Summary
Brown fat defends against hypothermia and obesity through thermogenesis mediated by mitochondrial UCP1. Recent data suggest that there are two distinct types of brown fat: classical brown fat derived from a myf-5 cellular lineage and UCP1-positive cells that emerge in white fat from a non-myf-5 lineage. Here we report the cloning of “beige” cells from murine white fat depots. Beige cells resemble white fat cells in having extremely low basal expression of UCP1, but like classical brown fat, they respond to cyclic AMP stimulation with high UCP1 expression and respiration rates. Beige cells have a gene expression pattern distinct from either white or brown fat and are preferentially sensitive to the polypeptide hormone irisin. Finally, we show that deposits of brown fat previously observed in adult humans are composed of beige adipose cells. These data illustrate a new cell type with therapeutic potential in mouse and human.
Introduction
The epidemic of obesity and diabetes has greatly increased the interest in brown fat. Adipocytes can be broadly divided in white and brown fat cells. While white fat cells are specialized to store chemical energy, brown adipocytes defend mammals against hypothermia, obesity and diabetes. Brown fat utilizes a high mitochondrial content and high mitochondrial UCP1 to uncouple respiration and dissipate chemical energy as heat. Rodents and other small mammals have copious brown fat deposits, but larger mammals often lose prominent brown fat depots after infancy. Recent data indicates that adult humans contain significant deposits of UCP1-positive brown fat that can be detected by PET-scanning methods, particularly in the supraclavicular and neck region (Cypess et al., 2009; Mirbolooki et al., 2011; Orava et al., 2011; van Marken Lichtenbelt et al., 2009; Virtanen et al., 2009). The physiological significance of adult human brown fat has not yet been fully explored.
It has been known for many years that some white adipose tissues contain cells that can express high levels of UCP1 and take on a multilocular appearance upon prolonged stimulation by cold or pathways that elevate intracellular cyclic AMP (Cousin et al., 1992; Young et al., 1984). Recent data has shown that classical brown fat, exemplified by the interscapular depots of rodents, is derived from a myf-5, muscle-like cellular lineage (Seale et al., 2008). The “brown-like” cells within white adipose depots are not derived from the myf-5 lineage and have been called beige cells or brite cells (Ishibashi and Seale, 2010; Petrovic et al., 2010; Seale et al., 2008). Interestingly, it has been reported that distinct genetic loci control the amounts of UCP1-positive cells in the white and classical brown fat depots (Coulter et al., 2003; Guerra et al., 1998; Koza et al., 2000; Xue et al., 2005; Xue et al., 2007), strongly suggesting these two types of thermogenic cells are regulated differently. The therapeutic potential of both kinds of brown fat cells is clear (Himms-Hagen et al., 1994; Seale et al., 2011) as genetic manipulations in mice that create more brown or beige fat have strong anti-obesity and anti-diabetic actions. For example, ectopic expression in WAT of PRDM16, a transcriptional coregulator that controls the development of brown adipocytes in classical BAT depots, or COX-2, a down-stream effector of β-adrenergic signaling, protects mice from diet-induced obesity and metabolic dysfunction (Seale et al., 2011; Vegiopoulos et al., 2010).
While classical brown fat cells have been isolated, cloned and characterized, beige fat cells have never been isolated or cloned. In fact, some studies have suggested that the “brown conversion” of white fat is an inherent property of most or all white fat cells, and may not be due to the presence of a distinct cell type with this predisposition (Cinti, 2002; Himms-Hagen et al., 2000). Importantly, the identity of brown adipose tissues in adult humans as either classical brown fat or beige fat is unknown.
Here we report the cloning of murine beige fat cells and describe their unique gene expression signature. While these cells have a very low basal level of UCP1 gene expression, comparable to bona fide white fat cells, they retain a remarkable ability to powerfully activate expression of this gene and turn on a robust program of respiration and energy expenditure that is equivalent to that of classical brown fat cells. Furthermore, we show here that the deposits of brown fat previously observed in adult humans have the gene expression pattern and immunohistochemical characteristics of beige fat. These data definitively demonstrate the existence and properties of a distinct type of adipose cell in both mice and humans.
Results
Multilocular, UCP1-positive cells are prominent in the subcutaneous white adipose depot of mice
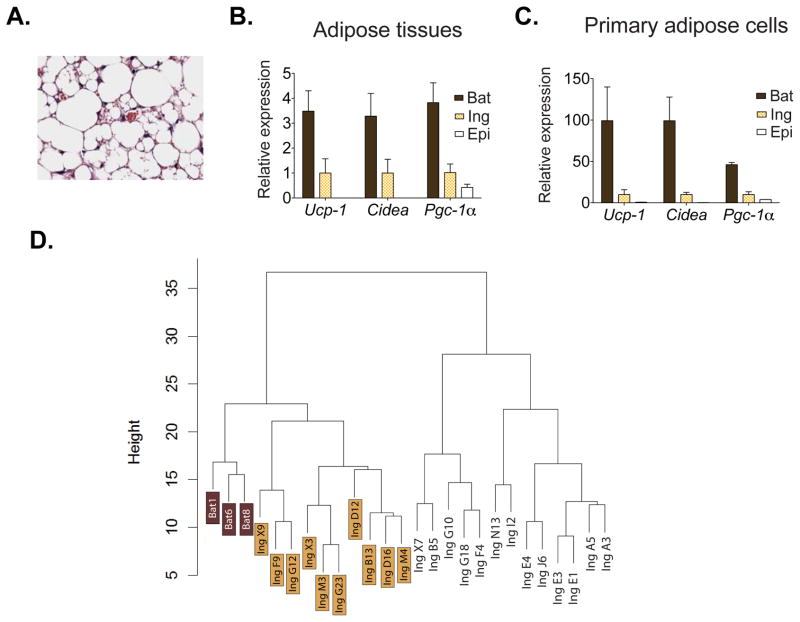
It has been observed that the subcutaneous white adipose depots of rodents have a higher propensity toward expression of UCP1 and other brown fat cell genes, compared to the visceral white adipose depots (Cousin et al., 1992). This propensity to activate these cells varies widely among different strains of inbred mice (Collins et al., 1997; Guerra et al., 1998). As shown in Figure 1A, multilocular brown fat-like cells are readily detectable in the inguinal subcutaneous fat depot of 129SVE mice maintained at ambient temperature, with no prior cold exposure. Gene expression analysis of this subcutaneous fat tissue showed levels of mRNA encoding UCP1 and other brown fat cell-enriched proteins, like CIDEA and PGC1α, that were intermediate between those of epididymal, visceral WAT and the classical (interscapular) BAT (Figure 1B). To determine whether these differences in gene expression were cell autonomous and could be maintained after differentiation in culture, cells from the stromal-vascular fraction (SVF) taken from these three depots were subjected to in vitro differentiation. Expression of these brown-selective genes maintained their interdepot differences after this procedure (Figure 1C). These data strongly suggest that at least part of the intermediate “brown” nature of this subcutaneous adipose depot is independent of extrinsic factors, such as innervation, blood flow, the level of oxygenation and nutrients.
Figure 1. Beige fat cells arise from a subset of preadipocytes in the subcutaneous adipose depot.
(A) Multilocular beige fat cells are prominent in the subcutaneous white adipose depot of 129SVE male mice at 7–9 weeks of age. Hematoxylin & Eosin stain show islets of multilocular fat cells within inguinal white fat depot (400X). (B) Total RNA was isolated from epididymal (Epi), inguinal (Ing) and interscapular (Bat) adipose tissues of 129SVE mice and assayed for mRNA expression for Ucp-1 and other brown fat-like genes by qPCR. Values are mean±SD (n=5). Expression levels of Ucp-1 and Cidea are undetected in epididymal depot. (C) Total RNA was isolated from fat cells differentiated from epididymal (Epi), inguinal (Ing) and interscapular (Bat) primary stromal vascular cells and assayed for mRNA expression for Ucp-1 and other brown fat-like genes by qPCR. Relative gene expression was normalized to adipsin mRNA levels. Values are mean±SD (n=3) (D) Cluster dendrogram of array expression data with RNA samples from differentiated cultures of 26 immortalized fat cell lines as described in text. Analysis details were described in the Supplemental Experimental Procedures. Height (y axis) is Euclidean distance. To mimic the sympathetic tone in vivo upon prolonged cold exposure when beige fat cells are functionally activated, we analyzed RNA from fully differentiated cells (day8), treated with 10 μM forskolin (FSK), a cAMP inducing agent, for 4 hrs.
Unbiased gene expression analysis identifies two distinct adipose cell types derived from the subcutaneous fat depot
To investigate the molecular identity of fat cells from the subcutaneous fat depot, we isolated the SVF from subcutaneous fat in the inguinal region, and then subjected these undifferentiated cells to a classical 3T3 immortalization protocol (Todaro and Green, 1963). These cultures were then subjected to cloning of individual cells by limiting dilution; we thus derived 305 new clonal cell lines (Figure S1A). Among these clonal lines were more than 20 that were highly and reproducibly adipogenic, as evidenced by lipid accumulation after the induction of differentiation (Figure S1B). For purposes of subsequent comparison, multiple adipogenic clones were also generated by the same methods from the interscapular brown fat depot. Importantly, these immortalized clonal lines retain expression of previously reported interscapular depot and inguinal depot-enriched genes (Billon and Dani, 2011; Gesta et al., 2006; Seale et al., 2007) (Figure S1C).
To investigate possible heterogeneity in the clonal cells derived from the subcutaneous adipose tissue, multiple cell lines were differentiated, treated with forskolin and their RNA was subjected to microarray analysis. We used the established class finding algorithm ISIS (von Heydebreck et al., 2001) to search for the existence of subgroups among these inguinal cell lines. As shown in Figure 1D, these 23 cell lines separated into 2 distinct clusters, with 10 cell lines in one group and 13 in the other. Once these groupings were identified and the differentially expressed gene sets were determined, we included analysis of the cell lines derived from the classical brown fat depots into the dataset; all samples were then hierarchically clustered using complete linkage of differentially expressed genes. This clustering (Figure 1D) indicates that one of the subsets of cells derived from the inguinal depot has a gene expression pattern more similar to the bona fide brown fat cell lines than to the other cluster of inguinal cell lines. The separation of these three groups of cells was further established by principle component analysis (PCA; Figure S1D). These data strongly support the notion that a distinct pool of progenitors in the inguinal depot can give rise to cells (beige cells) that are similar but not identical to classical brown fat cells.
Beige cells have characteristics of both white and brown fat cells
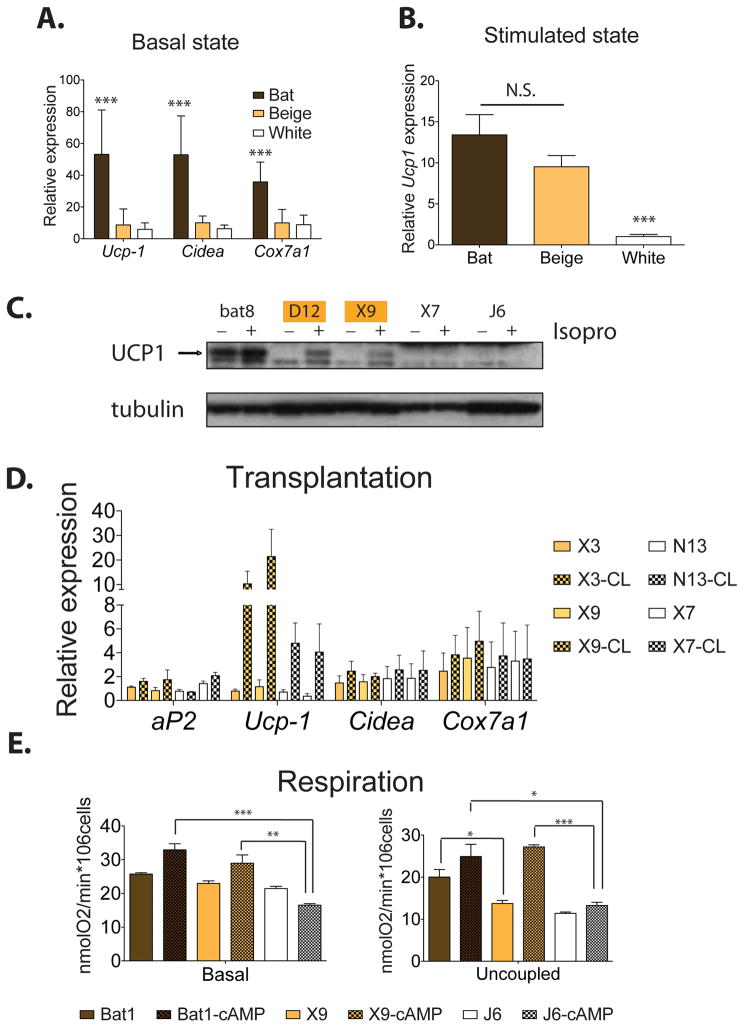
We further characterized several cell lines from both subgroups (beige and white) derived from the subcutaneous depot in terms of their molecular and functional properties. All these lines are reproducibly highly adipogenic and presented similar levels of adipogenesis and expression of fat cell specific markers (e.g. aP2, adiponectin, adipsin and PPARγ) (Figure S2A). Both groups of the inguinal-derived cells showed a similar, low basal level of gene expression for Ucp1, Cox7a1 and Cidea; these levels of gene expression were much lower than those observed in all of the cell lines derived from the classical brown fat (Figure 2A). However, upon cAMP stimulation, the beige lines responded with a very large induction of Ucp1 gene expression, reaching similar absolute levels to that observed in the interscapular BAT lines (Figure 2B and 2C). In fact, although the absolute levels of Ucp-1 mRNA were similar between beige and brown cells, the fold induction in the beige cell lines (>150 fold) was actually greater than that observed in the BAT cell lines (about 40 fold) (Figure S2B). It has been observed that some of the precursor cells within heterogeneous cultures derived from epididymal adipose tissue can show an elevated expression of Ucp-1 in response to PPARγ agonist treatment (Petrovic et al., 2010). In our clonal cell lines, basal expression levels of Ucp-1 are increased upon long-term TZD treatments in both beige and white cell lines, although this induction is stronger in the beige cells (8–10-fold vs. 4–5-fold, respectively) (Figure S2C).
Figure 2. Beige fat cells show comparable thermogenic potential to brown fat cells upon stimulation.
Representative lines from each group were further analyzed functionally. Bat1, bat6, bat8 for brown fat cells; X9, X3, D16, D12 for beige fat cells and N13, X7, J6, G18 for white fat cells. All these lines presented similar levels of adipogenesis and fat cell specific markers were measured via qPCR. See Figure S2A. Similar results were observed in more than three independent experiments. (A) Total RNA was isolated from the differentiated brown, beige and white clonal cell lines and was assayed for mRNA levels of brown fat-like genes (Ucp-1, Cidea and Cox7a1) by qPCR. (B) Total RNA was isolated from the differentiated brown, beige and white clonal cell lines treated with 10μM forskolin for 4 hours and was assayed for mRNA levels of Ucp-1. (C) Protein lysates were isolated from the differentiated brown, beige and white clonal lines either unstimulated or treated with 10 μM isoproterenol for 6 hours, and probed by immunoblot as indicated, with tubulin as a loading control. (D) Cell transplantations were done in the preadipose state as described in Experimental Procedures. After 6 weeks, fat pads were harvested and mRNA expression was analyzed by qPCR (n=5–8). For acute sympathetic stimulation, CL316,243, at 1mg kg−1, was injected intraperitoneally 5 hours before harvesting the transplanted fat pads. (E) Oxygen consumption in cultured brown, beige and white fat cells was assayed as described in Experimental Procedures. Oligomycin (ATP synthase inhibitor) was added to cells to measure the uncoupled respiration rate. The cells were treated with dibutyryl-cAMP for 12 hours before harvesting. Values are mean±SD (n=4). *, p<0.05; **, p<0.01; ***, p<0.001.
We next investigated the potential of these white and beige cells to express UCP1 in vivo, via transplantation experiments. Multiple beige and white inguinal lines can differentiate in vivo and form ectopic fat pads 4–6 weeks after transplantation into immunodeficient mice. Cidea, Cox7a1 and basal Ucp1 levels were comparable in transplanted fat pads derived from beige fat cell lines (X3 and X9) and white inguinal lines (N13 and X7) in the basal, unstimulated state (Figure 2D). To mimic sympathetic nervous system input, we treated mice with CL316,243, a selective β3-adrenergic agonist and harvested the transplanted fat pads 5 hours later. The fat pads from the beige lines displayed a 10–30 fold increase in Ucp-1 mRNA compared to basal level, whereas fat pads from white lines showed a 5 fold increase. Thus, these molecular differences in Ucp1 gene expression are a stable property of these cells and can be readily observed in an in vivo setting.
Mitochondrial respiration is central to adaptive thermogenesis. To assay the functional characteristics of these cells, we investigated respiratory function in representative lines of the white, beige and brown groups. In brown cells (bat1) without cAMP stimulation, uncoupled respiration (respiration insensitive to oligomycin) contributed to 78% of their basal respiration rate, significantly higher than the relative contribution of uncoupled respiration in the beige cells (X9, 60%). In the presence of cAMP, uncoupled respiration went up almost 2 fold in beige fat cells compared to a 1.2 fold induction in the brown fat cells. Importantly, the uncoupled respiration in the beige cells equaled or even exceeded that in the classical brown fat cells with the cAMP stimulation. The white cells (J6) had 53% of uncoupled respiration without stimulation and showed little cAMP-dependent increase in uncoupled respiration. Both the basal respiration and uncoupled respiration rates in the white fat cells are significantly lower than both the brown and beige fat cells in the presence of cAMP. These experiments revealed that the beige cells show high respiratory capacity and an uncoupled respiration that is even more cAMP-responsive that the brown cells (Figure 2E).
Beige fat cells express a unique gene expression profile
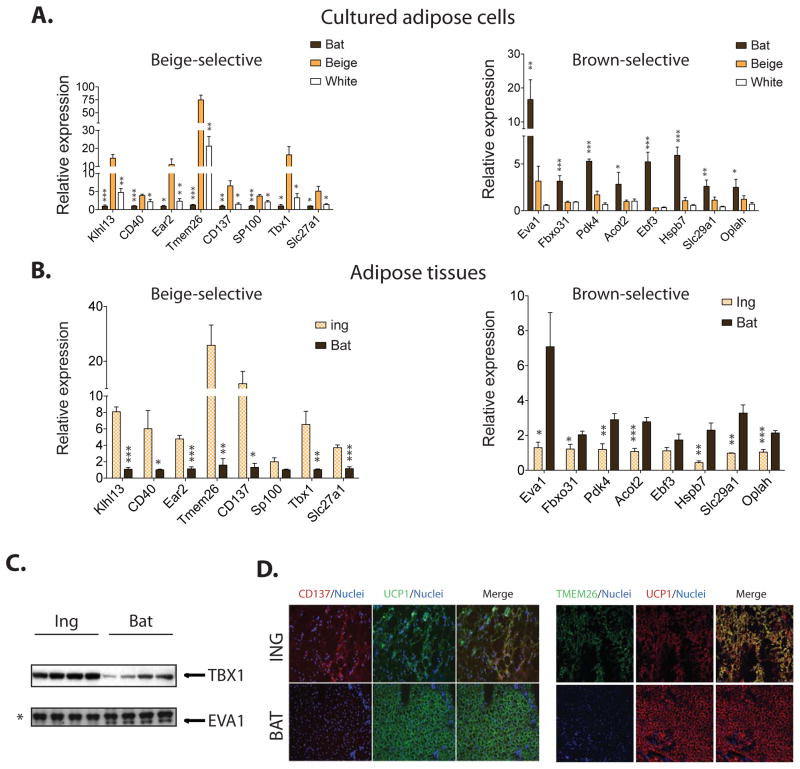
Analysis of differentially expressed genes between the beige and brown fat cells demonstrates that they have related but distinct gene expression profiles (Supplemental Experimental Procedures). The expression of a subset of the beige-selective and brown-selective genes was verified in the basal (not cAMP stimulated) state via qPCR analysis (Figure 3A). The genes whose expression is enriched in brown cell lines included markers identified in previous studies, such epithelial V-like antigen 1 (EVA1, also known as myelin protein zero-like 2, MPZL2) (Seale et al., 2007). Importantly, expression of certain beige-selective genes can distinguish beige fat cells from both brown fat cells as well as from the white cells derived from the inguinal depot. Figure 3A shows that genes expressed in a beige-selective manner include a developmental transcription factor (Tbx1), a component of lipid metabolism pathways (Slc27a1), as well as molecules known to be important in immune and inflammatory response pathways (CD40, CD137).
Figure 3. Beige and brown fat cells exhibit distinct gene expression profiles.
(A) Genes with a beige- or brown-selective expression pattern were first identified by microarray analysis as described in Supplemental Experimental Procedures. Relative mRNA expression for these genes was then measured by qPCR in differentiated brown, beige and white fat cells in an unstimulated state. (B) Analysis of beige- and brown-selective genes expression in adipose tissues isolated from inguinal WAT and interscapular BAT of 129 SVE mice. Values are mean±SD (n=5). *, p<0.05; **, p<0.01; ***, p<0.001. (C) Protein lysates of adipose tissues isolated from inguinal WAT and interscapular BAT of 129 SVE mice were probed with antibodies by immunoblot as indicated. Asterisk represents a nonspecific band as a loading control. (D) Microscopic and confocol images of mouse inguinal white and interscapular brown adipose tissue stained with antibodies recognizing CD137 (red) (microscopic images), TMEM26 (green) (confocal images) and UCP1 (green and red), with nuclei co-stained with DAPI as indicated. To induce the “browning” morphology and UCP1 expression in inguinal white adipose depot, 129SVE mice were injected intraperitoneally with CL316, 243, at 1mg kg−1 daily for 4 days. Inguinal and interscapular adipose tissues were harvested on the fifth day.
Since these gene signatures were established from the clonal cell lines, we returned to mice kept at ambient temperature to examine expression levels of putative beige- and brown-selective genes in the inguinal and interscapular adipose depots. Despite the inevitable heterogeneity in the fat tissues, mRNAs encoding beige fat cell markers such as TMEM26, CD137 and TBX1 were all expressed at higher levels in the inguinal fat depot, compared to the interscapular brown fat. Conversely Eva1 and other brown fat genes were expressed at higher levels in the interscapular brown fat depot (Figure 3B).
Antibodies were commercially available for some of these potential markers, including CD137, TMEM26 and TBX1 for beige cells, and EVA1 for brown cells. We confirmed that the protein expression was highly concordant with mRNA expression, as shown by immunoblot and immunohistochemistry in vivo (Figure 3C, 3D and Figure S3). Importantly, immunohistochemical analysis showed that the UCP1+ cells contained CD137 and TMEM26 from the inguinal depot but not from the interscapular brown fat depot. (Figure 3D and Figure S3)
Beige cell surface proteins can be used to select primary beige fat cell precursors
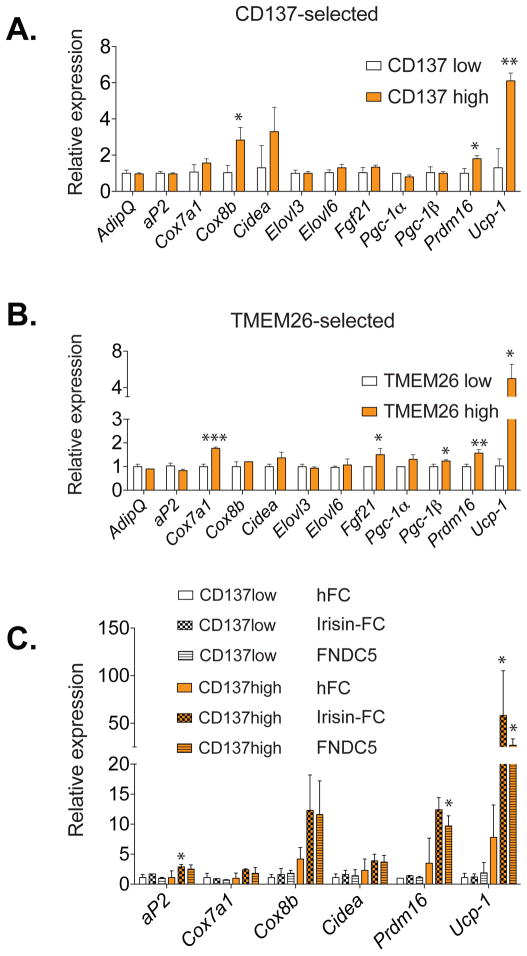
Some of the identified beige-selective markers are cell surface proteins, allowing us to use fluorescence-activated cell sorting (FACS) to separate primary cells from the stromal-vascular fraction (SVF) expressing high levels of CD137 or TMEM26 versus those expressing low levels of these proteins (Figure S4). While these cells differentiated equally well, the CD137+ group clearly show greatly elevated expression of Ucp-1, compared to the low CD137 cells (Figure 4A), in this unstimulated state. A very similar phenomenon was observed when we used another beige-selective marker, TMEM26, to sort these cells by FACS (Figure 4B). Many other thermogenic genes shown statistically significant or a trend towards enriched expression in beige-marker positive cells, including mitochondrial genes Cox7a1, Cox8b, transcriptional coregulators Prdm16, Pgc-1β and the thermogenic hormone Fgf21 (Figure 4A and 4B).
Figure 4. Isolation of beige precursor cells from inguinal stroma with beige-selective cell surface markers.
Total RNA was isolated from differentiated cultures of beige-selective marker CD137 (A) and TMEM26 (B) positive and negative cells purified from inguinal SV cells; these adipocytes were then assayed for mRNA levels of general adipogenic markers, Ucp-1 and other characteristic thermogenic/brown fat-like genes by qPCR. (C) Primary inguinal SV cells were purified as in (A) and differentiated into adipocytes for 6 days in the presence of FC fragment of human IgG (hFC) (100nM), fusion protein of irisin-FC (100nM) or recombinant FNDC5 (20nM). Total RNA was isolated and assayed for mRNA levels of general adipogenic marker aP2, thermogenic gene Ucp-1 and other brown-like genes by qPCR. Values are mean±SD (n=3). *, p<0.05, **, p<0.01, ***, p<0.001. Similar results were observed in more than three independent experiments.
We have recently shown that irisin, a polypeptide hormone secreted by muscle and increased with exercise, induced the “browning” of subcutaneous white adipose tissues; in contrast, this protein had little effect on the classical brown fat cells isolated from the interscapular depot (Bostrom et al., 2012). This differential regulation of a thermogenic program by irisin suggests that the response to this hormone could be a selective characteristic of beige cells. To test this hypothesis, we applied irisin, prepared either as a fusion protein with the FC fragment of human IgG, or FNDC5, the transmembrane precursor of irisin shown to be active in Bostrom et al, (2012) to the sorted primary inguinal precursor cells during differentiation. All cultures treated with irisin or vehicle differentiated well, with greater than 90% showing an adipocyte morphology and copious lipid droplets. As shown in Figure 4C, expression of Ucp-1 and other known brown-like genes such as Prdm16, Cox8b were robustly increased by both irisin-FC and FNDC5 treatments in the “CD137-high” expressing groups. Very little effect of these same polypeptides was observed on the expression of these genes in the “CD137-low” cellular population. These data strongly suggest that beige cell precursors have preferential sensitivity to the “browning” effects of irisin. It is also worth noting that expression of the fat cell gene and PPARγ target aP2 is slightly but significantly elevated in the irisin/FNDC5 treated primary beige adipocytes. Although both CD137 high and CD137 low cultures appeared to be very well differentiated, this data suggests that irisin might have subtle effects on the differentiation or PPARγ activity of the beige cell precursors.
Brown fat in adult humans has the molecular characteristics of murine beige rather than brown adipose cells
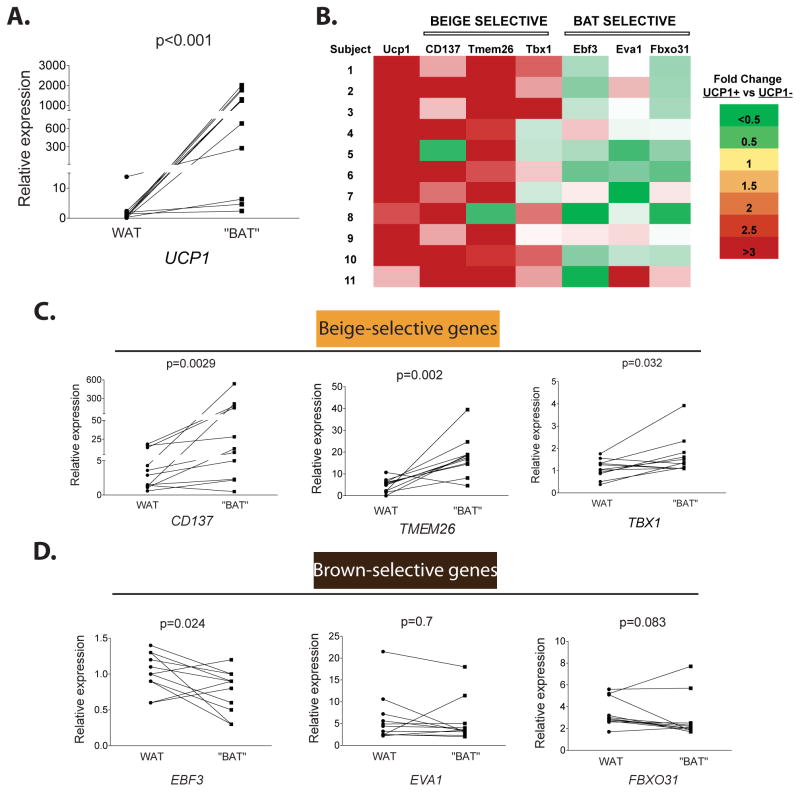
The gene expression signatures generated in this study provide a powerful tool for investigating the identity of the brown fat previously identified in adult humans. Human BAT biopsies from two independent cohorts of patients were analyzed for the gene expression levels of both beige- and brown-selective genes (Figure 5A, 5B and Figure S5). It was necessary to do this in a quantitative manner because all of the human brown fat biopsies were somewhat contaminated with visible white fat. Neighboring white fat that was free of detectable brown fat was also taken as a control. UCP1 mRNA expression levels were first determined to confirm BAT versus WAT identity; as expected, the UCP1 levels were several fold higher in putative BAT as compared to WAT samples (Figure 5A). The mRNA for genes characteristic of beige cells, such as CD137, TMEM26 and TBX1 were all found to be expressed at significantly higher levels in the human BAT versus WAT (Figure 5C); Conversely (and importantly), genes characteristic of the classical murine brown fat, such as EBF3, EVA1, and FBXO31, were not differentially expressed in the two tissues (Figure 5D).
Figure 5. “Brown” fat in human adults share molecular characteristics of murine beige rather than brown cells.
Expression of beige- and brown-selective genes in hWAT versus hBAT samples from total 11 subjects of two independent cohorts were measured by qPCR. (A) UCP1 expression levels were measured and confirmed to be higher in BAT as compared to WAT. (B) Heatmap summary of relative fold changes in expression of these genes in each subject. Relative gene expression level of 3 beige-selective (C), 3 brown-selective (D) are shown individually. P value was analyzed using Wilcoxom matched pairs signed ranks test.
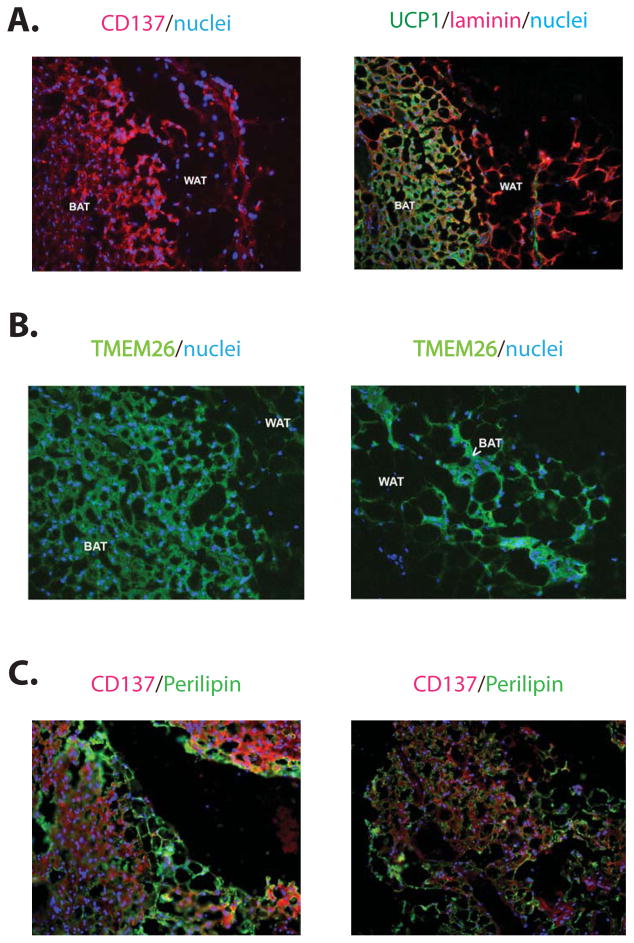
Since the biopsies of adult human BAT always contain a mixture of brown and white adipocytes, we also studied the molecular identity of these UCP1+ fat cells via immunohistochemistry. As shown in Figure 6A and 6B, UCP1-positive cells in these sections from the supraclavicular region also stained positively for the beige marker proteins CD137 and TMEM26. The WAT (perilipin-1+, UCP1−) in the neighboring areas does not express detectable CD137 (Figure 6C). Taken together, these results indicate that the brown adipose tissues identified in adult humans resemble murine beige fat much more closely than they resemble classical brown fat.
Figure 6. Beige selective markers are expressed in human “brown” fat cells residing in supraclavicular regions.
Microscopic images of adult supraclavicular adipose tissue stained with antibodies recognizing CD137 (red, A and C), UCP1 (green, A), TMEM26 (green, B) and perilipin (green, C) as indicated with nuclei stained with DAPI. It is worth noting that in (A) and (B) the expression of both CD137 and TMEM26 in multilocular (see the arrowhead in B), UCP1+ adipocytes (labeled with “BAT”), but the neighboring perilipin-1+, UCP1− adipocytes are CD137− (labeled with “WAT”, see C).
Discussion
The ability of brown fat to suppress obesity through increased energy expenditure has caused an explosion of interest in the development and function of brown adipocytes. The studies indicating that the brown fat cells that emerge in white fat depots come from a completely different cell lineage than those in the classical brown fat depots first suggested the possible existence of a new type of fat cell, termed beige or brite cells (Seale et al., 2008). This view has been strengthened by recent studies of primary cultures derived from epididymal adipose tissue, which showed that the thermogenic gene program expressed in response to PPARγ agonists was distinct from that stimulated in cultures of classical brown fat cells from the interscapular depot (Petrovic et al., 2010). Alternatively, this apparent morphological “transdifferentiation” from white fat to brown fat could, at least theoretically, represent a fundamental switch in cell identity (Himms-Hagen et al., 2000). To date, definitive evidence supporting either hypothesis has been lacking due to the heterogeneity of tissues in vivo and in primary stromal-vascular cultures.
Stem/progenitor cells have been reported to be isolated from different fat depots and skeletal muscle by FACS and their cell-intrinsic adipogenic capacities have been evaluated in the presence of various environmental cues (Joe et al., 2009; Joe et al., 2010; Lee et al., 2012; Rodeheffer et al., 2008; Schulz et al., 2011; Uezumi et al., 2010). Notably, Sca-1+/CD45-/Mac1- (referred as ScaPCs; (Schulz et al., 2011) and PDGFα+/CD34+/Sca-1+ (referred as PDGFRα+ cells; (Lee et al., 2012) have been suggested to have “brown” adipogenic potential. These studies use cell-surface markers to enrich for stem/progenitor cells that later commit for adipose lineage; however, knowledge about the molecular identity and functional phenotypes of these cells remain limited due to the heterogeneity of the cellular populations.
The studies presented here demonstrate that a subset of the precursor cells within subcutaneous adipose tissue give rise to beige cells, which are capable of expressing abundant UCP1 and a broad gene program that is distinct from either white or classical brown adipocytes. Roughly 40% of the differentiation-competent preadipocyte clones that we isolated from the subcutaneous cultures had the characteristics of beige cells. While 129 mice are rather prone to the “browning” of their white fat compared to certain other murine strains, these data certainly suggest that the beige adipocytes are not a rare cell type in the subcutaneous depots of mice. Further studies will help to answer important questions regarding the presence and/or abundance of beige precursor cells in various white fat depots of inbred mice and whether/how the beige precursor pool contributes to the metabolic status of these animals. It is tempting to speculate that at basal state, the beige precursor cells represent a higher percentage of adipose precursors in the subcutaneous depots than in the visceral depots. When animals are exposed to cold or receive chronic β-adrenergic stimulation, the pre-existing beige adipocytes (which may appear unilocular at the basal state) will go through phenotypic “transdifferentiation” and “browning” will appear morphologically and histochemically. . On the other hand, for the depots more resistant to “browning” (such as the abdominal WAT depots), beige precursor cells may have to go through a proliferation step before robust browning can take place. This notion is consistent with the observations that BrdU+, UCP1+ cells are abundant in the epididymal depot but not in the inguinal or retroperitoneal depots upon adrenergic stimulation (Himms-Hagen et al., 2000; Lee et al., 2012).
One important issue is how the beige cells differ functionally from the classical brown fat cells; the gene expression studies presented here give a first indication. The beige cells express very little of the thermogenic gene program, including UCP1, in the basal (unstimulated) state. In this regard they resemble bona fide white adipocytes. On the other hand, once stimulated, these cells activate expression of UCP1 to levels that are similar to those of the classic brown fat cells. Thus, the beige cells have the capability to switch between an energy storage and energy dissipation phenotype in a manner that other fat cells lack. It is worth noting that primary preadipose cells isolated by sorting for CD137 have a higher basal level of UCP1 than the beige immortalized cells. Whether this is a consequence of the immortalization procedure itself or is simply a function of the time apart from the β-adrenergic stimulation that takes place in vivo is not known. Further optimization of this protocol for isolation and purification of primary beige cells should provide a powerful tool for investigations of this cell type.
Infant humans have brown fat in the same interscapular location as the classical brown fat of rodents. This tissue disappears as humans mature and it is only recently that brown fat was fully recognized in adult humans (Cypess et al., 2009; Orava et al., 2011; Ouellet et al., 2012; van Marken Lichtenbelt et al., 2009; Virtanen et al., 2009). This brown fat is observed primarily in the supraclavicular and neck region, and along the spine. The cloning and subsequent gene expression analyses of the beige and brown rodent fat cells done here allowed us to critically determine the nature of the adult human BAT. Our data show that the selective molecular markers of beige fat cells, rather than those of the classical brown fat cells, are enriched in the human UCP1-positive tissues. If these UCP1-positive cells are functionally similar to the rodent beige cells, this might explain why a relatively low proportion of humans show PET-positive fat deposits until activated with a brief cold exposure. The existence of beige fat cells may represent an evolutionarily conserved cellular mechanism to provide flexibility in adaptive thermogenesis. It is likely that these beige adipocytes, rather than the classical brown fat cells, remain present in the adult state of larger mammals where hypothermia is a less frequent threat than in rodents.
The therapeutic potential of activating brown fat-mediated thermogenesis in human has yet to be fulfilled. Trials of drugs that increase the β-adrenergic activation of BAT have not been successful in humans, due to either lack of efficacy or to intolerable side-effects due to activation of β-adrenergic receptors in other tissues (Collins et al., 2004; Whittle et al., 2012). Clearly there is a need to develop more specific means to activate brown fat in humans in more specific ways. Since irisin is an endogenous circulating molecule that mediates some of the benefits of exercise, and activates beige fat cells in rodents, it could represent one way to do this in humans. Other polypeptide hormones that brown white adipose tissues in rodents, such as FGF21(Fisher et al., 2012) and ANF(Bordicchia et al., 2012), might also prove useful in humans with obesity and diabetes.
Experimental Procedures
General Note
Primary mouse stromal-vascular fractions from adipose tissues were isolated and differentiated as described (Seale et al., 2011). Derivation of clonal cell lines via limiting dilution was performed as described (Gupta et al., 2010). For adipocyte differentiation assay, confluent cultures of clonal lines were exposed to induction DMEM/F-12 GlutaMAX™ (Invitrogen) containing dexamethasone(5 μM), insulin (0.5 μg ml−1), isobutylmethylxanthine (0.5 mM), rosiglitazone (1μM), T3 (1nM) and 10% FBS. Four days after induction, cells were maintained in media containing insulin (0.5 μg ml−1), T3 (1nM) and 10% FBS until they are ready for collection.
Cell transplantations
Immortalized clonal inguinal cells (3×107) were implanted subcutaneously into 7–9-week-old male NCR nude mice (Taconic, n=5–8 mice per group), accordingly to the methods described previously (Seale et al., 2007). Six weeks after injection, the fat pads were isolated for the analysis of gene expression. For acute sympathetic stimulation, CL316,243, at 1mg kg−1, was injected intraperitoneally 5 hours before harvesting the transplanted fat pads.
Oxygen consumption assays
At day 6 of differentiation, oxygen consumption was measured at 37°C in cultured fat cells using a Mitocell S200 micro respirometry system. For cyclic-AMP-induced respiration assays, fully differentiated fat cells were incubated with 1mM dibutyryl cyclic AMP for 12 hr before measuring oxygen consumption. 1μM oligomycin (Sigma-Aldrich) was added to block ATP production, rendering uncoupled respiration. Basal and uncoupled (i.e. oligomycin-insensitive) respiration rates were normalized by total cell numbers. Similar results were observed when the results were normalized by total cell protein amount.
FACS analysis
FACS experiments were carried out on a FACSAria cell sorter at Beth Israel Deaconess Medical Center Flow Cytometry Core Facility. Briefly, primary SVF were isolated from inguinal depot of male 129SVE mice (4–6 weeks old) housed at ambient temperature. Subconfluent SV cultures passaged 2–3 times following the initial fractionation were trypsinized, centrifuged, resuspended in 1% FBS/PBS for labeling. Antibody incubations were performed on ice for 30 min for primary and 20min for secondary. Cells were washed and resuspended for sorting. Cells were initially selected by size, on the basis of forward scatter (FSC) and side scatter (SSC), followed by exclusion of immune cells (APC labeled F4/80 or CD19 positive cells). Cells were gated on both SSC and FSC singlets, ensuring that the staining of individual cells was analyzed. Next, the cells were separated on the basis of the cell-surface markers indicated. The following antibodies were used: rabbit anti-CD137 (abcam, ab64836); rabbit anti-TMEM26 (IMGENEX, IMG-6633A); Monoclonal antibody F4/80 Rat anti-Mouse (Invitrogen, MF48000); Monoclonal antibody CD19 Rat anti-Mouse (Invitrogen, RM7700); F(ab′)2 Donkey Anti-Rabbit IgG PE(ebioscience, 12-4739-81); F(ab′)2 Anti-Rat IgG APC (ebioscience, 17-4822-82).
Human subjects
Human brown fat biopsies from two independent cohorts of patients were analyzed for expression of beige- and brown-selective genes. Seven are from University of Turku, Finland and four from Maastricht University, the Netherlands. Details on subjects and procedures are as described previously (Orava et al., 2011; van Marken Lichtenbelt et al., 2009). Briefly, the site of biopsies of supraclavicular adipose tissue was decided by the cold exposure [18F]FDG-PET/CT image that showed activated BAT. A subcutaneous WAT sample was collected from the same incision. All subjects gave written informed consent before taking part in the study.
Statistical Analysis
All results except human studies are expressed as means ± SD. Two-tailed Student’s t test was used to determine p values. Statistical significance was defined as p < 0.05. Gene expression levels in human WAT versus BAT were analyzed using Wilcoxom matched pairs signed ranks test.
Supplementary Material
Highlights.
A subset of cloned precursor cells within white fat give rise to beige adipocytes
Beige adipocytes have a highly inducible thermogenic capacity upon stimulation
Beige adipocytes express distinct genes and are sensitive to irisin
“Brown” fat in human adults is largely composed of beige adipocytes
Acknowledgments
We thank Y. Wang (Center for Cancer Computational Biology at Dana-Farber Cancer Institute) for assistance with affymetrix gene chip analysis, S. White (Beth Israel Deaconess Medical Center Histology Core) for cryosectioning and A. Korde for technical help. We are grateful to Drs. P. Seale, R. Gupta, P. Cohen, S. Kleiner and J. Lo for useful discussions. B.M.S. is supported by NIH grants DK31405 and DK90861 and a grant from the JPB Foundation. P.S. is supported by a VICI research grant for innovative research from the Netherlands Organization for Scientific Research. J.W. was supported in part by a postdoctoral fellowship from the American Heart Association (Founders Affiliate #09POST2010078) and a Scientist Developmental Grant from the American Heart Association (National Center, #12SDG8070003).
Footnotes
Author Contributions J.W. and B.M.S. conceived and designed the experiments. J.W., P.B., L.M.S., L.Y., J.H.C., A.G., M.K., G.S. and J.H. performed experiments. All authors analyzed the data. P.N., W.D.M.L. and S.E. provided reagents and samples, and J.W. and B.M.S. wrote the manuscript.
Author Information: B.M.S and S.E. are shareholders and consultants to Ember Therapeutics, which has licensed the rights to irisin from the DFCI.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Billon N, Dani C. Developmental Origins of the Adipocyte Lineage: New Insights from Genetics and Genomics Studies. Stem Cell Rev. 2011 doi: 10.1007/s12015-011-9242-x. [DOI] [PubMed] [Google Scholar]
- Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinti S. Adipocyte differentiation and transdifferentiation: plasticity of the adipose organ. J Endocrinol Invest. 2002;25:823–835. doi: 10.1007/BF03344046. [DOI] [PubMed] [Google Scholar]
- Collins S, Cao W, Robidoux J. Learning new tricks from old dogs: beta-adrenergic receptors teach new lessons on firing up adipose tissue metabolism. Mol Endocrinol. 2004;18:2123–2131. doi: 10.1210/me.2004-0193. [DOI] [PubMed] [Google Scholar]
- Collins S, Daniel KW, Petro AE, Surwit RS. Strain-specific response to beta 3-adrenergic receptor agonist treatment of diet-induced obesity in mice. Endocrinology. 1997;138:405–413. doi: 10.1210/endo.138.1.4829. [DOI] [PubMed] [Google Scholar]
- Coulter AA, Bearden CM, Liu X, Koza RA, Kozak LP. Dietary fat interacts with QTLs controlling induction of Pgc-1 alpha and Ucp1 during conversion of white to brown fat. Physiol Genomics. 2003;14:139–147. doi: 10.1152/physiolgenomics.00057.2003. [DOI] [PubMed] [Google Scholar]
- Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Penicaud L, Casteilla L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci. 1992;103(Pt 4):931–942. doi: 10.1242/jcs.103.4.931. [DOI] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesta S, Bluher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, Boucher J, Lewis C, Kahn CR. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci U S A. 2006;103:6676–6681. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest. 1998;102:412–420. doi: 10.1172/JCI3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Arany Z, Seale P, Mepani RJ, Ye L, Conroe HM, Roby YA, Kulaga H, Reed RR, Spiegelman BM. Transcriptional control of preadipocyte determination by Zfp423. Nature. 2010;464:619–623. doi: 10.1038/nature08816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himms-Hagen J, Cui J, Danforth E, Jr, Taatjes DJ, Lang SS, Waters BL, Claus TH. Effect of CL-316,243, a thermogenic beta 3-agonist, on energy balance and brown and white adipose tissues in rats. Am J Physiol. 1994;266:R1371–1382. doi: 10.1152/ajpregu.1994.266.4.R1371. [DOI] [PubMed] [Google Scholar]
- Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol. 2000;279:C670–681. doi: 10.1152/ajpcell.2000.279.3.C670. [DOI] [PubMed] [Google Scholar]
- Ishibashi J, Seale P. Medicine. Beige can be slimming. Science. 2010;328:1113–1114. doi: 10.1126/science.1190816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe AW, Yi L, Even Y, Vogl AW, Rossi FM. Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells. 2009;27:2563–2570. doi: 10.1002/stem.190. [DOI] [PubMed] [Google Scholar]
- Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koza RA, Hohmann SM, Guerra C, Rossmeisl M, Kozak LP. Synergistic gene interactions control the induction of the mitochondrial uncoupling protein (Ucp1) gene in white fat tissue. J Biol Chem. 2000;275:34486–34492. doi: 10.1074/jbc.M002136200. [DOI] [PubMed] [Google Scholar]
- Lee YH, Petkova AP, Mottillo EP, Granneman JG. In Vivo Identification of Bipotential Adipocyte Progenitors Recruited by beta3-Adrenoceptor Activation and High-Fat Feeding. Cell Metab. 2012;15:480–491. doi: 10.1016/j.cmet.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirbolooki MR, Constantinescu CC, Pan ML, Mukherjee J. Quantitative assessment of brown adipose tissue metabolic activity and volume using 18F-FDG PET/CT and & beta3-adrenergic receptor activation. EJNMMI Res. 2011;1:30. doi: 10.1186/2191-219X-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T, Scheinin M, Taittonen M, Niemi T, Enerback S, et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 2011;14:272–279. doi: 10.1016/j.cmet.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Ouellet V, Labbe SM, Blondin DP, Phoenix S, Guerin B, Haman F, Turcotte EE, Richard D, Carpentier AC. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest. 2012;122:545–552. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285:7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, Cerletti M, McDougall LE, Giorgadze N, Tchkonia T, et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci U S A. 2011;108:143–148. doi: 10.1073/pnas.1010929108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121:96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro GJ, Green H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- Vegiopoulos A, Muller-Decker K, Strzoda D, Schmitt I, Chichelnitskiy E, Ostertag A, Berriel Diaz M, Rozman J, Hrabe de Angelis M, Nusing RM, et al. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science. 2010;328:1158–1161. doi: 10.1126/science.1186034. [DOI] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- von Heydebreck A, Huber W, Poustka A, Vingron M. Identifying splits with clear separation: a new class discovery method for gene expression data. Bioinformatics. 2001;17(Suppl 1):S107–114. doi: 10.1093/bioinformatics/17.suppl_1.s107. [DOI] [PubMed] [Google Scholar]
- Whittle AJ, Lopez M, Vidal-Puig A. Using brown adipose tissue to treat obesity - the central issue. Trends Mol Med. 2012;17:405–411. doi: 10.1016/j.molmed.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Xue B, Coulter A, Rim JS, Koza RA, Kozak LP. Transcriptional synergy and the regulation of Ucp1 during brown adipocyte induction in white fat depots. Mol Cell Biol. 2005;25:8311–8322. doi: 10.1128/MCB.25.18.8311-8322.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B, Rim JS, Hogan JC, Coulter AA, Koza RA, Kozak LP. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J Lipid Res. 2007;48:41–51. doi: 10.1194/jlr.M600287-JLR200. [DOI] [PubMed] [Google Scholar]
- Young P, Arch JR, Ashwell M. Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Lett. 1984;167:10–14. doi: 10.1016/0014-5793(84)80822-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.