Abstract
Background
Pirfenidone (PFD) is an anti-fibrotic agent with beneficial effects upon proinflammatory disorders. In this study, we further investigated PFD and long acting form, “deuterated (d)PFD” immune modulating properties by evaluating their effects on mouse dendritic cells (DCs).
Methods
The effects of PFD upon DCs were examined in vivo using an orthotopic mouse lung transplant model and in vitro utilizing isolated bone marrow derived DCs in response to lipopolysaccharide and allogeneic stimulation.
Results
In mouse lung transplants, PFD and dPFD treatment improved allograft lung function based on peak airway pressure, less infiltrates/consolidation on microCT scan imaging, and reduced lung rejection/injury. DC activation from lung allografts was suppressed with PFD and there appeared to be a greater effect of PFD upon CD11c+CD11b−CD103+ lung DCs. In addition, PFD reduced the expression of a number of proinflammatory cytokines/chemokines from lung allografts. In vitro, DCs treated with PFD showed decreased expression of MHC class II and co-stimulatory molecules and impaired DC’s capacity to stimulate T cell activation while antigen uptake was preserved. PFD directly inhibited the release of inflammatory cytokines from isolated DCs, and was associated with a reduction of stress protein kinases and attenuated LPS-dependent MAPKp38 phosphorylation.
Conclusion
PFD has lung allograft protective properties and in addition to its known effects on T cell biology, PFD’s immune modulating activities encompass inhibitory effects upon DC activation and function.
Keywords: pirfenidone, lung transplantation, dendritic cell, acute rejection, mouse
Introduction
Pirfenidone (5-methyl-1-phenyl-2-(1H)-pyridone) (PFD) is an orally active anti-fibrotic agent shown to be efficacious in fibroproliferative disease models, including pulmonary fibrosis (1–3). In clinical trials, PFD was found to have a short half-life (~4 hours), but had limited side effects and was well tolerated by most patients (4, 5). Because of its safety profile and potential to ameliorate both pro-inflammatory and pro-fibrotic processes, we evaluated its role in transplantation.
Our laboratory previously showed that PFD reduces the obstructive airway disease in mouse heterotopic tracheal transplants and transplant-mediated fibrosis in rat lung transplants (6–8). Moreover, PFD delayed acute rejection and prolonged mouse heterotopic cardiac allograft survival (9). PFD protective properties are believed to be from its inhibition of profibrotic and inflammatory cytokines (5, 10, 11). However, we also observed a modest but significant effect upon T cell activation (9), demonstrating immune modulating properties for PFD. Although PFD inhibition on T cell proliferation and activation was significant, the response we observed in vivo appeared more robust and may not be explained solely by its inhibitory effect upon T cells.
Dendritic cells (DCs) are professional antigen-presenting cells capable of inducing activation of both innate and adaptive immune systems. DCs sense their environment via their pattern recognition receptors and respond by secreting cytokines and chemokines thereby activating both innate and adaptive immunity. Upon exposure to foreign antigens, DCs uptake, process and present antigens to T cells (12). Stimulation of DC receptors initiates maturation, thereby loading antigen into MHC molecules, up-regulation of co-stimulatory molecules and MHC, and activation of T cells (13).
In this study, we further explored PFD actions upon inflammation in order to better understand its immune modulating properties. We hypothesized that PFD has inhibitory effects upon DC activation and abrogates its capacity to induce innate and adaptive immune response. Therefore, we examined the effects of PFD and long acting PFD “deuterated pirfenidone” (dPFD) in vivo using the mouse lung transplant model and in vitro using bone marrow derived DCs. We observed a remarkable inhibitory effect of PFD upon DC activation, maturation, and function.
Results
PFD reduces mouse lung allograft rejection/injury and DC activation
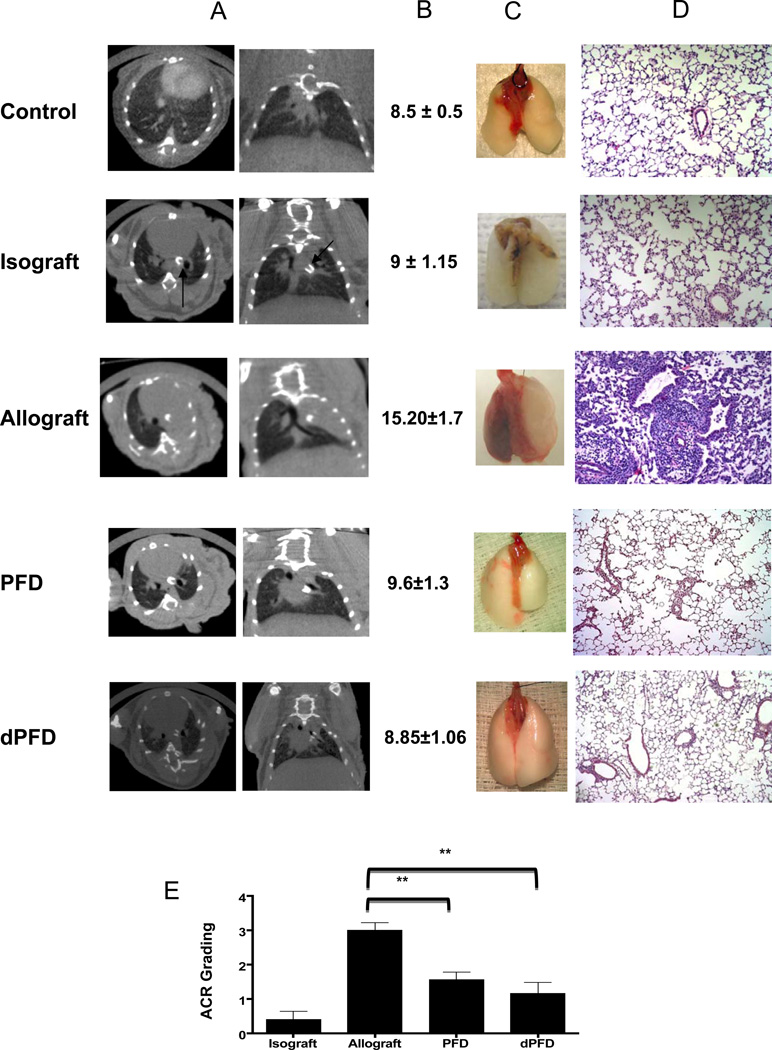
Murine lung transplants with a full MHC mismatch, BALB/c to C57BL/6, were utilized to examine PFD in vivo effects upon DCs. Lung allografts were initially evaluated by in vivo microCT imaging with mouse thorax microCTs illustrating consolidation of untreated lung allografts while PFD treated allografts show little disease (Figure 1A). Lung function based on peak airway pressure (PawP) with delivery of a fixed tidal volume was also improved in PFD and dPFD treated allografts (Figure 1B). PFD and dPFD treatment groups showed less acute cellular rejection (ACR) (Figure 1C and D), approximately A3 based on International Society of Heart and Lung Transplantation grading scale (14) in untreated allografts as compared to mean of 1.5 and 1.1 with PFD and dPFD respectively (Figure 1E).
Figure 1. PFD and dPFD inhibit acute allograft rejection.
Mouse lung transplants were performed and evaluated after 7 days. The different groups include untransplanted mouse lungs (Control), Isografts (C57BL/6 donor lung into C57BL/6 recipient), Allograft (BALB/c lungs transplanted into C57BL/6 mice treated with vehicle), PFD (400mg/kg/day) treated recipient, and dPFD (150 mg/kg/day) treated recipient. Each group represents 4–8 mice per experimental group and significance is based on p value < 0.05. A) Representative in vivo micro CTs of mouse thorax for a control, isograft, allograft, and PFD treated allograft-showing improvement in PFD and dPFD treated recipients as compared to untreated recipients. B) PawP (cmH2O) was significantly reduced in both the PFD and dPFD treated mice as compared to untreated allografts and no different from control or isografts. C) Representative gross pictures of both explanted naive right and left allograft lungs from the different groups with the allograft being hyperemic and smaller as compared to the other groups. D) Representative example of HE stains for each group with allograft showing an increased perivascular and peribronchial inflammatory infiltrate with both PFD and dPFD showing less cellular infiltrate. E) Graphic representation of ACR grade for the different groups with both PFD and dPFD recipients having a significant reduction of ACR as compared to vehicle treated allograft.
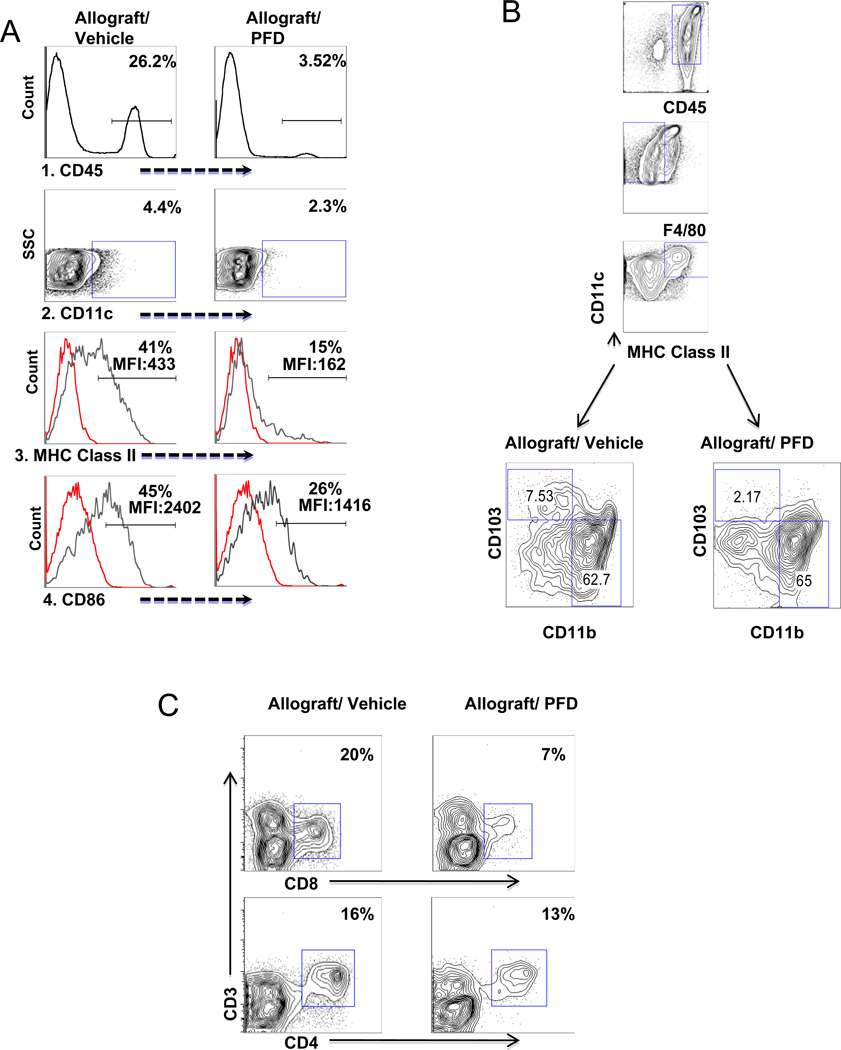
To better assess PFD’s effects upon DCs, we evaluated single cell suspensions of treated and untreated lung allografts for CD45, CD11c, MHC class II, and CD86 expression based on flow cytometry, 7 days after transplantation (Figure 2A). As expected, PFD resulted in a decrease in the commonly used marker for leukocytes, CD45+ cells, and more importantly reduced the percentage of DCs (CD11c+) within the CD45 population. Within CD11c+ cells there was a reduction in MHC class II and CD86 suggesting an inhibition of DC activation/maturation. Lung DCs were better characterized by isolating CD11c+ cells from PFD and vehicle treated lung allografts and analyzed based on CD45+ F4/80− CD11c+ MHCII+ cells gating and CD103 and CD11b surface expression. PFD treatment reduced CD103+ cells, which are thought to activate naïve CD8+ T cells in lung (15, 16) while no difference in the percentage of CD11b+ cells (Figure 2B) was noted. PFD reduced both CD4 and CD8 T cell with a further reduction in the percentage of CD3+CD8+ T cells but little difference in the percentage of CD3+CD4+ T cell (Figure 2C). Further evidence of PFD’s effects upon the inflammatory response was a decrease in a number of cytokines based on Luminex assay (see table in supplemental data) including CSF3, IFN-γ, IL-2, IL-1β, TNF-α, RANTES, IL-4, IL-5, IL-6, IL-13, IP-10, and MCP-1.
Figure 2.
A. PFD treatment reduces activation of DC in vivo
CD45+ and CD11c+ cells were isolated based on flow cytometry from both untreated and PFD treated mouse lung allografts seven days after transplantation. 1.) Anti-CD45 marker was used to determine total leukocytes in allografts. There is a decrease in percentage of CD45+ cells in transplanted lung with PFD treatment. 2.) CD11c+ expression on gated CD45+ cells showed a decrease of DCs in PFD treated allografts. 3. and 4.) To determine activation of DCs, the percentage/MFI of MHC class II and CD86 expression on the gated CD11c+ cells were analyzed and the results reveal a decrease in activation of DCs in PFD treated allografts. Illustrated are representative analyses of the MHC class II and CD86 with isotype controls for 4 PFD and untreated transplant samples.
B. PFD treatment reduce CD11b− CD103+ DC
Highly purified CD11c+ cells were sorted by magnetic cells sorting from both untreated and PFD treated mouse lung allografts seven days after transplantation. CD45+F4/80− CD11c+ MHC Class II+ cells were gated and surface expression of CD11b and CD103 analyzed. The result showed a decrease in CD11b−CD103+ cells but not CD11b high cells. Illustrated are representative analyses of each for 4 individual PFD treated and untreated transplant samples. Illustrated are representative analyses for 4 PFD and untreated transplant samples.
C. PFD treatment reduce lung CD8 T cells
Seven days after transplantation, single cell suspensions from lung allografts of both untreated and PFD treated mouse recipients were stained with anti-CD45, anti-CD3, anti-CD4 and anti-CD8. There was a decrease in the percentage of CD3+CD8+ T cells but not CD3+CD4+ T cells. Illustrated are representative analyses for 4 PFD and untreated transplant samples.
Pirfenidone abrogates dendritic cell activation/maturation
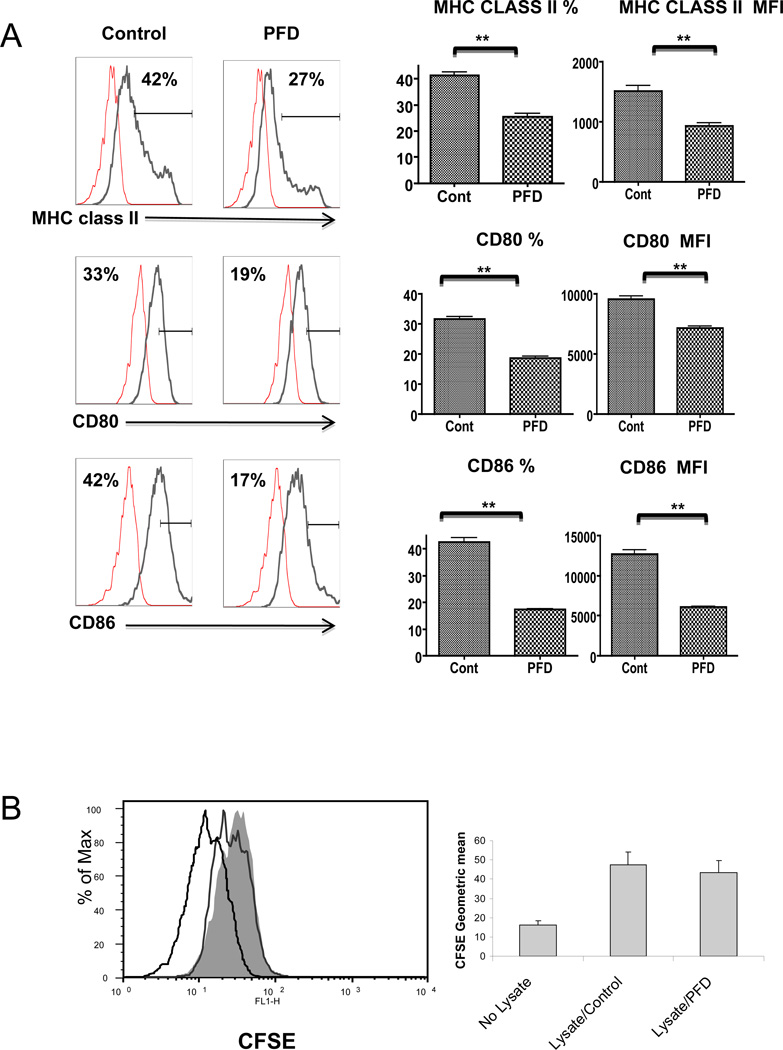
To further examine PFD’s effect on DC activation and maturation, in vitro studies were performed using a known activator of DCs, lipopolysaccharide (LPS) (17). As expected, LPS stimulated DC activation and maturation while PFD decreased the maturation markers, MHC class II, CD80, and CD86 expression (Figure 3A) following LPS stimulation. To determine if PFD altered DCs capacity for endocytosis, we examined the ability of these cells to uptake alloantigens. Lysates of CFSE labeled BALB/c spleen cells were incubated overnight with DCs from C57BL/6 mice, and endocytosis measured based on CFSE uptake within DCs. No significant difference was seen in DCs uptake of allogeneic cell lysates, with or without PFD exposure, indicating that PFD does not affect DC endocytosis (Figure 3B).
Figure 3.
A. PFD inhibits DC maturation and activation in vitro;
DCs were incubated with or without 2 mM PFD for 72 hours, stimulated with 1µg/ml LPS, and evaluated 24 h later based on MHC class II, CD80, and CD86 expression using flow cytometry. The PFD treated DCs express significantly less MHC class II and the co-stimulatory molecules CD80 and CD86 as compared to control/untreated cells. Presented are representative flow cytometry histograms showing mean fluorescence intensity (MFI) of the staining for each of the antibodies along with isotype control and graphs for percentage of MHC class II, CD80 and CD86 positive cells and MFI of each based on the mean +/− SEM of 4 independent experiments.
B. PFD does not inhibit DCs alloantigen uptake;
C57BL/6 BM derived DCs were incubated with or without 2mM PFD for 72 h. DCs were then exposed to CFSE pre-labeled BALB/c splenocyte lysates at 1:5 ratio overnight. DC ability to uptake was assessed by flow cytometry for CFSE labeling within DCs. PFD treatment had no significant effect on DCs ability to uptake alloantigen. A representative histogram is shown illustrating negative control (no CFSE), CFSE staining with (unshaded) and without (shaded) PFD treatment along with a graph illustrating the MFI mean +/− SE for the ability of C57BL/6 DC to endocytose CFSE pre-labeled BALB/c splenocyte lysate.
PFD inhibits DC -T cell interaction
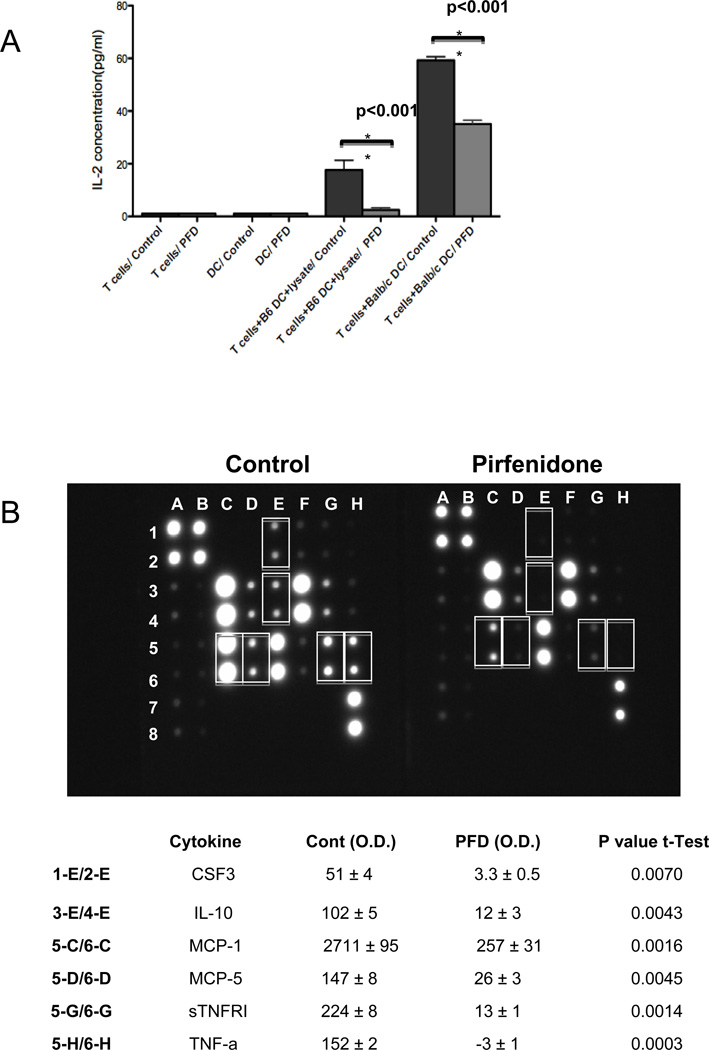
In transplanted tissue, donor (direct allorecognition) and recipient (indirect allorecognition) derived DCs stimulate T cells by presenting antigen via MHC class I/II and co-stimulatory molecules (18). To address whether PFD affects DC-T cell interactions, we examined both direct and indirect pathways of DCs to allo-specific T cell activation based on IL-2 secretion. C57BL/6 or BALB/c BM derived DCs were initially incubated with PFD, placed in media without PFD and exposed to previously sensitized C57BL/6 derived CD3+ T cells to stimulate an allogeneic response. As shown in Figure 4A, PFD exposure resulted in impaired T cell stimulation with limited IL-2 secretion indicating an inhibition of DCs ability to activate T cells via direct and indirect stimulation.
Figure 4.
A. PFD inhibits DC direct and indirect T cell activation
To determine the response of PFD to the indirect pathway, C57BL/6 +/− PFD pretreated DCs were incubated with BALB/c splenocyte lysate (1:5 ratio) and previously stimulated CD3+ T cells (1:1 ratio). For the direct pathway, BALB/c +/− PFD pretreated DCs were incubated with previously stimulated C57BL/6 alloreactive CD3+ T cells. The DC-T cell co-cultures were without PFD so PFD had no direct effect upon T cells. Cell supernatant was collected 24 hours after stimulation and evaluated for T cell activation based on IL-2 secretion as measured by ELISA (pg/ml). There is a significant decrease in IL-2 expression of T cells that were stimulated with PFD treated C57BL/6 and BALB/c DCs thereby demonstrating indirect and direct pathways inhibition. The graph illustrates the mean +/− SEM (n = 4–6 per group) of IL-2 from unstimulated T cells +/− PFD and stimulated (T cells + DC + lysate) +/− PFD.
B. PFD inhibits stimulated DC cytokine secretion
Isolated DCs were treated with or without 2mM PFD for 72 hours and stimulated with 1µg/ml LPS for 24 hours. The supernatant was collected and cytokines measured based on mouse multiple cytokine array panel and relative cytokine value assessed by densitometry for each individual cytokine spot. Represented is an example of one of the multiple cytokine array panels. Densitometry analysis of the PFD inhibited cytokines is presented in the table and is based on three independent experiments showing the mean +/− SEM and p value based on individual t-tests.
PFD inhibits DC cytokine production
As another measure of DC activation, we analyzed the effect of PFD on cytokine production of LPS-stimulated DCs. PFD inhibited the secretion of CSF3, IL-10, MCP-1, CCL12 (MCP-5), soluble tumor necrosis factor receptor I (sTNFRI), and TNF-α while there was no significant change in the levels of IL-6, IL-9, IL-12p40, IL-12p70, or RANTES (Figure 4B). This data showed that PFD selectively inhibits cytokine release from stimulated DCs.
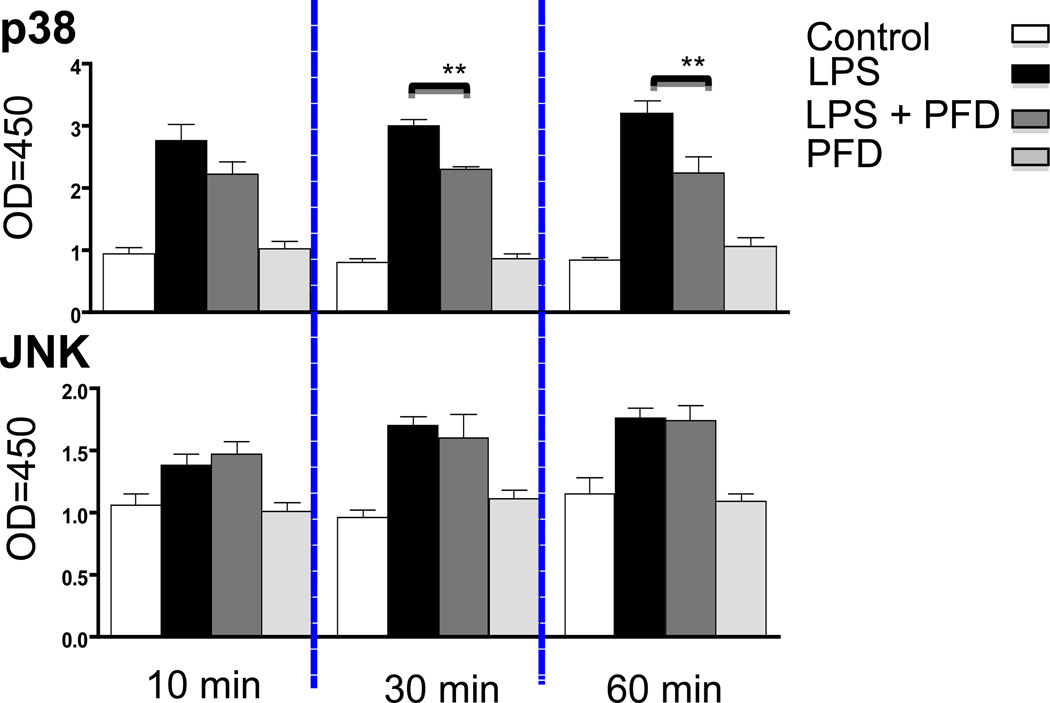
PFD selectively inhibits p38 Map kinase phosphorylation
The MAPK signaling pathway is activated under a variety of cellular stresses including LPS induced maturation and upregulation of DC surface antigens via two prototype families of MAPK, p38 and JNK (19, 20). We examined whether PFD actions may be mediated by altering p38 and JNK. In DCs, PFD attenuated LPS stimulated p38 phosphorylation after 30 and 60 minutes while it had little effect upon JNK stimulated phosphorylation (Figure 5). The relative increase of p38 phosphorylation in PFD treated cells to LPS was significantly less as compared to non-PFD exposed cells to LPS.
Figure 5. PFD inhibits p38 MAPK phosphorylation.
The effect of PFD on MAPK activation was measured using the p38 and JNK cell- based assay. Isolated DCs with or without 2mM PFD for 72 hours were stimulated with 1µg/ml LPS and p38 MAPK and JNK phosphorylation measured at 0, 10, 30, and 60 minutes. DCs stimulated with LPS showed an increase in both p38 and JNK phosphorylation while PFD treated DCs demonstrated a decrease in p38 MAPK phosphorylation at 30 and 60 min but there was no effect on the JNK phosphorylation. ** Represents p value < 0.05 (n = 3 independent experiments) for LPS versus LPS plus PFD treated DCs.
Discussion
PFD is best known as an anti-fibrotic agent and shown to be efficacious in blocking the development of fibroproliferative disorders (1, 21). PFD was initially thought to have little significant immunosuppressive properties. However, our laboratory and others have shown that it inhibits both inflammatory and profibrotic cytokine expression and has beneficial effects against immune-mediated injury models such as multiple sclerosis, asthma, and transplantation (6, 7, 22–24). Its immunosuppressive effects is partially related to its inhibition of proinflammatory cytokines; however, it is now clear that PFD has additional immune modulating activities with impairment of T cell activation, and this study demonstrated its inhibitory actions upon DCs. Using an in vivo model, mouse lung transplantation, not only showed that PFD reduced lung injury/rejection, but had an inhibitory effect upon lung DC activation. This was also seen systemically with reduced CD11c+ cells and activation markers in thoracic lymph nodes and spleen (data not shown).
Lung allograft DC profile was better characterized based on the two major myeloid CD11c+MHC class II+ DC populations in the lung, CD11b+CD103− and CD11b−CD103+ (25). Although we observed no difference in CD11b+CD103− DCs, there was an inhibition of CD103+ DCs. While the roles of CD103+ and CD103− DCs are not rigorous, CD103+ DCs are believed to preferentially cross-present antigen to CD8+ T cells while CD103− DCs present antigen to CD4+ T cells (15, 16). This may explain why we observed a reduction in CD8+ as compared to CD4+ T cells. Interestingly, mice with a targeted disruption of CD103 were also shown to have less rejection of islet allografts (26).
In this study, PFD was given one day prior to transplantation. We found that PFD given after transplantation had limited success in suppressing transplant-mediated injury of mouse lung allografts (data not shown). We also observed this in mouse heterotopic tracheal transplants (8). That PFD needs to be given early in the transplant process suggests its primary actions may be on DC activation, thereby, preventing the early innate and adaptive process rather than having a potent immune effect directly upon T cells with little if any benefit as a rescue agent.
PFD has a short half-life making delivery to mice or patients difficult (27). Recently, dPFD was developed (PCT US2008/010565) showing a longer half-life and increased levels for the same dose. The use of deuterium does not change an agent’s biochemical potency or selectivity to pharmacological targets; however, deuterium may alter its pharmacokinetics (28). We found that dPFD at approximately 1/3rd the dose was equally if not more efficacious than standard PFD.
We utilized different methods to assess mouse lung allograft injury including microCT imaging, lung function, and histology. This is the first report that we are aware of for mouse lung allograft imaging. The imaging studies coincided with histology showing minimal rejection in PFD treated allografts while untreated grafts showed severe rejection. A major advantage of this technique is that mouse lung allografts can be monitored over time since it is not a terminal outcome measure as are the other techniques
We found that PFD has an inhibitory action upon stimulated DC activation, maturation and function. This was not attributed to cell death due to drug toxicity since in the preliminary phase of the study we observed a similar ratio of cell viability with or without PFD based on forward and side scatter light properties and propidium iodide (data not shown). We also found a reduced response to the TLR2 (Zymosan) and TLR 3 (polyI;C) agonist after PFD treatment (data not shown). To go along with the more immature DC phenotype with PFD, DC endocytosis was not impaired by PFD. However, PFD had a remarkable inhibitory effect on DC’s ability to present antigen and stimulate T cell activation. This was addressed for both direct and indirect pathways. As expected we found that the direct response was more potent (18), and PFD inhibited both pathways with a nearly 50% reduction of IL-2 release for the direct pathway and nearly complete inhibition of the indirect pathway.
PFD inhibitory effects may be attributed not only to lower MHC and co-stimulation expression in PFD treated DC but also to lower inflammatory cytokine secretion from DCs. This was evident by a selective inhibition of proinflammatory cytokines from stimulated DCs in response to PFD including TNF-α, sTNFR1, CSF3, MCP-1 and MCP-5, IL-10. Interestingly we observed a decrease in the anti-inflammatory cytokine, IL-10, which participates in recovery phase of infection and reduces tissue damage (29, 30). However, there was no significant decrease of IL-10 from mouse lung allografts treated with PFD (see supplemental data), nor did we see an effect upon IL-10 secretion from T cells in response to PFD (9), so the overall effect of PFD upon IL-10 appears to be limited.
The inflammatory response along with DC activation and cytokine production is mediated through a number of signaling molecules. We initially performed a TLR signaling pathway PCR microarray and found PFD inhibited a number of kinases including MAPK signaling molecules. Therefore, we evaluated whether PFD alters a major MAPK signaling cascade. Previously, PFD was shown to inhibit p38 and JNK gene expression in cardiac tissue (31). We also observed 25–40% decrease in p38, JNK, and ERK protein levels with PFD exposure of DCs (data not shown). Similar to previous studies, LPS stimulation of DCs increased p38 MAPK and JNK activation/phosphorylation (32, 33), while PFD inhibited p38 phosphorylation but not JNK phosphorylation. The selective inhibition of the MAPK signaling pathways could be one explanation for the differential cytokine response in response to PFD.
This study illustrates that PFD has an inhibitory affect on stimulated DCs and this is a potential mechanism for its protection of lung allografts. Future studies are planned to more definitively substantiate the actions of PFD on DCs in transplantation by ex vivo treatment of DC with PFD and subsequent adoptive transfer. However, this work further supports the benefits of PFD in transplantation and its potential as a therapeutic agent. Compared to most therapies for transplantation, PFD has relatively minimal side effects. Despite the encouraging results of this study, we do not expect PFD to be effective long term as a single therapy, but as adjunct therapy, which will augment immunosuppresion without significant increase in drug toxicity. The other potential therapeutic benefit is its anti-fibro proliferative actions thereby reducing fibrosis, which is a hallmark of chronic rejection.
Material and Methods
Lung transplantation
BALB/c (H-2d) and C57BL/6 (H-2b), mice were purchased from Jackson Laboratory (Bar Harbor, ME). The Institutional Animal Care and Use Committees of the Children’s Hospital Boston approved all protocols. Orthotopic mouse lung transplantation was performed with modifications as previously described for rat lung transplantation (6, 7). Briefly, the mouse donor (BALB/c) was anesthetized with ketamine/xylazine, intubated orotracheally, and ventilated with 1–2% isoflurane/air at 120 breaths/min with a tidal volume of 8 ml/kg (Harvard Rodent Ventilator, Model 687; Harvard Apparatus, Boston, MA, USA). A thoracotomy was performed and lung flushed with ice-cold low-potassium dextran preservation solution (Perfadex®; Vitrolife, Gotenberg, Sweden). The left lung was isolated and cuffs placed into left pulmonary vein, bronchus and artery, respectively. The recipient (C57BL/6) was anesthetized with ketamine/xylazine, intubated, and ventilated with isoflurane/oxygen. A thoracotomy was performed and donor lung implanted using the three-cuffed hilar structures. After implantation, the incision was closed and recipient mouse extubated and monitored following surgery. Seven days after transplantation in vivo assessment of transplanted lung function was performed by measuring PawP as previously described (7). ACR was assessed by hematoxylin and eosin (H&E) staining of lung tissue and based on grading criteria (A0–A4) from the International Society of Heart and Lung Transplantation (14).
Experimental Groups
The experimental groups included untransplanted control (normal), isografts, vehicle treated allografts, PFD 400 mg/kg (CAS 53179-13-8 from the Department of Chemistry, University of Florida, Gainesville, FL), and d PFD 150 mg/kg (Organix Inc., Woburn, MA). PFD and dPFD solutions were prepared in 10% dimethyl sulfoxide (Sigma Aldrich, St Louis, MO) and given by subcutaneous injection one day before lung transplantation and continued for 7 days after surgery.
Computed tomography
Micro computed tomography (CT) imaging was performed using the Siemens MicroCAT II scanner with the radiation detector configured for a transaxial field of view 5.4 cm and axial field of view 8 cm. Animals were anesthetized (isoflurane or ketamine/zylazine), and positioned headfirst prone on the imaging table. Data were acquired with x-ray tube voltage of 80 kVp, tube currents of 500 uA and exposures per view of 300 ms for 200 views.
Lung DCs
Single cell suspensions were obtained from lungs 7 days post transplantation by enzyme digestion (Liberase Blendzyme 3, Roche Applied Science, Indianapolis, IN). Antibodies were purchased from BD Biosciences and EBiosciences. DC phenotyping was based on flow cytometry (LSR II BD Biosciences) using the following antibodies; anti-CD11c, anti-CD86, anti-CD45, and anti-I-A/I-E (MHC) while T cells stained for anti-CD45, anti-CD3, anti-CD4, and anti-CD8. In separate experiments, highly purified DCs were obtained by magnetic sorting of CD11c+ cells (Stem Cell Technologies, Vancouver, Canada), and stained with anti-CD103, anti-CD11b, anti-F4/80, anti-CD86, anti-I-A/I-E, and anti-CD45. Viable cells were selected based on forward and side scatter light properties and analyzed with FlowJo software (Tree Star software Inc, Portland, OR).
DC isolation and maturation
C57BL/6 bone marrow (BM) derived DCs were generated as previously described (34). Cells were plated in RPMI with 10% FBS supplemented with penicillin, streptomycin, glutamine and recombinant mouse granulocyte-macrophage colony stimulating factor (CSF2) (EBioscience). DCs (>95%) were obtained at day 7 by CD11c+ magnetic cell sorting (Stem Cell Technologies), and cultured with/without 2 mM PFD for 72 hours and stimulated with 1 µg/ml LPS (Escherichia coli 0127:B8, Sigma-Aldrich) for 24 hours.
DC in vitro endocytosis
BALB/c allogeneic splenocytes were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE, Sigma-Aldrich), and cell lysates obtained by three rapid freeze/thaw cycles. Endocytosis was measured by incubating CFSE cell lysates with DCs +/− PFD for 24 hours, washed with phosphate-buffered saline and uptake of labeled cell lysates analyzed by flow cytometry.
DC antigen presentation
DC antigen presentation in response to PFD was evaluated with modifications as previously described (35). BALB/c spleen cells were injected intraperitoneally into C57BL/6 mice, and two weeks later spleen CD3+ T cells isolated by flow cytometry sorter MoFlo (Beckman Coulter, Miami, FL). The indirect pathway was analyzed by incubating C57BL/6 derived DCs with sensitized syngeneic isolated CD3+ T cells at 1:1 ratio and BALB/c allogeneic splenocyte lysates at 1:5 ratio for 24 hours. The direct pathway was analyzed by incubating T cells with BALB/c BM derived DCs. T cell activation was assessed by enzyme-linked immunosorbent assay (ELISA) for secreted IL-2 (RayBiotech, Norcross, GA). Before co-culturing DCs with T cells, media were exchanged with FBS and PFD free media.
Cytokine production
C57BL/6 BM derived DCs were cultured with/without PFD for 72 hours, stimulated with LPS, and supernatant analyzed after 24 hours for cytokine release by Mouse Cytokine Antibody Array (RayBiotech). The relative amounts were determined by densitometry with membrane background measurements subtracted from blanks, and normalized to the positive control readings.
p38 and JNK phosphorylation assay
C57BL/6 DCs +/− PFD were stimulated with LPS for 0, 10, 30, and 60 min. Intracellular p38 mitogen-activated protein kinase (MAPK) and c-jun NH2-terminal kinase (JNK) activation were measured using phospho-p38 and phospho JNK ELISA (RayBiotech). To calculate p38 and JNK phosphorylation, the intensity of color measured at 450 nm and optical density value for each time point was normalized to 0 min.
Statistics
Data were expressed as mean ± standard error of the mean (SEM), and statistical analyses performed using Prism statistical program (Graph Pad, San Diego, CA). One-way ANOVA with Tukey's Multiple Comparison Test were used to evaluate differences between groups, and student t-test to compare two groups. P values less than 0.05 were considered significant.
Supplementary Material
Acknowledgments
Grant: NIH, NIAID, R03 AI074646; NHLBI, RO1 HL088191 (Gary Visner)
List of Non-standardized Abbreviations
- ACR
acute cellular rejection
- CT
computed tomography
- DC
dendritic cells
- PFD
pirfenidone
- dPFD
deuterated PFD
- PawP
peak airway pressure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author’s contribution:
Peyman Bizargity- participated in research design, performance of research, data analysis, and writing of the paper
Kaifeng Liu- participated in research design and performance of research
Liqing Wang- participated in performance of research and data analysis
Wayne W. Hancock- participated in research design and writing of the paper
Gary A. Visner- participated in research design, data analysis, and writing of the paper
References
- 1.Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171(9):1040. doi: 10.1164/rccm.200404-571OC. [DOI] [PubMed] [Google Scholar]
- 2.Azuma A, Taguchi Y, Ogura T, et al. Exploratory analysis of a phase III trial of pirfenidone identifies a subpopulation of patients with idiopathic pulmonary fibrosis as benefiting from treatment. Respir Res. 12:143. doi: 10.1186/1465-9921-12-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spagnolo P, Del Giovane C, Luppi F, et al. Non-steroid agents for idiopathic pulmonary fibrosis. Cochrane Database Syst Rev. (9):CD003134. doi: 10.1002/14651858.CD003134.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Nagai S, Hamada K, Shigematsu M, Taniyama M, Yamauchi S, Izumi T. Open-label compassionate use one year-treatment with pirfenidone to patients with chronic pulmonary fibrosis. Intern Med. 2002;41(12):1118. doi: 10.2169/internalmedicine.41.1118. [DOI] [PubMed] [Google Scholar]
- 5.Raghu G, Johnson WC, Lockhart D, Mageto Y. Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone: results of a prospective, open-label Phase II study. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1061. doi: 10.1164/ajrccm.159.4.9805017. [DOI] [PubMed] [Google Scholar]
- 6.Liu H, Drew P, Cheng Y, Visner GA. Pirfenidone inhibits inflammatory responses and ameliorates allograft injury in a rat lung transplant model. J Thorac Cardiovasc Surg. 2005;130(3):852. doi: 10.1016/j.jtcvs.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Liu H, Drew P, Gaugler AC, Cheng Y, Visner GA. Pirfenidone inhibits lung allograft fibrosis through L-arginine-arginase pathway. Am J Transplant. 2005;5(6):1256. doi: 10.1111/j.1600-6143.2005.00876.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhou H, Latham CW, Zander DS, Margolin SB, Visner GA. Pirfenidone inhibits obliterative airway disease in mouse tracheal allografts. J Heart Lung Transplant. 2005;24(10):1577. doi: 10.1016/j.healun.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Visner GA, Liu F, Bizargity P, et al. Pirfenidone inhibits T-cell activation, proliferation, cytokine and chemokine production, and host alloresponses. Transplantation. 2009;88(3):330. doi: 10.1097/TP.0b013e3181ae3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iyer SN, Gurujeyalakshmi G, Giri SN. Effects of pirfenidone on transforming growth factor-beta gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Ther. 1999;291(1):367. [PubMed] [Google Scholar]
- 11.Spond J, Case N, Chapman RW, et al. Inhibition of experimental acute pulmonary inflammation by pirfenidone. Pulm Pharmacol Ther. 2003;16(4):207. doi: 10.1016/S1094-5539(03)00026-9. [DOI] [PubMed] [Google Scholar]
- 12.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(7161):419. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 13.Liu G, Zhang L, Zhao Y. Modulation of immune responses through direct activation of Toll-like receptors to T cells. Clin Exp Immunol. 160(2):168. doi: 10.1111/j.1365-2249.2010.04091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26(12):1229. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 15.del Rio ML, Bernhardt G, Rodriguez-Barbosa JI, Forster R. Development and functional specialization of CD103+ dendritic cells. Immunol Rev. 234(1):268. doi: 10.1111/j.0105-2896.2009.00874.x. [DOI] [PubMed] [Google Scholar]
- 16.del Rio ML, Rodriguez-Barbosa JI, Kremmer E, Forster R. CD103- and CD103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to CD4+ and CD8+ T cells. J Immunol. 2007;178(11):6861. doi: 10.4049/jimmunol.178.11.6861. [DOI] [PubMed] [Google Scholar]
- 17.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85(2):85. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 18.Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J Immunol. 1999;162(1):352. [PubMed] [Google Scholar]
- 19.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 20.Xie J, Qian J, Wang S, Freeman ME, 3rd, Epstein J, Yi Q. Novel and detrimental effects of lipopolysaccharide on in vitro generation of immature dendritic cells: involvement of mitogen-activated protein kinase p38. J Immunol. 2003;171(9):4792. doi: 10.4049/jimmunol.171.9.4792. [DOI] [PubMed] [Google Scholar]
- 21.Lasky J. Pirfenidone. IDrugs. 2004;7(2):166. [PubMed] [Google Scholar]
- 22.Dosanjh A, Ikonen T, Wan B, Morris RE. Pirfenidone: A novel anti-fibrotic agent and progressive chronic allograft rejection. Pulm Pharmacol Ther. 2002;15(5):433. doi: 10.1006/pupt.2002.0367. [DOI] [PubMed] [Google Scholar]
- 23.Hirano A, Kanehiro A, Ono K, et al. Pirfenidone modulates airway responsiveness, inflammation, and remodeling after repeated challenge. Am J Respir Cell Mol Biol. 2006;35(3):366. doi: 10.1165/rcmb.2005-0452OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker JE, Giri SN, Margolin SB. A double-blind, randomized, controlled study of oral pirfenidone for treatment of secondary progressive multiple sclerosis. Mult Scler. 2005;11(2):149. doi: 10.1191/1352458505ms1134oa. [DOI] [PubMed] [Google Scholar]
- 25.Jakubzick C, Tacke F, Ginhoux F, et al. Blood monocyte subsets differentially give rise to CD103+ and CD103− pulmonary dendritic cell populations. J Immunol. 2008;180(5):3019. doi: 10.4049/jimmunol.180.5.3019. [DOI] [PubMed] [Google Scholar]
- 26.Feng Y, Wang D, Yuan R, Parker CM, Farber DL, Hadley GA. CD103 expression is required for destruction of pancreatic islet allografts by CD8(+) T cells. J Exp Med. 2002;196(7):877. doi: 10.1084/jem.20020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giri SN, Wang Q, Xie Y, et al. Pharmacokinetics and metabolism of a novel antifibrotic drug pirfenidone, in mice following intravenous administration. Biopharm Drug Dispos. 2002;23(5):203. doi: 10.1002/bdd.311. [DOI] [PubMed] [Google Scholar]
- 28.Kushner DJ, Baker A, Dunstall TG. Pharmacological uses and perspectives of heavy water and deuterated compounds. Can J Physiol Pharmacol. 1999;77(2):79. [PubMed] [Google Scholar]
- 29.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170(6):2081. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 29:71. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 31.Lee KW, Everett THt, Rahmutula D, et al. Pirfenidone prevents the development of a vulnerable substrate for atrial fibrillation in a canine model of heart failure. Circulation. 2006;114(16):1703. doi: 10.1161/CIRCULATIONAHA.106.624320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arrighi JF, Rebsamen M, Rousset F, Kindler V, Hauser C. A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-alpha, and contact sensitizers. J Immunol. 2001;166(6):3837. doi: 10.4049/jimmunol.166.6.3837. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto M, Sato S, Hemmi H, et al. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol. 2003;4(11):1144. doi: 10.1038/ni986. [DOI] [PubMed] [Google Scholar]
- 34.Inaba K, Swiggard WJ, Steinman RM, Romani N, Schuler G, Brinster C. Isolation of dendritic cells. Chapter 3. Curr Protoc Immunol. 2009 doi: 10.1002/0471142735.im0307s86. Unit 3 7. [DOI] [PubMed] [Google Scholar]
- 35.Harding CV, Ramachandra L. Presenting exogenous antigen to T cells. Chapter 16. Curr Protoc Immunol. doi: 10.1002/0471142735.im1602s88. Unit 16 2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.