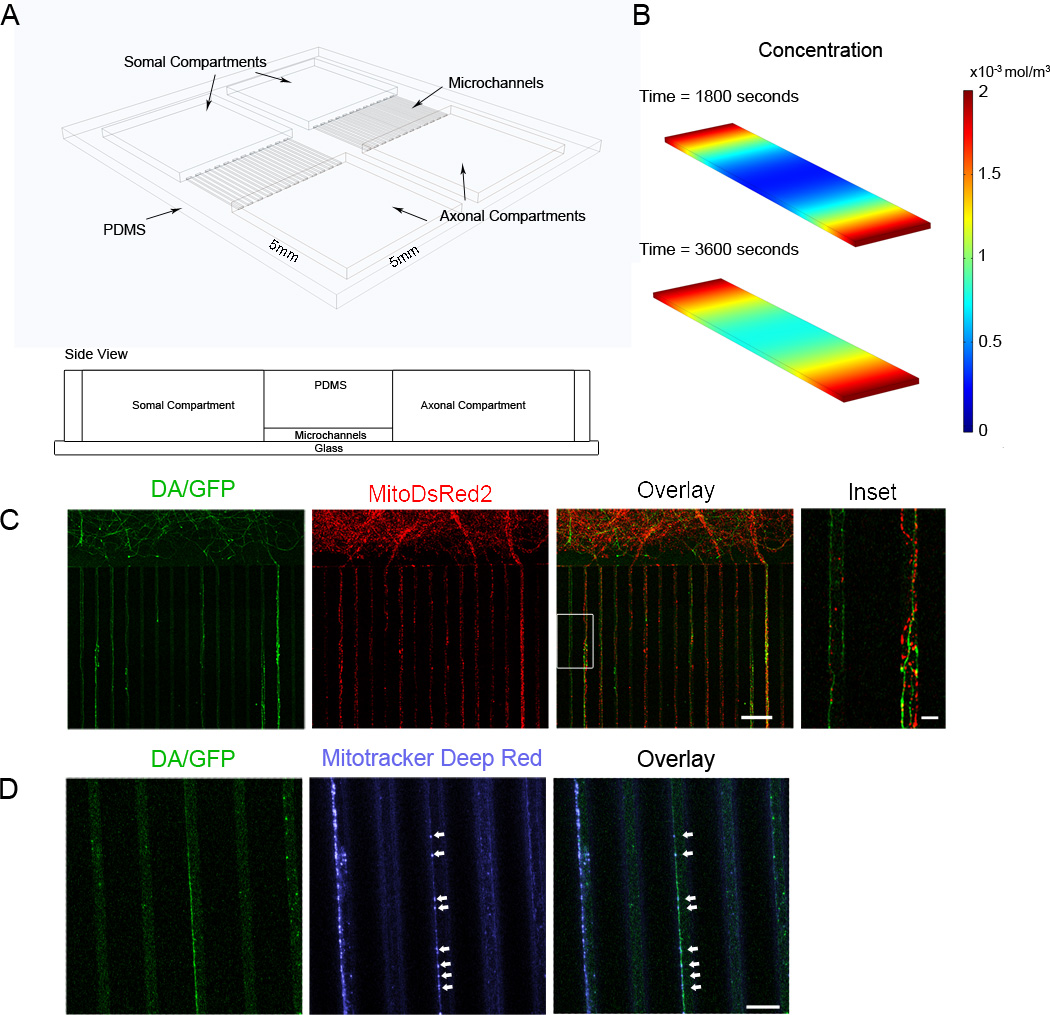
We previously tried culturing midbrain DA neurons within the Jeon microfluidic device. The somal compartment of the Jeon device is enclosed within the PDMS and plating/feeding the cells within the compartment is done through two large wells connecting directly to the somal compartment. But after repeated attempts, we found that very few of the midbrain cells remained within the somal compartment of the Jeon device and most of the cells were found within the larger loading wells away from the microchannels. The cells that remained within the somal compartment did not survive (Kim-Han, Antenor-Dorsey et al. 2011). It is possible that even with careful technique and extended plating time, shear forces that occur during loading may damage the fragile midbrain cultures and may remove them from the somal compartment. Additionally, the somal compartment of the Jeon device holds only a very small volume of cell culture media, hence it may take a long time for nutrients to diffuse from the media compartment to the cell compartment and for waste to diffuse out. Also, dead cells are trapped within the cell compartments and cannot be removed after plating without risking further shear damage to the surviving cells. Finally, the enclosed nature of the device makes toxin treatment of DA axons or cell bodies difficult as excessive force may dislodge the cells. Because some PD toxins oxidize within a few minutes, rapid, homogeneous delivery of toxin is critical for consistent results. Modeling diffusion of 2 µM MPP+ through an enclosed somal compartment show that uneven distribution of the toxin at 1 hour (Figure 1B). Therefore to improve viability of midbrain cells within our microdevice, we adopted an "open compartment" design where we have direct access to and can directly load the cells right next to the microchannels in a large compartment for convenient cell seeding, media sampling, cell/axon labeling and more efficient waste/nutrient exchange (Figure 1A). The new design reduces exposure of the cells to shear forces during plating and feeding, and ensures that the cells have sufficient access to a large volume of media through the entire culture period. Using the open chamber microdevice, DA/GFP cultures, seeded at 70,000 cells per device, were successfully plated and maintained for up to 4 weeks. Axons begin extending into the microchannels by DIV 6 and generally take two weeks (Fig. 1C) to completely cross the microchannels into the axonal compartment. The micro-channels between the somal and axonal compartments provided both a means for orienting axons as they extend and for providing fluidic isolation between the compartments so that drugs can be applied separately to either the axons or cell bodies. This isolation is maintained by a hydrostatic pressure difference created by a volume difference (~30 µL) between the compartments. In the micro-channels, we saw that a large proportion (~30%) of micro-channels contained DA/GFP axons so that imaging of mitochondria from many different neurons is possible. Based on morphological assessments, there was no evidence of dendrite infiltration in the micro-channels, nor did neurites in the axonal chamber stain for the dendritic marker MAP2 (Kim-Han, Antenor-Dorsey et al. 2011) (data not shown). Over time (>DIV14), axons continued to fasciculate such that establishing directionality and uniqueness became problematic. Thus all measurements were performed on either DIV13 or 14.
Figure 1. Schematic of “open” compartment microdevice and visually tracking axonal mitochondria within the microdevice.
(A) Two compartments (5 mm × 5 mm) are connected by a parallel array of microchannels, which are 5 µm high, 10 µm wide, and 900 µm long. Two microdevices can fit side by side within a single 35 mm culture dish. (B) Simulation of 2 µM of MPP+ diffusing into the somal compartment from both feeder wells of the Jeon microfluidics device in COMSOL. Diffusion is very slow and even after 1 hour, the drug has not fully equilibrated in the somal compartment. Time is in seconds. (C) The majority of the axons fully cross the microchannels by DIV 14. In the magnified inset of the overlay image (right), mitochondria labeled with mitoDsRed2 that co-localize with GFP expression are assumed to be from dopaminergic axons whereas mitochondria that do not co-localize with GFP signal are assumed to be from non-dopaminergic axons. Scale bar indicates 100 µm. Inset scale bar indicates 10 µm. (D) Mitochondria from both dopaminergic (arrows) and non-dopaminergic axons can also be tracked within the microchannels using Mitotracker Deep Red. The direction of transport can be clearly distinguished as the axons grow from the somal to the axonal compartment (top to bottom). Dye adsorbs into the microchannels and causes them to fluoresce; generating background noise that can significantly reduces the signal to noise ratio. Scale bar indicates 40 µm.
A major advantage of the open chamber system is that the micro-channels “straightened” the axons making distinguishing between anterograde and retrograde movement very simple. No axon was observed to reverse directions within the micro-channels and grow toward the somal compartment. The alignment provided by the micro-channels is a significant improvement compared to the random growth of the axons in the Campenot compartments. In the Campenot, the axons are often likely to cross other axons and change direction (i.e. growing back towards the somal compartment), making it difficult to identify the transport direction even when using landmarks, such as the growth cone. Establishing polarity is important for understanding the disruption of axonal transport processes, as anterograde and retrograde transport are mediated by different proteins and may be targeted in different ways during degeneration. Previously, we found a difference between anterograde and retrograde transport of mitochondria after exposure to MPP+; specifically, there is a decrease in anterograde transport speed and an increase in retrograde transport speed. These data are consistent with a model in which impaired mitochondria stop traveling towards the growth cone and instead return to the cell body for repair (Kim-Han, Antenor-Dorsey et al. 2011). Since we would like to address the mechanisms underlying these events having a culture platform that allows for the isolation of aligned axons and visualization of interior transport processes can immeasurably aid this process.
3.2 Mitochondria of oriented DA/GFP axons can be tracked and measured within microchannels
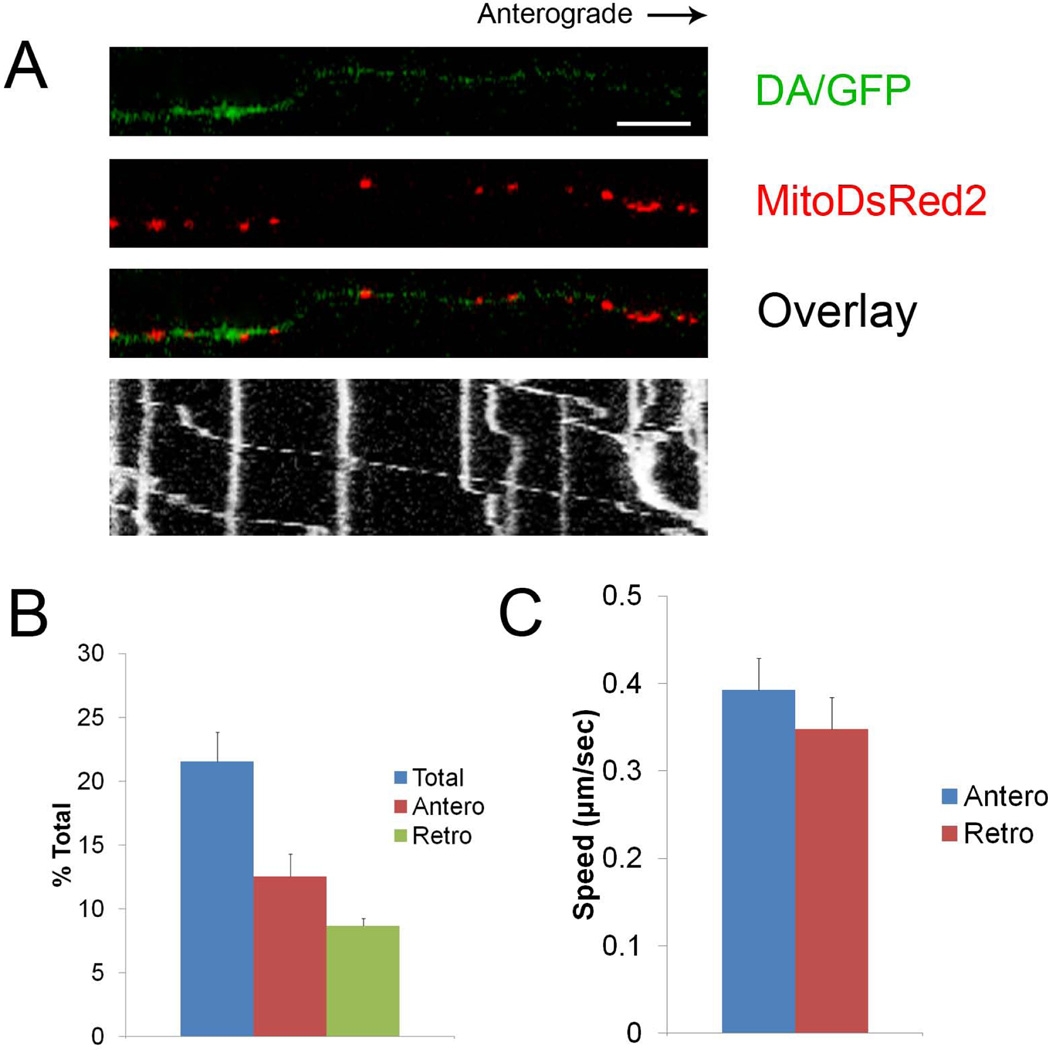
Using an optically transparent and biologically compatible elastomer, PDMS, it is possible to visualize and track the movement of mitochondria in live cells by either transducing the cells with the mito-targeted DsRed2 lentiviral vector (Fig. 1C) or by using a mitochondria-specific dye, such as MitoTracker Deep Red FM (50 nM; Fig. 1D). In the case of MitoDsRed2, the level of transduction can be manipulated by using varying amounts of virus whereas with the dye all cells have an equal probability of being labeled. We did not observe toxicity with either method, and there were no changes to the mitochondrial morphology or transport speed (not shown). MitoDsRed2-labeled mitochondria can be observed and tracked visually by DIV 5 in the microchannels. Alternatively, MitoTracker Deep Red labeled organelles in both somal and axonal compartments within 15 minutes. However, mitochondria labeling inside of the micro-channels was not immediately apparent. The latter required incubating the cells with the dye for 20 minutes, washing out the excess dye and then letting the cells incubate overnight in order to label the mitochondria within the micro-channels. In addition, MitoTracker Deep Red tends to adhere to PDMS and can render it fluorescent. This leads to background noise in the channels, which can make distinguishing individual mitochondria difficult. Reducing dye concentrations did not overcome the problem but instead led to inconsistent labeling. In contrast, MitoDsRed2 had no “noise” problems and once labeled, the mitochondria can be clearly tracked within the microchannels using a confocal microscope. Time-lapse images of mitochondrial movement along DA axons can then be used to generate kymographs (Fig. 2A). These kymographs can then be used to calculate the proportion of moving mitochondria (Fig 2B) as well as speed of mitochondria moving in either the anterograde or retrograde direction (Fig. 2C). Measurements of these key DA mitochondria parameters such as motility were essentially the same between the Campenot chamber (Kim-Han, Antenor-Dorsey et al. 2011) and the microdevice (21±2.3%). Mitochondrial speeds between the two devices were also comparable to what was previous reported; anterograde speeds in the microdevice was 0.39±0.03 µm/s while the retrograde speed was 0.34±0.03 µm/s.
Figure 2. Properties of axonal mitochondria transport can be measured within microchannels.
(A) Kymograph of mitoDsRed2 labeled DA mitochondria movement within the microchannels under normal culturing conditions. Scale bar indicates 10 µm. (B) The kymographs were then used to calculate the proportion of moving mitochondria from 12 axons over 3 independent experiments as well as the (C) mitochondrial movement speed in both anterograde and retrograde directions (over 60 mitochondria were examined). Error bars indicate standard error of mean (SEM) for B and C.
3.3 Conclusion
In summary, the methods presented by this research provide a technique for real time tracking and measurement of the movement of labeled mitochondria in oriented DA axons. This technique could be extended to the study of other processes, such as vesicular transport and microtubule fragmentation, which may also participate in the onset of axonal degeneration. The microdevice platform shown here displays improved culture conditions for sensitive neurons, such as those from the midbrain and promotes oriented axon growth into a separate axonal compartment for analysis. Using this culture system, we may better understand the mechanisms of axonal degeneration that may underlie the pathophysiology of major neurodegenerative diseases, such as Parkinson’s disease.
Highlights.
We developed a microdevice for studying mitochondria transport
Dopaminergic neurons from midbrain cultures extended axons into the microchannels
Axonal mitochondria were visualized within the microchannels
Acknowledgements
The authors would like to thank Steve Harmon for his help in the cell harvest and Dennis Oakley for technical assistance during imaging. This work was funded by NIH grants R21 NS067561(SSE) and NS39084 (KOM).
Abbreviations
- PD
Parkinson’s disease
- PDMS
polydimethylsiloxane
- DA
dopaminergic
- MPP+
1-methyl-4-phenylpyridinium
- PDL
poly-d-lysine
- MTR
Mitotracker Deep Red
- FBS
fetal bovine serum
- DIV
day in vitro
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declares no conflict of interest
References
- Araki T, Sasaki Y, et al. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305(5686):1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- Blum D, Torch S, et al. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson's disease. Prog Neurobiol. 2001;65(2):135–172. doi: 10.1016/s0301-0082(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Campenot RB. Local control of neurite development by nerve growth factor. Proc Natl Acad Sci U S A. 1977;74(10):4516–4519. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HC, Ulane CM, et al. Clinical progression in Parkinson disease and the neurobiology of axons. Ann Neurol. 2010;67(6):715–725. doi: 10.1002/ana.21995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivins KJ, Bui ET, et al. Beta-amyloid induces local neurite degeneration in cultured hippocampal neurons: evidence for neuritic apoptosis. Neurobiol Dis. 1998;5(5):365–378. doi: 10.1006/nbdi.1998.0228. [DOI] [PubMed] [Google Scholar]
- Kim-Han JS, Antenor-Dorsey JA, et al. The Parkinsonian mimetic, MPP+, specifically impairs mitochondrial transport in dopamine axons. J Neurosci. 2011;31(19):7212–7221. doi: 10.1523/JNEUROSCI.0711-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YT, Karthikeyan K, et al. Neuro-optical microfluidic platform to study injury and regeneration of single axons. Lab Chip. 2009;9(17):2576–2581. doi: 10.1039/b903720a. [DOI] [PubMed] [Google Scholar]
- Mayeux R. Epidemiology of neurodegeneration. Annu Rev Neurosci. 2003;26:81–104. doi: 10.1146/annurev.neuro.26.043002.094919. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Blurton-Jones M, et al. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat Methods. 2005;2(8):599–605. doi: 10.1038/nmeth777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Rhee SW, et al. Microfluidic chambers for cell migration and neuroscience research. Methods Mol Biol. 2006;321:167–177. doi: 10.1385/1-59259-997-4:167. [DOI] [PubMed] [Google Scholar]
- Yu W, Sun Y, et al. The PINK1/Parkin pathway regulates mitochondrial dynamics and function in mammalian hippocampal and dopaminergic neurons. Hum Mol Genet. 2011;20(16):3227–3240. doi: 10.1093/hmg/ddr235. [DOI] [PMC free article] [PubMed] [Google Scholar]