Abstract
Coffin-Siris syndrome (CSS) is a rare, clinically heterogeneous disorder often considered in the setting of cognitive/developmental delay and 5th finger/nail hypoplasia. Due to the clinical variability of facial and other features, this diagnosis is often difficult to confirm clinically and the existence of this disorder as a specific diagnosis has been at times an issue of debate.
In an effort to further delineate the spectrum and key phenotypic features, we reviewed 80 previously reported cases to define features in patients that most closely correlated with a convincing diagnosis. There appear to be two subtypes of CSS, one which displays the “classic” coarse facial features previously described; another displays “variant” facial features which are less striking. Using these features, we defined an algorithm to rank the confidence of diagnosis and applied it to fifteen additional patients who had been previously characterized by chromosome microarray. This approach will also facilitate uniform categorization for whole-exome analysis.
Keywords: Coffin-Siris syndrome, fifth digit hypoplasia, developmental delay, cognitive delay, algorithm
INTRODUCTION
Coffin and Siris [1970] first described three unrelated individuals with severe mental retardation, delayed growth, and hypoplastic fifth digit nails and phalanges. Since then, approximately 80 individuals have been reported in the literature with overlapping phenotypes. However, considerable difficulty lies in the diagnosis of patients with CSS due to the variability of the phenotype. Many of the features seen in patients diagnosed with CSS are not specific to the syndrome, and can be seen in other disorders with multiple congenital anomalies and dysmorphic facial features. To assist in clarifying the diagnosis, Fleck et al [2001], proposed minimal clinical criteria for the diagnosis of CSS. These criteria include some degree of developmental delay, hirsutism, coarse facial features, and hypoplastic terminal phalanges or nails to the fifth digits of the hands or feet.
Unfortunately, to date there have been no consistent clinical criteria to assist in the diagnosis. While several reports have postulated specific gene regions in isolated patients with chromosome anomalies that cause coarse features, cognitive delay, and hypoplastic fifth digits, no consistent candidate regions or genes have been elucidated. Here we describe an additional 15 patients who were referred with a consideration of CSS. These patients serve to illustrate the difficult elements of making this diagnosis. To help categorize these patients as well as the larger diagnostic group of CSS, we performed a detailed assessment of previously reported patients in the literature and developed an algorithm to facilitate diagnosis and categorization. We then evaluated these 15 additional patients to assess the utility of this tool. This analysis suggests that many patients who are considered for the diagnosis of CSS should have additional diagnostic considerations, and that many of the published cases seem to fit into one of two subcategories that we have outlined below. Although intended for categorization for gene identification through potential exome sequencing, this algorithm may also be beneficial to clinicians to determine the likelihood of the diagnosis of CSS.
METHODS
Human Subjects
All individuals enrolled in the study were suspected by clinical geneticists to have a diagnosis of Coffin-Siris syndrome. All patients and family members were enrolled in the study under an Institutional Review Board-approved protocol of informed consent at The Children’s Hospital of Philadelphia.
Genome-wide copy number analysis
Whole genome SNP genotyping was performed using Illumina (San Diego, CA) Infinium HumanHap550 Beadchip arrays according to the manufacturer protocols. Copy number calling was performed using custom algorithms [Shaikh et al., 2009] and PennCNV [Wang et al., 2007]. Inspection of copy number variants was performed for 8q24 by analyzing allele frequency and log R ratio values using Illumina BeadStudio (ver. 3.1.3).
Mutation Screening
Candidate genes were screened for mutations in the coding exons and intron-exon boundaries using PCR of genomic DNA followed by sequencing. Primers were designed using ExonPrimer [Lucarelli et al., 2006]. Primer sequences and PCR conditions are available upon request. Sequencing was performed using BigDye Terminator v3.1 cycle sequencing and analyzed on an ABI 3730 (Applied Biosystems, Carlsbad, CA).
Literature Review
Cases reported in the literature were identified using PubMed searches with the keywords “Coffin-Siris syndrome” or “fifth-digit syndrome.” Thirty-nine English language journal articles reporting 80 individuals with presumed or suspected Coffin-Siris syndrome were identified in the years 1970-2011. The individuals in these reports were then analyzed for the presence of the facial features and other physical findings listed in Table I; reports in which a particular feature was not mentioned were not included in that particular finding’s literature total. Categorization of individuals in the algorithm (Fig. 2) was derived from the analysis of the most prevalent facial features and other findings seen in the literature.
Table I. Prevalence of features in the literature and our cohort.
P – proband; AB – affected brother; PC – paternal cousin; S – sister; CDL # – Cornelia de Lange study number; IUGR – intrauterine growth retardation; SGA – small for gestational age; HEENT – head, ears, eyes, nose, throat; GU – genitourinary. (x/x) denotes individuals noted to have characteristic when feature is cited in report.
| Feature Gender |
Literature | 1P Male |
1AB Male |
1PC Female |
2P Male |
2S Female |
3P Female |
4P Male |
5P Female |
6P Female |
7P Male |
8P Female |
9P Male |
10P Male |
11P Male |
12P Female |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | ||||||||||||||||
| IUGR/SGA | 33/67 | - | + | + | - | + | + | + | ||||||||
| Failure to Thrive | 38/45 | + | + | + | + | + | ||||||||||
| Developmental Delay | 57/75 | + | + | - | + | + | + | + | + | + | + | + | + | |||
| Cognitive Delay | 42/45 | + | + | + | + | + | ||||||||||
| Hypotonia | 38/42 | + | + | + | + | |||||||||||
| Short Stature | 25/38 | + | + | + | + | + | ||||||||||
| Hypertrichosis | 70/75 | + | + | + | ||||||||||||
| Microcephaly | 31/54 | + | + | + | ||||||||||||
|
| ||||||||||||||||
| HEENT | ||||||||||||||||
| Craniofacial abnormalities | 4/4 | + | + | + | ||||||||||||
| Vision problems | 19/27 | + | - | + | ||||||||||||
| Ptosis | 17/28 | + | - | - | - | + | + | |||||||||
| Hearing loss | 8/21 | + | + | + | - | + | ||||||||||
| Ear anomalies | 33/42 | + | + | + | - | + | + | - | + | + | ||||||
| Periorbital fullness | 8/8 | |||||||||||||||
| Tear duct anomalies | 6/18 | + | ||||||||||||||
| Cleft Palate | 9/31 | - | - | - | - | - | - | - | - | - | - | - | ||||
| High palate | 19/25 | + | + | - | + | |||||||||||
| Dental anomalies | 23/24 | - | + | + | + | + | + | + | + | |||||||
| Sparse Scalp Hair | 48/70 | + | + | + | + | + | + | + | ||||||||
| Low Posterior Hairline | 5/6 | - | + | + | + | |||||||||||
|
| ||||||||||||||||
| Facial Features | ||||||||||||||||
| Long eyelashes | 23/25 | + | + | + | + | + | + | + | + | - | + | + | + | |||
| Bushy eyebrows | 51/66 | - | + | + | - | + | + | + | - | + | ||||||
| Depressed nasal bridge | 39/51 | - | + | + | - | + | + | + | - | - | + | |||||
| Anteverted nares | 7/7 | + | + | + | + | + | + | |||||||||
| Broad nasal tip/root | 38/51 | - | - | - | - | + | + | + | + | + | ||||||
| Coarse facies | 40/46 | - | - | - | - | + | + | + | ||||||||
| Wide mouth | 52/63 | + | - | - | - | + | + | + | ||||||||
| Full lip/Thick vermillion | 43/56 | - | - | - | - | + | + | + | + | |||||||
| Short Philtrum | 10/31 | - | + | - | + | - | ||||||||||
|
| ||||||||||||||||
| Gastrointestinal | ||||||||||||||||
| Intestinal ulcer | 8/11 | - | - | - | - | |||||||||||
| Intestinal malrotation | 2/4 | - | - | + | ||||||||||||
| Feeding difficulties | 49/59 | + | + | + | + | - | + | + | + | |||||||
|
| ||||||||||||||||
| Musculoskeletal | ||||||||||||||||
| Umbilical/Inguinal hernias | 15/30 | + | + | |||||||||||||
| Delayed Bone Age | 19/42 | |||||||||||||||
| Spinal abnormalities | 25/38 | + | ||||||||||||||
| Hypoplastic fifth finger/toe phalanx/nail | 74/80 | + | + | + | + | + | + | + | + | + | - | + | + | + | + | |
| Other digit/nail hypoplasia | 10/12 | + | + | |||||||||||||
| Hyperextensible/lax joints | 26/33 | + | ||||||||||||||
|
| ||||||||||||||||
| Other | ||||||||||||||||
| Recurrent infections | 36/45 | |||||||||||||||
| Renal anomalies | 8/28 | + | + | + | ||||||||||||
| GU anomalies | 15/35 | + | + | + | + | |||||||||||
| Seizures/tics | 10/27 | + | + | + | + | + | ||||||||||
| Agenesis of the corpus callosum | 7/30 | + | + | |||||||||||||
| Dandy-Walker malformation | 5/53 | |||||||||||||||
| Congenital Heart Disease | 31/68 | + | + | + | + | |||||||||||
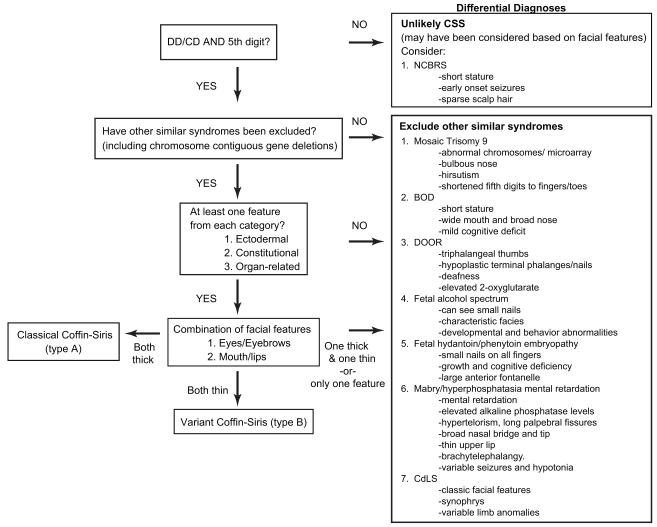
Figure 2.
Coffin-Siris diagnostic algorithm. DD = developmental delay; CD = cognitive delay; CSS = Coffin-Siris syndrome; BOD = brachymorphism-onychodysplasia-dysphalangism; DOOR = deafness, onychodystrophy, osteoodystrophy, and mental retardation; NCBRS = Nicolaides-Baraitser syndrome; CdLS = Cornelia de Lange syndrome
RESULTS
Clinical summary of patients
Many of the clinical features are summarized in Table I. Below we give brief descriptions of their clinical presentations.
Family 1 consists of three affected individuals from a Palestinian family, with two brothers and one female paternal cousin. The family history revealed several routes of consanguinity, tracing back three generations, suggesting recessive inheritance.
The proband was a 9-year-old male, born at 28 weeks gestation weighing 1515g, with a length of 42cm and a HC of 29cm (all + 2SD). Complications in the neonatal period included tracheo-laryngomalacia, a patent foramen ovale (PFO), genitourinary (GU) anomalies including scrotal hernia and glandular hypospadias, bilateral aplasia of the 5th distal phalanges of the hands toes, and hypoplasia of the distal phalanges of the 2nd fingers and the 4th and 5thtoes. He displayed hypotonia, hyperextensible joints, sparse scalp hair, long curved eyelashes, and profound growth and developmental delay. He had severe sensorineural hearing loss (70/80 dB). A metabolic work-up, including urine organic acids, as well as a karyotype were within normal limits. The patient died at 9 years of age due to unknown causes.
The proband’s 2-year-old younger brother was born at 28 weeks gestation after prenatal ultrasounds showed increased nuchal translucency. At birth he had severe microcephaly (23cm, −3.5SD) with a small triangular face, micrognathia, and hypoplastic distal phalanges of the 5th toes. The neonatal period was complicated by respiratory and feeding difficulties, with resultant placement of a gastrostomy tube. He also received antiepileptics for seizures. At 7 months he displayed global growth and developmental delay, with sparse scalp hair and eyebrows. He also had a high and narrow palate, thin upper lip vermillion, small, low-set ears, a small umbilical hernia, and GU anomalies which included a hydrocoele and hypospadias. Metabolic testing was normal. The patient died at 2.5 years of age, also of an unknown cause.
A 10-year-old paternal cousin was born at 35 weeks gestation with a birthweight of 1610g length of 41cm, and HC of 29cm (all −2SD). She had camptodactyly of the fifth fingers bilaterally, hypoplastic nails of the 5th toes, a pre-auricular skin tag, and a facial skin tag. Her neonatal period was complicated by feeding difficulties and GI reflux, with subsequent insertion of a gastrostomy tube. She additionally had multiple admissions for respiratory difficulties. Physical examination shortly after birth revealed microcephaly, hyperextensible joints, sparse scalp hair, and dysplastic, narrow auricles. Over time she was noted to have failure to thrive and developmental delay. Metabolic testing revealed no abnormalities. An echocardiogram and upper GI revealed no abnormalities.
This familial presentation could be consistent with autosomal recessive inheritance, which has been previously proposed [Haspeslagh et al., 1984; Bonioli et al., 1995; Flynn and Milunsky, 2006]. Single-nucleotide polymorphism (SNP) 550K array analysis of this family demonstrated a 758kb de novo duplication on chromosome 3p26.3 in the proband not seen in the other affected children. In addition, homozygosity mapping demonstrated 11 shared regions of 500kb or greater (on chromosomes 4, 12, 13, 16, and 17), with the only region shared among all three individuals involving a 591kb region on chromosome 12q24.11. Candidate genes in this shared region included Ubiquitin-protein ligase E3B (UBE3B) although sequencing of UBE3B revealed no mutations. Of note, this pattern of an affected paternal cousin could also demonstrate autosomal dominant inheritance with reduced penetrance, as has previously proposed as well [Flynn and Milunsky, 2006].
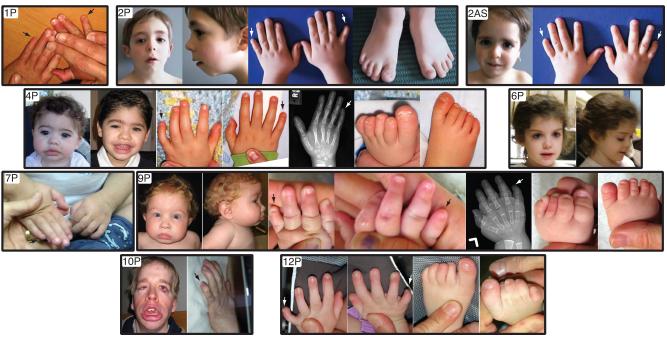
Family 2 consists of an Italian boy, now 14 years old, and his younger sister, now 12, with hypoplasia of the fifth fingers (Fig. 1). A similar pattern of digital hypoplasia was also noted in the father and paternal grandmother. In addition, the children were noted to have mild cognitive deficits not noted in other family members. A 550K SNP array of this family revealed a mosaic duplication on 2pter-p22.2 in only the proband, and a 2.3 Mb deletion on chromosome 20p12.3 in the proband, sister, father and paternal grandmother. This deleted region contains the gene BMP2, implicated in bone and cartilage formation [Johnson and Tabin, 1997] as well as brachydactyly [Dathe et al., 2009] and cleft palate [Sahoo et al., 2011]. Sequencing of BMP2 coding exons revealed no mutations. While the mild cognitive deficits appear to affect only the proband and sister, the 20p12.3 deletion seems appears to segregate with the digital anomalies, and the CSS features in these children may represent the overlap of two separate phenotypes.
Figure 1.
Photos of individuals. Illustrated are the varied facial features and hypoplastic fifth digits of the hands and/or feet. Radiographs of two probands are also shown, demonstrating the hypoplastic distal fifth phalanges of the hands. Individual patients photographs are outlined in a bold black line. Numbers in the photo correlate with those in the text and in Table I. P=proband, AS=affected sibling. Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.
Proband 3 was a female infant born at 35 weeks gestational age weighing 3100g (+ 2SD) with a length of 50.8cm (+2SD). She was noted prenatally with polyhydramnios and hydronephrosis, and postnatally had feeding difficulties requiring G-tube placement. Physical exam was significant for fine, arched eyebrow, long eyelashes, slight anteverted nares, a high, narrow palate with downturned corners of the mouth, a bifid uvula and retrognathia. She displayed bridged palmar creases bilaterally, as well as small 5th fingernails and absent 5th toenails. She died at 1 year of age after complications from a respiratory infection. A 610K SNP array was normal.
Proband 4 is a 7-year-old male, born at 40 weeks gestation with a birthweight of 3238 g (50%) and 50.4cm (75%). He has feeding difficulties necessitating tube feeds, laryngomalacia, hypotonia, a small VSD and ASD, and motor and language deficits. He sat at age 12 months and at 4 years was unable to walk or talk. Physical exam was significant for hypermobile joints, long, thick eyelashes, broad nasal bridge, and small, widely spaced teeth. He had small hands and feet, with hypoplastic nails of the 5th toes bilaterally and mild hypoplasia of the 5th digit fingernails. Bone age was not delayed (Fig. 1). A 550K SNP array was also normal.
Proband 5 is a 10-year-old Irish female with hyperactive behaviors, a poor sleep pattern, and developmental delay. She has seizures controlled by medication. She has wide-spaced teeth, sparse, curly hair, thick eyebrows, downturned mouth, and thick lips. Her back is hypertrichotic, and she had significantly delayed eruption of her secondary dentition, which did not appear until 9 years of age. She was ascertained through suspicion of CSS based on her facial features, and it is unclear is there were definitive digital anomalies present. A 660K SNP array was normal.
Proband 6 is a 6-year-old female with hyperactivity, absent speech and autistic features. She has a lower lip pit, thick curly hair, with sparseness in the temporal areas of the scalp, with hirsutism over other areas of her body. She has hypoplasia of the terminal phalanges of her hands. A 660K SNP array was normal.
Proband 7 is a 3-year-old boy with hypotonia, history of pericarditis, patent foramen ovale and patent ductus arteriosus, and mild hearing loss of an unknown nature. At 3 years, height was −5SD, weight was −3SD, and his head circumference was −1SD. He has sparse, dry hair, thick eyebrows, synophrys, long eyelashes, a wide mouth with thick, protruding lips, and thick auricles. He has a pectus excavatum and a hirsute back. As with proband 5, it was unclear if he displayed fifth digit anomalies (Fig. 1), but was referred for what appeared to be typical Coffin-Siris facial features. A 550K SNP array revealed a de novo 3Mb deletion on chromosome 6q25.3, as well as a region of homozygosity from 2q37.2-ter.
Proband 8 was a 4-year-old girl from the Philippines. She was born at 39 weeks gestation, with a birthweight 1800g (−4SD). At four years of age, she weighed 4600g (−6SD), was 67cm in height (−10SD), with a head circumference of 37.2cm (−4.5SD). She had sensorineural hearing loss, and at age 4 was unable to walk or talk. In addition, she displayed premature thelarche, with Tanner III breast development, without any development of adrenarche. She had microbrachycephaly, arched eyebrows with synophrys, long, thick eyelashes, sparse scalp hair, and posteriorly rotated ears with thickened helices. Her facial features included upturned/anteverted nares, a long, smooth philtrum with thin upper lip, downturned mouth, small teeth and micrognathia. She had both clinodactyly and camptodactyly of the 5th digits to the hand and syndactyly of digits 3-4 to her left hand. A 660K SNP array revealed a de novo 26Mb deletion from 2q24.3-2q35.2, as the cause of her features.
Proband 9 is a 2-year-old male with IUGR, seizures, strabismus, and feeding issues with GERD. He demonstrated severe developmental and growth delay. He is microbrachycephalic, with anteverted nares, a high-arched palate, thick vermillion of the lips, widely spaced teeth, and hypoplastic genitalia. Skeletal survey revealed absent or hypoplastic terminal phalanges of the hands and feet and most of the middle phalanges of the feet. X-rays showed a delayed bone age (Fig. 1). A 550K SNP array revealed a 523kb duplication on 9p23 inherited from his unaffected father.
Proband 10 is a 29-year-old male with significant developmental and cognitive deficits as well as profound growth delays. He was born at 41.5 weeks with a birthweight of 2380g (−3SD). He has a history of schwannomatosis. He has mixed hearing loss, ptosis to the right eye, and a history of GERD and gastric outlet obstruction. Facial features are significant for thick vermillion of the lips, coarse facies, long, thick eyelashes, low-set ears, broad nasal bridge, high palate with small, widely spaced teeth. The facial features appear to have coarsened significantly over time as compared to childhood photographs (Fig. 1). His back is hypertrichotic. His 5th digits to the hands and feet display absent terminal phalanges, present from birth. A 610K SNP array showed no pathogenic deletions or duplications.
Proband 11 is a 2-year-old male, born at 32 weeks to a 29 year-old G10P3 mother with a history of multiple prior miscarriages or IUFDs. He was born IUGR with a birthweight of 1136g (−2.5SD), and a length of 39.3cm (−1.5SD). He displayed micrognathia, a high arched palate, and sparse scalp hair. He had feeding difficulties resulting in gastrostomy tube placement, as well as an ASD, VSD, bilateral inguinal hernias, and a sacrococcygeal appendage. His left fifth finger was hypoplastic with an absent nail, and had absent fifth toenails bilaterally. He rolled at 6 months, and at 8 months could not sit independently. A 550K SNP array was normal.
Proband 12 is a 7-year-old female born with IUGR, intestinal malrotation and Meckel’s diverticulum, and eventually required a gastrostomy tube. She has a history of an inguinal hernia, ASD, VSD, congenital ptosis, and complex partial seizures. An MRI revealed complete absence of the corpus callosum. Physical exam is significant for anteverted nares, arched eyebrows, a long, smooth philtrum, thick eyebrows, and low-set posteriorly rotated ears. The nails on her 4th and 5th digits of her right hand and both of her 5th toes are hypoplastic (Fig. 1). At 4 years, she demonstrated simple sign language, and was able to walk with a walker. A 550K SNP array revealed a 559kb duplication containing no genes on chromosome 8q21.1, seen in her unaffected mother, and a 1.6Mb duplication on Xp21 from her unaffected father.
DISCUSSION AND REVIEW OF LITERATURE
Here we describe 15 individuals referred to us for possible CSS. Although all have features suggestive of this syndrome, like those reported in the literature, there is a large degree of clinical variability.
Furthermore, we identified several varied chromosomal aberrations in three patients, suggestive of a chromosomal basis of their features, rather than CSS as diagnosis. Literature reports may be limited to a resolution of 5 Mb secondary to karyotyping. Also consistent with published reports, none of these anomalies overlap from one family to another, nor do they overlap with anomalies from previously reported cases (Table II).
Table II.
Chromosomal changes in the literature and this cohort
| Chromosomal Change | Features consistent with CSS | Reference |
|---|---|---|
| Partial Trisomy 9 | Hypoplastic 5th digit nails Hirsutism Coarse facial features |
Kushnick and Adessa (1976) |
| t(1;7)(q21.3;q34), balanced | Coarse facial features GI disturbances Hypoplastic fifth toenails Absent fifth digit fingernails |
Mcpherson et al (1997) |
| t(7;22)(q32;q11.2), balanced | Feeding difficulties Microcephaly Hirsutism Hypoplastic fifth digits Coarse facial features |
McGhee et al (2000) |
| t(12;14)(q24;q32) | Coarse facial features Broad nasal bridge Hypoplastic nails of the hands Recurrent infections Developmental and cognitive delay Sparse scalp hair |
Patel et al (1987) |
| Dup 3p26.3 (758 kb) (de novo in proband only) Shared homozygosity on 12q24.11 (591kb; proband, brother, cousin) |
Sparse scalp hair Developmental/cognitive delay IUGR Hypoplastic 5th digits Feeding difficulties |
Family 1, Current report |
| Del 20p12.3 (2.3 Mb), inherited Dup 2p22.2-pter (39 Mb, mosaic, de novo in proband) |
Hypoplastic 5th digits Developmental delay |
Family 2, Current report |
| Del 6q25.3 (3.0 Mb), de novo Homozygosity 2q37.2ter (7.0 Mb) |
Hypoplastic 5th digits Developmental delay Hypotonia Wide mouth Hypertrichosis Ptosis Thick eyebrows Hearing loss Congenital heart disease |
Proband 7, Current report |
| Del 2q24.3-2q32.2 (26Mb, presumed de novo) |
Sparse scalp hair Hypoplastic fifth digits IUGR FTT Feeding difficulties Hearing loss Long eyelashes |
Proband 8, Current report |
IUGR – intra-uterine growth retardation; FTT – failure to thrive
While the genetic etiology of CSS is not known, literature reports propose that it follows Mendelian inheritance. Several translocations have been reported in individuals with CSS, and in particular, translocations involving 7q32-7q34 have been reported and postulated as a candidate region, although none of our families demonstrated anomalies in this region. [Patel et al., 1987; McPherson et al., 1997; McGhee et al., 2000]. Furthermore, both autosomal dominant [Flynn and Milunsky, 2006; Haspeslagh et al., 1984] and autosomal recessive [Bonioli et al., 1995; Flynn and Milunsky, 2006] modes of transmission have been proposed. Six literature reports describe familial cases [Carey and Hall, 1978; Franceschini et al., 1986; Flynn and Milunsky, 2006; Bonioli et al., 1995; Kirel et al., 2000; Haspeslagh et al., 1984]. A previous report of what was described as Coffin-Lowry syndrome now appears to be regarded as likely Coffin-Siris syndrome, involving two affected sisters and displaying a possible autosomal recessive inheritance pattern [Mattei et al., 1981].
To help define a specific set of features useful for the confirmation and categorization of these patients, we undertook an exhaustive review of the literature, looking at a combination of well-described, lesser reported, and some unique features that we observed in our cohort. The prevalence of these features in both the literature and our cohort are outlined in Table I.
Core features
In 2001, Fleck et al. performed patient surveys and reviewed the literature to establish a set of minimal diagnostic criteria for the CSS. They proposed these criteria were 1) some degree of developmental delay, 2) hypertrichosis, 3) coarse facial features, 4) hypoplastic or absent fifth digit nails, and 5) hypoplastic 5th digits.
Digital anomalies
In our experience, as well as reviewing the literature, hypoplasia or absence of fifth digit nails or phalanges appears to be the salient feature, which prompts clinicians to consider the diagnosis of CSS. Almost all individuals reported in the literature to date display some degree of fifth finger or toe anomalies. While this finding alone may not lead the clinician to suspect CSS, this unusual feature in addition to one or more of the main findings is strongly suggestive of CSS (see Fig. 2).
Developmental Deficits
The degree of developmental deficits found in patients with CSS ranges, but global developmental deficits usually present in the moderate to severe range. Several reviews [Fleck et al., 2001; Swillen et al., 1995], found that expressive language delay was more affected than receptive language, and that their IQs ranged from 40-69, with one individual at 97. Children averaged sitting at 12 months, walking at 30 months, speaking their first word at 24 months, and attaining skills involved with daily aspects of living around early school age. Autism also has been described [Hersh et al., 1982] although additional individuals with cognitive or developmental abnormalities may display autistic features that are components of their development changes. Although all the individuals reported to date appear to have developmental or cognitive deficits, this may represent a selection bias rather than an inherent feature, since those without any delays may not present to medical care, nor would CSS be considered.
Hair findings
Hypertrichosis/hirsutism is seen frequently in literature cases (93%). Thick eyebrows and long eyelashes are also frequent findings, and as described later, along with thick lips, comprise the “classical/Type A” CSS subtype as described below. Paradoxically, sparse hair to the temporal and frontal areas of the scalp is also a common finding. Thin eyebrows, although less common, appear in the “variant/Type B” subtype, with thin lips.
Facial Features
Individuals with CSS have been described as having “coarse” facial features, with increased thickening of the skin and loss of normal facial angulation. Specifically in the literature, these include a broad nose with depressed nasal bridge, thick lip vermillion, and overall thickening of the face. Other facial features that have been described include abnormally formed or placed ears, a wide mouth, ptosis, and a short philtrum. The absence or milder expression of these features does not exclude a diagnosis of CSS; rather it may be indicative of the heterogeneous phenotype. After reviewing all the individuals described and photographed in the literature, the following pattern has emerged: thick eyebrows and thick lip vermillion tend to appear together, and we have classified these individuals as “classical” CSS (Type A). Thin lip vermillion (particularly upper) and “penciled” eyebrows also appear together, and we have classified these patients as “variant” (Type B) CSS. The significance of these facial findings for diagnosis is elaborated below (Fig. 2).
Additional features
The following features are described in many individuals with CSS, but are not crucial for the diagnosis. Percentages of individuals reported in the literature with a given feature are described, if the case reports describe the presence or absence of that feature.
Neurologic
The neurological anomalies seen in CSS consist of both brain and skull anomalies. Microcephaly is seen in the majority of the individuals in the literature (60%). Gross abnormalities of the brain include Dandy-Walker variant (10%), [Fleck et al., 2001; Imai et al., 2001], simplification of the gyri [DeBassio et al., 1985] and agenesis of the corpus callosum [Coulibaly et al., 2010] (23%). Microscopic abnormalities of the brainstem and cerebellum [DeBassio et al., 1985] and disorganization of the neurons in the cerebral cortex [Coulibaly et al., 2010] have also been reported.
Endocrine and Growth
Endocrine abnormalities are rare, but include individual reports of hypoglycemia without other laboratory abnormalities [Imaizumi et al., 1995], premature thelarche [Brunetti-Pierri et al., 2003; Flynn and Milunsky, 2006] and pituitary hypoplasia with growth hormone deficiency [Baban et al., 2008]. While Proband 8 also displays premature thelarche, the other phenotypic characteristics do not appear to overlap with the probands described in the literature with thelarche. Additionally, if the proband featured in the Flynn and Milunsky [2006] report were to be analyzed with our current algorithm (below), the features would not meet our defined criteria for classification of CSS. The short stature (66%) in other individuals with CSS has not been attributable to hormone deficiencies. Many of the patients reported are IUGR at birth (50%), with subsequent weights and heights below the 5th centile or reported as failure to thrive (84%).
Gastrointestinal
Gastrointestinal anomalies frequently involve difficulty feeding and swallowing (83%), often necessitating a gastrostomy or permanent feeding tubes. Anomalies within the gastrointestinal system that have been reported include diaphragmatic hernia [Delvaux et al., 1998] neonatal intussusceptions [Coffin and Siris, 1970], partial gastric outlet obstruction [Bodurtha et al., 1986] and one individual with multiple gastrointestinal malformations, including a redundant duodenum and heterotopic pancreas [Kellermayer et al., 2007].
Hearing loss and vision anomalies
Hearing loss was reported in approximately 30% of cases that described the quality of hearing in the literature. Additional HEENT anomalies include choanal atresia [de Jong and Nelson, 1992] and a variety of ocular anomalies. A review of ophthalmologic abnormalities reported in the literature from between 1970 and 1985 and found vision anomalies affecting approximately 70% of those individuals studied, with the most common anomalies including strabismus, nystagmus, and cataracts [Pallotta, 1985].
Other features
There are rare reports of malignancies within the CSS population; these include neuroblastoma [Pollono et al., 2009] and medulloblastoma [Rogers et al., 1988].
GU anomalies that are frequent include hypospadias and undescended testes (43%); internal reproductive anomalies are rare, but have been reported [Goyal et al., 2010]. Renal anomalies include horseshoe kidneys or other malformations (28%).
Differential Diagnosis
Due to the difficulty of the diagnosis and the number of features that overlap with other diagnoses, we feel that the diagnosis of CSS requires the consideration of several specific disorders.
The findings of hypoplastic fifth digit nails and fifth digits are one of the more unique features described in CSS, and likely draws the clinician to its possibility as a diagnosis. Several specific entities with 5th finger hypoplasia have been reported, including a patient with partial trisomy 9 whose features were similar to those seen in CSS, with a wide, bulbous nose, moderate hirsutism, and hypoplasia of the fifth finger and nails [Kushnick and Adessa, 1976].
Additional reports have outlined the similarities of CSS to Brachymorphism-Onychodysplasia-Dysphalangism (BOD) syndrome [Brautbar et al., 2009; Verloes et al., 1993; Ounap et al., 1998; Elliott and Teebi, 2000]. BOD is characterized by short stature, multiple tiny dysplastic nails, short fifth fingers, a wide mouth and broad nose, and mild intellectual deficit [Senior, 1971]. While many of the above features are shared in patients with CSS, the degree of cognitive deficits in CSS is typically significantly more severe than in BOD.
Due to similarities in the digital anomalies, DOOR syndrome (deafness, onychodystrophy, osteodystrophy, and “mental retardation”; OMIM #220500) should also be considered. Individuals felt to have DOOR syndrome have been reported to have triphalangeal thumbs, hypoplastic terminal phalanges, and/or nail anomalies. Probands have also been reported to display several systemic features seen in those thought to have CSS, including deafness and neurological abnormalities. Elevated levels of 2-oxyglutarate have been reported in proband’s with DOOR [Patton et al., 1987; Rajab et al., 2000], which may distinguish it from other entities.
Coarse facial features with hirsutism and organ anomalies warrant consideration of storage disorders, particularly the mucopolysaccharidoses, and infants who are IUGR or FTT, in addition to those with multiple congenital anomalies, generate a wealth of differential diagnoses. Small nails can be seen in teratogenic conditions as well, including fetal alcohol syndrome (FAS) and fetal hydantoin syndrome secondary to phenytoin use; subsequently a thorough social history must be taken.
There are several cases in the literature where individuals have been reported to have syndromes resembling CSS, but not necessarily fitting typical features of the syndrome. Rabe et al [1991] reported two individuals who presented with delayed development, coarse facial features, a broad nose and anteverted nares, and hypoplastic fifth digits. They were both found to have elevated levels of alkaline phosphatase (>1000 U/Liter, normal < 600) unattributable to other causes. Subsequent reports of similar individuals [Thompson et al., 2010; Kruse et al., 1988; Gomes and Hunter, 1970] have identified this entity as Mabry syndrome (OMIM 239300). Mutations in the PIGV have recently been implicated as causative [Krawitz et al., 2010]. Based on this and other differential diagnoses for which there are associated lab findings, testing including metabolic labs (particularly urine organic acids), lysosomal studies, and alkaline phosphatase levels may be indicated to rule out these disorders.
Other syndromes with similar features include Cornelia de Lange syndrome (CdLS), characterized by distinctive facies, hirsutism, variable limb anomalies, and cognitive delay. Although hypoplastic fifth digit is not a classic limb finding in CdLS, the facial features and hirsutism may be difficult to distinguish from Coffin-Siris, especially in milder cases. 4q-syndrome is a chromosomal deletion syndrome in which individuals display a characteristic volar, curved fifth digit nail with or without accompanying fifth phalangeal hypoplasia.
Nicolaides-Baraitser syndrome, (NCBRS), (OMIM 601538), is arguably the most phenotypically similar to CSS; affected individuals display dysmorphic facial features, early-onset seizures, sparse scalp hair, short stature, and intellectual disability [Sousa et al., 2009]. The digit anomalies that have been reported include brachydactyly, but not hypoplastic digits or nails.
Summary of Diagnostic Approach
After reviewing the frequency of various findings in patients diagnosed with Coffin-Siris as well as individuals in our cohort, there appears to be a consistent spectrum of features. Fleck et al’s minimal criteria for the syndrome are thorough, but many of the children reported have facial features which are not necessarily coarse, or do not display hypertrichosis of the face and body. We note that the facial features seem to fall into two categories with a spectrum connecting them. This includes individuals at one end of the spectrum displaying thinner, “penciled” eyebrows, anteverted nares with a narrow, bulbous nasal tip, and a thin upper lip vermillion. At the other end, individuals display the characteristic bushy eyebrows, broad nose with broad nasal tip, and thick and fleshy lips. Patients may have varying degrees of coarseness as they fall in the spectrum. General body hypertrichosis, sparse scalp hair, and presence of major organ anomalies, as well as degree of developmental or cognitive delay do not seem to be independent of facial features.
Based on this insight from the literature, we have developed an algorithm derived from the most frequently seen features in the literature (Fig. 2). The two most consistent features of the syndrome are fifth finger phalanx/nail hypo/aplasia and cognitive or developmental deficits based on the current definitions of CSS. If either of these features is absent, we feel confident that a diagnosis of CSS can be functionally excluded.
Also, since the differential diagnosis for CSS can be broad, we encourage the clinician to exclude some specific overlapping syndromes for which there is available molecular testing, as well as performing a microarray to rule out small intragenic deletions or duplications.
Systemic features, categorized into constitutional, ectodermal, and organ-related, are also varied in patients diagnosed with CSS. The features for each sub-category are outline in table III. The presence of one or more systemic anomalies helps contribute to the certainty of the diagnosis of CSS, but absence does not necessarily exclude it. Subsequently, each feature is equal in weight. An individual needs to have at least one feature in each systemic category for the diagnosis of CSS.
Table III.
Classification of features
|
Facial Features
|
Eyes/Eyebrows:
|
| Systemic Features |
Ectodermal
|
Constitutional
|
Organ-related
|
IUGR- intrauterine growth retardation; FTT – failure to thrive; GI – gastrointestinal; GU - genitourinary
The facial features outlined in our algorithm have two distinct types. Coarser facial features, specifically thick eyebrows and thick lip vermillion, are seen most frequently in the literature; these characteristics fit with what we describe as subtype “classical/Type A” CSS, with other features such as thin eyebrows and thin upper lip vermillion weigh, under the subtype of “variant/Type B” end of the dysmorphic spectrum for the syndrome. If the individual demonstrates a combination of thick and thin features, or displays neither, the clinician should reconsider the possible diagnoses.
While the numbers are small, Family 1, would be classified as the “Type B/Variant” CSS using our algorithm. Family 2, would not fulfill diagnostic criteria based on our algorithm given the mild degree of reported cognitive delay in the proband and sister and none in the father and paternal grandmother.
The Coffin-Siris syndrome continues to be a diagnostic difficulty among clinicians. However, with increasingly advanced genomic technologies, specifically with the advent of whole exome sequencing, the molecular basis of CSS can potentially be elucidated. Here we have proposed an algorithm-based approach that will help define certainty of the diagnosis of CSS both in this era prior to understanding a molecular basis, as well as in the process of interpreting sequence variants as a cause of CSS, and beyond to help determine genotype-phenotype correlations.
ACKNOWLEDGMENTS
The authors are exceedingly grateful to the individuals and their families who participated in this study. The authors also acknowledge Adva Sadeh with Emek Medical Center and Ashleigh Wheatley with University of Manitoba and Children’s Hospital for their assistance in the acquisition of clinical information. No authors have reported any conflicts of interest. This work was supported, in part, by the following NIH grants: NIH/NICHD K08HD055488 (MAD), T32GM008638 (SAS) and by a grant from Tuscany Region (AM).
REFERENCES
- Baban A, Moresco L, Divizia MT, Rossi A, Ravazzolo R, Lerone M, De Toni T. Pituitary hypoplasia and growth hormone deficiency in Coffin-Siris syndrome. Am J Med Genet A. 2008;146:384–388. doi: 10.1002/ajmg.a.32111. [DOI] [PubMed] [Google Scholar]
- Bodurtha J, Kessel A, Berman W, Hartenberg M. Distinctive gastrointestinal anomaly associated with Coffin-Siris syndrome. J Pediatr. 1986;109:1015–1017. doi: 10.1016/s0022-3476(86)80288-8. [DOI] [PubMed] [Google Scholar]
- Bonioli E, Palmieri A, Bertola A, Bellini C. Autosomal recessive mode of inheritance of a Coffin-Siris like syndrome. Genet Couns. 1995;6:309–312. [PubMed] [Google Scholar]
- Brautbar A, Ragsdale J, Shinawi M. Is this the Coffin-Siris syndrome or the BOD syndrome? Am J Med Genet A. 2009;149:559–562. doi: 10.1002/ajmg.a.32671. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Esposito V, Salerno M. Premature thelarche in Coffin-Siris syndrome. Am J Med Genet A. 2003;121A:174–176. doi: 10.1002/ajmg.a.20158. [DOI] [PubMed] [Google Scholar]
- Carey JC, Hall BD. The Coffin-Siris syndrome: five new cases including two siblings. Am J Dis Child. 1978;132:667–671. doi: 10.1001/archpedi.1978.02120320027005. [DOI] [PubMed] [Google Scholar]
- Coffin GS, Siris E. Mental retardation with absent fifth fingernail and terminal phalanx. Am J Dis Child. 1970;119:433–9. doi: 10.1001/archpedi.1970.02100050435009. [DOI] [PubMed] [Google Scholar]
- Cohn DM, Pagon RA, Hudgins L, Schwartz CE, Stevenson RE, Friez MJ. Partial ATRX gene duplication causes ATR-X syndrome. Am J Med Genet A. 2009;149:2317–2320. doi: 10.1002/ajmg.a.33006. [DOI] [PubMed] [Google Scholar]
- Coulibaly B, Sigaudy S, Girard N, Popovici C, Missirian C, Heckenroth H, Tasei AM, Fernandez C. Coffin-Siris syndrome with multiple congenital malformations and intrauterine death: towards a better delineation of the severe end of the spectrum. Eur J Med Genet. 2010;53:318–321. doi: 10.1016/j.ejmg.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Dathe K, Kjaer KW, Brehm A, Meinecke P, Nurnberg P, Neto JC, Brunoni D, Tommerup N, Ott CE, Klopocki E, Seemann P, Mundlos S. Duplications involving a conserved regulatory element downstream of BMP2 are associated with brachydactyly type A2. Am J Hum Genet. 2009;84:483–492. doi: 10.1016/j.ajhg.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong G, Nelson MM. Choanal atresia in two unrelated patients with the Coffin-Siris syndrome. Clin Genet. 1992;42:320–322. doi: 10.1111/j.1399-0004.1992.tb03265.x. [DOI] [PubMed] [Google Scholar]
- DeBassio WA, Kemper TL, Knoefel JE. Coffin-Siris syndrome. Neuropathologic findings. Arch Neurol. 1985;42:350–353. doi: 10.1001/archneur.1985.04060040060012. [DOI] [PubMed] [Google Scholar]
- Delvaux V, Moerman P, Fryns JP. Diaphragmatic hernia in the Coffin-Siris syndrome. Genet Couns. 1998;9:45–50. [PubMed] [Google Scholar]
- Elliott AM, Teebi AS. New autosomal dominant syndrome reminiscent of Coffin-Siris syndrome and Brachymorphism-Onychodysplasia-Dysphalangism syndrome. Clin Dysmorphol. 2000;9:15–9. doi: 10.1097/00019605-200009010-00003. [DOI] [PubMed] [Google Scholar]
- Fleck BJ, Pandya A, Vanner L, Kerkering K, Bodurtha J. Coffin-Siris syndrome: review and presentation of new cases from a questionnaire study. Am J Med Genet. 2001;99:1–7. doi: 10.1002/1096-8628(20010215)99:1<1::aid-ajmg1127>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Flynn MA, Milunsky JM. Autosomal dominant syndrome resembling Coffin-Siris syndrome. Am J Med Genet A. 2006;140:1326–1330. doi: 10.1002/ajmg.a.31287. [DOI] [PubMed] [Google Scholar]
- Franceschini P, Silengo M Cirillo, Bianco R, Biagioli M, Guala A, Bell G Lopez. The Coffin-Siris syndrome in two siblings. Pediatr Radiol. 1986;16:330–333. doi: 10.1007/BF02386876. [DOI] [PubMed] [Google Scholar]
- Gomes WJ, Hunter JL. Mental retardation, cataracts, and unexplained hyperphosphatasia. Arch Dis Child. 1970;45:726–727. doi: 10.1136/adc.45.243.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal D, Yadav DK, Shukla U, Sethi SK. Coffin-Siris syndrome with Mayer-Rokitansky-Kuster-Hauser syndrome: a case report. J Med Case Reports. 2010;4:354. doi: 10.1186/1752-1947-4-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haspeslagh M, Fryns JP, van den Berghe H. The Coffin-Siris syndrome: report of a family and further delineation. Clin Genet. 1984;26:374–378. doi: 10.1111/j.1399-0004.1984.tb01074.x. [DOI] [PubMed] [Google Scholar]
- Hersh JH, Bloom AS, Weisskopf B. Childhood Autism in a female with Coffin Siris Syndrome. J Dev Behav Pediatr. 1982;3:249–252. doi: 10.1097/00004703-198212000-00016. [DOI] [PubMed] [Google Scholar]
- Imai T, Hattori H, Miyazaki M, Higuchi Y, Adachi S, Nakahata T. Dandy-Walker variant in Coffin-Siris syndrome. Am J Med Genet. 2001;100:152–155. doi: 10.1002/ajmg.1231. [DOI] [PubMed] [Google Scholar]
- Imaizumi K, Nakamura M, Masuno M, Makita Y, Kuroki Y. Hypoglycemia in Coffin-Siris syndrome. Am J Med Genet. 1995;59:49–50. doi: 10.1002/ajmg.1320590111. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Tabin CJ. Molecular models for vertebrate limb development. Cell. 1997;90:979–990. doi: 10.1016/s0092-8674(00)80364-5. [DOI] [PubMed] [Google Scholar]
- Kellermayer R, Kitagawa S, Redel CA, Cass DL, Belmont JW, Klish W. Upper gastrointestinal malformations in Coffin-Siris syndrome. Am J Med Genet A. 2007;143A:1519–1521. doi: 10.1002/ajmg.a.31865. [DOI] [PubMed] [Google Scholar]
- Kirel B, Kural N, Yakut A, Adapinar B. Triplets with growth failure, microcephaly, mental retardation, nail hypoplasia and corpus callosum agenesis: is it a variant of Coffin-Siris or a new syndrome? Turk J Pediatr. 2000;42:171–176. [PubMed] [Google Scholar]
- Krawitz PM, Schweiger MR, Rodelsperger C, Marcelis C, Kolsch U, Meisel C, Stephani F, Kinoshita T, Murakami Y, Bauer S, Isau M, Fischer A, Dahl A, Kerick M, Hecht J, Kohler S, Jager M, Grunhagen J, de Condor BJ, Doelken S, Brunner HG, Meinecke P, Passarge E, Thompson MD, Cole DE, Horn D, Roscioli T, Mundlos S, Robinson PN. Identity-by-descent filtering of exome sequence data identifies PIGV mutations in hyperphosphatasia mental retardation syndrome. Nat Genet. 2010;42:827–829. doi: 10.1038/ng.653. [DOI] [PubMed] [Google Scholar]
- Kruse K, Hanefeld F, Kohlschutter A, Rosskamp R, Gross-Selbeck G. Hyperphosphatasia with mental retardation. J Pediatr. 1988;112:436–439. doi: 10.1016/s0022-3476(88)80331-7. [DOI] [PubMed] [Google Scholar]
- Kushnick T, Adessa GM. Partial trisomy 9 with resemblance to Coffin-Siris syndrome. J Med Genet. 1976;13:237–239. doi: 10.1136/jmg.13.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucarelli M, Narzi L, Piergentili R, Ferraguti G, Grandoni F, Quattrucci S, Strom R. A 96-well formatted method for exon and exon/intron boundary full sequencing of the CFTR gene. Anal Biochem. 2006;353:226–235. doi: 10.1016/j.ab.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Mattei JF, Laframboise R, Rouault F, Giraud F. Coffin-Lowry Syndrome in Sibs. Am J Med Genet. 1981;8:315–319. doi: 10.1002/ajmg.1320080310. [DOI] [PubMed] [Google Scholar]
- McGhee EM, Klump CJ, Bitts SM, Cotter PD, Lammer EJ. Candidate region for Coffin-Siris syndrome at 7q32-->34. Am J Med Genet. 2000;93:241–243. doi: 10.1002/1096-8628(20000731)93:3<241::aid-ajmg16>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- McPherson EW, Laneri G, Clemens MM, Kochmar SJ, Surti U. Apparently balanced t(1;7)(q21.3;q34) in an infant with Coffin-Siris syndrome. Am J Med Genet. 1997;71:430–433. doi: 10.1002/(sici)1096-8628(19970905)71:4<430::aid-ajmg11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Ounap K, Justus I, Lipping-Sitska M. Two sisters with growth failure, microcephaly, peculiar facies and apical dystrophy: the presentation of brachymorphism-onychodysplasia-dysphalangism syndrome? Clin Dysmorphol. 1998;7:45–50. [PubMed] [Google Scholar]
- Pallotta R. Ocular anomalies in Coffin-Siris syndrome. Ophthalmic Paediatr Genet. 1985;6:349–52. [PubMed] [Google Scholar]
- Patel ZM, Mulye VR, Raghavan K, Shah SB. 12;14 translocation in Coffin Siris syndrome. Indian Pediatr. 1987;24:435–438. [PubMed] [Google Scholar]
- Patton MA, Krywawych S, Winter RM, Brenton DP, Baraitser M. DOOR syndrome (deafness, onycho-osteodystrophy, and mental retardation): elevated plasma and urinary 2-oxoglutarate in three unrelated patients. Am. J. Med. Genet. 1987;26:207–215. doi: 10.1002/ajmg.1320260131. [DOI] [PubMed] [Google Scholar]
- Pollono D, Drut R, Cecotti N, Pollono A. Neuroblastoma in a patient with Coffin-Siris syndrome. Fetal Pediatr Pathol. 2009;28:185–191. doi: 10.1080/15513810902984129. [DOI] [PubMed] [Google Scholar]
- Rabe P, Haverkamp F, Emons D, Rosskamp R, Zerres K, Passarge E. Syndrome of developmental retardation, facial and skeletal anomalies, and hyperphosphatasia in two sisters: nosology and genetics of the Coffin-Siris syndrome. Am J Med Genet. 1991;41:350–354. doi: 10.1002/ajmg.1320410317. [DOI] [PubMed] [Google Scholar]
- Rajab A, Riaz A, Paul G, Al-Khusaibi S, Chalmers R, Patton MA. Further delineation of the DOOR syndrome. Clin. Dysmorph. 2000;9:247–251. doi: 10.1097/00019605-200009040-00003. [DOI] [PubMed] [Google Scholar]
- Rogers L, Pattisapu J, Smith RR, Parker P. Medulloblastoma in association with the Coffin-Siris syndrome. Childs Nerv Syst. 1988;4:41–44. doi: 10.1007/BF00274083. [DOI] [PubMed] [Google Scholar]
- Sahoo T, Theisen A, Sanchez-Lara PA, Marble M, Schweitzer DN, Torchia BS, Lamb AN, Bejjani BA, Shaffer LG, Lacassie Y. Microdeletion 20p12.3 involving BMP2 contributes to syndromic forms of cleft palate. Am J Med Genet A. 2011;155:1646–1653. doi: 10.1002/ajmg.a.34063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior B. Impaired growth and onychodysplasia. Short children with tiny toenails. Am J Dis Child. 1971;122:7–9. doi: 10.1001/archpedi.1971.02110010043002. [DOI] [PubMed] [Google Scholar]
- Shaikh TH, Gai X, Perin JC, Glessner JT, Xie H, Murphy K, O’Hara R, Casalunovo T, Conlin LK, D’Arcy M, Frackelton EC, Geiger EA, Haldeman-Englert C, Imielinski M, Kim CE, Medne L, Annaiah K, Bradfield JP, Dabaghyan E, Eckert A, Onyiah CC, Ostapenko S, Otieno FG, Santa E, Shaner JL, Skraban R, Smith RM, Elia J, Goldmuntz E, Spinner NB, Zackai EH, Chiavacci RM, Grundmeier R, Rappaport EF, Grant SF, White PS, Hakonarson H. High-resolution mapping and analysis of copy number variations in the human genome: a data resource for clinical and research applications. Genome Res. 2009;19:1682–1690. doi: 10.1101/gr.083501.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa SB, Abdul-Rahman OA, Bottani A, Cormier-Daire V, Fryer A, Gillessen-Kaesbach G, Horn D, Josifova D, Kuechler A, Lees M, MacDermot K, Magee A, Morice-Picard F, Rosser E, Sarkar A, Shannon N, Stolte-Dijkstra I, Verloes A, Wakeling E, Wilson L, Hennekam RCM. Nicolaides–Baraitser syndrome: Delineation of the phenotype. Am J Med Genet Part A. 2009;149A:1628–1640. doi: 10.1002/ajmg.a.32956. [DOI] [PubMed] [Google Scholar]
- Swillen A, Glorieux N, Peeters M, Fryns JP. The Coffin-Siris syndrome: data on mental development, language, behavior and social skills in 12 children. Clin Genet. 1995;48:177–182. doi: 10.1111/j.1399-0004.1995.tb04084.x. [DOI] [PubMed] [Google Scholar]
- Thompson MD, Nezarati MM, Gillessen-Kaesbach G, Meinecke P, Mendoza-Londono R, Mornet E, Brun-Heath I, Squarcioni CP, Legeai-Mallet L, Munnich A, Cole DE. Hyperphosphatasia with seizures, neurologic deficit, and characteristic facial features: Five new patients with Mabry syndrome. Am J Med Genet A. 2010;152:1661–1669. doi: 10.1002/ajmg.a.33438. [DOI] [PubMed] [Google Scholar]
- Verloes A, Bonneau D, Guidi O, Berthier M, Oriot D, Van Maldergem L, Koulischer L. Brachymorphism-onychodysplasia-dysphalangism syndrome. J Med Genet. 1993;30:158–161. doi: 10.1136/jmg.30.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Li M, Hadley D, Liu R, Glessner J, Grant SF, Hakonarson H, Bucan M. PennCNV: an integrated hidden Markov model designed for high-resolution copy number variation detection in whole-genome SNP genotyping data. Genome Res. 2007;17:1665–1674. doi: 10.1101/gr.6861907. [DOI] [PMC free article] [PubMed] [Google Scholar]