Abstract
Chromogranin A (CgA) is a member of the granins, a family of acidic proteins found in abundance in (neuro)endocrine cells (e.g. in chromaffin cells) and in some tumors. Like other granins, CgA has a granulogenic role in secretory granule biogenesis and is stored in these organelles. CgA is partially processed differentially in various cell types to yield biologically active peptides, such as vasostatin, pancreastatin, catestatin and serpinins. In this review we describe the roles of CgA and several of its derived peptides. CgA, which is elevated in the blood of cancer patients, inhibits angiogenesis and exerts protective effects on the endothelial barrier function in tumors, thus affecting response to chemotherapy. Recent studies indicate that the serpinins promote cell survival and myocardial contractility and relaxation. Other peptides such as pancreastatin was found to have significant effects on inhibition of glucose stimulated insulin secretion and glucose up-take, induction of glycogenolysis in hepatocytes and inhibition of lipogenesis. In contrast, catestatin has opposite effects to that of pancreastatin in glucose metabolism and lipogenesis. Catestatin appears to also play a significant role in cardiac function, blood pressure regulation, and mutations in the catestatin domain of the CgA gene are associated with hypertension in humans.
Keywords: Chromogranin A, catestatin, vasostatin, serpinin, cancer, hypertension, diabetes
Introduction
Granins are a family of highly acidic proteins found in secretory granules of endocrine and neuroendocrine cells. The most ubiquitously distributed and abundant members of this family are chromogranin A, B (CgA, CgB), secretogranin II (SgII), while other members such as SgIII, VGF, 7B2, and proSAAS are more restricted in their distribution. These proteins have a granulogenic function in secretory granule biogenesis and some are processed to various peptides that have diverse activities such as regulation of glucose balance and energy expenditure, action as inhibitors of prohormone processing enzymes (proprotein convertases) and regulation of neural pathways that control emotional behavior (for reviews, see Helle et al., 2007; Bartolomucci et al., 2011).
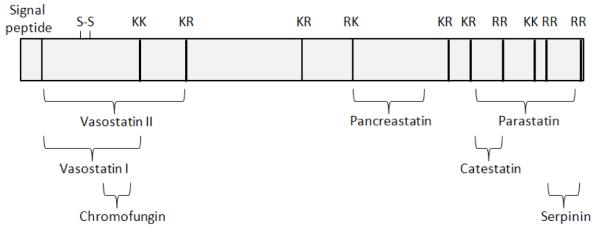
In this minireview, we focus on some of the roles of CgA and its derived peptides in health and disease. Fig 1 shows a schematic diagram of the structure of CgA and its derived peptides. Specifically, we will review the role of CgA in regulating endothelial barrier, tumor angiogenesis and vascular structure and permeability. The anti-apoptotic effect of the serpinins, the newest members of CgA-derived peptides will be described. Additional roles of the serpinins, vasostatin and catestatin in cardiac function will be discussed. The function of catestatin and another CgA-derived peptide, pancreastatin, in the regulation of gluconeogenesis and lipidogenesis in metabolic syndrome will be reviewed. Finally the role of catestatin in hypertension, including the regulation of blood pressure, and the variations in the CgA gene, such as single nucleotide polymorphisms (SNPs) in the catestatin domain, that are associated with hypertension susceptibility in humans will be discussed.
Fig. 1.
Schematic of structure of human CgA showing the various biologically active peptides derived from this precursor.
Chromogranin A in regulation of endothelial barrier function and tumor biology
It is well established that tumor blood vessels are critical for tumor growth and progression as well as for the delivery of anticancer therapies. Tumor blood vessels are characterized by highly disorganized and dilated architecture, excessive branching, shunts and fenestrations, and may lack basement membrane or have fewer pericytes, when compared to normal vessels (Marcucci and Corti, 2011). Furthermore, alteration in endothelial cell-cell adhesion and barrier function can lead to heterogeneous permeability and increased interstitial fluid pressure in tumor tissues (Marcucci and Corti, 2011). These features may cause, in certain tumor areas, transient vascular compression, irregular blood flow, poor perfusion and, consequently, hypoxia, which is a potent stimulus for the activation of pro-angiogenic mechanisms. In addition, these features may also cause uneven delivery and penetration of drugs in tumors, thereby limiting the response to chemotherapy (Corti et al., 2011; Marcucci and Corti, 2011). A growing body of evidence, discussed below, suggests that chromogranin A (CgA), a protein present in variable amounts in the blood of cancer patients, may play a role in the regulation of tumor angiogenesis, vascular structure and permeability, and that it may also affect the response to certain therapies.
Human CgA is a 439-residue long protein stored in the secretory granules of many normal and neoplastic cells of the diffuse neuroendocrine system (Taupenot et al., 2003; Helle et al., 2007; Portela-Gomes et al., 2010). In addition, certain tumors, such as non-small cell lung cancer, prostate, breast, gastric and colorectal cancer can undergo neuroendocrine differentiation and present focal expression of CgA (Taupenot et al., 2003; Corti, 2010; Portela-Gomes et al., 2010). CgA is exocytotically released, together with the co-stored hormones, in the extracellular environment and then in circulation (Helle et al., 2007). Increased levels of circulating CgA have been detected in patients with carcinoids or with other neuroendocrine tumors, and in subpopulations of patients with non-small cell lung cancer, prostate or breast cancer (O’Connor and Bernstein, 1984; O’Connor and Deftos, 1986; Corti et al., 1996; Gregorc et al., 2007; Corti, 2010). Elevated serum CgA levels have been detected also in patients with heart failure, renal failure, hypertension, rheumatoid arthritis, atrophic gastritis, inflammatory bowel disease, sepsis and other inflammatory diseases, or in subjects treated with proton pump inhibitors (Corti, 2010). Thus, CgA levels higher than normal values can occur not only in patients with neuroendocrine tumors or with tumors that undergo neuroendocrine differentiation, but also in patients with non-neuroendocrine tumors, for a variety of reasons.
We have previously shown that mammary adenocarcinoma and lymphoma cell lines genetically engineered to secrete CgA are characterized by reduced growth rate, compared to non-secreting parental cells, when implanted subcutaneously in mice, but not when cultured in vitro, suggesting that CgA can affect host/tumor interactions (Colombo et al., 2002; Veschini et al., 2011). We have also observed that a fragment of CgA spanning the N-terminal residues 1-78, called vasostatin-1 (VS-1, see Fig 1), can inhibit a series of effects induced by vascular endothelial growth factor (VEGF) on endothelial cells, including ERK phosphorylation, cell proliferation, motility, migration, sprouting, invasion and capillary-like structure formation (Belloni et al., 2007). VS-1 can also inhibit, in endothelial cells, the nuclear translocation of HIF-1alpha, a master regulator of angiogenesis (Veschini et al., 2011). These findings suggest that VS-1 is an important regulator of vessel formation in tumors. Accordingly, mammary adenocarcinomas genetically engineered to release VS-1 in their microenvironment are characterized by reduced vascular density and more regular vessels (Veschini et al., 2011).
CgA and VS-1 are also important modulators of the endothelial barrier function. Indeed we have found that these molecules are potent inhibitors of VEGF- and thrombin-induced endothelial cell permeability (Ferrero et al., 2004). Furthermore, CgA and VS-1 can inhibit a series of effects exerted by TNF on endothelial cells, including disassembly of vascular endothelial (VE)-cadherin adherence junctions, gap formation and vascular leakage (Ferrero et al., 2004; Blois et al., 2006; Dondossola et al., 2011). The concept that CgA exerts protective effects on the endothelial barrier function is also supported by the observation that systemic administration of CgA (1 μg) to lymphoma-bearing mice restricts the TNF-induced penetration of patent blue, a synthetic dye, in tumor tissues (Dondossola et al., 2011). Considering that new vessel formation and endothelial barrier alteration in tumor tissues are necessary for the delivery of oxygen, nutrients and growth factors to tumor cells, it is possible that CgA and VS-1, by regulating angiogenesis and endothelial barrier function, might contribute to regulate tumor growth. However, blood vessels are also crucial for the delivery of chemotherapeutic drugs to tumor cells. Thus, it is also possible that CgA and VS-1 might affect the delivery of drugs to tumors and, consequently, their efficacy.
The results of a recent study, performed in animal models, suggest that indeed CgA and VS-1 can affect the delivery of drugs to tumors, at least when chemotherapy is combined with NGR-TNF (Dondossola et al., 2011). NGR-TNF is a cytokine-peptide fusion protein, originally developed by our group, consisting of TNF fused to the CNGRCG peptide (Curnis et al., 2000). This peptide enables selective delivery of TNF to tumor blood vessels, because of its capability to recognize an aminopeptidase N (CD13) isoform over-expressed in the tumor neovasculature (Curnis et al., 2000; Curnis et al., 2002b; Curnis et al., 2002a; Corti et al., 2008; Corti and Curnis, 2011). Targeted delivery of ultra-low doses of NGR-TNF (picograms) to tumor vessels is sufficient to alter the endothelial barrier function and to increase the penetration of various chemotherapeutic drugs into murine solid tumors, including doxorubicin, gemcitabine, cisplatin, melphalan and paclitaxel (Curnis et al., 2002a; Sacchi et al., 2006). Because of this property, NGR-TNF is currently tested in combination with chemotherapy in various phase II and III clinical studies (Corti et al., 2011). We have recently observed that CgA can inhibit the synergism of NGR-TNF with doxorubicin and melphalan in murine RMA-lymphoma and B16-melanoma models (Dondossola et al., 2011). In these models, pathophysiologically relevant levels of circulating CgA inhibited the NGR-TNF-induced penetration of chemotherapeutic drugs in tumor tissues, by enhancing the endothelial barrier function and by reducing drug extravasation (Dondossola et al., 2011). Remarkably, two-fold enhancement of endogenous circulating CgA, obtained by pharmacological treatment with omeprazole, significantly reduced the NGR-TNF-induced penetration of doxorubicin in tumors (Dondossola et al., 2011). Considering the wide use of proton pump inhibitors in patients, these findings may have important clinical implications, and may stimulate further studies to assess whether plasma CgA and VS-1 levels can predict the response to NGR-TNF/chemotherapy in patients.
Although a growing body of evidence suggest that CgA and VS-1 can inhibit angiogenesis and NGR-TNF/chemotherapy synergism, a recent study, performed by other investigators, has shown that the CgA352-372 fragment of CgA (called catestatin) can induce, opposite to VS-1, pro-angiogenic effects (Theurl et al., 2010). This finding highlights the complexity of the CgA system and the importance of proteolytic processing as a regulatory mechanism, which may vary in different tumors, in different patients, in different tumor areas, and possibly also during tumor progression. Future studies aimed at developing new assays capable to quantify the circulating levels of each fragment in patients and at characterizing their biological activity will undoubtedly help to elucidate the role of this protein in cancer and other inflammatory diseases.
Serpinins: Function in cell survival and neuroprotection
a. Forms and localization of serpinin peptides
The serpinins are a family of peptides processed from the C-terminus of CgA. Cleavage of the penultimate pair of basic residues at the C-terminus of CgA yields an extended form, serpinin-Arg-Arg-Gly which has been found in rat heart (Tota et. al. 2012). The Arg-Arg pair of amino acids is cleaved to generate serpinin, a 26 amino acid peptide, (PEDQELESLSAIEAELEKVAHQLQAL, hCgA411-436) found in a mouse pituitary cell line, AtT-20 (Koshimizu et al., 2011b) and human pheochromocytoma (Hook et al., 2010). A form of serpinin modified at the N-terminal to yield pyroglutamic-serpinin (pGlu-ELESLSAIEAELEKVAHQLQAL) has been isolated from rat heart (Loh et al, unpublished data) and AtT-20 cells (Koshimizu et al., 2011a). Immunoreactive pGlu-serpinin has also been found in various regions of mouse brain using a specific antibody in immunocytochemistry studies. Immunostaining was found predominantly in mouse brain neuronal processes and terminals (Loh, Y.P. et al. unpublished data) and observed along and at the tips of processes in AtT-20 cells (Koshimizu et al., 2011a). These serpinin peptides are released from secretory granules in AtT-20 endocrine cells in an activity-dependant manner.
b. Serpinin peptides in cell survival and neuroprotection
Serpinin peptides have various biological functions. Studies on the role of serpinin in granule biogenesis revealed that it acts extracellularly in an autocrine/paracrine fashion in (neuro)endocrine cells. Serpinin upon being released from secretory granules bind to a congnate receptor, likely a G-Protein coupled receptor to activate adenylate cyclase and cAMP production. This leads to activation of protein kinase A and translocation of the transcription factor sp1 into the nucleus where it up-regulates protease-nexin 1 (PN-1) transcription. Increased PN-1 expression has the effect of stabilizing granule proteins in the Golgi complex, leading to increased levels of these proteins and enhanced secretory granule biogenesis to replenish those that have been released (Koshimizu et al., 2011b). Subsequent studies showed that pGlu-serpinin is more potent in activating PN-1 transcription (Koshimizu et al., 2011a). These studies demonstrated that CgA-derived peptides, such as the serpinins, can serve as extracellular signal transduction molecules that ultimately have effects on gene transcription. Given these findings, we examined the action of serpinin peptides in neuroprotection and survival of pituitary cells.
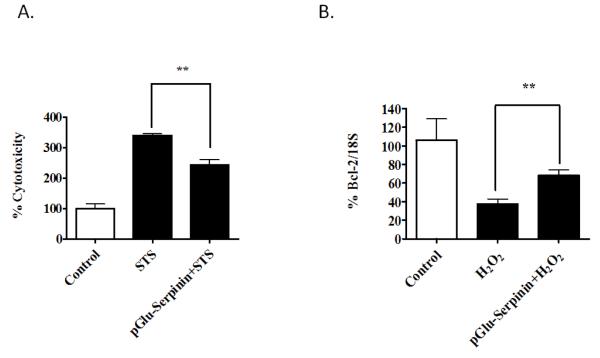
Studies on AtT-20 pituitary cells revealed that treatment of cells with 50uM H2O2 resulted in cell death due to oxidative stress. However when cells were incubated with serpinin or pGlu-serpinin, together with H2O2, the induced apoptosis was inhibited. The cell survival effect of pGlu-serpinin was found to be 1000 times more potent then serpinin in these cells challenged with H2O2. Similar experiments using rat cortical neurons incubated with 50uM H2O2 also resulted in neuronal cell death which was inhibited by co-incubation with 10nM p-Glu serpinin. Mouse cerebellar neurons cultured in low K+ (5mM) induced apoptosis, which was also inhibited with 10nM serpinin treatment (Koshimizu et al., 2011a). Additionally, pGlu-serpinin protected rat hippocampal neurons from staurosporine (STS)-induced cell death (Fig 2A). To determine if the neuroprotective effect of serpinin peptides involves up-regulation of gene transcription, we analyzed the mRNA levels of the anti-apoptotic protein, Bc12, in cortical neurons, during H2O2-induced oxidative stress (Fig 2B). Cell death induced by mitochondrial leakage due to pore formation is determined by a balance between pro-and anti-apoptotic proteins in the Bcl2 family (Chipuk and Green, 2008). Our study showed that Bcl2 mRNA expression was increased in pGlu-serpinin treated neurons after oxidative stress compared to untreated neurons. This result suggests that the neuroprotective effect of pGlu-serpinin involves activation of a signal transduction pathway leading to up-regulation of transcription of Bcl2 mRNA. The demonstration that serpinin peptides can up-regulate gene transcription such as Bcl2 and PN-1 (Koshimizu et al., 2011b) indicate that these peptides can act as signaling molecules to mediate important physiological functions such as neuroprotection, regulation of secretory granule biogenesis and cardiac activity (see next section).
Fig. 2.
(A) Neuroprotective role of pGlu-Serpinin against Staurosporine (STS) in primary cultured rat hippocampal neurons. Primary E18 rat hippocampal neurons were cultured for 5 days. Then neurons were treated with 10 nM pGlu-serpinin or vehicle for 24 h. Subsequently, cytotoxicity was induced by 200 nM STS. Lactic acid dehydrogenase release assay was used to determine cytotoxicity after 24 h treatment of cells with STS. **p<0.01 (t-test). (B) pGlu-serpinin up-regulated Bcl-2 mRNA expression after H2O2. - induced oxidative stress. Primary E 18 rat cortical neurons were cultured for 5 days. and then treated with 10 nM pGlu-serpinin or vehicle for 24 h. Neurons were then treated with 50 μM H2O2. to induce cell death. 24 h later, the neurons were collected for qPCR assay to assess the mRNA level of bcl-2 in different groups. **p<0.01 (t-test).
CgA-derived peptides in cardiac function
Very recent findings indicate that the newly discovered C-terminal CgA-derived serpinin peptides are present in the heart, and influence myocardial contractility (inotropy) and relaxation (lusitropy), providing innovative insight into the CgA-elicited regulation of cardiac function (Tota et al., 2011). In fact, while both vasostatin 1 (VS-1) and catestatin (CST) directly suppress myocardial inotropy and lusitropy, exerting remarkable anti-adrenergic and anti-endothelin-1 modulation, the serpinin peptides appear to act in an opposite, β-agonist-like manner (for review of VS-1 and CST in cardiobiology, see Angelone et al., 2008; Tota et al., 2008, also section on CST and hypertension below). Here, we focus on the role of serpinin peptides in cardiac function.
a. Serpinin peptides act as novel myocardial β-adrenergic like agonists
In the rat heart extracts CgA is processed at the C-terminus to different serpinin-related peptides which, on the basis of their HPLC elution profiles, are identified as serpinin, pGlu-serpinin and the extended serpinin peptide, Ala29Gly. Two major peaks consistent with the Ala29Gly extended serpinin and pGlu-serpinin (pGlu23Leu) are evidenced by immunoreactivity, while serpinin (Ala26Leu) is detectable only in small amounts. The predominant peak identified (sandwich ELISA for pGlu-serpinin specifically) is pGlu-serpinin, which concentration in rat heart is 103.8 ± 14.7 pg/g (Tota et al., 2011).
On the Langendorff perfused rat heart serpinin and pGlu-serpinin, tested from 1 to 165 nM., concentration-dependently induced positive inotropic effect, beginning at 11 nM and 1 nM, respectively, and enhanced relaxation (positive lusitropy), without affecting heart rate (HR) (Tota et al., 2012). In addition, unlike pGlu-serpinin, serpinin elicits coronary vasodilation. In contrast, serpinin Ala29Gly at all concentrations does not affect cardiac performance. The finding that the peptide-induced positive inotropism and lusitropism are unaffected by Tyramine (which enhances norepinephrine outflow by displacing it from its storage vesicles) excludes that norepinephrine release from sympathetic nerve terminals is involved in the mechanism of action of these peptides. Notably, both pGlu-serpinin and serpinin exhibit a striking ß-adrenergic-like agonist profile, acting through a β1-Adrenergic Receptor/Adenylate Cyclase/cAMP/PKA pathway. By interacting as allosteric modulators of the ß-adrenergic receptor independently from the ligand binding site (experiments with the β1 antagonist Nebivolol, and the more selective CGP20712A), they activate the typical signaling cascade triggered by the ß-adrenergic agonists. Similarly to other CgA-derived peptides, no direct receptor or binding partner has been so far detected for serpinin or pGlu-serpinin. We may therefore speculate that, in common with the mechanism of other cAMP-elevating agents and the serpinin signaling involved in granule biogenesis, serpinin and pGlu-serpinin can bind to a G protein-coupled receptor (GPCR). In agreement with this hypothesis, they increase at nanomolar range the cardiac cAMP levels, whereas both their elicited inotropic and lusitropic actions are abolished by selective inhibition of AD (MDL123330A) and PKA (Kt5720). Of particular relevance in relation to the β-adrenergic agonist-like properties of p-Glu-serpinin and serpinin, is that their cardiotropic influence is dependent on several PKA-induced phosphorylated proteins i.e. the major down-stream targets of the AD-cAMP signal-transduction pathway. Among these proteins, the sarcoplasmic reticulum Ca++ pump (SERCA) and its associated regulatory protein phospholanban (PLN) are fundamental regulators of inotropy and lusitropy. In fact, SERCA-dependent Ca++ uptake within SR induces Ca++ removal during diastole, hence affecting relaxation and the subsequent contraction. At the same time, Ca++ sensitivity for SERCA is increased by PKA-induced phosphorylation of PLN, thus contributing to the β-dependent inotropic and lusitropic effects. We demonstrated that SERCA inhibition by thapsigargin abolishes the pGlu-serpinin-elicited inotropic and lusitropic effects, while pGlu-serpinin elicits PNL phosphorylation at Ser16 residue, which is another known downstream target of the β-adrenergic-PKA cascade. The emerging picture is that, like the ß-agonists, the serpinin peptides, through their increased cAMP levels, strikingly affect myocardial mechanical performance regulating the inotropy/lusitropy interplay. In fact, they enhance the rate and extent of tension development during systole and, consequently, stroke volume; at the same time, they speed up myocardial relaxation with consequent shortening of the overall duration of diastole. Under conditions of ß-adrenergic-induced stimulation of HR (tachycardia), this ß-adrenergic-cAMP-dependent regulation of inotropy and lusitropy permits the heart to relax from a stronger systolic contraction in a shorter time, allowing it to adequately fill during the shortened diastole.
In conclusion, it is conceivable that the serpinin peptides may contribute to cardiac homeostasis by counterbalancing the VS-1- and CST-induced cardio-suppressive and anti-adrenergic effects. The temporal scale and the sequential proteolytic events of this intracardiac CgA processing are completely unknown. Future studies will test the hypothesis that in the rodent heart, excitatory (adrenergic) stimuli can activate an intracardiac autocrine/paracrine loop (CgA -> proteolytic processing-> serpinin fragment production-> cardiac actions) with consequent short-medium term regulation of cardiac performance.
Pancreastatin (PST) and catestatin (CST) in metabolic syndrome
Obesity is a global health problem and is believed to cause an insulin-resistant state in adipose tissue, liver, and muscle, which is a strong risk factor for the development of type 2 diabetes (T2DM). Syndrome X or metabolic syndrome is a constellation of several metabolic disorders including insulin resistance, hyperinsulinemia, and dyslipidemia with elevated plasma triglyceride and/or decreased high-density lipoprotein levels (Olefsky and Glass, 2010).
a. PST and metabolic syndrome
The first chromogranin/secretogranin-derived peptide to be discovered was the CgA-derived peptide, PST, which was initially identified in porcine pancreas as a C-terminally amidated 49-mer peptide (pCgA240-288) (Tatemoto et al., 1986). In human plasma, the major form detected was a 52 amino acid PST (hCgA250-301). Amongst CgA peptides, PST is the least conserved having only 54% homology between human and mouse (Bartolomucci et al., 2011). PST homology cannot be ascertained in non-mammalian vertebrates (Bartolomucci et al., 2011). After proteolytic cleavage from CgA, PST requires C-terminal amidation by the peptide α-amidating monooxygenase for activation. PST exerts multiple, potentially dysglycemic actions, which are given below.
PST inhibition of glucose stimulated insulin secretion. PST inhibits insulin secretion in vivo in mice, rats, dogs and pigs, as well as in vitro from isolated rat islets and in the perfused rat pancreas (Ahren et al., 1996). It is generally agreed that PST inhibits the 1st phase of glucose-stimulated insulin secretion (Ahren et al., 1996). However, reports are also available showing PST inhibition of insulin secretion during both the 1st and 2nd phase of secretion or only the 2nd phase of secretion. Of note, PST fails to inhibit insulin secretion induced by other agents such as forskolin (Ahren et al., 1996).
PST inhibition of glucose uptake. The main function of insulin is to facilitate entry of glucose into muscle, adipose and other tissues. Being an anti-insulin peptide, PST dose dependently inhibits basal and insulin-stimulated glucose transport as well as translocation of GLUT4 in primary rat adipocytes (Gonzalez-Yanes and Sanchez-Margalet, 2000). A recent study on primary mouse adipocytes supports the above finding (Gayen et al., 2009b). In humans, brachial arterial infusion of PST reveals that under basal condition PST inhibits glucose uptake by ~50% (O’Connor et al., 2005). Since there was no change in forearm plasma flow it is believed that PST action is metabolic rather than hemodynamic.
PST effects on hepatic glucose metabolism. The major functions of insulin in liver are to stimulate glycogenesis and to inhibit gluconeogenesis and glycogenolysis. Being an anti-insulin peptide, PST inhibits (by ~45%) insulin-stimulated glycogen synthesis in primary hepatocytes (Sanchez-Margalet et al., 2000; Sanchez-Margalet et al., 2010). PST, however, exerted little to no effects on insulin-stimulated glycolysis. As opposed to insulin, PST activates glycogenolysis in the rat liver without the modification of glucagon or insulin levels, suggesting a direct effect on liver metabolism (Sanchez-Margalet et al., 2000; Sanchez-Margalet et al., 2010). Consistent with in vivo studies, PST induces glycogenolysis in isolated hepatocytes, which is comparable to that of glucagon in potency, but was independent of cAMP production and dependent on calcium (Sanchez-Margalet et al., 2000; Sanchez-Margalet et al., 2010).
PST effects on lipid metabolism. The metabolic pathways for utilization of fats and carbohydrates are deeply and intricately intertwined. Insulin augments lipid synthesis by forcing adipose tissue to take in blood lipids, and converting it to triglycerides. In addition, insulin decreases lipolysis as fatty acids inhibit insulin stimulated glucose transport in muscles. As an anti-insulin peptide, PST inhibits lipogenesis and induces lipolysis in rat adipocytes (Sanchez-Margalet et al., 2000; Sanchez-Margalet et al., 2010). Consistent with the above findings, CgA-KO mice display augmented expression of lipogenic genes such as Srebp1c (sterol regulatory element-binding protein 1c), Ppar-γ (peroxisome proliferator-activated receptor gamma), and Gpat (glycerol-3-phosphate acyltransferase) (Gayen et al., 2009b). In rat adipocytes, PST stimulates (by 3-fold) glycerol and free fatty acids release from adipocytes, which however, is completely inhibited by insulin (Sanchez-Margalet et al., 2000; Sanchez-Margalet et al., 2010). In humans, PST augments free fatty acid efflux from the forearm into the circulation, resulting in the A-V differences (by ~6.4-fold) as well as the overall spillover (by ~4.5-fold) (O’Connor et al., 2005). This is consistent with the lipolytic action of PST in adipocytes (Sanchez-Margalet et al., 2000; Sanchez-Margalet et al., 2010). Insulin reverses forearm free fatty acid metabolism in humans, from spillover toward uptake, which was not antagonized by PST (O’Connor et al., 2005).
PST generation of insulin resistance. Since PST antagonizes the effects of insulin on glucose and lipid metabolism coupled with its inhibitory effect on insulin secretion, it is hypothesized that PST plays a crucial role in the development of insulin resistance. Therefore, one would expect increased sensitivity to insulin in CgA null mice because of lack of PST. Consistent with this, CgA-KO mice maintain euglycemia with only ~20% of wild-type insulin (Gayen et al., 2009b). This was confirmed by glucose and insulin tolerance tests as well as by hyperinsulinemic-euglycemic clamp test. The latter study indicates increased insulin sensitivity in liver (Gayen et al., 2009b).
Pathophysiological and metabolic role of PST. Clinical data show increased PST levels (nM range) in T2DM, gestational diabetes, and hypertension (Sanchez-Margalet et al., 2000; Sanchez-Margalet et al., 2010), which point to a possible role of PST in the pathophysiology of insulin resistance. While PST is elevated ~3.7-fold in T2DM, the PST level was comparable in lean and modestly insulin-resistant state of obesity (O’Connor et al., 2005). Of note, PST did not change during substantial (~7 kg) weight loss, indicating that increased PST in T2DM is not simply a response to insulin resistance, but might instead be pathophysiological in T2DM (O’Connor et al., 2005). PST is co-stored and co-released with catecholamines in response to stress. Both PST and catecholamines would promote glycogenolysis and favor catabolic metabolism and supply energy to the whole body. Thus, PST and catecholamines can act in concert to maintain homeostasis during stress situation (Sanchez-Margalet et al., 2010). PST appears to have little to no effect on the regulation of blood pressure (BP).
b. CST and metabolic syndrome
As opposed to PST, CST (human CgA352-372; bovine CgA344-364) (Mahata et al., 1997) is highly conserved in mammals with ~86% homology between human and mouse. Human CST bears significant homology with sub-mammalian vertebrates: 38% with jungle fowl, 33% with frog and ~19% with zebrafish (Bartolomucci et al., 2011). Although the anti-hypertensive and anti-adrenergic effects of CST are well established (Mahapatra et al., 2005; Gayen et al., 2009a; Mahata et al., 2010), recent studies seem to indicate that CST acts as an insulin-sensitizing peptide. It thus appears that PST and CST have opposing roles in metabolic syndrome and glucose homeostasis is maintained by a balance between the anti-insulin action of PST and the insulin-sensitizing effect of CST.
CST inhibition of gluconeogenesis. One of the main functions of insulin is to inhibit gluconeogenesis. As opposed to PST, chronic CST treatment results in 50% inhibition of hepatic glucose production as determined by hyperinsulinemic-euglycemic clamp study in CgA-KO mice (unpublished observation).
CST inhibition of lipogenesis and stimulation of fatty acid oxidation. In adipocytes, insulin promotes lipogenesis by stimulating the uptake of glucose and lipoprotein-derived fatty acids and by inducing ADD-1 (adipocyte determination and differentiation factor 1)/SREBP-1c, which regulates genes promoting fatty acid synthesis and lipogenesis, not only in adipocytes but also in hepatocytes. Insulin also diminishes triglyceride breakdown by inhibiting lipolysis. Although CST inhibits both lipogenesis and lipolysis, it also stimulates fatty acid oxidation (unpublished observation).
CST and hypertension
a. CST in regulation of BP
Circulating levels of CST decrease in patients with essential hypertension (O’Connor et al., 2002) and targeted ablation of the CgA gene in mice increases BP, which can be “rescued” by replacement with CST, indicating a direct role of CST in preventing hypertension (Mahapatra et al., 2005). The potential mechanisms underlying the hypotensive action of CST are as follows: (a) Vasodilation: Existing literature reveals that CST acts as a potent vasodilator in vivo in rat (Kennedy et al., 1998) as well as in human beings (Fung et al., 2010). (b) Decreased peripheral sympathetic tone: Since CST replacement normalizes heightened adrenergic tone in CgA-KO mice, it is hypothesized that CST restoration of elevated BP in CgA-KO mice likely results from CST inhibition of catecholamine secretion from chromaffin cells (Gayen et al., 2009a). (c) Decreased central sympathetic tone: Intrathecal administration of CST results in attenuation of nicotine-induced elevation of mean arterial pressure, which indicates that CST also antagonizes central nicotinic acetylcholine receptors to decrease sympathetic tone (Gaede et al., 2009). (d) Decreased reactive oxygen (ROS) and increased nitric oxide (NO): Since hypertensive and hyperadrenergic CgA-KO mice display increased ROS and decreased NO, and CST replacement normalizes BP and adrenergic tone, it is believed that CST decreases BP in these mice by decreasing ROS and increasing NO (Gayen et al., 2010). (e) Direct cardiosuppressive action: In the Langendorff-perfused rat heart preparation, CST decreases both left ventricular pressure and cardiac contractility (Angelone et al., 2008). (f) Modulation of brainstem circuitry that regulates BP: The brainstem circuitry that regulates BP resides in the ventrolateral medulla consisting of caudal ventrolateral medulla (CVLM) and rostral ventrolateral medulla (RVLM). The CVLM is an essential component of the brainstem circuitry that maintains sympathetic tone and integrates responses from the periphery, and the central nervous system. Baroreceptor afferents synapse on a subpopulation of nucleus of the solitary tract (NTS) neurons that excite GABAergic inhibitory neurons in the CVLM. Excitation of these GABAergic inhibitory neurons in the CVLM acts to restrain the activity of excitatory bulbospinal pre-sympathetic neurons in the RVLM. The RVLM is the primary regulator of the sympathetic nervous system, sending excitatory fibers (glutamatergic) to the sympathetic preganglionic neurons located in the intermediolateral nucleus of the spinal cord. Activation of neurons in the CVLM is crucial for normal operation of the sympathetic baroreceptor reflex. It appears from recent studies that microinjection of CST into RVLM of bilaterally vagotomized rats is sympathoexcitatory and results in increase in mean arterial pressure (MAP) and barosensitivity (Gaede and Pilowsky, 2010). Complementing the effects in RVLM, recent studies indicate that microinjection of CST into CVLM of bilaterally vagotomized rats is most likely to be sympathoinhibitory, causing decrease in basal arterial pressure, sympathetic nerve activity, heart rate and sympathetic baroreflex (Gaede and Pilowsky, 2011).
Genetic variation in CgA gene associated with hypertension
Variation in the coding region: Resequencing of the human CgA gene in 180 individuals (2n=360 chromosomes) revealed 3 non-synonymous (change in amino acids) variants or SNPs (single nucleotide polymorphisms) in the CST domain (Wen et al., 2004). Although 2 of the variants (Pro370Leu and Arg374Gln) were relatively rare, 1 variant, Gly364Ser had an allele frequency of ~3% to ~4%. Gly364Ser variant displayed profound alterations in autonomic activity in both the parasympathetic and sympathetic branches and may have provided protection against future development of hypertension especially in females (Rao et al., 2007).
Variation in the non-coding region (3′-UTR): CgA is overexpressed in hypertension, and that a common (~27% frequency) genetic variant in the CgA 3′-UTR (C+87T) is strongly associated with human essential hypertension, accounting for up to ~12/~9 mm Hg of BP variation within the population, especially in men (Chen et al., 2008). The 3′-UTR variant also predicts environmental stress-induced increments in BP, which suggests a mechanism for early effects of the gene on a pathogenic series of events eventuating in ultimate population profile of sustained BP elevation.
Conclusions
In this minireview, we have highlighted very recent findings of new physiological functions of CgA and CgA- derived peptides, of which catestatin, serpinins, vasostatin and pancreastatin have been most intensely studied. The role of these four peptides in tumor biology, metabolic syndrome, neuroprotection, cardiac function and hypertension emphasizes their importance in health and disease states. The finding that serpinins are molecules that can activate a signal transduction pathway(s), likely by binding to a G-protein coupled receptor, leading to modulation of gene transcription such as BCl2 is particularly exciting. This suggests that the serpinins can execute many cellular actions such as neuroprotection and cardiac inotropic activity, acting in an autocrine/paracrine fashion. Of significance is that some of these CgA-derived peptides have opposing effects, for example: serpinin and vasostatin/catestatin have positive and negative myocardial inotropy and lusitropy respectively, while catestatin and pancreastatin have opposite effects on gluconeogenesis. Hence, regulation of processing of CgA to generate different peptides under different physiological conditions is critical for counterbalance effects to maintain homeostasis. Future research on the mechanisms of action of these CgA-derived peptides and elucidation of their receptors will also be important in developing these peptides as potential therapeutics.
Acknowledgements
We thank Dr. Niamh Cawley (NIH) for helpful discussions. This work was supported in part by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, USA (YPL, YC). S.K.M. is supported by grants from the VA Merit Review and the National Institutes of Health. B.T. was supported by a grant from the University of Calabria (Cosenza), Italy. A.C. was supported by a grant from Associazione Italiana per la Ricerca sul Cancro (AIRC) of Italy.
List of Abbreviations
- ADD1
adipocyte determination and differentiation factor 1
- BP
blood pressure
- CST
catestatin
- CVLM
caudal ventrolateral medulla
- GLUT4
glucose transporter type 4
- Gpat
glycerol-3-phosphate acyltransferase
- MAP
mean arterial pressure
- NO
nitric oxide
- NTS
nucleus of the solitary tract
- Ppar-γ
peroxisome proliferator-activated receptor gamma
- ROS
reactive oxygen species
- SNP
single nucleotide polymorphism
- Srebp1c
sterol regulatory element-binding protein 1c
- UTR
untranslated region
References
- Ahren B, Bertrand G, Roye M, Ribes G. Pancreastatin modulates glucose-stimulated insulin secretion from the perfused rat pancreas. Acta physiologica Scandinavica. 1996;158:63–70. doi: 10.1046/j.1365-201X.1996.525291000.x. [DOI] [PubMed] [Google Scholar]
- Angelone T, Quintieri AM, Brar BK, Limchaiyawat PT, Tota B, Mahata SK, Cerra MC. The antihypertensive chromogranin a peptide catestatin acts as a novel endocrine/paracrine modulator of cardiac inotropism and lusitropism. Endocrinology. 2008;149:4780–4793. doi: 10.1210/en.2008-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomucci A, Possenti R, Mahata SK, Fischer-Colbrie R, Loh YP, Salton SR. The extended granin family: structure, function, and biomedical implications. Endocrine reviews. 2011;32:755–797. doi: 10.1210/er.2010-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belloni D, Scabini S, Foglieni C, Veschini L, Giazzon A, Colombo B, Fulgenzi A, Helle KB, Ferrero ME, Corti A, Ferrero E. The vasostatin-I fragment of chromogranin A inhibits VEGF-induced endothelial cell proliferation and migration. Faseb J. 2007;21:3052–3062. doi: 10.1096/fj.06-6829com. [DOI] [PubMed] [Google Scholar]
- Blois A, Srebro B, Mandala M, Corti A, Helle KB, Serck-Hanssen G. The chromogranin A peptide vasostatin-I inhibits gap formation and signal transduction mediated by inflammatory agents in cultured bovine pulmonary and coronary arterial endothelial cells. Regulatory peptides. 2006;135:78–84. doi: 10.1016/j.regpep.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Chen Y, Rao F, Rodriguez-Flores JL, Mahata M, Fung MM, Stridsberg M, Vaingankar SM, Wen G, Salem RM, Das M, Cockburn MG, Schork NJ, Ziegler MG, Hamilton BA, Mahata SK, Taupenot L, O’Connor DT. Naturally occurring human genetic variation in the 3′-untranslated region of the secretory protein chromogranin A is associated with autonomic blood pressure regulation and hypertension in a sex-dependent fashion. Journal of the American College of Cardiology. 2008;52:1468–1481. doi: 10.1016/j.jacc.2008.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo B, Curnis F, Foglieni C, Monno A, Arrigoni G, Corti A. Chromogranin A expression in neoplastic cells affects tumor growth and morphogenesis in mouse models. Cancer research. 2002;62:941–946. [PubMed] [Google Scholar]
- Corti A. Chromogranin A and the tumor microenvironment. Cellular and molecular neurobiology. 2010;30:1163–1170. doi: 10.1007/s10571-010-9587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti A, Curnis F. Tumor vasculature targeting through NGR peptide-based drug delivery systems. Current pharmaceutical biotechnology. 2011;12:1128–1134. doi: 10.2174/138920111796117373. [DOI] [PubMed] [Google Scholar]
- Corti A, Curnis F, Arap W, Pasqualini R. The neovasculature homing motif NGR: more than meets the eye. Blood. 2008;112:2628–2635. doi: 10.1182/blood-2008-04-150862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti A, Pastorino F, Curnis F, Arap W, Ponzoni M, Pasqualini R. Targeted drug delivery and penetration into solid tumors. Medicinal research reviews. 2011 doi: 10.1002/med.20238. [DOI] [PubMed] [Google Scholar]
- Corti A, Gasparri A, Chen FX, Pelagi M, Brandazza A, Sidoli A, Siccardi AG. Characterisation of circulating chromogranin A in human cancer patients. British journal of cancer. 1996;73:924–932. doi: 10.1038/bjc.1996.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnis F, Sacchi A, Corti A. Improving chemotherapeutic drug penetration in tumors by vascular targeting and barrier alteration. The Journal of clinical investigation. 2002a;110:475–482. doi: 10.1172/JCI15223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnis F, Sacchi A, Borgna L, Magni F, Gasparri A, Corti A. Enhancement of tumor necrosis factor alpha antitumor immunotherapeutic properties by targeted delivery to aminopeptidase N (CD13) Nature biotechnology. 2000;18:1185–1190. doi: 10.1038/81183. [DOI] [PubMed] [Google Scholar]
- Curnis F, Arrigoni G, Sacchi A, Fischetti L, Arap W, Pasqualini R, Corti A. Differential binding of drugs containing the NGR motif to CD13 isoforms in tumor vessels, epithelia, and myeloid cells. Cancer research. 2002b;62:867–874. [PubMed] [Google Scholar]
- Dondossola E, Gasparri AM, Colombo B, Sacchi A, Curnis F, Corti A. Chromogranin A restricts drug penetration and limits the ability of NGR-TNF to enhance chemotherapeutic efficacy. Cancer research. 2011;71:5881–5890. doi: 10.1158/0008-5472.CAN-11-1273. [DOI] [PubMed] [Google Scholar]
- Ferrero E, Scabini S, Magni E, Foglieni C, Belloni D, Colombo B, Curnis F, Villa A, Ferrero ME, Corti A. Chromogranin A protects vessels against tumor necrosis factor alpha-induced vascular leakage. Faseb J. 2004;18:554–556. doi: 10.1096/fj.03-0922fje. [DOI] [PubMed] [Google Scholar]
- Fung MM, Salem RM, Mehtani P, Thomas B, Lu CF, Perez B, Rao F, Stridsberg M, Ziegler MG, Mahata SK, O’Connor DT. Direct vasoactive effects of the chromogranin A (CHGA) peptide catestatin in humans in vivo. Clin Exp Hypertens. 2010;32:278–287. doi: 10.3109/10641960903265246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaede AH, Pilowsky PM. Catestatin in rat RVLM is sympathoexcitatory, increases barosensitivity, and attenuates chemosensitivity and the somatosympathetic reflex. American journal of physiology. 2010;299:R1538–1545. doi: 10.1152/ajpregu.00335.2010. [DOI] [PubMed] [Google Scholar]
- Gaede AH, Pilowsky PM. Catestatin, a chromogranin A derived peptide, is sympathoinhibitory and attenuates sympathetic barosensitivity and the chemoreflex in rat CVLM. American journal of physiology. 2011 doi: 10.1152/ajpregu.00409.2011. [DOI] [PubMed] [Google Scholar]
- Gaede AH, Lung MS, Pilowsky PM. Catestatin attenuates the effects of intrathecal nicotine and isoproterenol. Brain research. 2009;1305:86–95. doi: 10.1016/j.brainres.2009.09.088. [DOI] [PubMed] [Google Scholar]
- Gayen JR, Gu Y, O’Connor DT, Mahata SK. Global disturbances in autonomic function yield cardiovascular instability and hypertension in the chromogranin a null mouse. Endocrinology. 2009a;150:5027–5035. doi: 10.1210/en.2009-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayen JR, Saberi M, Schenk S, Biswas N, Vaingankar SM, Cheung WW, Najjar SM, O’Connor DT, Bandyopadhyay G, Mahata SK. A novel pathway of insulin sensitivity in chromogranin A null mice: a crucial role for pancreastatin in glucose homeostasis. The Journal of biological chemistry. 2009b;284:28498–28509. doi: 10.1074/jbc.M109.020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayen JR, Zhang K, RamachandraRao SP, Mahata M, Chen Y, Kim HS, Naviaux RK, Sharma K, Mahata SK, O’Connor DT. Role of reactive oxygen species in hyperadrenergic hypertension: biochemical, physiological, and pharmacological evidence from targeted ablation of the chromogranin a (Chga) gene. Circulation. 2010;3:414–425. doi: 10.1161/CIRCGENETICS.109.924050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Yanes C, Sanchez-Margalet V. Pancreastatin modulates insulin signaling in rat adipocytes: mechanisms of cross-talk. Diabetes. 2000;49:1288–1294. doi: 10.2337/diabetes.49.8.1288. [DOI] [PubMed] [Google Scholar]
- Gregorc V, Spreafico A, Floriani I, Colombo B, Ludovini V, Pistola L, Bellezza G, Vigano MG, Villa E, Corti A. Prognostic value of circulating chromogranin A and soluble tumor necrosis factor receptors in advanced nonsmall cell lung cancer. Cancer. 2007;110:845–853. doi: 10.1002/cncr.22856. [DOI] [PubMed] [Google Scholar]
- Helle KB, Corti A, Metz-Boutigue MH, Tota B. The endocrine role for chromogranin A: a prohormone for peptides with regulatory properties. Cell Mol Life Sci. 2007;64:2863–2886. doi: 10.1007/s00018-007-7254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook V, Bark S, Gupta N, Lortie M, Lu WD, Bandeira N, Funkelstein L, Wegrzyn J, O’Connor DT, Pevzner P. Neuropeptidomic components generated by proteomic functions in secretory vesicles for cell-cell communication. AAPS J. 2010;12:635–645. doi: 10.1208/s12248-010-9223-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BP, Mahata SK, O’Connor DT, Ziegler MG. Mechanism of cardiovascular actions of the chromogranin A fragment catestatin in vivo. Peptides. 1998;19:1241–1248. doi: 10.1016/s0196-9781(98)00086-2. [DOI] [PubMed] [Google Scholar]
- Koshimizu H, Cawley NX, Yergy AL, Loh YP. Role of pGlu-Serpinin, a novel chromogranin a-derived peptide in inhibition of cell death. J Mol Neurosci. 2011a;45:294–303. doi: 10.1007/s12031-011-9521-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshimizu H, Cawley NX, Kim T, Yergey AL, Loh YP. Serpinin: a novel chromogranin A-derived, secreted peptide up-regulates protease nexin-1 expression and granule biogenesis in endocrine cells. Mol Endocrinol. 2011b;25:732–744. doi: 10.1210/me.2010-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahapatra NR, O’Connor DT, Vaingankar SM, Hikim AP, Mahata M, Ray S, Staite E, Wu H, Gu Y, Dalton N, Kennedy BP, Ziegler MG, Ross J, Mahata SK. Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. The Journal of clinical investigation. 2005;115:1942–1952. doi: 10.1172/JCI24354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahata SK, Mahata M, Fung MM, O’Connor DT. Catestatin: a multifunctional peptide from chromogranin A. Regulatory peptides. 2010;162:33–43. doi: 10.1016/j.regpep.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahata SK, O’Connor DT, Mahata M, Yoo SH, Taupenot L, Wu H, Gill BM, Parmer RJ. Novel autocrine feedback control of catecholamine release. A discrete chromogranin a fragment is a noncompetitive nicotinic cholinergic antagonist. The Journal of clinical investigation. 1997;100:1623–1633. doi: 10.1172/JCI119686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcucci F, Corti A. How to improve exposure of tumor cells to drugs - Promoter drugs increase tumor uptake and penetration of effector drugs. Advanced drug delivery reviews. 2011 doi: 10.1016/j.addr.2011.09.007. [DOI] [PubMed] [Google Scholar]
- O’Connor DT, Bernstein KN. Radioimmunoassay of chromogranin A in plasma as a measure of exocytotic sympathoadrenal activity in normal subjects and patients with pheochromocytoma. The New England journal of medicine. 1984;311:764–770. doi: 10.1056/NEJM198409203111204. [DOI] [PubMed] [Google Scholar]
- O’Connor DT, Deftos LJ. Secretion of chromogranin A by peptide-producing endocrine neoplasms. The New England journal of medicine. 1986;314:1145–1151. doi: 10.1056/NEJM198605013141803. [DOI] [PubMed] [Google Scholar]
- O’Connor DT, Kailasam MT, Kennedy BP, Ziegler MG, Yanaihara N, Parmer RJ. Early decline in the catecholamine release-inhibitory peptide catestatin in humans at genetic risk of hypertension. Journal of hypertension. 2002;20:1335–1345. doi: 10.1097/00004872-200207000-00020. [DOI] [PubMed] [Google Scholar]
- O’Connor DT, Cadman PE, Smiley C, Salem RM, Rao F, Smith J, Funk SD, Mahata SK, Mahata M, Wen G, Taupenot L, Gonzalez-Yanes C, Harper KL, Henry RR, Sanchez-Margalet V. Pancreastatin: multiple actions on human intermediary metabolism in vivo, variation in disease, and naturally occurring functional genetic polymorphism. The Journal of clinical endocrinology and metabolism. 2005;90:5414–5425. doi: 10.1210/jc.2005-0408. [DOI] [PubMed] [Google Scholar]
- Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annual review of physiology. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- Portela-Gomes GM, Grimelius L, Wilander E, Stridsberg M. Granins and granin-related peptides in neuroendocrine tumours. Regulatory peptides. 2010;165:12–20. doi: 10.1016/j.regpep.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Rao F, Wen G, Gayen JR, Das M, Vaingankar SM, Rana BK, Mahata M, Kennedy BP, Salem RM, Stridsberg M, Abel K, Smith DW, Eskin E, Schork NJ, Hamilton BA, Ziegler MG, Mahata SK, O’Connor DT. Catecholamine release-inhibitory peptide catestatin (chromogranin A(352-372)): naturally occurring amino acid variant Gly364Ser causes profound changes in human autonomic activity and alters risk for hypertension. Circulation. 2007;115:2271–2281. doi: 10.1161/CIRCULATIONAHA.106.628859. [DOI] [PubMed] [Google Scholar]
- Sacchi A, Gasparri A, Gallo-Stampino C, Toma S, Curnis F, Corti A. Synergistic antitumor activity of cisplatin, paclitaxel, and gemcitabine with tumor vasculature-targeted tumor necrosis factor-alpha. Clin Cancer Res. 2006;12:175–182. doi: 10.1158/1078-0432.CCR-05-1147. [DOI] [PubMed] [Google Scholar]
- Sanchez-Margalet V, Gonzalez-Yanes C, Santos-Alvarez J, Najib S. Pancreastatin. Biological effects and mechanisms of action. Advances in experimental medicine and biology. 2000;482:247–262. doi: 10.1007/0-306-46837-9_20. [DOI] [PubMed] [Google Scholar]
- Sanchez-Margalet V, Gonzalez-Yanes C, Najib S, Santos-Alvarez J. Metabolic effects and mechanism of action of the chromogranin A-derived peptide pancreastatin. Regulatory peptides. 2010;161:8–14. doi: 10.1016/j.regpep.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Efendic S, Mutt V, Makk G, Feistner GJ, Barchas JD. Pancreastatin, a novel pancreatic peptide that inhibits insulin secretion. Nature. 1986;324:476–478. doi: 10.1038/324476a0. [DOI] [PubMed] [Google Scholar]
- Taupenot L, Harper KL, O’Connor DT. The chromogranin-secretogranin family. The New England journal of medicine. 2003;348:1134–1149. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- Theurl M, Schgoer W, Albrecht K, Jeschke J, Egger M, Beer AG, Vasiljevic D, Rong S, Wolf AM, Bahlmann FH, Patsch JR, Wolf D, Schratzberger P, Mahata SK, Kirchmair R. The neuropeptide catestatin acts as a novel angiogenic cytokine via a basic fibroblast growth factor-dependent mechanism. Circulation research. 2010;107:1326–1335. doi: 10.1161/CIRCRESAHA.110.219493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tota B, Angelone T, Mazza R, Cerra MC. The chromogranin A-derived vasostatins: new players in the endocrine heart. Curr Med Chem. 2008;15:1444–1451. doi: 10.2174/092986708784567662. [DOI] [PubMed] [Google Scholar]
- Tota B, Pasqua T, Gentile S, Koshimizu H, Cawley NX, Cerra MC, Loh YP, Angelone T. C-terminal Chromogranin A-derived serpinin and pyroglutaminated serpinin as novel cardiac ß-adrenergic agonists; 62nd Meeting of The Italian Physiological Society; Sorrento, (Italy): Acta Physiologica. 2011.p. 154. [Google Scholar]
- Tota B, Gentile S, Pasqua T, Bassino E, Koshimizu H, Cawley NX, Cerra MC, Loh YP, Angelone T. The novel Chromogranin A-derived serpinin and pyroglutaminated serpinin peptides are positive cardiac beta-adrenergic-like inotropes (Submitted) 2012 doi: 10.1096/fj.11-201111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veschini L, Crippa L, Dondossola E, Doglioni C, Corti A, Ferrero E. The vasostatin-1 fragment of chromogranin A preserves a quiescent phenotype in hypoxia-driven endothelial cells and regulates tumor neovascularization. Faseb J. 2011;25:3906–3914. doi: 10.1096/fj.11-182410. [DOI] [PubMed] [Google Scholar]
- Wen G, Mahata SK, Cadman P, Mahata M, Ghosh S, Mahapatra NR, Rao F, Stridsberg M, Smith DW, Mahboubi P, Schork NJ, O’Connor DT, Hamilton BA. Both rare and common polymorphisms contribute functional variation at CHGA, a regulator of catecholamine physiology. American journal of human genetics. 2004;74:197–207. doi: 10.1086/381399. [DOI] [PMC free article] [PubMed] [Google Scholar]