Abstract
TH-302, a hypoxia-activated anticancer prodrug was evaluated for anti-tumor activity and changes in dynamic contrast enhanced (DCE)- and diffusion weighted (DW)-magnetic resonance imaging (MRI) in a mouse model of pancreatic cancer. TH-302 monotherapy resulted in a significant delay in tumor growth compared to vehicle treated controls. TH-302 treatment was also associated with a significant decrease in the volume transfer constant (Ktrans) compared to vehicle treated controls one day following the first dose measured using DCE-MRI. This early decrease in Ktrans following the first dose as measured is consistent with selective killing of the hypoxic fraction of cells which are associated with enhanced expression of HIF-1α that regulates expression of permeability & perfusion factors including VEGF-A. No changes were observed in DW-MRI following treatment with TH-302, which may indicate this technique is not sensitive enough to detect changes in small hypoxic fractions of the tumor targeted by TH-302. These results suggest that changes in tumor permeability and/or perfusion may be an early imaging biomarker for response to TH-302 therapy.
Keywords: TH-302, Dynamic Contrast Enhanced MRI, diffusion weighted MRI, tumor hypoxia
Introduction
Hypoxia, defined as reduced oxygen levels, occurs in 50–60% of locally advanced solid tumors. It is well established that hypoxia in the local tumor environment contributes to more aggressive disease due to increased chemotherapy and radiation resistance, decreased apoptosis, increased glycolysis, angiogenesis and metastasis [1]. Pancreatic cancers are among the most hypoxic of all solid tumors, which may contribute to the dismal 5-year survival rate for patients estimated at 4–5% [2]. One strategy for selectively targeting hypoxic tumor regions and minimizing systemic exposure is the use of hypoxia-activated prodrugs (HAP) [3]. These drugs are designed to release and/or activate a cytotoxin to kill both the hypoxic tumor cells and nearby oxygenated cells, termed the “bystander effect".
TH-302 (Threshold Pharmaceuticals, Redwood City CA) is a second generation tumor-selective HAP comprised of a 2-nitroimidazole hypoxia trigger linked to a dibromo isophosphoramide mustard toxin that induces DNA cross linking [4]. TH-302 is selectively activated only under extreme hypoxic conditions, which improves its selectivity for tumor tissue and avoids toxicity in normal tissues [5, 6]. The activation of TH-302 both in vitro and in vivo results in dose-dependent cytotoxicity mediated primarily by induction of apoptosis (via caspase-3, -8, and -9), inhibition of the cell cycle through down-regulation of cyclins (D1/2/3, CDK4/6, p21cip-1, p27kip-1, and pRb), and decreased expression of pro-angiogenic factors (HIF-1α and VEGF-A) [5]. In nude mice bearing pancreatic MiaPaCa2 xenograft tumors, administration of TH-302 alone and in combination with gemcitabine resulted in enhanced survival times [24]. Early Phase I/II clinical studies investigating TH-302 alone and in combination with gemcitabine have demonstrated acceptable safety and encouraging signs of clinical benefit in patients with pancreatic tumors [7]. Currently, a Phase 2 clinical trial of TH-302 combined with gemcitabine is being conducted in patients with metastatic pancreatic cancer to investigate clinical efficacy (NCT01144455).
In order to individualize the clinical use of TH-302, identification of biomarkers to predict and monitor early response to therapy is needed. The anti-tumor activity of TH-302 is influenced by the efficacy of drug delivery to the tumor which depends on vessel patency, amount of tumor hypoxia and related intracellular reductases necessary to activate the prodrug, and the molecular context of the tumor including DNA repair capacity. The use of hypoxia biomarkers to predict response has been limited by sampling bias and biomarker stability caused by the heterogeneous and cyclic nature of oxygen gradients in tumors. Additionally the investigation of predictive and response biomarkers in pancreatic cancer is further complicated by the difficulty of obtaining primary tumor tissue samples [8]. A promising alternative is the use of biomedical imaging techniques to interrogate disease phenotypes and monitor response to therapy. Most imaging modalities are non-invasive and can be performed longitudinally to provide pharmacodynamic information before, during, and after therapy. Magnetic resonance imaging (MRI) is particularly appropriate for this interrogation of therapy response in tumor tissues, because MRI is sensitive to many physiological characteristics of soft tumor tissues [9].
To investigate the value of non-invasive imaging for assessing the early response to TH-302, we monitored changes induced by TH-302 using MiaPaCa2 flank xenograft tumors with Dynamic Contrast Enhanced Magnetic Resonance Imaging (DCE-MRI). This imaging method is used to obtain a semi-quantitative assessment of decreases in tumor vascular permeability and/or perfusion in pre-clinical studies of anti-vascular and anti-angiogenic chemotherapies by analyzing decreases in the pharmacokinetic transport rate constant (Ktrans) of a MRI tracer agent [10]. Some DCE-MRI studies have measured a significant decrease in Ktrans as early as one day after initiating chemotherapy, which occurs well before changes in therapy-induced vascular remodeling or tumor size can be detected [11, 12]. We also collected diffusion-weighted MRI (DW-MRI) data during the same imaging study of TH-302. This imaging modality measures the Apparent Diffusion Coefficient (ADC) of water, which is sensitive to spatial barriers such as cell membrane, providing a qualitative assessment of changes in cell density and/or membrane integrity [13]. Some pre-clinical DW-MRI studies have detected an early response to cytotoxic chemotherapies before necrosis or changes in tumor size were detected [14]. Both of these MRI methods are employed in a clinical setting, thus, positive results from our pre-clinical MRI studies may be used to support the development of clinical trials.
Materials and Methods
Cell Culture
The MiaPaCa2 cells were obtained from the ATCC and maintained in the DMEM culture media supplemented with 10% fetal bovine serum (FBS), 500 U/mL penicillin, and 5000 U/mL streptomycin. To verify cell line authenticity, genomic DNA was extracted (using a kit purchased from Sigma GIN70-KT) and diluted appropriately in Tris-EDTA, and submitted to the UA Genomics Core (Human Origins Gentoyping Lab) for analysis. Autosomal short tandem repeat (STR) typing was conducted across the 13 core STRs in CODIS and referenced against allelic peaks in cell lines of previously confirmed genotype. Cells were tested for mycoplasma contamination on a quarterly basis using the MycoAlert mycoplasma detection assay kit from Lonza (Basal, Switzerland) and found to be negative. All cells were grown in 5% CO2 at 37°C in a humidified tissue culture incubator.
Flank MiaPaCa2 Xenograft Model
Female SCID mice were housed and maintained under specific pathogen-free conditions in accordance with the guidelines of the American Association for Laboratory Animal Care. All experiments met the current regulations and standards of the U.S. Department of Agriculture, the U.S. Department of Health and Human Services, the National Institutes of Health, and the University of Arizona Animal Care Facility. All the experiments were conducted by the Arizona Cancer Center Experimental Mouse Shared Service. For each mouse, 10×106 cells were injected subcutaneously in 0.1 mL saline in the right flank. Mice were weighed and tumors were measured every 2–3 days using electronic calipers. Tumor volume was calculated using the formula: tumor volume = (dlong × dshort2/2). Mice were sacrificed when tumors reached 2000 mm3.
Immunohistochemistry
Control MiaPaCa2 flank xenograft tumors were injected with 60 mg/kg pimonidazole 2 hours prior to sacrifice. After sacrifice, tumors were excised and immediately fixed in 10% formalin in phosphate-buffered saline. Tissues were transferred to 70% ethanol within 24 hours and then embedded in paraffin within 3 days. Hematoxylin and eosin (H&E) stains were performed on three micron sections of tissue cut from the formalin fixed, paraffin embedded (FFPE) blocks.
Immunohistochemistry (IHC) was performed using carbonic anhydrase-IX (CAIX) rabbit monoclonal antibody (Abcam, Inc., #ab15086) diluted 1:500, vascular endothelial growth factor-A (VEGF-A) mouse monoclonal antibody (Santa Cruz Biotech #sc-7269) diluted 1:70, and anti-pimonidazole mouse monoclonal antibody (MAb1, clone 4.3.11.3, Hyoxyprobe Inc, Burlington, MA) diluted 1:50. The anti-pimonidazole antibody is directed against the covalent adducts formed between the activated intermediate of pimonidazole (Hypoxyprobe™-1) and sulphydryl groups in proteins, peptides and amino acids. Tissue sections were stained on a Discovery XT Automated Immunostainer (Ventana Medical Systems, Inc, Tucson, Arizona). All steps were performed with this instrument using VMSI validated reagents, including deparaffinization, cell conditioning (antigen retrieval with a borate-EDTA buffer), primary antibody staining, detection and amplification using a a biotinylated-streptavidin-HRP and Diaminobenzidine (DAB) system and hematoxylin counterstaining. VEGF-A was detected using a goat anti-rabbit secondary antibody and an UltraMap DAB detection kit (VMSI). CAIX was detected using an anti-rabbit HRP kit, and pimonidazole adducts detected using an anti-mouse HRP kit. Following staining on the instrument, slides were dehydrated through graded alcohols to xylene and cover slipped with mounting medium (Richard Allan #4112). Images were captured using a Paxcam 3 camera with PAX-it Digital Image Management & Image Analysis. Images were standardized for light intensity. One pathologist evaluated each case using a semi-quantitative histological scoring method as previously described [15]. Briefly, staining intensity for neoplastic cells was scored as: 0 negative, 1 weak, 2 moderate, and 3 intense. In addition, the percentage of positive neoplastic cells was evaluated. The overall scores were calculated by multiplying the intensity by the corresponding percentage of positive cells, resulting in values ranging from 0 to 300.
Magnetic Resonance Imaging
A total of thirteen mice underwent MRI studies, among which seven mice were treated with TH-302 and six mice were treated with vehicle as a control. Tumors were allowed to grow for 22–23 days to an average volume of 250 mm3 before initiating MRI studies. Mice were treated with TH-302 (50 mg/kg qd×5 days/week×2 weeks, i.p.) or vehicle control. The mice did not show signs of physiological distress, or significant weight loss in response to therapy, vehicle treatments, or imaging procedures.
MRI was performed one day prior to starting therapy (Day -1), one day after starting therapy (Day 1) and 10 days after starting therapy (Day 10). To perform each MRI study, a mouse was anesthetized with 1.5–2% isoflurane. A 100% O2 carrier gas was used for anesthetization to offset pCO2 build-up during hypoventilation. A 27 G catheter was inserted in the tail vein and physiological monitoring leads were connected to monitor respiration rate and core body temperature during the MRI session. The mouse was kept at 37.0 ± 0.2°C during the MRI studies using warmed air that is controlled by an automated temperature-feedback system (SA Instruments, Inc.). Each MRI scan session required about 1.25 hours to complete. At the end of the MRI scan session, the mouse was allowed to recover from the gas anesthesia.
DCE-MRI studies were conducted by acquiring a set of 3 contiguous axial image slices through the xenograft flank tumor, 5 coronal image slices through the renal artery and 3 axial image slices through the femoral artery. The orientation of these image slices were determined by acquiring T2-weighted MR images with a Rapid Acquisition with Relaxation Enhancement (RARE) MRI protocol that used the following parameters: 1.0 second TR; 8.2 msec TE; 0.5 mm slice thickness; 128×128 data matrix; 3.5×3.5 cm field of view; 273×273 mm in-plane resolution; 1 average; a RARE factor of 4. The imaging of the renal and femoral arteries acquired an Arterial Input Function (AIF) in order to measure the concentration of a contrast agent in the blood stream. A parametric map of endogenous T1 relaxation time was obtained by acquiring a series of T1-weighted MR images using the same slice geometry and a RARE MRI protocol with the following parameters: 0.375, 0.75, 1.5, 3.0, and 6.0 second TR; 9.07 msec TE; 1 average; a RARE factor of 2. Finally, to acquire a dynamic series of MR images for DCE-MRI analysis, a RARE MRI protocol was used with the same slice geometry and the following parameters: 250 msec TR; 8.2 msec TE; 2 averages; a RARE factor of 2. A total of 65 image sets were acquired for a total acquisition time of 34.66 min and a temporal resolution of 32 sec/image. After the fifth image set was acquired, 0.2 mmol/kg Gd-DTPA (Magnevist ) was injected through the tail vein catheter in a total volume of 0.25 mL during 60 seconds. Using a customized program wrote in Matlab (Mathworks, Inc.), DCE-MRI results were analyzed using a general kinetic model as described by Tofts et al. [16] to obtain the fractional plasma volume (ve) of the tumor and the volume transfer constant Ktrans.
Diffusion-Weighted (DW) MRI studies were conducted using a diffusion-weighted radial echo pulse sequence to measure the Apparent Diffusion Coefficient (ADC). The following parameters were used: 1000 msec TR; 40 msec TE; 1.5 mm slice thickness; 128×202 data matrix; 5.12×5.12 cm field of view; 400×253 µm in-plane resolution; 1 average; 25, 450, and 750 s/mm2 b-values; 20.2 min total acquisition time. To account for the potential anisotropic nature of tumor tissue, the direction of the diffusion-weighting gradients was continuously iterated along the read, phase and slice directions. Hence, the resulting images were isotropically diffusion-weighted [18]. DW-MRI results were processed using ParaVision 5.0 (Bruker Biospin, Inc.). ADC maps were obtained by fitting the signal intensity to a monoexponential decay over the b value range (25, 450, 750 s/mm2).
Statistical Analysis
The tumor growth rate for each mouse was estimated by fitting a least square linear regression of the cube root of tumor volume over time. The cube root of observed tumor volume was used to induce linearity in the data. A two-sample t-test was employed to test for treatment effects.
All parametric maps were tested for normality using a Lilliefors test, because none of them was normal at the 95% confidence level, the median of all the pixels in the regions of interest of each parametric map were used to summarize the data. The effect of therapy on the parametric maps was studied using a Kruskal-Wallis test when more than two groups were compared and a Wilcoxon Rank-Sum test when only two groups were compared. All statistical tests were performed at the 95% confidence level as implemented in Matlab (MathWorks, Natick, MA) or Excel, (MicroSoft Corp, Redmond,WA).
Results
Evidence for tumor hypoxia in pancreatic xenograft tumor models
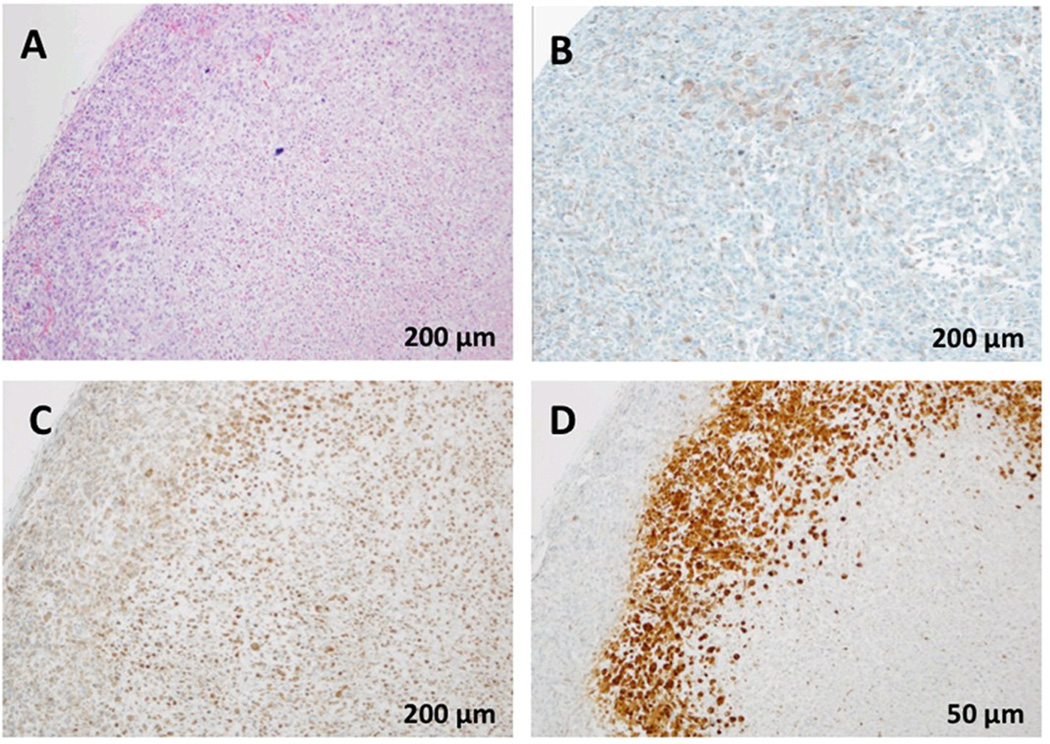
A standard hematoxylin and eosin stain was performed to verify cellular architecture and viability (Figure 1A). Using immunohistochemistry, we detected high expression of carbonic anhydrase-IX (CAIX) in the MiaPaCa2 tumor xenograft (Figure 1B). CAIX is partially regulated via hypoxia inducible transcription factor-1 alpha (HIF-1α), and is used as an endogenous molecular biomarker of tumor hypoxia [8]. One of the consequences of tumor hypoxia is increased angiogenesis, partially through upregulation of VEGF-A. Thus we used immunohistochemistry to investigate the expression of VEGF-A in the MiaPaCa2 xenograft model (Figure 1C). VEGF-A staining was predominantly cytoplasmic throughout the tumor (Figure 1A. The VEGF-A staining had a high average staining score of 103 in vehicle treated tumors (N=3). To determine whether VEGF-A expression was associated with tumor hypoxia, we assessed hypoxia in the MiaPaCa2 xenograft using a chemical probe, pimonidazole (Hypoxyprobe™-1). This probe is activated in hypoxic regions and forms adducts that can be detected using an anti-pimonidazole antibody by IHC [19]. The MiaPaCa2 tumors showed marked regions of anti-pimonidazole staining (Figure 1D), and these hypoxic regions qualitatively correlated with regions that showed higher VEGF-A expression. These results suggest that VEGF-A is constitutively expressed in MiaPaCa2 tumors, but may also be upregulated in response to hypoxia.
Figure 1.
Immunohistochemical staining for: A) hematoxylin and eosin (H&E), B) Carbonic Anhydrase IX (CA IX), C) Vascular endothelial growth factor A (VEGF-A), and D) pimonidazole adducts, which is a biomarker for hypoxia, in MiaPaCa2 xenograft tumor tissue.
Therapeutic activity of TH-302 in MiaPaCa2 Flank Xenograft Tumors
TH-302 is in Phase II clinical trials for the treatment of pancreatic cancer. Our investigation showed significant growth delay during the post-treatment period in the MiaPaCa2 flank xenograft model treated with TH-302 as a monotherapy (p=0.0008 vs. vehicle) (Figure 2). Interestingly, median growth rates between vehicle control and TH-302 treatment groups were not significantly different (p>0.2) throughout the treatment period, but diverged at the end of the treatment cycles.
Figure 2.
Effect of treatment with TH-302 on MiaPaCa2 flank xenograft tumor model. The errors bars show the standard error of the mean (Control, N=10 and TH-302, N=9).
Dynamic Contrast Enhanced-MRI (DCE-MRI)
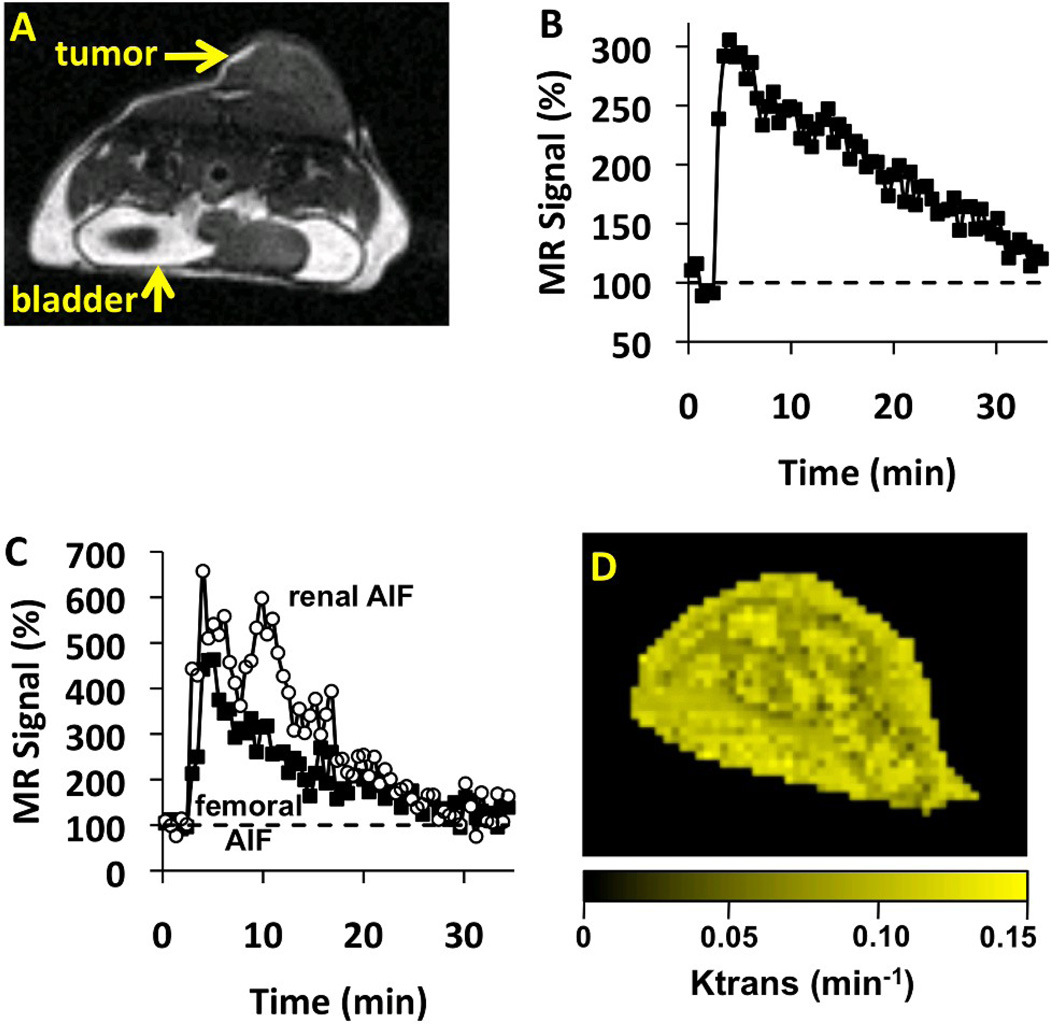
Initial T2-weighted MR images were used to identify the location of the tumor. These images showed that the tumor tissue was uniform, without a "rim and core" that has been observed in other xenograft tumors (Figure 3A), which may be due to their small size at the time of imaging. A strong 3-fold signal change was observed in the tumor after injecting the contrast agent for DCE-MRI scan sessions, followed by a return to baseline signal levels within ~35 minutes (Figure 3B). A signal change was not observed during two DCE-MRI scan sessions, which was attributed to non-patent catheter as evidenced by high back-pressure during injection. A 95% success rate (37 patent catheters, 39 total catheterizations) is typical for our DCE-MRI methodology. The DCE-MRI data were used to calculate Ktrans on a pixel-wise basis for one image slice of the tumor tissue (Figure 3D). Similar to T2-weighted MR images, the parametric maps of Ktrans did not show evidence of a "rim and core" within the tumor.
Figure 3.
The effect of TH-302 therapy on Ktrans measured with DCE-MRI. A) An anatomical image shows the location of the tumor. Dark bands below the tumor were caused by excitation of orthogonal slices that imaged the renal artery. B) After injecting the agent, a strong change in MRI signal was observed in the tumor. C) The Arterial Input Function (AIF) from the femoral artery showed less variability than the renal AIF. D) The parametric map of Ktrans values showed good spatial homogeneity.
The high quality of these Ktrans measurements was facilitated by using an AIF derived from a femoral artery that had excellent signal-to-noise because the leg was immobilized to eliminate motion artifacts (Figure 3C). For comparison, Ktrans was also measured using an AIF derived from the renal artery, but abdominal motion affected this AIF in most MRI studies, which reduced the precision of the Ktrans measurements. The small size of these arteries exacerbated problems caused by motion. Furthermore, the identification of the femoral artery was facilitated by positioning the leg to be perpendicular to the axial image orientation, while the renal artery was more difficult to identify due to its oblique and more variable orientation.
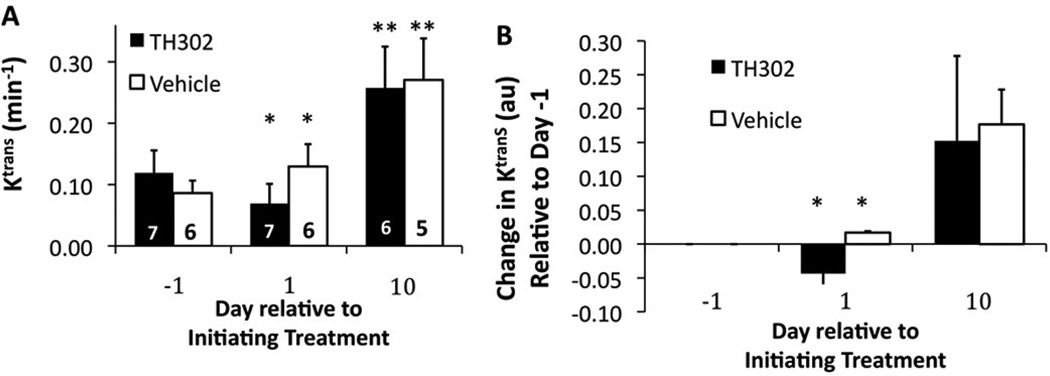
The median of the pixel-wise Ktrans values decreased 44.3 % from Day -1 to Day 1 for TH-302-treated mice, and then increased by 121.7 % on Day 10 relative to "baseline" Day -1 (Figure 4). This same measurement increased 52.1 % from Day -1 to Day 1 for vehicle-treated mice, and then increased 220.1 % on Day 10 relative to Day -1. Each of these temporal changes within each treatment group was statistically significant (p < 0.05). In addition, the TH-302-treated and vehicle-treated groups showed a statistically significant difference on Day 1 (p<.01), but not on Day -1 or Day 10 (p > 0.10 and p >0.5 respectively).
Figure 4. The effect of treatment on vascular permeability (Ktrans).
The average Ktrans of the tumor was measured with Dynamic Contrast Enhancement MRI. Error bars represent the standard deviation of cohorts of 5 to 7 mice. Statistically significant differences relative to the same cohort at Day -1 are indicated by asterisks (*, p< 0.05; **, p< 0.01). A) The median value of Ktrans and B) the relative changes in median Ktrans values showed that TH-302 caused a significant decrease in Ktrans relative to vehicle after one day of treatment, but not after eight days of treatment.
Diffusion Weighted-MRI (DW-MRI)
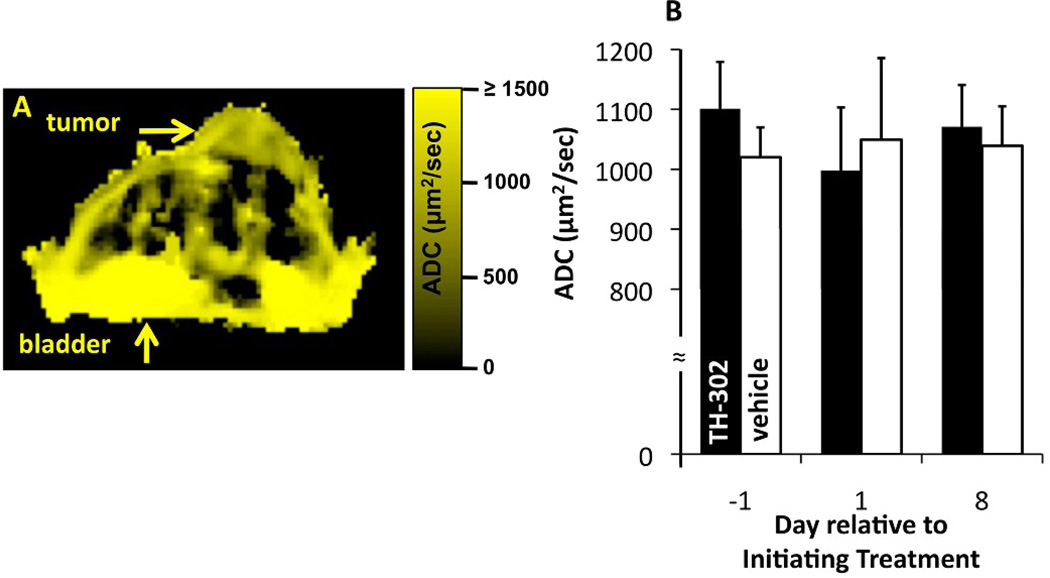
The DW-MRI results were used to calculate the ADC on a pixel-wise basis for the same image slice of the tumor tissue that was used for DCE-MRI analyses (Figure 5). Similar to the Ktrans results, the ADC parametric maps showed no evidence for a "rim and core" within the tumor, and instead showed a uniform spatial distribution throughout each tumor (Figure 5A). The median of the pixel-wise ADC values showed no statistically significant difference between Days -1, 1, and 10 for each treatment group (p >0.6 control group and p>0.23 for TH-302 group), and also showed no difference (p>0.68) between treatment groups regardless of the treatment day (Figure 5B). Standard deviations of these average ADC values within each treatment group on each day were 5–13%, which demonstrated good measurement consistency.
Figure 5.
The effect of TH-302 therapy on ADC measured with DW-MRI. A) The parametric map of ADC values showed good spatial homogeneity, especially relative to the torso. The anatomical image that corresponds to this ADC map is shown in Figure 2A. B) The median ADC did not change following TH-302 therapy. Error bars represent the standard deviation of each cohort. All median ADC values were statistically equivalent (p> 0.05).
Discussion
TH-302 treatment was associated with a delayed but significant decrease in tumor growth rate in the MiaPaCa2 flank xenograft model. These results suggest that selectively targeting the hypoxic tumor cell fraction, even if that fraction is small, results in a decreased growth rate compared to control tumors. These results are similar to the reported effect of TH-302 treatment on tumor growth rate in a MiaPaCa2 orthotopic tumor model [4].
Our studies focused on the investigation of two MRI techniques to monitor the early response to TH-302 therapy, including DCE-MRI, and Diffusion Weighted-MRI. DCE-MRI results indicated that TH-302 caused a substantial decrease in tumor vascular permeability and/or perfusion within one day after starting treatment, which preceded c hanges in tumor growth that occurred after the treatment period ends. (Figure 4E). This reduction could be due to selective targeting of the hypoxic tumor fraction resulting in a decrease in hypoxia-mediated VEGF-A production, which has been shown to be correlated with changes in Ktrans [19]. Interestingly, Ktrans was not significantly decreased after 10 days of TH-302 treatment. This could be a consequence of compensatory mechanisms regulating permeability & perfusion factors including VEGF-A. Future studies should investigate additional xenograft tumor models, and measure how the decrease in Ktrans is sustained between the first and tenth day after initiating TH-302 treatment.
DW MRI was investigated because it indirectly assesses changes in cell membrane integrity. TH-302 is activated to become an alkylating agent that induces caspase-mediated apoptosis and necrosis. No difference in the ADC was observed between the control and therapy groups or within each group on different days (Figure 5B). Additionally, a difference in tumor growth rate was not detected during the treatment period, but the tumors of mice in the control group grew faster after the last day of therapy and reached 2000 mm3 about 40 days before the TH-302 group.
DW MRI has previously been applied to assess the response to therapy of an orthotopic model of pancreatic cancer [20]. Orthotopic models of MiaPaCa2 pancreatic cancer were treated with gemcitabine, an antibody therapy that targets death receptor 5, or a combination of gemcitabine and the antibody therapy. The antibody therapy and combination therapy caused an increase in ADC and a decrease in tumor volume, but gemcitabine did not induce changes. Only tumors with apoptotic cell densities greater than 20% showed a significant increment in ADC, which suggests that a minimum number of cells must be killed before changes in cellularity and/or cell density become apparent by DW MRI. Therefore, one interpretation of the results of our study is that DW MRI is insufficient to detect the level of selective cell killing caused by TH-302 in the hypoxic regions of the tumor. To further investigate the sensitivity of DW MRI for cell killing, future studies should conduct DW MRI evaluations between the first and tenth day after initiating TH-302 treatment, and after tumor growth shows a change in response to TH-302. Because TH-302 and gemcitabine combination therapy are being investigated in early phase I/II clinical trials with pancreatic cancer patients, a future study should also conduct DCE MRI and DW MRI evaluations with this combination therapy with a pre-clinical model [7, 20].
Other studies have compared the utility of DCE MRI and DW MRI for assessing treatment response in pre-clinical cancer models. For example, studies investigating PX-478, an investigational HIF-1α targeting agent, induced a dramatic reduction in tumor blood vessel permeability and/or perfusion within two hours after treatment, while tumor cellularity significantly decreased 24 and 36 hours after treatment, as measured with DCE MRI and DW MRI, respectively [21]. These results showed a rapid effect from the chemotherapy, which supports the assertion that a significant therapeutic effect is needed before DW MRI can detect a change in the tumor. Furthermore, the changes observed with DCE MRI after administering the HIF-1α-targeted therapy were temporary, which is consistent with our study and suggests that DCE MRI may have best utility as an early response biomarker relative to longer-term assessments of therapeutic effects. Finally, other studies have compared the utility of DCE MRI and DW MRI for assessing treatment response in patients, which demonstrates that our study with a pre-clinical model can be translated to the clinic [22, 23].
In conclusion, TH-302 is a promising therapeutic strategy for the treatment of pancreatic cancer. Our studies suggest that DCE-MRI may have potential as a response biomarker for TH-302 therapy even when DW MRI is not able to detect early changes in tumor physiology.
Acknowledgements
This work was supported by the Arizona Cancer Center and the National Cancer Institute under grants P50 CA95060, PO1 CA017094, R01 CA125627, and P30 CA023074. JCR is supported through the US Army Medical Research and Materiel Command under grant number W81XWH-09-1-0053. The authors would like to thank Ms. Christy Howison and the Arizona Cancer Center Experimental Mouse Shared Service for assistance with animal handling, the Arizona Cancer Center, TACMASS and Biometry Shared Services and Ms. Michelle Benson for assistance with data analysis. We would also like to thank Dr. Charles Hart from Threshold Pharmaceuticals, for providing TH-302 and for his intellectual contribution and review of this manuscript. Finally, we thank the late Dr. John Curd for his support and guidance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Cassavaugh J, Lounsbury KM. Hypoxia-mediated biological control. J Cell Biochem. 2011;112:735–744. doi: 10.1002/jcb.22956. [DOI] [PubMed] [Google Scholar]
- 2.Duffy JP, Eibl G, Reber HA, Hines OJ. Influence of hypoxia and neoangiogenesis on the growth of pancreatic cancer. Mol Cancer. 2003;2:12–21. doi: 10.1186/1476-4598-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denny WA. Hypoxia-activated prodrugs in cancer therapy: progress to the clinic. Future Oncol. 2010;6:419–428. doi: 10.2217/fon.10.1. [DOI] [PubMed] [Google Scholar]
- 4.Duan JX, Jiao H, Kaizerman J, Stanton T, Evans JW, Lan L, Lorente G, Banica M, Jung D, Wang J, Ma H, Li X, Yang Z, Hoffman RM, Ammons WS, Hart CP, Matteucci M. Potent and highly selective hypoxia-activated achiral phosphoramidate mustards as anticancer drugs. J Med Chem. 2008;51:2412–2420. doi: 10.1021/jm701028q. [DOI] [PubMed] [Google Scholar]
- 5.Hu J, Handisides DR, Van Valckenborgh E, De Raeve H, Menu E, Vande Broek I, Liu Q, Sun JD, Van Camp B, Hart CP, Vanderkerken K. Targeting the multiple myeloma hypoxic niche with TH-302, a hypoxia-activated prodrug. Blood. 2010;116:1524–1527. doi: 10.1182/blood-2010-02-269126. [DOI] [PubMed] [Google Scholar]
- 6.Li S, Zhang J, Li J, Chen D, Matteucci M, Curd J, Duan JX. Inhibition of both thioredoxin reductase and glutathione reductase may contribute to the anticancer mechanism of TH-302. Biol Trace Elem Res. 2010;136:294–301. doi: 10.1007/s12011-009-8544-1. [DOI] [PubMed] [Google Scholar]
- 7.Weiss GJ, Infante JR, Chiorean EG, Borad MJ, Bendell JC, Molina JR, Tibes R, Ramanathan RK, Lewandowski K, Jones SF, Lacouture ME, Langmuir VK, Lee H, Kroll S, Burris HA., 3rd Phase 1 Study of the Safety, Tolerability, and Pharmacokinetics of TH-302, a Hypoxia-Activated Prodrug, in Patients with Advanced Solid Malignancies. Clin Cancer Res. 2011;17:2997–3004. doi: 10.1158/1078-0432.CCR-10-3425. [DOI] [PubMed] [Google Scholar]
- 8.Evans CE, Mattock K, Humphries J, Saha P, Ahmad A, Waltham M, Patel A, Modarai B, Porter L, Premaratne S, Smith A. Techniques of assessing hypoxia at the bench and bedside. Angiogenesis. 2011;14:119–124. doi: 10.1007/s10456-011-9205-5. [DOI] [PubMed] [Google Scholar]
- 9.Evelhoch JL, Gillies RJ, Karczmar GS, Koutcher JA, Maxwell RJ, Nalcioglu O, Raghunand N, Ronen SM, Ross BD, Swartz HM. Applications of magnetic resonance in model systems: cancer therapeutics. Neoplasia. 2000;2:52–65. doi: 10.1038/sj.neo.7900078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yankeelov TE, Gore JC. Dynamic Contrast Enhanced Magnetic Resonance Imaging in Oncology: Theory, Data Acquisition, Analysis, and Examples. Curr Med Imaging Rev. 2009;3:91–107. doi: 10.2174/157340507780619179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jordan BF, Runquist M, Raghunand N, Gillies RJ, Tate WR, Powis G, Baker AF. The thioredoxin-1 inhibitor 1-methylpropyl 2-imidazolyl disulfide (PX-12) decreases vascular permeability in tumor xenografts monitored by dynamic contrast enhanced magnetic resonance imaging. Clin. Cancer Res. 2005;11:529–536. [PubMed] [Google Scholar]
- 12.Davis TW, O'Neal JM, Pagel MD, Zweifel BS, Mehta PP, Heuvelman DM, Masferrer JL. Synergy between celecoxib and radiotherapy results from inhibition of cyclooxygenase-2-derived prostaglandin E2, a survival factor for tumor and associated vasculature. Cancer Res. 2004;64:279–285. doi: 10.1158/0008-5472.can-03-1168. [DOI] [PubMed] [Google Scholar]
- 13.Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, Dzik-Jurasz A, Ross BD, Van Cauteren M, Collins D, Hammoud DA, Rustin GJ, Taouli B, Choyke PL. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009;11:102–125. doi: 10.1593/neo.81328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morse DL, Galons JP, Payne CM, Jennings DL, Day S, Xia G, Gillies RJ. MRI-measured water mobility increases in response to chemotherapy via multiple cell-death mechanisms. NMR Biomed. 2009;20:602–614. doi: 10.1002/nbm.1127. [DOI] [PubMed] [Google Scholar]
- 15.Huang CI, Kohno N, Ogawa E, Adachi M, Taki T, Miyake M. Correlation of reduction in MRP-1/CD9 and KAI1/CD82 expression with recurrences in breast cancer patients. Am J Pathol. 1998;153:973–983. doi: 10.1016/s0002-9440(10)65639-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, Larsson HB, Lee TY, Mayr NA, Parker GJ, Port RE, Taylor J, Weisskoff RM. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10:223–232. doi: 10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 17.von Marschall Z, Cramer T, Höcker M, Burde R, Plath T, Schirner M, Heidenreich R, Breier G, Riecken EO, Wiedenmann B, Rosewicz S. De novo expression of vascular endothelial growth factor in human pancreatic cancer: evidence for an autocrine mitogenic loop. Gastroenterology. 2000;119:1358–1372. doi: 10.1053/gast.2000.19578. [DOI] [PubMed] [Google Scholar]
- 18.Trouard TP, Theilmann RJ, Altbach MI, Gmitro AF. High-resolution diffusion imaging with DIFRAD-FSE (diffusion-weighted radial acquisition of data with fast spin-echo) MRI. Magn Reson Med. 1999;42:11–18. doi: 10.1002/(sici)1522-2594(199907)42:1<11::aid-mrm3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 19.Ali Meser M, Janic Branislava, Babajani-Feremi Abbas, Varma Nadimpalli RS, Iskander ASM, Anagli John, Arbab Ali S. Changes in vascular permeability and expression of different angiogenic factors following anti-angiogenic treatment in rat glioma. PLoS One. 2010;5(1):e8727. doi: 10.1371/journal.pone.0008727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim H, Morgan DE, Buchsbaum DJ, Zeng H, Grizzle WE, Warram JM, Sotckard CR, McNally LR, Long JW, Sellers JC, Forero A, Zinn KR. Early therapy evaluation of combined anti-death receptor 5 antibody and gemcitabine in orthotopic pancreatic tumor xenografts by diffusion-weighted magnetic resonance imaging. Cancer Research. 2008;68:8369–8376. doi: 10.1158/0008-5472.CAN-08-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jordan BF, Runquist M, Raghunand N, Baker A, Williams R, Kirkpatrick L, Powis G, Gillies RJ. Dynamic Contrast-Enhanced and Diffusion MRI Show Rapid and Dramatic Changes in Tumor Microenvironment in Response to Inhibition of HIF-1A Using PX-478. Neoplasia. 2005;7(5):475–485. doi: 10.1593/neo.04628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yankeelov TE, Lepage M, Chakravarthy A, Broome EE, Niermann KJ, Kelley MC, Meszoely I, Mayer IA, Herman CR, McManus K, Price RR, Gore JC. Integration of quantitative DCE-MRI and ADC mapping to monitor treatment response in human breast cancer: initial results. Magn Reson Imaging. 2007;25:1–13. doi: 10.1016/j.mri.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrett T, Gill AB, Kataoka MY, Priest AN, Joubert I, McLean MA, Graves MJ, Stearn S, Lomas DJ, Griffiths JR, Neal D, Gnanapragasam VJ, Sala E. DCE and DW MRI in Monitoring Response to Androgen Deprivation Therapy in Patients With Prostate Cancer: A Feasibility Study. Magn Reson Med. 2011 doi: 10.1002/mrm.23062. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 24.Liu Q, Sun JD, Wang J, Ahluwalia D, Baker AF, Cranmer LD, Ferraro D, Wang Y, Duan JX, Ammons WS, Curd JG, Matteucci MD, Hart CP. TH-302, a hypoxia-activated prodrug with broad in vivo preclinical combination therapy efficacy: optimization of dosing regimens and schedules. Cancer Chemother Pharmacol. 2012 Mar 2; doi: 10.1007/s00280-012-1852-8. [Epub ahead of print] PMID: 22382881. [DOI] [PMC free article] [PubMed] [Google Scholar]