Abstract
Mannose binding lectin (MBL) mediated complement pathway is an important constituent of innate immune response in several infections including neuroinflammatory and neurodegenerative diseases. Although there are Enzyme-Linked Immunosorbent Assays (ELISAs) for estimating MBL, MBL-associated serine protease -2 (MASP-2) and functional MBL-MASP-2 (fMBL) proteins for the plasma, serum and cell supernatants there are no established methods for their estimation in the cerebrospinal fluid (CSF). We developed sensitive ELISAs for the detection of MBL, fMBL and MASP-2 in the CSF. First, we adapted standard ELISAs for the detection of these proteins in the CSF. Second, we used a biotinyl-tyramide based horseradish peroxidase (HRP) signal amplification for the sensitive detection of these proteins in the CSF. In summary, using modified ELISA and biotinyl-tyramide based HRP signal amplification, we successfully detected MBL, fMBL and MASP-2 proteins in the CSF samples with high sensitivity and reproducibility.
Keywords: Cerebrospinal Fluid, MBL, Functional MBL, MASP-2, ELISA
1. INTRODUCTION
Mannose-binding lectin (MBL), also called mannose-binding protein is coded by the MBL2 gene. MBL is an acute-phase protein synthesized by the liver and is released into the bloodstream where it recognizes and binds to mannose residues or carbohydrates on pathogens such as bacteria and viruses. MBL binding to the mannose residues on the surface of infectious agents activates the MBL-associated serine protease -2 (MASP-2) (Møller-Kristensen et al., 2003) which in turn triggers the complement activation pathway. Although these can be detected in plasma and serum samples, there are currently no established methods to estimate the expression of MBL, MASP-2 and functional MBL/MASP-2 (fMBL) in the cerebrospinal fluid (CSF) because of their low expression levels. Several studies failed to detect these proteins in the CSF (Munts et al, 2008; Terai I., 1997) except for one in Alzheimer’s disease that used an in-house ELISA protocol (Lanzrein et al., 1998). Developing sensitive ELISAs for the detection of MBL, fMBL and MASP-2 in the CSF will help in evaluating the lectin based innate and adaptive immune response in central nervous system and developing therapeutic targets and drug regimens in several brain infections, neuropinflammatory and neurodegenerative diseases like Alzheimer’s disease, multiple sclerosis and Huntington disease.
In order to develop ELISAs for the sensitive detection of MBL, MASP-2 and fMBL in the cerebrospinal fluids, as a first step we adapted the standard plasma/serum ELISA protocols for estimating these proteins in the CSF samples. Additionally, in the second step we further modified the CSF ELISA protocols for the sensitive detection of MBL, MASP-2 and fMBL using enzyme horseradish peroxidase (HRP) signal amplification system that had been used earlier for plasma, serum and cell supernatant samples (Bobrow et al., 1989; Bobrow et al., 1991).
2. MATERIAL AND METHODS
ELISA kits for MBL, functional MBL/MASP-2 (fMBL) and MASP-2 (Catalog #s HK323, HK327, HK326 respectively, Cell Sciences Inc. Canton, MA, USA) were used. These kits have been used for the detection of MBL (Kirkpatrick et al., 2006), fMBL (Petersen et al., 2001) and MASP-2 (Møller-Kristensen et al., 2003; Schlapbach et al., 2007) in plasma, serum and cell culture supernatant samples only. ELISA Tyramide-Signal Amplification System (ELAST) (Catalog no.NEP116001EA, Perkin Elmer Inc., Waltham, MA) was used to amplify the HRP signals from CSF ELISAs for all three proteins. Human CSF specimens with or without multiple sclerosis disease were obtained from the Human Brain and Spinal Fluid Resource Center, VA West Los Angeles Healthcare Center (Los Angeles, CA) sponsored by NINDS/NIMH, National Multiple Sclerosis Society and Department of Veterans Affairs. Results from twenty CSF samples from patients with multiple sclerosis disease and 7 control healthy individuals without any disease were analyzed in this study.
2.1. Development of ELISAs for the detection of MBL, fMBL and MASP-2 in cerebrospinal fluid
Samples and reagents were brought to room temperature (RT) at 18–25°C. Plate activation buffer, wash buffer, dilution buffer, binding buffer or tracer were diluted or reconstituted according to the recommended protocols. A general protocol for any of the three ELISA kits for MBL, fMBL and MASP-2 included the following steps.
Plate activation buffer (150 µl) was added to all the wells in an ELISA plate and was incubated for 30 minutes at RT. For MASP-2 kit plate activation step was not needed. During incubation, dilution series for MBL, fMBL and MASP-2 standards were made by diluting the provided respective standards in binding buffer. Eight polypropylene tubes, numbered 1–8 were used. Tube 8 was set aside with 500 µl binding buffer to be used as control value. Volume of standard (listed on batch control sheet in respective kits) was transferred to tube 1 and dilutions were made for tubes 2–7 as recommended by kit protocol. Tubes 1–8 were used as the standards. Standard curves for MBL included dilution series of 100, 40, 16, 6.4, 2.56, 1.02, 0.41ng/ml, for fMBL it included dilution series of 15, 9, 5.4, 3.2, 1.9, 1.2, 0.7, 0 U/ml) and for MASP-2, it included the dilution series of 100, 50, 25, 12.5, 6.3, 3.1, 1.6 ng/ml. The plate adhesive cover and wash wells were washed 4× with 200 µl wash buffer. A multichannel pipette was used to load 100 µl of each standard and sample, and an adhesive cover was applied to the ELISA plate and incubated for 1 hour at 37°C in dark. A multichannel pipette was used to aspirate standards/samples and washed with wash buffer as stated above. For fMBL assays only (not for MBL and MASP-2 assays) 100 µl diluted complement component 4 (C4) was added to each well. The ELISA plate was covered with adhesive cover and was incubated for 1 hour and 30 minutes at 37°C followed by wash with wash buffer as stated above. Diluted tracer (100 µl) (biotinylated antibody for MBL, MASP-2 assays or peroxidase conjugated antibody to human complement component 4 degradation fragment c (C4c) for fMBL assay) was added to all wells. The plate was covered with adhesive cover and incubated for 1 hour at RT followed by wash as stated above. Diluted streptavidin-peroxidase conjugate was added to each well, plate was covered and was incubated for 1 hour at RT followed by wash as stated above. TMB (Tetramethylbenzidine) substrate (100 µl) was added to wells. Plate was covered, kept in dark and was incubated at RT for 20–30 minutes. Finally, 100 µl stop solution was added to wells and read at 450nm by an ELISA reader.
2.2. Development of sensitive ELISAs for the detection of MBL, fMBL and MASP-2 in cerebrospinal fluid based on signal amplification
HRP signal amplification protocol integrated in the CSF ELISAs included treatment of samples with biotinyl-tyramide followed by streptavidin-HRP and substrate TMB addition steps. The ELISA was performed with 1:4 diluted CSF samples as usual up to streptavidin-peroxidase conjugate step and subsequent washing. No substrate was added. Ten ml of Biotinyl-Tyramide Working Solution (BTWS) was prepared by adding 5 ml of 2× Amplification Diluent Concentrate to 5 ml deionized water (1:1) and subsequently 100 µl of Biotinyl-Tyramide Solution was added in 10 ml of Amplification Diluent. Since 100 µl per well was required, 10 ml was sufficient for a complete 96-well plate.
BTWS (100 µl) was added to each well and was incubated for 10 minutes at RT. The wells were washed 4× with PBS-T (PBS-Tween 20). The Streptavidin-HRP was diluted 1:500 (2 µl/ml) in 1% BSA-PBS-T and 20 µl Streptavidin-HRP was used in 10 ml of 1% BSA-PBS-T. Streptavidin-HRP (100 µl) was added to each well and incubated for 5 minutes at RT followed by Wash 4× with PBS-T. TMB substrate (100 µl) was added to the wells, plate was covered, kept in dark and was incubated at RT for 20–30 minutes. Stop solution (100 µl) was added to the wells and read at 490 nm by an ELISA reader.
3. RESULTS AND DISCUSSION
Our results show that we successfully detected MBL, fMBL and MASP-2 in the CSF samples by modified ELISA protocols and further increased the sensitivity of these assays by using biotinyl-tyramide based horseradish peroxidase (HRP) enzyme signal amplification.
The standard curves for MBL, fMBL and MASP-2 from the optimized standard ELISA and ELISAs after HRP signal amplification in the CSF were strikingly similar (Figure 1) suggesting that there were no quantitative differences in two assays with or without the signal amplification. Additionally, coefficient of correlations were similar in the standard MBL ELISA (r2 = 0.9958) and after signal amplification (r2 = 0.9966) (Figure 1). For the fMBL assays, coefficient of correlations were comparable for ELISA (r2 = 0.9989) and after signal amplification (r2 = 0.9982) protocols. For the MASP-2 assays, standard ELISA and ELISAs after signal amplification ELAST had similar coefficients of correlations (r2 = 0.9989 and 0.9984 respectively). Three users performed these CSF assays and user variations were between 5–10% for MBL, fMBL and MASP-2. Inter-assay and intra-assay variations of 5–10% and 2–5% respectively of reported concentrations in this manuscript were observed by triplicate determinations of the MBL, fMBL, MASP-2 concentrations in 3–6 CSF samples.
Figure 1.
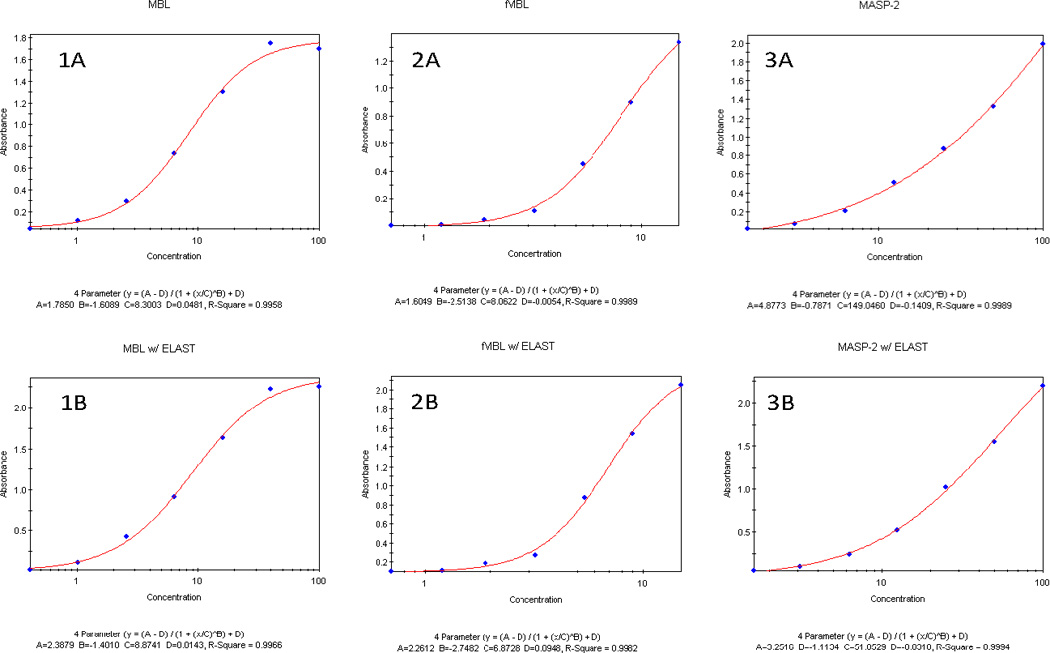
Comparative standard curves for quantifying MBL, functional MBL/MASP-2 (fMBL) and MASP-2 in the plasma by optimized standard ELISAs using Cell Sciences/ Hycult kits (panels 1A, 2A, 3A) compared to ELISAs of CSF after HRP signal amplification with ELAST reagent (panels 1B, 2B, 3B) respectively.
After the integration of HRP signal amplification step in the CSF ELISAs, concentration range of 1.64 – 56.58 ng/ml for MBL (only 5–7 ng/ml detected in Terai et al) , 4.86 – 30.95 U/ml for fMBL and 21.70 – 44.27 ng/ml concentrations for the MASP-2 were observed respectively with a standard variation of 5–10%. With the signal amplification protocol, we could use 1:4 dilutions, thus saving precious CSF samples.
The HRP signal amplification is based on the catalyzed reporter deposition technology (Bobrow et al., 1989; Bobrow et al., 1991) where the ELISA reporter enzyme, HRP, catalyzes the covalent binding of biotinyl-tyramide to the solid surface of a well and subsequent addition of sreptavidin-HRP cause additional HRP to bind to the solid phase leading to signal amplification on addition of the chromogenic TMB substrate. To our knowledge, this is the first report of applying this method for sensitive detection of MBL, fMBL and MASP-2 in the cerebrospinal fluid samples.
In conclusion, using modified ELISA assay with biotinyl-tyramide based HRP signal amplification we successfully detected MBL, fMBL and MASP-2 proteins in the cerebrospinal fluid samples with high reproducibility and sensitivity.
ACKNOWLEDGEMENTS
This work was supported in part by NIMH/NIH (5R01MH085608) and California HIV/AIDS Research Program IDEA Grant (ID07-SD-160). KKS conceived, designed and supervised the study, participated in the assays and analyses; and drafted and finalized the manuscript for publication. JYK optimized the assays for MBL, fMBL and MASP-2. RMA and DYY carried out several MBL, fMBL and MASP-2 assays with the CSF samples. This paper is subject to the NIH Public Access Policy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bobrow MN, Harris TD, Shaughnessy KJ, Litt GJ. Catalyzed reporter deposition, a novel method of signal amplification. Application to immunoassays. J. Immunol Methods. 1989;125:279–285. doi: 10.1016/0022-1759(89)90104-x. [DOI] [PubMed] [Google Scholar]
- Bobrow MN, Shaughnessy KJ, Litt GJ. Catalyzed reporter deposition, a novel method of signal amplification. II. Application to membrane immunoassays. J Immunol Methods. 1991;137:103–112. doi: 10.1016/0022-1759(91)90399-z. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick BD, Huston CD, Wagner D, Noel F, Rouzier P, Pape JW, Bois G, Larsson CJ, Alston WK, Tenney K, Powden C, O'Neill JP, Sears CL. Serum mannose-binding lectin deficiency is associated with cryptosporidiosis in young Haitian children. Clin Infect Dis. 2006;43:289–294. doi: 10.1086/505396. [DOI] [PubMed] [Google Scholar]
- Lanzrein AS, Jobst KA, Thiel S, Jensenius JC, Sim RB, Perry VH, Sim E. Mannan-binding lectin in human serum, cerebrospinal fluid and brain tissue and its role in Alzheimer's disease. Neuroreport. 1998;9:1491–1495. doi: 10.1097/00001756-199805110-00045. [DOI] [PubMed] [Google Scholar]
- Møller-Kristensen M, Jensenius JC, Jensen L, Thielens N, Rossi V, Arlaud G, Thiel S. Levels of mannan-binding lectin-associated serine protease-2 in healthy individuals. J Immunol Methods. 2003;282:159–167. doi: 10.1016/j.jim.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Munts AG, Zijlstra FJ, Nibbering PH, Daha MR, Marinus J, Dahan A, van Hilten JJ. Analysis of cerebrospinal fluid inflammatory mediators in chronic complex regional pain syndrome related dystonia. Clin J Pain. 2008;24:30–34. doi: 10.1097/AJP.0b013e318156d961. [DOI] [PubMed] [Google Scholar]
- Petersen SV, Thiel S, Jensen , Steffensen R, Jensenius JC. An assay for the mannan-binding lectin pathway of complement activation. J Immunol Methods. 2001;257:107–116. doi: 10.1016/s0022-1759(01)00453-7. [DOI] [PubMed] [Google Scholar]
- Schlapbach LJ, Aebi C, Otth M, Leibundgut K, Hirt A, Ammann RA. Deficiency of mannose-binding lectin-associated serine protease-2 associated with increased risk of fever and neutropenia in pediatric cancer patients. Pediatr. Infect. Dis. J. 2007;26:989–994. doi: 10.1097/INF.0b013e31811ffe6a. [DOI] [PubMed] [Google Scholar]
- Terai I, Kobayashi K, Fujita T, Hagiwara K. Human serum mannose binding protein (MBP): development of an enzyme-linked immunosorbent assay (ELISA) and determination of levels in serum from 1085 normal Japanese and in some body fluids. Biochem. Med. Metab. Biol. 1993;50:111–119. doi: 10.1006/bmmb.1993.1052. [DOI] [PubMed] [Google Scholar]
- Terai I, Kobayashi K, Matsushita M, Fujita T. Human serum mannose-binding lectin (MBL)-associated serine protease-1 (MASP-1): determination of levels in body fluids and identification of two forms in serum. Clin. Exp. Immunol. 1997;110:317–323. doi: 10.1111/j.1365-2249.1997.tb08334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


