Abstract
Doxorubicin (DOX) is an effective antineoplastic agent used for the treatment of a variety of cancers. Unfortunately, its use is limited as this drug induces cardiotoxicity and heart failure as a side effect. There is no report which describes whether transplanted embryonic stem (ES) cells or their conditioned medium (CM) in DOX induced cardiomyopathy (DIC) can repair and regenerate myocardium. Therefore, we transplanted ES cells or CM in DIC to examine apoptosis, fibrosis, cytoplasmic vacuolization and myofibrillar loss and their associated Akt and ERK pathway. Moreover, we also determined activation of endogenous c-kit+ve cardiac stem cells (CSCs), levels of HGF and IGF-1, growth factors required for c-kit cell activation, and their differentiation into cardiac myocytes, which also contributes in cardiac regeneration and improved heart function. We generated DIC in C57Bl/6 mice (cumulative dose of DOX 12mg/kg body weight, i.p), and animals were treated with ES cells, CM or cell culture medium in controls. Two weeks post-DIC, ES cells or CM transplanted hearts showed a significant (p<0.05) decrease in cardiac apoptotic nuclei and their regulation with Akt and ERK pathway. Cardiac fibrosis observed in the ES cell or CM groups was significantly less compared with DOX and cell culture medium groups (p<0.05). Next, cytoplasmic vacuolization and myofibrillar loss was reduced (p<0.05) following treatment with ES cells or CM. Moreover, our data also demonstrated increased levels of c-kit+ve CSCs in ES cells or CM hearts and differentiated cardiac myocytes from these CSCs, suggesting endogenous cardiac regeneration. Importantly, the levels of HFG and IGF-1 were significantly increased in ES cells or CM transplanted hearts. In conclusion, we reported that transplanted ES cells or CM in DIC hearts significantly decreases various adverse pathological mechanisms as well as enhances cardiac regeneration that effectively contributes to improved heart function.
Introduction
Doxorubicin (DOX) is an antineoplastic antibiotic vastly used antitumor agent for a diversity of malignancies (22,27). However, the clinical use of this drug is restricted due to a severe, dose-dependent, acute cardiotoxicity that may progress to irreversible chronic cardiomyopathy and congestive heart failure. DOX induced cardiomyopathy (DIC) has been well published in human and animal studies. Even though, the precise mechanism of DIC is unclear, antitumor activity of DOX is relayed to be distinct from the mechanisms of induced cardiomyopathy. DIC appears to involve multifactorial and complex disease mechanisms, however, oxidative stress plays a major role in this cardiotoxicity. DIC is characterized by contractile dysfunction and rhythm disturbances that lead to congestive heart failure in a time dependent manner (22,27). Moreover, development of heart failure includes; a) death of both cardiac myocytes and non-myocyte myocardial cells; b) myofibril loss and vacuolar degeneration; and c) fibrosis. These changes result in rearrangement of heart tissue, increased wall stress and insufficient systolic contractility in cardiac myocytes (12,22,27,38). Importantly, the collagen synthesis that is increased in DIC is also associated with concurrent extracellular matrix (ECM) degradation via activation of matrix metalloproteinases (MMPs) (12,33,38). Numerous studies have demonstrated inhibiting doxorubicin induced cardiac myocyte apoptosis in DIC using various agents such as erythropoietin and antioxidants (11-13). However, DIC is a major health problem, therefore, accurate identification of novel therapeutic approaches is still warranted.
Over the past decade, cell transplantation studies have demonstrated significant interest as a potential option to treat distinct heart diseases including DIC (1,9,39). Published studies demonstrate significant improvement in cardiac function in DIC following adult stem cell transplantation (1,9,39). Moreover, these studies proclaimed minimal or no successful engraftment of transplanted cells (1,39). There is no data yet attainable that describes the ability of embryonic stem (ES) cells or their factors released to repair and regenerate DIC, however, ES cells have a distinct advantage as they are unique in the potential to differentiate into many body cell types compared with their counterpart adult stem cells (28). Therefore, in the present study we hypothesized that transplanted ES cells or their conditioned medium (CM), containing cytoprotective factors, will inhibit DIC. We present data in this study on transplanted ES cells or CM that inhibit cardiac apoptosis, fibrosis, cytoplasmic vacuolization and myofibril loss, typical characteristics of DIC. Additionally, we also determined significant increased levels of hepatocyte growth factor (HGF) and insulin growth factor (IGF-1) in hearts transplanted with ES cells or CM, required in activating c-kit+ve cardiac stem cells (CSCs). Moreover, increased numbers of c-kit+ve CSCs were predominantly observed in the same HGF and IGF-1 hearts. Finally, we observed significant improvement in cardiac function following ES cells or CM transplantation, suggesting multiple mechanisms are required to blunt DIC.
MATERIALS AND METHODS
ES Cells and Preparation of CM
Mouse ES cells expressing green fluorescence protein (GFP) were maintained using our standard protocol as previously described (30,32). In brief, cells were plated 24 hours on gelatinized tissue culture plates in Dulbecco’s Modified Eagle Medium (DMEM) containing essential ingredients such as fetal bovine serum (FBS), leukemia inhibitory factor (LIF), glutamine, penicillin/streptomycin, sodium pyruvate and β-mercaptoethanol. For ES-CM, cell culture medium was replaced with cell culture medium without LIF. After 48 hours released factors containing cell supernatant was removed, filtered (0.2um filter, Millipore, USA) and used to inject sterile CM as we previously published (30,32).
Animals and Experimental Protocol
All the animals described in this study are approved by University of Central Florida animal care committee. C57Bl6 mice were divided into five different study groups: Control, normal saline, DOX, DOX+Cell culture (CC) medium, DOX+ES cells or DOX+CM. There were n=6-8 animals in each of the five study groups.
DOX was administered in 3 equal injections in mice (4mg/kg/injection i.p), on alternate days over the period of one week for a cumulative dose of 12 mg/kg. For treatment groups, CC medium (400μl/injection and the total of three injections), ES cells (5×105 ES cells/injection and total of three injections) or ES-CM (400μl/injection and the total of three injections) was injected during DOX treatment on alternative days. For example: DOX or normal saline in controls 400μl/injection was given on MON, WED and FRI, whereas treatment groups, CC medium, ES-cells or ES-CM were given on TUE, THUR and SAT. After the last injection of treatment groups, animals were examined for heart function using echocardiography and were sacrificed at day (D) 14 by administering pentabarbitol (40 mg/kg, IP). Furthermore, half of the heart tissue was fixed in 4% paraformaldehye whereas the other half was saved in RNA later to perform biochemical and ELISA assays.
Identification of Apoptotic Nuclei and Caspase 3 Staining
Apoptotic nuclei in heart sections with or without cell transplantation groups were identified using a commercially available Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL assay kit; TMR red; Roche Applied Biosystems, IN) as per detailed instructions available with the kit. Moreover, we have published this protocol in our previous studies (29). Next, we identified co-labeling of TUNEL positive apoptotic nuclei with pro-apoptotic caspase-3 as well as with sarcomeric cardiac α-actin to determine apoptosis does occur in cardiac myocytes. In brief, TUNEL stained sections were stained with primary antibodies; active anti-caspase-3 rabbit polyclonal (1:1000; Cell Signaling) and sarcomeric cardiac α-actin mouse monoclonal antibodies (1:30; Sigma). Following incubations, sections were washed with PBS and labeled with secondary antibodies; anti-rabbit Alexa 568 (Invitrogen) or anti-mouse Alexa 488 (MOM kit, vector laboratories). Total nuclei were stained blue with DAPI (4’,6-diamidino-2-phenylindole) present in the mounting Vectashield medium. Positive stained cells were examined and photomicrographs were prepared with an Olympus fluorescence microscope and a LEICA laser scanning confocal microscope. Red stained apoptotic nuclei present in the left ventricular of the heart were examined and quantifications were determined by two blind observers in 4-5 random areas in 1-2 heart sections from 6-8 different hearts as published (29). To determine percent apoptotic nuclei per section we calculated using the formula; number of apoptotic nuclei /total number of nuclei per section and then multiply by 100. Percentage apoptotic nuclei data was examined under 20x magnification.
Caspase 3 Activity
Heart homogenates were prepared from different DOX and ES cell treated groups. Caspase-3 activity was performed using a caspase-3 colorimetric activity assay kit from BioVision (CITY, CA) as we reported recently (5). Protein concentrations were measured in the supernatant using a Bio-Rad assay. Caspase-3 activity was performed as per the instructions provided in the kit and measured at 405 nm in a microtiter plate reader. Caspase 3 activity was calculated and plotted in the form of arbitrary units (A.U).
Phosphorylated Akt Activity
Phosphorylated Akt (p-Akt) and phosphorylated extracellular signal regulated kinases 1/2 (p-ERK 1/2) activities were examined using commercially available kits (Exalpha Biologicals, Inc., Maynard, MA). In brief, tissues were homogenized as described beforehand and p-Akt and p-ERK 1/2 were quantified according to manufacturer’s instructions. The reactions were developed and the color reactions were measured at 450 nm using a microtiter plate reader for each ELISA. P-Akt and p-ERK 1/2 protein data were plotted as ng/mg tissue and arbitrary units (A.U.), respectively.
Determination of HGF and IGF-1 in Heart Tissue in DIC
Hearts were isolated, homogenates were prepared with the assay buffer provided with the kit. Using our standard Bio-Rad assay, total protein concentrations were determined. Commercially available kits HGF (B-Bridge International, CA), and IGF-1 (R&D, Minneapolis, USA) were purchased and used to measure HGF and IGF-1 levels in the DIC hearts with or without treatment groups. These kits are based on the ELISA sandwich assay which was performed in accordance with the kit instructions. HGF and IGF-1 concentrations in the samples were analyzed from the standard curve performed according to the kit directions.
Determination of Myofibrillar Loss, Cytoplasmic Vacuolization and Fibrosis
Hearts were rapidly removed, placed in iced saline, fixed in 4% buffered formalin and embedded in paraffin as described previously (29). 5 μm serial sections were cut and deparaffinized by incubation in xylene. Rehydration was accomplished by sequential incubation in 100%, 95% and 70% ethanol for 5 min each at room temperature, followed by washing in distilled water and then phosphate buffer saline (PBS) for 5-10 min. Heart sections were stained with commonly used H&E and Mason’s trichrome protocols. Two to three H&E stained heart sections were examined completely for the evaluation of myofibrillar loss and cytoplasmic vacuolization. Qualitative data scale, developed to determine cytoplasmic vacuolization and myofibrillar loss, varies from 0-2. If left ventricular (LV) heart section showed no signs of myofibrillar loss and cytoplasmic vacuolization then score was graded as 0, whereas if significant portion of the section had myofibrillar loss and cytoplasmic vacuolization then slide was graded as 2. To determine cardiac fibrosis, sections were stained with Masson’s Trichrome. Cardiac fibrosis predominantly present in the left ventricle was examined and quantified by measuring the total blue area per mm2 in each section using freely available imageJ, NIH program.
Identification of c-kit+ve Cells and their Differentiation into Cardiac Myocytes
Sections were deparaffinized as described above. To block non-specific staining, sections were incubated in humidified chamber in 10% normal goat serum (NGS) from 30 min to 1hour at room temperature. Following incubation, sections were washed and incubated with primary rabbit polyclonal antibody anti-c-kit (Santa Cruz Biotechnologies), and sarcmomeric cardiac α-actin mouse monoclonal antibody (Sigma), diluted with 10% NGS, for an hour at 37°C. Sections were washed in PBS and incubated with secondary antibody Alexa Fluor 568 or antimouse fluorescein isothiocyanate (FITC) available in the MOM kit (Vector, laboratories) for 1 hour. Heart sections were washed and mounted with Vectashield antifade medium (containing nuclear stain DAPI; Vector Laboratories). Quantitative analysis of c-kit+ve cells was determined on heart sections from 4-6 different hearts using Olympus and confocal fluorescence microscope.
Echocardiographic Analysis
We routinely performed 2D-echocardiography on mice using a Sonos 5500 ultrasonograph with a 15-MHz transducer (Philips, Andover MA). Mice were sedated with ketamine (100 mg/kg, IM), the chest was shaved and maintained on a heated platform to control temperature, and placed in a supine or left lateral decubitus position to measure left ventricular (LV) functions using 2D-echocardiography. For quantification of LV dimensions and wall thickness, we digitally recorded 2D clips and M-mode images in a short axis view from the mid-LV at the tips of the papillary muscles. End diastolic and systolic LV diameter as well as anterior and posterior wall (AW and PW respectively) thickness was measured on M-mode images using the leading edge-to- leading edge convention. LV fractional shortening and mass were calculated from LV cross sectional area in 2D short axis view as: [(LVDdiastole-LVDsystole/ LVDdiastole)] *100 and LV mass with formula [1.05× (PWdiastole+AWdiastole+LVDdiastole)3-(LVDdiastole)3]. Relative wall thickness was calculated as 2* PWdiastole/LVDdiastole.
Data Analysis
Values were presented as means ± SEM. One way analysis of variance (ANOVA) was performed followed by Dunn’s test. Differences between values were considered statisticaly significant when p<0.05.
Results
Effects of Transplanted ES Cells or CM on Apoptosis in DIC
Figure 1 (A-O) shows apoptotic positive nuclei with TUNEL staining. Right panel histogram shows there was a significant (p<0.05) increase in TUNEL-positive nuclei in DOX and DOX+CC medium treated hearts compared with normal saline controls. This significant increase in apoptotic nuclei were attenuated in DOX+CM or DOX+ES cell groups, suggesting protective effects of CM or ES cell treatments (p<0.05, Figure 1, P). Next, we wanted to investigate whether apoptotic nuclei are present in the cardiac myocytes as well as stained positive with active caspase-3 staining, considered as a hall mark of apoptosis. Therefore, heart sections from all groups were stained with TUNEL, colabeled with sarcomeric cardiac α-actin and active caspase 3 antibody. Figure 2, A-E shows TUNEL stained nuclei were positive with active caspase-3 as well as suggesting apoptosis does occur in cardiac myocytes as confirmed with sarcomeric cardiac α-actin. Moreover, we performed a caspase 3 activity assay as shown in Figure 2F. Significant increased caspase 3 activity in DOX and DOX+CC hearts compared with controls were attenuated following CM or ES-cells transplantation. However, apoptosis and caspase 3 activities was not significant between DOX+CM and DOX+ES cells groups.
Figure 1.
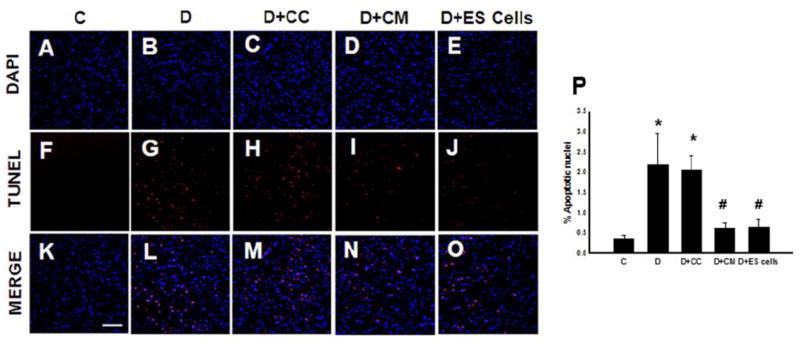
Transplanted conditioned medium (CM) or embryonic stem (ES) cells inhibit cardiac myocyte apoptosis at 2 weeks post doxorubicin-induced cardiomyopathy (DIC). Representative photomicrographs of total nuclei stained with DAPI in Blue (A-E) and Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) stained apoptotic nuclei in Red (F-J) and merged nuclei in Pink (K-O), (Scale bar = 50μm). Right panel histogram (P) shows quantitative apoptotic nuclei per section from Control (C), DOX (D), D+ culture medium (D+CC), D+CM and D+ES cell groups. Data is from the n=6-9 animals. *p<0.05 compared to C, and #p<0.05 vs D and D+CC.
Figure 2.
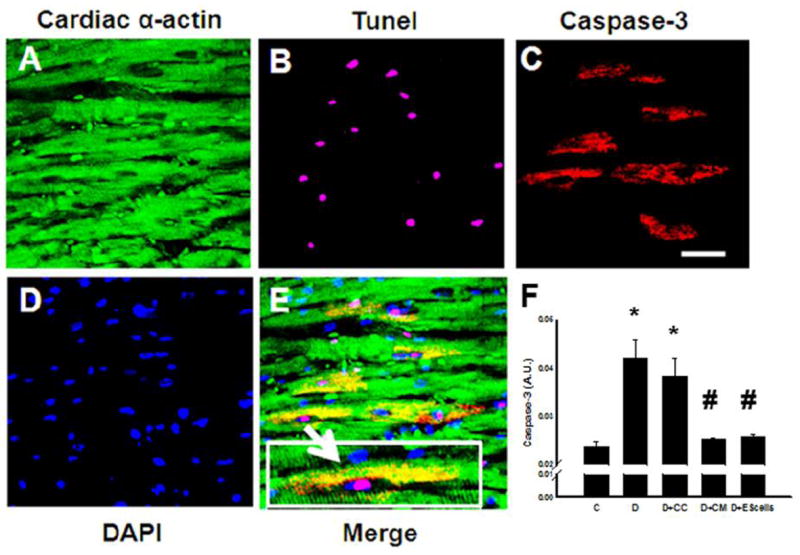
Effects of transplanted CM or ES cell on cardiac myocyte apoptosis and caspase 3 activity. Representative photos of heart section labeled with sarcomeric cardiac α-actin (A), apoptotic nuclei stained with TUNEL in purple (B), section stained with active caspase 3 antibody (C), total nuclei stained with DAPI in blue (D), and merged image (E) demonstrating apoptosis appearing in cardiac myocytes and positive with active caspase 3. Boxed area in panel E, demonstrates colocalization of sarcomeric cardiac α-actin, active caspase 3 (yellow area), TUNEL positive nucleus, and DAPI. Bottom right histogram (F) shows total apoptotic nuclei from 5-8 different animals each group. *p<0.001 vs. C, #p <0.05 vs. D and D+CC. A.U. = arbitrary units. Scale bar = 25μm.
CM or ES Cells Regulate Akt and ERK Pathways in DIC
Apoptosis has been regulated with increased or decreased expressions of Akt and ERK signaling pathways in ischemic heart diseases (8,17,32). Therefore, we examined effects of these pathways in the DIC and their modulation with transplanted CM or ES cells. Phosphorylated Akt was significantly reduced in the DOX and DOX+CC groups compared with normal controls, suggesting a decrease in cell survival. Moreover, hearts transplanted with CM or ES cells, had significant increased activation of p-Akt compared to DOX and DOX+CC (Figure 3A). Moreover, our data suggests phosphorylated ERK1/2 is significantly increased in DOX and DOX+CC groups compared with control. A significant decrease in ERK1/2 was observed in hearts transplanted with CM or ES cells (p<0.05, Figure 3B).
Figure 3.
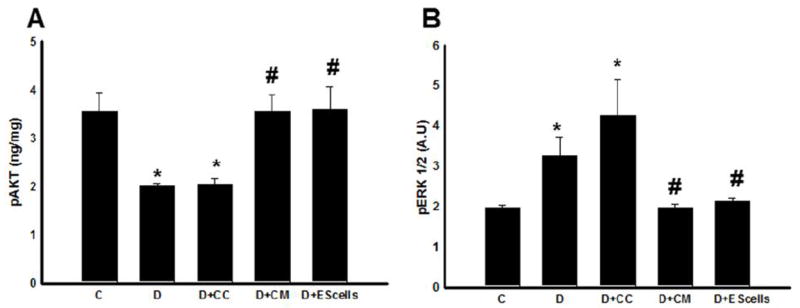
Phospho-Akt and extracellular signal regulated kinase (ERK) alterations following CM or ES cell transplantation in the DIC. (A) shows performed quantitative analysis of phospho-Akt regulation. (B) examines the quantitative levels of phosphorylated ERK up-regulation. *p<0.05 compared to C, and #p<0.05 vs D and D+CC. Data set are from heart homogenates of n=4-6 animals/group. A.U. = arbitrary units.
CM or ES Cells Inhibit Fibrosis, Cytoplasmic Vacuolization and Myofibrillar Loss
To determine the effect of transplanted CM or ES cells on cardiac fibrosis, we stained heart sections with Masson’s Trichrome. In Figure 4, A-E, the blue areas show fibrotic tissue present in the DOX treated hearts with or without CM or ES cells. Our quantitative data suggests that DOX and DOX+CC hearts demonstrated a significant (p<0.05) increase in blue area, an indicative of cardiac fibrosis (Figure 4 F). However, this increased fibrosis was reduced following CM or ES cell transplantation (Figure 4 F). Therefore, relative increased amounts of viable cardiac muscle were observed in the DOX+CM or ES cells (Figure 4, D and E) compared with DOX or DOX+CC groups (Figure 4, B and C).
Figure 4.
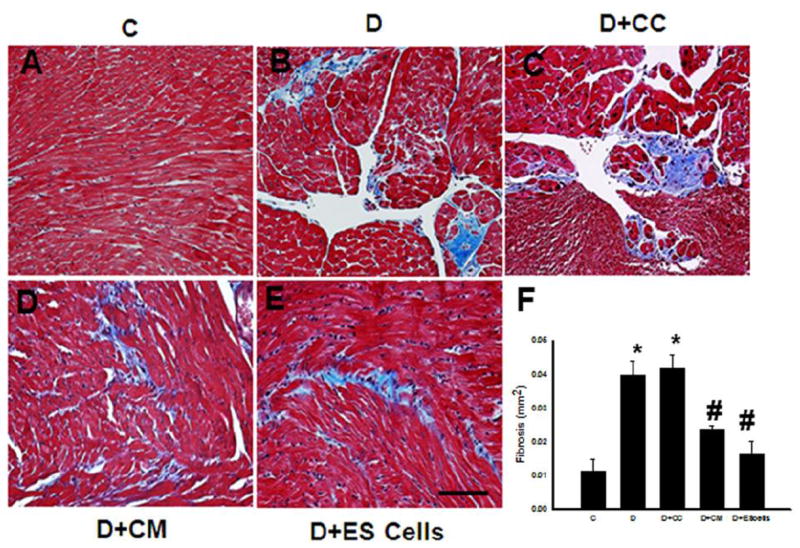
Effects of transplanted CM or ES cell on cardiac fibrosis. Photomicrographs show histological sections stained with Mason’s trichrome after 2 weeks of DOX treatment of different hearts from each group. Blue area shows extent of fibrosis in different conditions. Control, C (A), DOX, D (B), D+CC (C), D+CM (D) and D+ES cells (E). Scale bar = 50μm. (F) histogram shows quantitative cardiac fibrosis in post DIC with or without treatment groups. Data is from 6-8 animals. *p<0.05 compare to C, and #p<0.05 vs D and D+CC.
Cyoplasmic vacuolization and myofibrillar loss, a well-recognized characteristic of DIC, was examined to strengthen our findings. In normal saline treated control, hearts show full length cardiac myocytes with intact cytoplasmic structure as well as well-organized myofibrillars (Figure 5A). In contrast, DOX or DOX+CC treated hearts demonstrate clear evidence of loss of cytoplasm as well as myofibrils in many cardiac myocytes (Figure 5B and C) and this increased cytoplasmic vacuolization and myofibrillar loss was significantly reduced following CM or ES cells treatments (Figure 5 D and E). Moreover, our semi-quantitative data suggests significant (p<0.05) increase in cytoplasmic vacuolization and myofibrillar loss in DOX and DOX+CC hearts (Figure 5, F and G). Following treatments with CM or ES cells in DIC, hearts showed significantly reduced amounts of cytoplasmic vacuolization and myofibrillar, suggesting a decrease in cardiomyopathy (Figure 5 F and G).
Figure 5.
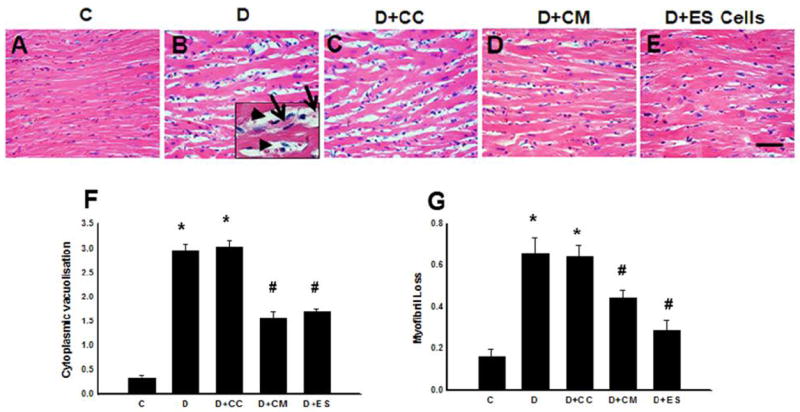
Effects of transplanted CM or ES cell on cytoplasmic vacuolization and myofibrillar loss. Photomicrographs show histological sections stained with H&E staining after 2 weeks of DOX treatment of different hearts from each group. In the representative enlarged area the arrow head indicates cytoplasmic vacuolization whereas the arrow shows myofibrillar loss. These cytoplasmic vacuolization and myofibrillar loss areas in cardiac myocytes were determined in different conditions. C (A), D (B), D+CC (C), D+CM (D) and D+ES cells (E). (F) histogram shows semi-quantitative cytoplasmic vacuolization, and (G) determines myofibrillar loss in post DIC with or without treatment groups. Data is from 5-8 animals. *p<0.05 compare to C, and #p<0.05 vs D and D+CC. Scale bar = 50μm.
c-kit+ve Cells and their Progenitor Cells following CM or ES Cell Transplantation
To determine activated cardiac endogenous c-kit+ve cells and their differentiation into cardiac myocytes to define their role in cardiac regeneration, we examined c-kit+ve cells and their co-labeling with sarcomeric cardiac α-actin to stain cardiac myocytes in heart sections with or without CM or ES cell transplantation in DIC. Figure 6, A-D, shows positively stained c-kit+ve cells as well as c-kit+ve cells combined with cardiac myocyte specific sarcomeric cardiac α-actin, suggesting some of the c-kit+ve cells differentiate into cardiac myocytes. Quantitative c-kit+ve cells in DOX or DOX +CC significantly increased (p<0.05) compared with normal saline controls, suggesting injury released cytokine and growth factors may have triggered c-kit+ve cells (Figure 6E). Moreover, c-kit+ve cells were further significantly increased in CM or ES cells groups compared with respective controls (Figure 6E).
Figure 6.
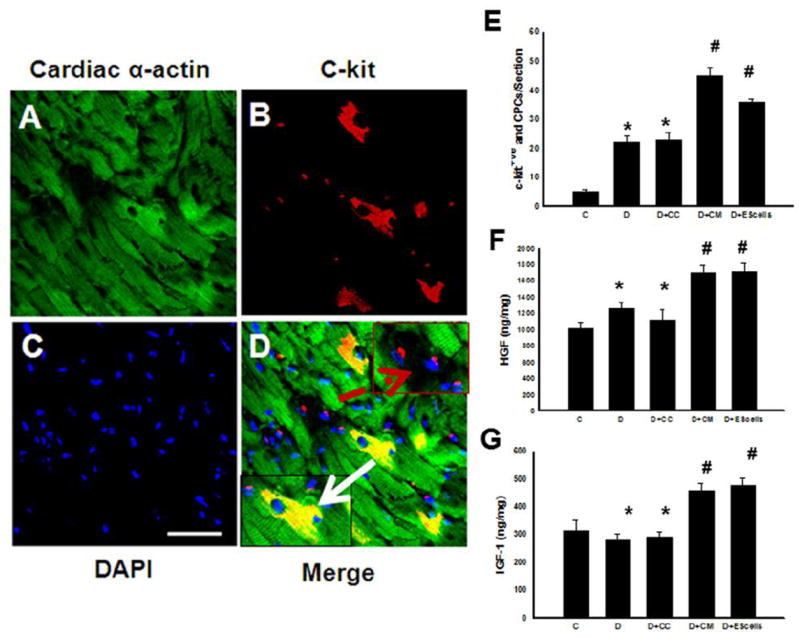
Effects of transplanted CM or ES cell on cardiac stem cells (CSCs) and their differentiation into cardiac myocytes. Heart section stained with sarcomeric cardiac α-actin showing all stained cardiac myocytes in green (A), resident CSCs were identified by anti-c-kit antibody labeling in red (B), and nuclei are stained with DAPI in blue (C). Merged image indicating presence of CSCs and the positive staining with cardiac α-actin, (D, green, red and blue). Bottom left arrow indicates differentiated CSCs whereas dashed arrow in right top panel shows CSCs positive with c-kit staining. (E) histogram shows quantitative cardiac progenitor cells in post-DIC hearts from C, D, D+CC, D+CM and D+ES cells groups. Data is from 4-5 animals. *p<0.05 compared to C, and #p<0.05 vs D and D+CC. (F) histogram shows hepatocyte growth factor (HGF) data analysis performed in different DIC groups. (G) Histogram shows levels of insulin-like growth factor (IGF)-1 in the DIC hearts with or without treatment groups. Data is from 4-5 animals.*p< non-significant compared to C, and #p<0.05 vs D and D+CC. Scale bar = 50μm.
Levels of HGF and IGF-1 Following CM or ES cells Transplantation
Following an increase in c-kit+ve cells in CM or ES cell groups in DIC, we determined the levels of HGF and IGF-1, a growth factor considered to be an activator of c-kit+ve cells. Our data demonstrates that heart tissue homogenates prepared from CM or ES cell treated hearts have significant increased levels of HGF and IGF-1 (Figure 6, F and G). Thus, increased HGF and IGF-1 levels suggest that these factors released into DIC hearts following CM or ES cell transplantation may also contribute to the stimulation and differentiation of the CSCs that play a role in regeneration.
No Evidence of Teratoma Formation Following ES cell Transplantation in DOX-Cardiomyopathy
To determine whether teratoma were present following ES cell transplantation, H&E and Masson’s Trichrome stained heart, lung and liver sections were examined at 2 weeks post-DOX treatment. No evidence of teratoma formation was observed at 2 weeks in the heart, lung and liver. Figure 7, is representative of these sections at 2 weeks post-DOX treated hearts.
Figure 7.
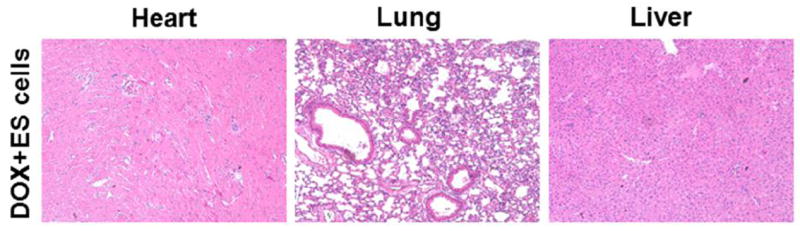
Representative photomicrographs of H&E –stained heart sections from DOX+ES cells group shows no evidence of teratoma formation in the heart, lung and liver post-DIC at 2 weeks. 10x.
Improvement in LV Function Following CM or ES cells Transplantation
M-mode echocardiography was used to determine the effect of CM or cell transplantation on LV size and function in mice at 2 weeks post-DOX treatment. Fractional shortening was improved by CM and ES cell transplanted hearts compared with DOX or DOX+CC groups (Figure 8A). Additionally, CM and ES cells also demonstrated significantly improved ejection fraction (Figure 8B). Moreover, the increased LVIDs in DOX and DOX+CC groups was significantly (p<0.05, Figure 8C) blunted following CM or ES cells transplantation.
Figure 8.
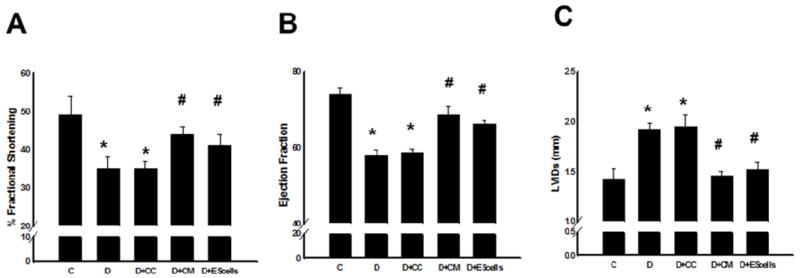
Transplanted CM or ES cells in DIC improve cardiac function at 2 weeks. Left panel, A, shows average echocardiographic left ventricular fractional shortening, middle panel, B, ejection fraction and right panel, C, left ventricular interior systolic diameter (LVIDs) for different treatment groups. *p<0.05 compared to C, and #p<0.05 vs D and D+CC.
Discussion
Cell therapy has been widely examined in various animal and human cardiac diseases (9,15,16,20,21,24,25). Animal studies have successfully manifested significant improvement in cardiac function (24,25). Human embryonic stem cells derived vascular cells and bone marrow derived multipotent progenitor cells following transplantation in the porcine infarcted heart improved cardiac function (36,37). Moreover, bone marrow derived stem cells or combined therapy with cardiomyocytes inhibits doxorubicin induced heart failure in rabbits (7,15,16). Unfortunately, adult stem cell transplanted clinical studies provided contentious reports on the improvement of cardiac function (9,21). Therefore, the use of ES cells may be an optimal cell type, with a wide potential for cell differentiation and unique in characteristics as released factors from these cell types are quite different compared with adult stem cells. Moreover, there is no data yet available which determines the effects of CM or ES cells on DIC. Therefore, we hypothesize that transplanted CM or ES cells will inhibit multiple pathological mechanisms in DIC and will improve associated cardiac function.
In the present study, we used a very clinical relevant DIC cardiomyopathy model, which is well established by various investigators (4,26,27). Importantly, DOX as administered in the present study demonstrates cardiac apoptosis, regulation of apoptotic mediated Akt/ERK pathways, cardiac fibrosis, cytoplasmic vacuolization, myofibrillar loss and decreases in cardiac function in terms of fractional shortening and ejection fraction as deliberated by echocardiography. These alterations construct DIC as observed in cancer patients treated with DOX (4,26,27). Therefore, we report that the DIC model in the present study is in accordance with published reports (4,26,27). The major findings of the present study is that transplantation of the CM or ES cells in DIC results in significant attenuation of various pathological mechanisms such as; 1) inhibition of cardiac myocyte apoptosis, 2) inhibited apoptosis involving phosphoinositol-3-kinase (PI3K)/Akt and ERK pathways, 3) significantly attenuated cardiac fibrosis, 4) inhibition of cytoplasmic vacuolization and myofibrillar loss, 5) activation of c-kit+ve CSCs and their role in cardiac regeneration, 6) increased levels HGF and IGF-1 growth factors, required for c-kit+ve cells activation, and 7) ultimately, improved cardiac function.
Apoptosis is an elemental part of normal organ development (2,14). Recently, presence of apoptosis has been notified to regulate various diseases, including DIC (2,13,14). We and others have reported that apoptosis is a significant contributor in the development and progression of DIC (4,13,14,22). The data on apoptosis in this investigation was established with TUNEL staining as well as by measuring caspase 3 activities. We also demonstrate that hearts transplanted with CM or ES cells have a significantly reduced amount of apoptosis. Additionally, we show that apoptosis does occur in cardiac myocytes as evidenced by co-labeling of active caspase 3 with TUNEL positive nuclei. Moreover, our data authenticates with the previously published data in DIC and infarcted hearts (2,4,13,14,22). As per the best of our knowledge, we are the first to report that transplanted CM or ES cells suppress apoptosis in DIC.
Next, to comprehend mechanisms by which CM or ES cells inhibit apoptosis in DIC, we explored the role of PI3K/Akt and ERK pathways. The PI3K/Akt pathway controls regulation of various cellular processes, including cell growth, proliferation, and survival (6,23). In the present study, we imply that DOX treatment significantly decreases levels of Akt. This reduction in Akt was reversed following treatment with CM or ES cells, suggesting a role of Akt in cell survival in DIC hearts. In contrast, the role of ERK pathway is not well defined in assorted heart diseases and considered controversial as it has been shown to be actively involved in cell survival, hypertrophy and cell death (18,23,32). However, the expression levels of ERK1/2 have been increased in the rat hearts at 4 hours post-DOX treatment whereas this level was decreased at 24 hours and 3 weeks post treatment (19). Our data suggests up regulation of ERK pathway in DOX treated hearts, suggesting ERK may induce hypertrophy or cell death at 2 weeks in DIC. Interestingly, this increase in ERK pathway was significantly reversed in the hearts treated with CM or ES cells. Varied difference in ERK1/2 in post-DIC can be explained as 1) we used injected DOX 12mg/kg cumulative dose over the period of one week whereas previous report injected 15mg/kg over the period of two weeks (19) which may have generated some variation in the induced cardiomyopathy which also alters cell signaling. Moreover, authors examined levels of ERK1/2 at 1-24 hours and 3 weeks post-DIC (19) compared with our study as we report ERK1/2 levels at 2 weeks post-DIC.
Next, we scrutinized whether transplanted CM or ES cells can inhibit cardiac fibrosis in the post-DIC hearts. Cardiac fibrosis, a major contributor to end stage heart failure, is the result of heightened levels of various proteinases such as MMPs as well as a decrease in their inhibitors (tissue inhibitor metalloproteinases; TIMPs) which leads to ECM degradation, collagen protein deposition, hardening of the contractile heart muscle, and eventually, cardiac dysfunction (10). We report in the current study that fibrosis considerably exists in DIC hearts. However, presence and amount of fibrosis in DIC compared with infarcted hearts is substantially different (10). Observed fibrosis in the DIC hearts was patchy and widely distributed. In contrast, infarcted hearts exhibit a transmural infarction and significant extent of fibrosis in the confined area (10). The accurate mechanism for the stimulation of fibrosis in the DIC hearts remains obscure, whereas transforming growth factor β1 (TGFβ 1) actively induces fibrosis in the infarcted hearts (10). Next, our data for the first time reports that cardiac fibrosis in the DIC hearts was significantly attenuated following CM or ES cell transplantation. We published recently that factors released from ES cells contain anti-fibrotic factor TIMP-1 and inhibit apoptosis in the H9c2 cells (30,32). We also published that apoptosis and fibrosis in the infarcted hearts was significantly reduced following CM transplantation (31). Moreover, inhibited apoptosis in the H9c2 cells was mediated by Akt pathway but not by ERK pathway (32). However, we suggest that DIC involves both Akt and ERK pathways in the regulation of cardiac remodeling in the present study. Therefore, this raises further questions whether inhibited apoptosis and fibrosis in DIC following CM or ES cell transplantation involves similar mechanisms.
Cytoplasmic vacuolization and myofibrillar loss in cardiac myocytes is well established and recorded characteristics of DIC were ascertained in animal models and patients (26,27). Our present study also demonstrates the appearance of cytoplasmic vacuolization and myofibrillar loss in DIC. Next, we scrutinized the effects of transplanted CM or ES cells in DIC. We showed that CM or ES cells significantly inhibit cytoplasmic vacuolization and myofibrillar loss in DIC compared with respective controls. Our data is in compliance with the previously published report on the carbon monoxide/heme oxygenase (CO/HO) system that inhibits cytoplasmic vacuolization and myofibrillar loss in DIC (34). In the present study, we confirmed evidence of cytoplasmic vacuolization and myofibrillar loss and extended our findings by proving for the first time that CM or ES cells attenuate cytoplasmic vacuolization and myofibrillar loss in the DIC.
In our present study, we were unable to identify transplanted ES cells in the heart (data not shown), however, there were significant improvement in cardiac function, therefore, we examined the role of c-kit + ve CSCs. We proposed that autocrine or paracrine factors released from ES cells will activate and differentiate resident CSCs into mature cardiac cell types and regenerate the DOX-injured myocardium. CSCs are undifferentiated, self-renewing, clonogenic and capable of differentiating into all three major heart cell types both in vitro and in vivo (3). These cells have been identified and proven to regenerate infarcted myocardium (3). In the present study we demonstrate increased levels of c-kit + ve CSCs following CM or ES cell transplantation compared with their controls. Our data is in accordance with the previous published studies showing c-kit + ve cells in the infarcted heart were up regulated and differentiated into cardiac myocytes following treatment with HGF and IGF-1 growth factors (35). Therefore, we extended our findings and determined whether levels of HGF and IGF-1 are being affected in the DIC with or without CM or ES cell treatment. To our surprise, we observed significant increased levels of HGF and IGF-1 growth factors in CM and ES cell treated hearts compared with DOX, DOX+CC and normal saline groups, suggesting these released factors might have played a significant role in their activation. Therefore, further studies are required to determine if more than two growth factors reported in this study are involved in the inhibition of multiple pathological conditions observed in DIC.
Moreover, we determined whether transplantation of CM or ES cells contributes to improved cardiac function in DIC. In the present study, we have demonstrated that 2 weeks post-DIC, mice transplanted with CM or ES cells had significantly improved cardiac function compared with DIC.
In conclusion, this is the first study proposing that CM or ES cells have the competence to inhibit multiple pathological conditions such as cardiac myocyte apoptosis, Akt and ERK pathways, cardiac fibrosis, cytoplasmic vacuolization and myofibrillar loss as well as up regulation of c-kit + ve CSCs and their differentiation into cardiac myocytes along with improved cardiac function in DIC.
Acknowledgments
The authors would like to thank Crystal Rocher for proof reading the Ms.
Funding
This work was supported, in part, from grants from the National Institutes of Health [1R01HL090646-01, and 5R01HL094467-02 to DKS].
Footnotes
Conflict of Interest: none declared.
Reference List
- 1.Agbulut O, Menot ML, Li Z, Marotte F, Paulin D, Hagege AA, Chomienne C, Samuel JL, Menasche P. Temporal patterns of bone marrow cell differentiation following transplantation in doxorubicin-induced cardiomyopathy. Cardiovasc Res. 2003;58:451–459. doi: 10.1016/s0008-6363(03)00281-5. [DOI] [PubMed] [Google Scholar]
- 2.Anversa P, Cheng W, Liu Y, Leri A, Redaelli G, Kajstura J. Apoptosis and myocardial infarction. Basic Res Cardiol. 1998;93(Suppl 3):8–12. doi: 10.1007/s003950050195. [DOI] [PubMed] [Google Scholar]
- 3.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 4.De Beer EL, Bottone AE, Voest EE. Doxorubicin and mechanical performance of cardiac trabeculae after acute and chronic treatment: a review. Eur J Pharmacol. 2001;415:1–11. doi: 10.1016/s0014-2999(01)00765-8. [DOI] [PubMed] [Google Scholar]
- 5.Fatma S, Selby DE, Singla RD, Singla DK. Factors Released from Embryonic Stem Cells Stimulate c-kit-FLK-1(+ve) Progenitor Cells and Enhance Neovascularization. Antioxid Redox Signal. 2010;13:1857–1865. doi: 10.1089/ars.2010.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garbade J, Dhein S, Lipinski C, Aupperle H, Arsalan M, Borger MA, Barten MJ, Lehmann S, Walther T, Mohr FW. Bone marrow-derived stem cells attenuate impaired contractility and enhance capillary density in a rabbit model of Doxorubicin-induced failing hearts. J Card Surg. 2009;24:591–599. doi: 10.1111/j.1540-8191.2009.00844.x. [DOI] [PubMed] [Google Scholar]
- 8.Gautreau A, Poullet P, Louvard D, Arpin M. Ezrin, a plasma membrane-microfilament linker, signals cell survival through the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci USA. 1999;96:7300–7305. doi: 10.1073/pnas.96.13.7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haider HK, Ashraf M. Bone marrow cell transplantation in clinical perspective. J Mol Cell Cardiol. 2005;38:225–235. doi: 10.1016/j.yjmcc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Jugdutt BI, Menon V, Kumar D, Idikio H. Vascular remodeling during healing after myocardial infarction in the dog model: effects of reperfusion, amlodipine and enalapril. J Am Coll Cardiol. 2002;39:1538–1545. doi: 10.1016/s0735-1097(02)01805-3. [DOI] [PubMed] [Google Scholar]
- 11.Kim KH, Oudit GY, Backx PH. Erythropoietin protects against doxorubicin-induced cardiomyopathy via a phosphatidylinositol 3-kinase-dependent pathway. J Pharmacol Exp Ther. 2008;324:160–169. doi: 10.1124/jpet.107.125773. [DOI] [PubMed] [Google Scholar]
- 12.Kumar D, Kirshenbaum L, Li T, Danelisen I, Singal P. Apoptosis in isolated adult cardiomyocytes exposed to adriamycin. Ann N Y Acad Sci. 1999;874:156–168. doi: 10.1111/j.1749-6632.1999.tb09233.x. [DOI] [PubMed] [Google Scholar]
- 13.Kumar D, Kirshenbaum LA, Li T, Danelisen I, Singal PK. Apoptosis in adriamycin cardiomyopathy and its modulation by probucol. Antioxid Redox Signal. 2001;3:135–145. doi: 10.1089/152308601750100641. [DOI] [PubMed] [Google Scholar]
- 14.Kumar D, Lou H, Singal PK. Oxidative stress and apoptosis in heart dysfunction. Herz. 2002;27:662–668. doi: 10.1007/s00059-002-2430-3. [DOI] [PubMed] [Google Scholar]
- 15.Li TS, Mikamo A, Takahashi M, Suzuki R, Ueda K, Ikeda Y, Matsuzaki M, Hamano K. Comparison of cell therapy and cytokine therapy for functional repair in ischemic and nonischemic heart failure. Cell Transplant. 2007;16:365–374. doi: 10.3727/000000007783464858. [DOI] [PubMed] [Google Scholar]
- 16.Li TS, Takahashi M, Ohshima M, Qin SL, Kubo M, Muramatsu K, Hamano K. Myocardial repair achieved by the intramyocardial implantation of adult cardiomyocytes in combination with bone marrow cells. Cell Transplant. 2008;17:695–703. doi: 10.3727/096368908786092702. [DOI] [PubMed] [Google Scholar]
- 17.Liu XW, Bernardo MM, Fridman R, Kim HR. Tissue inhibitor of metalloproteinase-1 protects human breast epithelial cells against intrinsic apoptotic cell death via the focal adhesion kinase/phosphatidylinositol 3-kinase and MAPK signaling pathway. J Biol Chem. 2003;278:40364–40372. doi: 10.1074/jbc.M302999200. [DOI] [PubMed] [Google Scholar]
- 18.Lorenz K, Schmitt JP, Vidal M, Lohse MJ. Cardiac hypertrophy: targeting Raf/MEK/ERK1/2-signaling. Int J Biochem Cell Biol. 2009;41:2351–2355. doi: 10.1016/j.biocel.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Lou H, Danelisen I, Singal PK. Involvement of mitogen-activated protein kinases in adriamycin-induced cardiomyopathy. Am J Physiol Heart Circ Physiol. 2005;288:H1925–H1930. doi: 10.1152/ajpheart.01054.2004. [DOI] [PubMed] [Google Scholar]
- 20.Menasche P. Myoblast-based cell transplantation. Heart Fail Rev. 2003;8:221–227. doi: 10.1023/a:1024705214292. [DOI] [PubMed] [Google Scholar]
- 21.Menasche P. Cellular transplantation: hurdles remaining before widespread clinical use. Curr Opin Cardiol. 2004;19:154–161. doi: 10.1097/00001573-200403000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 23.Mullonkal CJ, Toledo-Pereyra LH. Akt in ischemia and reperfusion. J Invest Surg. 2007;20:195–203. doi: 10.1080/08941930701366471. [DOI] [PubMed] [Google Scholar]
- 24.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 25.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339:900–905. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- 27.Singal PK, Iliskovic N, Li T, Kumar D. Adriamycin cardiomyopathy: pathophysiology and prevention. FASEB J. 1997;11:931–936. doi: 10.1096/fasebj.11.12.9337145. [DOI] [PubMed] [Google Scholar]
- 28.Singla DK. Embryonic stem cells in cardiac repair and regeneration. Antioxid Redox Signal. 2009;11:1857–1863. doi: 10.1089/ARS.2009.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singla DK, Lyons GE, Kamp TJ. Transplanted embryonic stem cells following mouse myocardial infarction inhibit apoptosis and cardiac remodeling. Am J Physiol Heart Circ Physiol. 2007;293:H1308–H1314. doi: 10.1152/ajpheart.01277.2006. [DOI] [PubMed] [Google Scholar]
- 30.Singla DK, McDonald DE. Factors released from embryonic stem cells inhibit apoptosis of H9c2 cells. Am J Physiol Heart Circ Physiol. 2007;293:H1590–H1595. doi: 10.1152/ajpheart.00431.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singla DK, Singla RD, Lamm S, Glass C. TGF-{beta}2 treatment enhances cytoprotective factors released from embryonic stem cells and inhibits apoptosis in infarcted myocardium. Am J Physiol Heart Circ Physiol. 2011;300:H1442–H1450. doi: 10.1152/ajpheart.00917.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singla DK, Singla RD, McDonald DE. Factors Released from Embryonic Stem Cells inhibit Apoptosis in H9c2 cells through P1-3kinase/Akt but not ERK pathway. Am J Physiol Heart Circ Physiol. 2008;295:H907–H913. doi: 10.1152/ajpheart.00279.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spinale FG. Matrix metalloproteinases: regulation and dysregulation in the failing heart. Circ Res. 2002;90:520–530. doi: 10.1161/01.res.0000013290.12884.a3. [DOI] [PubMed] [Google Scholar]
- 34.Suliman HB, Carraway MS, Ali AS, Reynolds CM, Welty-Wolf KE, Piantadosi CA. The CO/HO system reverses inhibition of mitochondrial biogenesis and prevents murine doxorubicin cardiomyopathy. J Clin Invest. 2007;117:3730–3741. doi: 10.1172/JCI32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Urbanek K, Rota M, Cascapera S, Bearzi C, Nascimbene A, De Angelis A, Hosoda T, Chimenti S, Baker M, Limana F, Nurzynska D, Torella D, Rotatori F, Rastaldo R, Musso E, Quaini F, Leri A, Kajstura J, Anversa P. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res. 2005;97:663–673. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Jameel MN, Li Q, Mansoor A, Qiang X, Swingen C, Panetta C, Zhang J. Stem cells for myocardial repair with use of a transarterial catheter. Circulation. 2009;120:S238–S246. doi: 10.1161/CIRCULATIONAHA.109.885236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiong Q, Hill KL, Li Q, Suntharalingam P, Mansoor A, Wang X, Jameel MN, Zhang P, Swingen C, Kaufman DS, Zhang J. A fibrin patch-based enhanced delivery of human embryonic stem cell-derived vascular cell transplantation in a porcine model of postinfarction left ventricular remodeling. Stem Cells. 2011;29:367–375. doi: 10.1002/stem.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Y, McLaughlin D, Robinson E, Harvey AP, Hookham MB, Shah AM, McDermott BJ, Grieve DJ. Nox2 NADPH oxidase promotes pathologic cardiac remodeling associated with Doxorubicin chemotherapy. Cancer Res. 2010;70:9287–9297. doi: 10.1158/0008-5472.CAN-10-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou YL, Zhang HF, Li XL, DI RM, Yao WM, Li DF, Feng JL, Huang J, Cao KJ, Fu M. Increased stromal-cell-derived factor 1 enhances the homing of bone marrow derived mesenchymal stem cells in dilated cardiomyopathy in rats. Chin Med J (Engl) 2010;123:3282–3287. [PubMed] [Google Scholar]


