Abstract
Photoperiodism is a biological phenomenonin which environmental day length is monitored to ascertain time of year to engage in seasonally-appropriate adaptations. This trait is common among organisms living outside of the tropics. White-footed mice (Peromyscus leucopus) are small photoperiodic rodents which display a suite of adaptive responses to short day lengths, including reduced hippocampal volume, impairments in hippocampal-mediated memory, and enhanced hypothalamic-pituitary-adrenal axis reactivity. Because these photoperiodic changes in brain and behavior mirror some of the etiology of post-traumatic stress disorder (PTSD), we hypothesized that photoperiod may also alter fear memory and neuronal morphology within the hippocampus - basolateral amygdala - prefrontal cortex fear circuit. Ten weeks of exposure to short days increased fear memory in an auditory-cued fear conditioning test. Short days also increased dendritic spine density of the neurons of the basolateral amygdala, without affecting morphology of pyramidal neurons within the infralimbic region of the medial prefrontal cortex. Taken together, photoperiodic phenotypic changes in brain morphology and physiology induced by a single environmental factor, exposure to short day lengths, affect responses to fearful stimuli in white-footed mice. These results have potential implications understanding seasonal changes in fear responsiveness, as well as for expanding translational animal models for studying gene-environment interactions underlying psychiatric diseases, such as PTSD.
Keywords: PTSD, BLA, spine density, auditory fear conditioning, predator avoidance
1. Introduction
Photoperiodism is the biological ability to measure environmental day length (photoperiod) to ascertain the time of year and engage in seasonally appropriate adaptations of physiology and behavior. These seasonal changes in physiology and behavior can be induced in a laboratory setting by simply exposing animals to different static day lengths [1]. White-footed mice (Peromyscus leucopus) display a suite of changes in physiology and behavior induced by exposure to short days, including changes in behavior, brain volume and functional connectivity, and enhanced HPA axis reactivity [2–4]. In recent years, photoperiodic rodent models are finding more utility as models of human pathologies involving alterations in brain structure and function [5–6].
Among human post-traumatic stress disorder (PTSD) patients, reduced hippocampal volume is associated with susceptibility to PTSD, but not severity of symptoms [7], amygdala activity is associated with symptom severity [8], and PTSD patients have enhanced HPA axis negative feedback [7]. Additionally, the medial prefrontal cortex (mPFC) is hyporesponsive to fear-related stimuli in patients suffering from PTSD [9–10]. Translational rodent studies have identified these three brain regions as critical components of the neural circuit underlying associative fear memory. The basolateral amygdala (BLA), hippocampus, and mPFC all play critical roles in fear memory: complex modulation of reciprocal connections among the mPFC, hippocampus, and basolateral amygdala (BLA) are integral to associative fear memory [7, 11–13].
Although standard laboratory rodents (Rattus norvegicus and Mus musculus) have been widely used to model PTSD and other human psychiatric disorders, to fully understand combined factors underlying the development, maintenance, and treatment of these diseases, more diverse animal models are needed [5]. Toward this end, photoperiod-induced changes in the brain of white-footed mice mirror several components of the etiology of PTSD. For example, in common with the human PTSD condition, short day exposed white-footed mice have reduced hippocampal volume [4] and increased HPA axis feedback [3]. To our knowledge, photoperiod–mediated changes in fear memory and responses in this, and other, photoperiodic rodent species remain largely undescribed. A preliminary study in our lab indicated that, unlike short-day induced impairments in hippocampal-mediated spatial learning and memory, white-footed mice exposed to short days may have enhanced nonspatial fear memory in the passive avoidance test [4].
Based on the commonality of photoperiodic changes in white footed mice with the etiology of PTSD described above, and on preliminary data demonstrating short day enhancement of fear memory, we hypothesized that exposure to short days would enhance fear memory and alter neuronal morphology in brain regions implicated in associative fear memory. To test our hypothesis, we exposed male white-footed mice to either short or long day lengths for ten weeks to induce maximal photoperiodic responses, tested them in an auditory fear conditioning test, and examined the neuronal morphology in the BLA and the infralimbic region of the mPFC (IL) using Golgi-Cox staining.
2. Materials and methods
2.1. Animals
Nineteen adult (>55 d of age) male Peromyscus leucopus, from our breeding colony maintained at the Ohio State University, were randomly assigned to either long (LD; 16L:8D, n=10) or short day lengths (SD; 8L:16D, n=9) for 10 weeks to establish photoperiod-induced changes prior to behavioral testing [2, 4]. Mice were housed in standard polycarbonate cages (32×18×14 cm), maintained at constant temperature (21 ± 4°C) and relative humidity (50 ± 5%), provided ad libitum access to filtered tap water and food (Harlan Teklad 8640, Indianapolis, IN, USA), and received care from the Ohio State University Laboratory Animal Resource staff for the duration of the study. All procedures proposed were approved by the Ohio State University Institutional Animal Care and Use Committee and are in compliance with guidelines established by the National Institutes of Health [14].
2.2. Behavioral Test
2.2.1. Auditory fear conditioning
To assess tone-conditioned fear acquisition and retention, 19 (n = 10 LD; n = 9 SD) mice were assessed using the Near-IR Video Fear Conditioning System (Med Associates Inc., St. Albans, VT, USA). For acquisition of the tone-conditioned fear, during the light phase mice were brought directly from their vivarium rooms and were placed in the test chamber illuminated with white light for a 2 min habituation period with 68dB white noise. Mice were then exposed to a series of 8 conditional stimuli (80dB tone, CS) for 6 s with the last 2 s paired with a 0.75 mA foot shock (unconditioned stimulus, US). Mice remained in the chamber for an additional 60 s after the last CS/US pairing before being returned to their home cages. Freezing behavior was recorded by the software for the 2 minute baseline, during the first 4 s of each tone, during the 30 s interval between CS presentations, and for the 60 s after the final CS. To assess contextual fear retention, 24 h after the acquisition session, mice were placed in the original unmodified chamber and freezing behavior to the unmodified chamber was recorded for180 s, and mice were returned to their home cages. Four hours after the contextual fear retention test, mice were tested for retention of the CS-US pairing with the following modifications to alter context. Mice were transported from their vivarium rooms via sound- and light-attenuating boxes to a staging area. From there, mice were brought into the testing room lit with dim red light and placed into the chambers. To avoid context-dependent freezing, the chamber was modified via the addition of a smooth plastic floor, a semi-circular unlit testing chamber, lights were extinguished, and a gauze pad with a drop of vanilla extract was placed in the chamber to present the CS in a novel environment. Mice were then tested for retention of the CS/US pairing by using the procedure described above for the acquisition trial above without receiving the foot shock (US).
2.3. Sample collection and histology
Twenty hours after the completion of auditory fear conditioning, under deep isoflurane anesthesia, mice were exsanguinated via the retro-orbital sinus, plasma was collected from the blood samples as previously described [4], and samples were stored at −80°C for corticosterone assay. Immediately after blood collection, mice were rapidly decapitated and brains were processed to study neuronal morphology (after [4, 15]) using a commercially available Golgi-Cox impregnation kit (FD NeuroTechnologies, Ellicott City, MD, USA) according to the manufacturer’s instructions. Briefly, after impregnation, brains were cut into 100 µm coronal sections and thaw mounted on to gelatin-coated slides. Slides were then developed, counterstained with cresyl violet acetate, dehydrated, cleared with xylenes, and coverslipped with Permount (Fisher).
2.3.1. Dendritic arborization analysis
Pyramidal neurons (n = 4 – 6 for each mouse) in the infralimbic cortex (IL), identified by its cytoarchitecture and neuroanatomical position medial to the forceps minor and cingulum between 1.3 to 1.9 mm anterior to bregma [16], were traced at 400× and quantified using neuronal tracing software (Neurolucida, Microbrightfield, VT, USA). Neurons were traced only if they met the following criteria: 1) completely and uniformly impregnated with Golgi stain, 2) all dendrites were intact and visible, and 3) not obscured by other stained neurons (after 4). The basolateral amygdala (BLA), identified by its location bounded by the branched arms of the external capsule between 0.8 and 2.0 mm posterior to bregma [16], did not contain sufficient numbers of neurons that met the above criteria for analysis. Representative values for each parameter measured by the software (see results) from each animal were calculated by averaging values from all neurons traced. Representative values calculated for each animal were then used for further analysis.
2.3.2. Dendritic spine density analysis
Dendritic spines of the neurons were traced at 1000× using Neurolucida software (Microbrightfield, VT, USA). Within the BLA, for each animal average spine density was calculated by selecting six neurons, and an unbranched, unbroken, and consistently stained dendritic segment at least 50 µm away from the soma from each neuron was quantified. Any protrusion originating from the dendritic shaft was classified as a spine, and all spines along a continuous 80 µm segment were counted for spine density analysis (after 17). For the IL, average spine densities were quantified for each animal from both basilar and apical dendrites, selected as above. For each neuron a total of 80 µm of basilar and 80 µm apical dendrite were quantified for spine density. For each brain region, the average spine density calculated from each animal was then used for further comparative analysis.
2.3.3. Corticosterone assay
Frozen plasma samples were thawed on ice and assayed for corticosterone using a commercially available double antibody RIA kit according to manufacturer’s instructions (Cat# 07120102; MP Biomedicals, Costa Mesa, CA, USA). The intra-assay coefficient of variation was 7.9%.
2.3.4. Statistics
Repeated measures ANOVA were used to compare auditory fear responses over time and dendritic arborization measures (Sholl analysis). Student’s t tests were used for comparisons between photoperiods for physiological data, spine density analysis, and for follow up testing of specific time points within trials for auditory fear testing after a main effect was identified by ANOVA. Data with unequal variance were log transformed prior to analysis. All analyses were performed using SPSS software (v19; IBM, NY, USA) and differences were considered statistically significant at p ≤ 0.05.
3. Results
3.1. Physiological measures
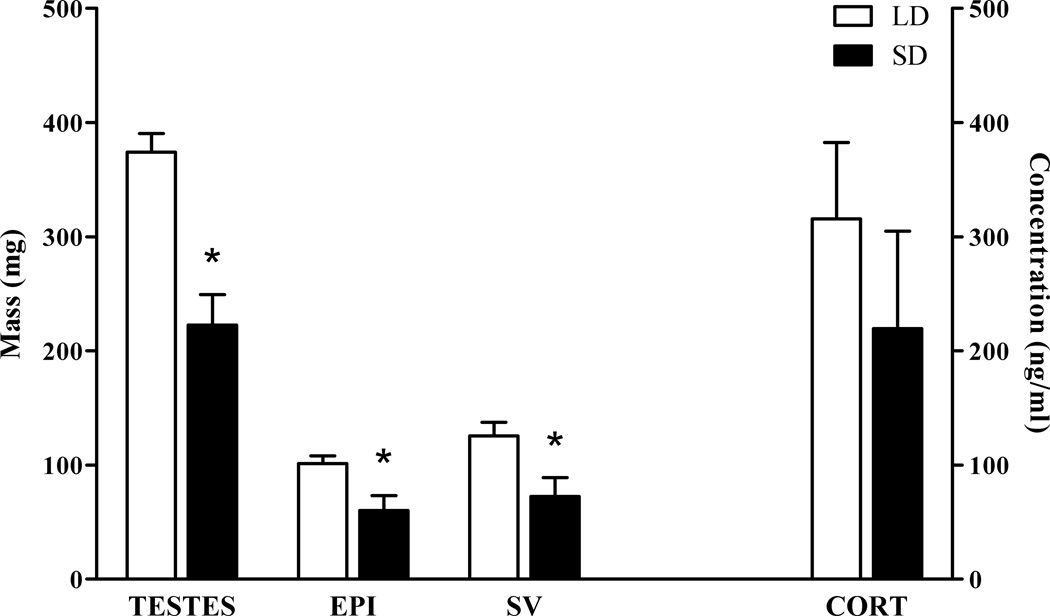
Exposure to short day lengths did not affect body mass (t(17) = 0.185, p> 0.05) or inguinal fat pad mass (t(17) = 0.036, p > 0.05). Compared to LD-exposed counterparts, exposure to SD reduced masses of all reproductive tissues assessed (paired testes, t(15) = 4.934, p< 0.001; epididymides, t(17) = 2.872, p< 0.05; seminal vesicles, t(17)= 2.655, p< 0.05 Figure 1 left). Short day exposure did not affect basal corticosterone concentrations at the terminal bleed (t(16) = 0.904, p> 0.05; Figure 1 right).
Figure 1.
SD exposure reduced the mass of the reproductive tissues (left), but did not alter basal plasma corticosterone concentrations (right). * p < 0.05 Student’s t test.
3.2. Behavioral measures
3.2.1. Auditory-cued fear conditioning
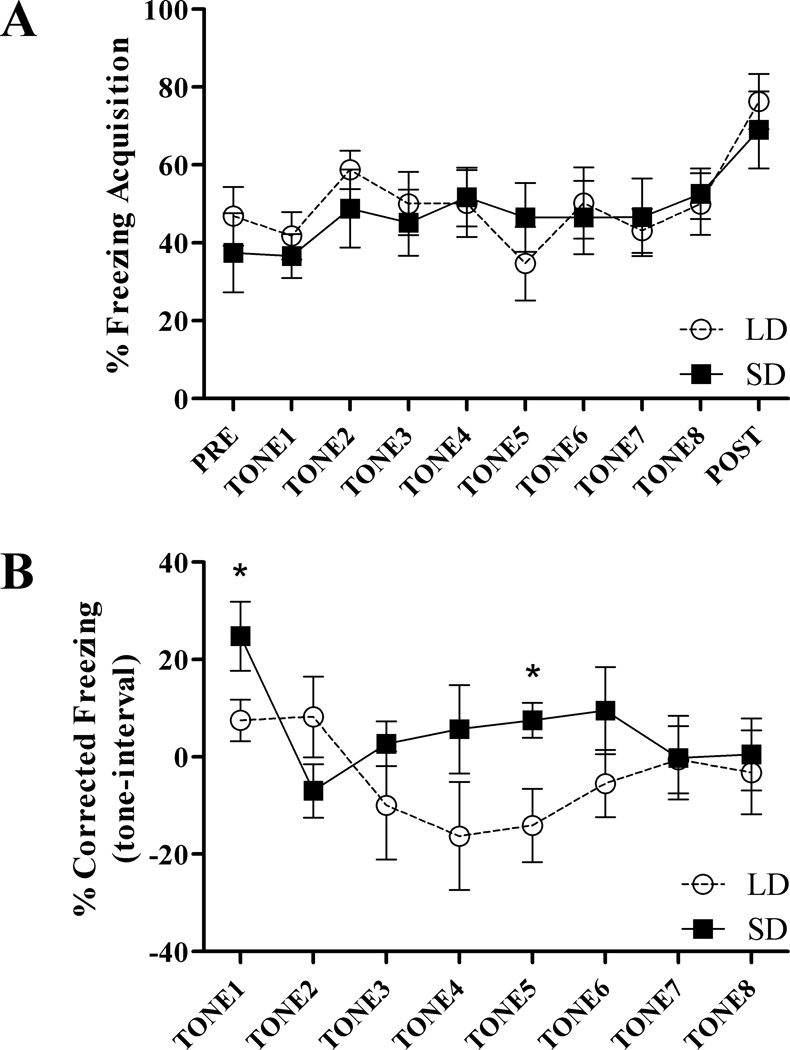
LD and SD mice did not differ in their freezing responses during acquisition of the CS-US pairing across trials (repeated measures ANOVA: F(1,15) = 0.056, p > 0.05: Figure 2A). Comparing baseline to post CS-US presentation, both SD and LD mice increased freezing behavior across acquisition (LD, t(8) = −2.714, p < 0.05; SD t(7) = −3.457, p < 0.05), however photoperiod did not affect increases in freezing between pre and post-stimulus (Figure 2A). There were no differences due to photoperiod in freezing to context 24 h after acquisition (t(17) = 0.777, p > 0.05; not shown). Because of individual variance in inter-trial interval freezing behavior, freezing responses during retention tone trials were corrected by subtracting freezing during the 30s immediately prior to tone presentation from freezing during the tone. Compared to mice exposed to LD, SD exposure increased freezing to tone across retention trials (repeated measures ANOVA: F(1,17) = 8.160, p < 0.05). Follow up, within-trial, Student’s t test comparisons show that SD mice froze significantly more than LD counterparts during the initial (t(17) = −2.668, p < 0.05) and the fifth (t(17) = −2.839, p < 0.05) tone presentation during retention trials (Figure 2B).
Figure 2.
SD exposure enhances fear memory in Peromyscus leucopus. A) LD and SD mice did not differ in freezing responses during acquisition in the auditory-cued fear conditioning test. Both LD and SD mice increased freezing across acquisition. B) Freezing responses to tone 24 h after acquisition are increased in SD mice compared to LD mice (p < 0.05 repeated measures ANOVA). Follow up within-trial comparisons show that SD mice froze significantly more than LD counterparts during the initial tone presentation and during tone 5. * p < 0.05 Student’s t test.
3.3. Neuronal morphology
3.3.1. Dendriticspine density
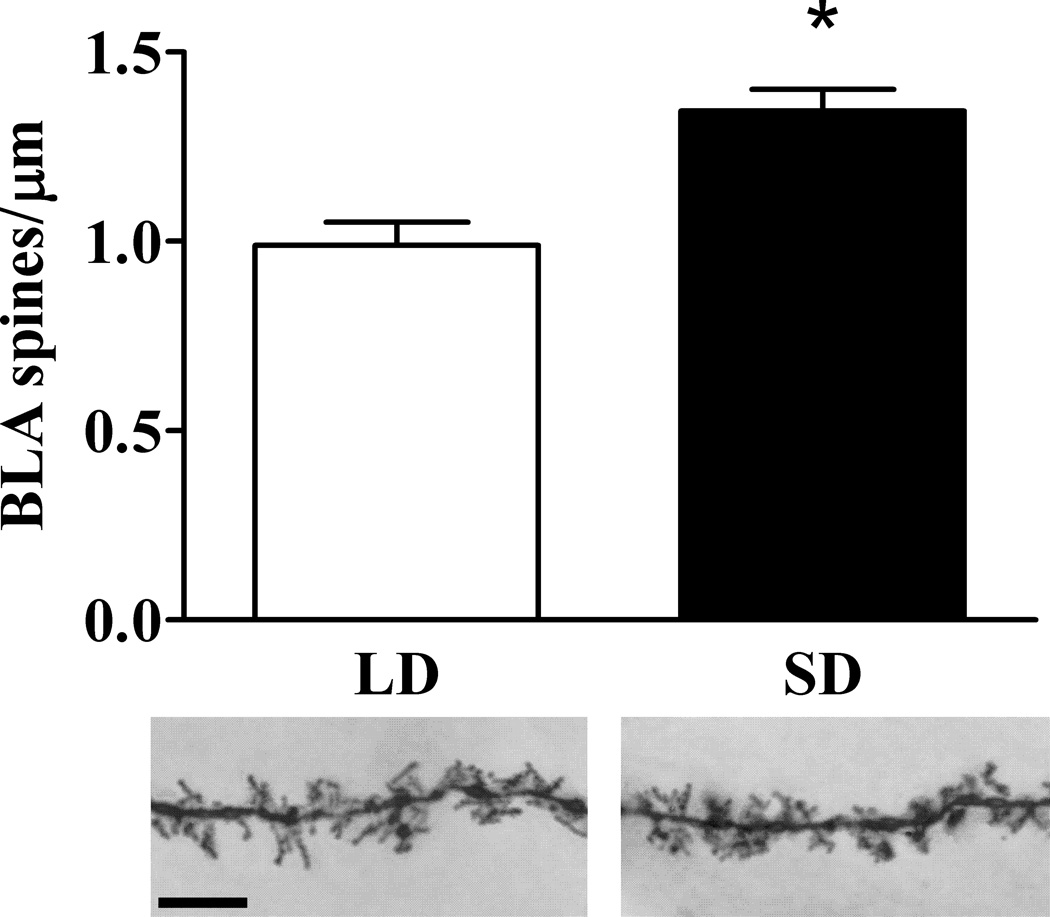
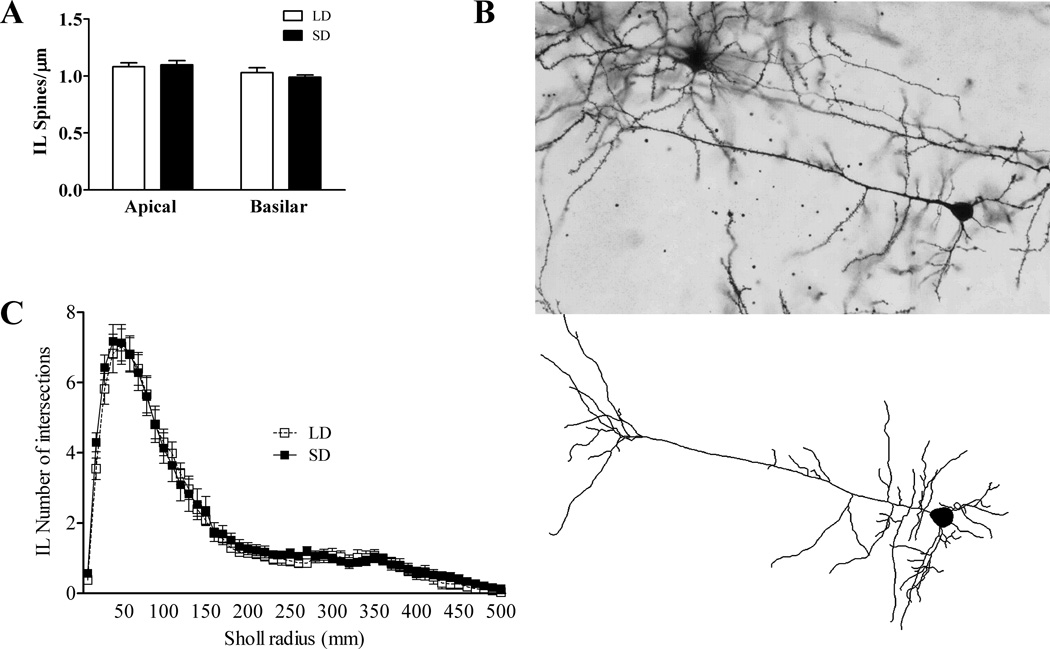
Compared to LD mice, mice exposed to SD had increased spine density on dendrites in the BLA (t(10) = −4.196, p < 0.01; Figure 3). In the IL, there were no differences in spine density due to photoperiod in either basilar (t(13) = −0.332, p > 0.05) or apical dendrites (t(13) = 0.753, p > 0.05; Figure 4A).
Figure 3.
Effects of SD exposure and fear conditioning on dendritic spine density in the basolateral amygdala. Upper panel: SD mice have increased dendritic spine density in the BLA. Lower panel: 1000× photomicrographs of representative dendritic segments of BLA neurons from LD (left) and SD (right) mice. Scale bar = 10 µm. * p < 0.05 Student’s t test.
Figure 4.
Effects of SD exposure and fear conditioning on pyramidal neuron morphology in the infralimbic cortex. A) SD exposure did not alter spine density on either apical or basilar dendrites. B) 200× photomicrograph of a representative IL pyramidal neuron (upper) and its Nuerolucida reconstruction upon which Sholl analysis was performed (lower). C) Exposure to SD did not alter complexity of pyramidal cell dendritic arborization (Sholl analysis) of infralimbic cortical pyramidal neurons.
3.3.2. IL pyramidal neuronal morphology
No differences due to photoperiod were found in IL pyramidal neurons for cell body perimeter (t(14) = 1.002, p> 0.05), cell body area (t(14) = 1.332, p> 0.05), basilar dendrite length (t(14) = −0.066, p> 0.05), apical dendrite length (t(14) = −0.061, p> 0.05), or total dendrite length (t(14) = −0.074, p> 0.05)(data not shown). No differences were observed due to photoperiod in number of intersections (repeated measures ANOVA:F(1,14) = 410.144, p> 0.05;Figure 4C) or dendrite length (repeated measures ANOVA: F(1,14) = 761.671, p> 0.05; not shown) via Sholl analysis of dendritic arborization complexity.
4. Discussion
The present study in white-footed mice demonstrates three novel findings. 1) Exposure to short day lengths enhances associative fear memory in the auditory-cued fear conditioning. 2) Photoperiod and fear conditioning interact to increase spine density of the neurons in the BLA. However, 3) neither photoperiod nor the interaction of photoperiod and auditory-cued fear conditioning alter the morphology of pyramidal neurons within the IL.
Exposure to short days in white-footed mice reduces hippocampal volume, alters hippocampal dendritic spine density, and impairs LTP within the hippocampus [2, 4]. Pursuant to these day length induced morphological and physiological changes, SD mice have impaired hippocampal-dependent spatial learning and memory [2, 4, 15]. Non-hippocampal learning and memory, assessed by several behavioral tests, was previously reported to be unaffected by photoperiod [4]. However, in the passive avoidance fear memory test as previously demonstrated [4], although SD mice did not differ from their LD counterparts in latency to step through during training or testing phases, only SD mice increased their latency to step through after training, indicating there may be photoperiodic alterations in fear-related memory. We have extended these findings to show SD-induced enhancement of fear memory in the auditory-cued fear test (Figure 2B); supporting our hypothesis that SD exposure enhances fear memory. Additionally, increased spine density in the dendrites of the BLA neurons is associated with increased fear memory in SD mice (Figure 3). The BLA is critical for encoding fear memory and imparting emotional valence on memories [13, 17–19]. Furthermore, enhanced spine density within the BLA is associated with enhanced auditory fear memory [20]. Thus, SD exposure causes hippocampal atrophy and functional impairments in the hippocampus, whereas the amygdala becomes hypertrophic and functionally enhanced by exposure to short day lengths. Although time-of-day differences have been reported in fear learning [21–23], the differences are generally confined to contextual fear, rather than tone-cued fear [21–22]. The current findings are not likely due to differences in circadian patterns of fear learning and memory between SD and LD mice as we conducted our acquisition trials during the light phase when fear learning is strongest [21–22], and all of our testing occurred at the same circadian time for both groups, thus limiting any circadian effects.
What is the role of the IL area of the mPFC in photoperiodic alteration in fear memory? The IL/mPFC is critical for orchestrating the balance of the BLA and the hippocampus in the formation and extinction of emotionally charged memories, such as fear conditioning. Reciprocal connections between the mPFC and the BLA regulate encoding and extinction of fear memories [12]. The mPFC-hippocampus pathway also coordinates extinction of fear memories [20, 24–25], and the BLA-hippocampus pathway is critical for modulating fear memory encoding and recall [26–28]. Well-orchestrated coordination of all the three brain regions is critical for encoding, recall, and extinction of fear memories [11, 13, 29]. Although SD mice do show cognitive inflexibility in spatial reversal learning [4], presumably mediated by the IL [30], the current neuroanatomical findings (Figure 4) do not support a specific role for altered IL function in the SD enhancement of fear memory. However, the role of photoperiodic impairment in the mPFC-hippocampus pathway in cognitive flexibility in both spatial fear learning remains to be described.
One of the hallmarks of photoperiodic responses in rodents is the involution of the gonads by exposure to short day lengths (Figure 1) via inhibition of the hypothalamic-pituitary-gonadal (HPG) axis, which results in low gonadal sex steroid concentrations (reviewed in [1]). Gonadal sex steroids can influence neuronal morphology in the regions above implicated in fear memory [31–32]. Although gonadectomy can recapitulate some of the appropriate photoperiodic responses, gonadal steroids are not the sole contributing factor to the suite of behavioral and physiological responses in photoperiodic rodents (reviewed in [1]). Indeed, gonadal steroids enhance fear memory; estrogens facilitate fear conditioning by upregulating CRH expression in the amygdala [33], and androgens (testosterone and dihydrotestosterone) also facilitate conditioned fear, but the enhanced effects are mediated in the hippocampus, and not the amygdala [34]. Thus, reduced sex steroid concentrations in SD mice should lead to impaired fear memory, which argues strongly against a significant role of gonadal steroids in SD enhancement of fear memory. However, we currently have ongoing experiments to study the effects of sex steroids on hippocampal- and amygdala-mediated memory.
In addition to inhibiting the HPG axis, short days enhance HPA axis responsiveness and negative feedback in white footed mice [3]. Although photoperiod does not affect baseline corticosterone concentrations (Figure 1; [3]), SD mice display increased HPA axis responsiveness to stressors and increased negative HPA axis feedback, potentially regulated at the level of the hippocampus by SD elevation of hippocampal glucocorticoid and mineralocortocoid receptors [3]. Photoperiod alterations in the HPG and HPA axis have adaptive significance for this species. To survive the energetic bottleneck of reduced food availability and increased thermogenic demands during the short days of winter, energy is conserved by reduction of reproductive tissue mass and reproduction-associated behaviors (reviewed in [1]). Additionally, activation of the HPG axis (glucocorticoid response to stressors) to mobilize energy for the fight-or-flight response is energetically expensive, thus increased regulation (efficiency) of the HPA axis in short days may be an adaptive response to conserve energy [3].
In the North American temperate zone home range of white-footed mice, photoperiodic changes in habit alter behavior and distribution [35]. Short day mice inhabit an environment that is both energetically demanding and devoid of dense understory cover, thus the alterations in endocrine responses to stress (HPA axis) and behavioral responses to fearful stimuli (fear memory) described above may provide an adaptive advantage to survive in their winter habitat. During the short days of winter forest understory is greatly reduced and leaf cover is minimal [36], and small photoperiodic mammals, including white-footed mice, alter their habitat use and movement to areas within their home range with maximum leaf understory density for predator avoidance [37–40]. To our knowledge, the effect of photoperiod on predator avoidance has never been directly tested on white-footed mice, however short days increase predator avoidance behavior in other rodent species [41–42]. In short days, enhanced fear memory may be advantageous for survival by offsetting the interaction of reduced available ground cover for predator avoidance with impaired hippocampal-mediated spatial navigation [2, 4]. Thus, it is possible that the photoperiodic differences in fear responses reported here are ecologically relevant and are an integral part of a suite of adaptive responses to short days, which include changes in physiology and hippocampal function.
In summary, short day exposed white-footed mice have reduced hippocampal volume [4], increased HPA axis negative feedback [3], increased fear memory (Figure 2), and increased connectivity within the BLA (Figure 3). Among human PTSD patients, reduced hippocampal volume is associated with susceptibility to PTSD, but not severity of symptoms [7], amygdala activity is positively correlated with symptom severity [8], and PTSD patients have enhanced HPA axis negative feedback [7]. One of the greatest research challenges of mechanisms involved in psychiatric disorders is the difficulty in replicating the symptoms of the disease in animal models [43], and it is important to develop new ethologically-relevant models for translational research of psychiatric disorders [5, 44]. In the laboratory setting, a simple day length manipulation in white-footed mice, exposure to short days, concurrently replicates three main features of PTSD and enhances fear memory responses. Taken together, the photoperiodic modulation of brain fear circuits and fear memory in white-footed mice described in this, and previous studies, may argue for the potential utility of this species as an additional, yet unique, animal model to research how a single environmental factor (day length) can interact with genes to alter phenotype to resemble a human psychiatric disorder, such as PTSD.
Highlights.
Shorts day lengths enhance fear memory in white-footed mice (Peromyscus leucopus)
Short days increase spine density in the basolateral amygdala.
Short days do not alter dendritic spines or arborization in the infralimbic PFC.
Acknowledgements
We thank Sallion Wolfe for her expert animal care and James Regennitter for technical assistance. This work was supported by NIH R01MH057535.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walton JC, Weil ZM, Nelson RJ. Influence of photoperiod on hormones, behavior, and immune function. Front Neuroendocrinol. 2011;32:303–319. doi: 10.1016/j.yfrne.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walton JC, Chen Z, Weil ZM, Pyter LM, Travers JB, Nelson RJ. Photoperiod-mediated impairment of long-term potentiation and learning and memory in male white-footed mice. Neuroscience. 2011;175:127–132. doi: 10.1016/j.neuroscience.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pyter LM, Adelson JD, Nelson RJ. Short days increase hypothalamic-pituitary-adrenal axis responsiveness. Endocrinology. 2007;148:3402–3409. doi: 10.1210/en.2006-1432. [DOI] [PubMed] [Google Scholar]
- 4.Pyter LM, Reader BF, Nelson RJ. Short photoperiods impair spatial learning and alter hippocampal dendritic morphology in adult male white-footed mice (Peromyscus leucopus) Journal of Neuroscience. 2005;25:4521–4526. doi: 10.1523/JNEUROSCI.0795-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shekhar A, McCann UD, Meaney MJ, Blanchard DC, Davis M, Frey KA, et al. Summary of a National Institute of Mental Health workshop: developing animal models of anxiety disorders. Psychopharmacology (Berl) 2001;157:327–339. doi: 10.1007/s002130100859. [DOI] [PubMed] [Google Scholar]
- 6.Workman JL, Nelson RJ. Potential Animal Models of Seasonal Affective Disorder. Neuroscience and Biobehavioral Reviews. 2010;35:669–679. doi: 10.1016/j.neubiorev.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Dickie EW, Brunet A, Akerib V, Armony JL. Neural correlates of recovery from post-traumatic stress disorder: a longitudinal fMRI investigation of memory encoding. Neuropsychologia. 2011;49:1771–1778. doi: 10.1016/j.neuropsychologia.2011.02.055. [DOI] [PubMed] [Google Scholar]
- 9.Gold AL, Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, et al. Decreased regional cerebral blood flow in medial prefrontal cortex during trauma-unrelated stressful imagery in Vietnam veterans with post-traumatic stress disorder. Psychol Med. 2011:1–10. doi: 10.1017/S0033291711000730. [DOI] [PubMed] [Google Scholar]
- 10.Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog Brain Res. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- 11.Guimarais M, Gregorio A, Cruz A, Guyon N, Moita MA. Time determines the neural circuit underlying associative fear learning. Front Behav Neurosci. 2011;5:89. doi: 10.3389/fnbeh.2011.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vouimba RM, Maroun M. Learning-induced changes in mPFC-BLA connections after fear conditioning, extinction, and reinstatement of fear. Neuropsychopharmacology. 2011;36:2276–2285. doi: 10.1038/npp.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 14.Institute of Laboratory Animal Resources (U.S.) Guide for the care and use of laboratory animals. 7th ed. Washington, D.C.: National Academy Press; 1996. [Google Scholar]
- 15.Workman JL, Bowers SL, Nelson RJ. Enrichment and photoperiod interact to affect spatial learning and hippocampal dendritic morphology in white-footed mice (Peromyscus leucopus) European Journal of Neuroscience. 2009;29:161–170. doi: 10.1111/j.1460-9568.2008.06570.x. [DOI] [PubMed] [Google Scholar]
- 16.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Compact 2nd ed. Amsterdam; Boston: Elsevier Academic Press; 2004. [Google Scholar]
- 17.Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143:387–393. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Maren S. Long-term potentiation in the amygdala: a mechanism for emotional learning and memory. Trends Neurosci. 1999;22:561–567. doi: 10.1016/s0166-2236(99)01465-4. [DOI] [PubMed] [Google Scholar]
- 19.McGaugh JL. Memory consolidation and the amygdala: a systems perspective. Trends Neurosci. 2002;25:456. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- 20.Koseki H, Matsumoto M, Togashi H, Miura Y, Fukushima K, Yoshioka M. Alteration of synaptic transmission in the hippocampal-mPFC pathway during extinction trials of context-dependent fear memory in juvenile rat stress models. Synapse. 2009;63:805–813. doi: 10.1002/syn.20657. [DOI] [PubMed] [Google Scholar]
- 21.Rudy JW, Pugh CR. Time of conditioning selectively influences contextual fear conditioning: further support for a multiple-memory systems view of fear conditioning. J Exp Psychol Anim Behav Process. 1998;24:316–324. doi: 10.1037//0097-7403.24.3.316. [DOI] [PubMed] [Google Scholar]
- 22.Eckel-Mahan KL, Phan T, Han S, Wang H, Chan GC, Scheiner ZS, et al. Circadian oscillation of hippocampal MAPK activity and cAmp: implications for memory persistence. Nat Neurosci. 2008;11:1074–1082. doi: 10.1038/nn.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaudhury D, Colwell CS. Circadian modulation of learning and memory in fear-conditioned mice. Behav Brain Res. 2002;133:95–108. doi: 10.1016/s0166-4328(01)00471-5. [DOI] [PubMed] [Google Scholar]
- 24.Takita M, Izaki Y, Jay TM, Kaneko H, Suzuki SS. Induction of stable long-term depression in vivo in the hippocampal-prefrontal cortex pathway. Eur J Neurosci. 1999;11:4145–4148. doi: 10.1046/j.1460-9568.1999.00870.x. [DOI] [PubMed] [Google Scholar]
- 25.Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Huff NC, Frank M, Wright-Hardesty K, Sprunger D, Matus-Amat P, Higgins E, et al. Amygdala regulation of immediate-early gene expression in the hippocampus induced by contextual fear conditioning. J Neurosci. 2006;26:1616–1623. doi: 10.1523/JNEUROSCI.4964-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seidenbecher T, Laxmi TR, Stork O, Pape HC. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science. 2003;301:846–850. doi: 10.1126/science.1085818. [DOI] [PubMed] [Google Scholar]
- 29.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li L, Shao J. Restricted lesions to ventral prefrontal subareas block reversal learning but not visual discrimination learning in rats. Physiol Behav. 1998;65:371–379. doi: 10.1016/s0031-9384(98)00216-9. [DOI] [PubMed] [Google Scholar]
- 31.Cooke BM, Woolley CS. Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol. 2005;64:34–46. doi: 10.1002/neu.20143. [DOI] [PubMed] [Google Scholar]
- 32.McEwen BS. Stress, sex, and neural adaptation to a changing environment: mechanisms of neuronal remodeling. Ann N Y Acad Sci. 2010;1204(Suppl):E38–E59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jasnow AM, Schulkin J, Pfaff DW. Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Horm Behav. 2006;49:197–205. doi: 10.1016/j.yhbeh.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Edinger KL, Lee B, Frye CA. Mnemonic effects of testosterone and its 5alpha-reduced metabolites in the conditioned fear and inhibitory avoidance tasks. Pharmacol Biochem Behav. 2004;78:559–568. doi: 10.1016/j.pbb.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 35.King JA. Biology of Peromyscus (Rodentia) Stillwater, Okla.: American Society of Mammalogist; 1968. [Google Scholar]
- 36.Bratton SP. Resource Division in an Understory Herb Community - Responses to Temporal and Microtopographic Gradients. American Naturalist. 1976;110:679–693. [Google Scholar]
- 37.Barnum SA, Manville CJ, Tester JR, Carmen WJ. Path Selection by Peromyscus leucopus in the Presence and Absence of Vegetative Cover. Journal of Mammalogy. 1992;73:797–801. [Google Scholar]
- 38.Litvaitis JA, Sherburne JA, Bissonette JA. Influence of Understory Characteristics on Snowshoe Hare Habitat Use and Density. J Wildlife Manage. 1985;49:866–873. [Google Scholar]
- 39.Bowers MA, Dooley JL. Predation Hazard and Seed Removal by Small Mammals - Microhabitat Versus Patch Scale Effects. Oecologia. 1993;94:247–254. doi: 10.1007/BF00341324. [DOI] [PubMed] [Google Scholar]
- 40.Kaufman DW, Peterson SK, Fristik R, Kaufman GA. Effect of Microhabitat Features on Habitat Use by Peromyscus leucopus. Am Midl Nat. 1983;110:177–185. [Google Scholar]
- 41.Borowski Z. Individual and seasonal differences in antipredatory behaviour of root voles - a field experiment. Can J Zool. 2002;80:1520–1525. [Google Scholar]
- 42.Herman CS, Valone TJ. The effect of mammalian predator scent on the foraging behavior of Dipodomys merriami. Oikos. 2000;91:139–145. [Google Scholar]
- 43.Miller MM, McEwen BS. Establishing an agenda for translational research on PTSD. Ann N Y Acad Sci. 2006;1071:294–312. doi: 10.1196/annals.1364.023. [DOI] [PubMed] [Google Scholar]
- 44.Clinchy M, Schulkin J, Zanette LY, Sheriff MJ, McGowan PO, Boonstra R. The Neurological Ecology of Fear: Insights Neuroscientists and Ecologists Have to Offer one Another. Front Behav Neurosci. 2010;4:21. doi: 10.3389/fnbeh.2011.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]