Abstract
Objectives
To measure spatial acuity on a right-left discrimination task in 2-to-3-year-old children who use a unilateral cochlear implant (UCI) or bilateral cochlear implants (BICIs); to test the hypothesis that BICI users perform significantly better when they use two CIs than when using a single CI, and that they perform better than the children in the UCI group; to determine how well children with CIs perform compared with children who have normal acoustic hearing; to determine the effect of intensity roving on spatial acuity.
Design
Three groups of children between 26-to-36 months of age participated in this study: 8 children with normal acoustic hearing (mean age: 30.9 months), 12 children who use a UCI (mean age: 31.9 months), and 27 children who use BICIs (mean age: 30.7 months). Testing was conducted in a large sound-treated booth with loudspeakers positioned on a horizontal arc with a radius of 1.2 m. The observer-based psychophysical procedure was used to measure the children’s ability to identify the hemifield containing the sound source (right vs. left). Two methods were used for quantifying spatial acuity, an adaptive-tracking method and a fixed-angle method. In Experiment 1 an adaptive tracking algorithm was used to vary source angle, and the minimum audible angle (MAA; smallest angle at which right-left discrimination performance is better than chance) was estimated. All three groups participated in Experiment 1. In Experiment 2 source angles were fixed at ±50°, and performance was evaluated by computing the number of standard deviations above chance. Children in the UCI and BICI groups participated in Experiment 2.
Results
In Experiment 1, when stimulus intensity was roved by 8 dB, MAA thresholds were 3.3º to 30.2º (mean = 14.5º) and 5.7º to 69.6º (mean = 30.9º) in children who have normal acoustic hearing and the BICI group, respectively. When the intensity level was fixed for the BICI group, performance did not improve. Within the BICI group, 5/27 children obtained MAA thresholds within one standard deviation of their peers who have normal acoustic hearing; all 5 had greater than 12 months of bilateral listening experience. In Experiment 2, BICIs provided some advantages when the intensity level was fixed. First, the BICI group outperformed the UCI group. Second, children in the BICI group who repeated the task with their first CI alone had statistically significantly better performance when using both devices. In addition, when intensity roving was introduced, a larger percentage of children who had 12 or more months of BICI experience continued to perform above chance than children who had less than 12 months of BICI experience. Taken together, the results suggest that children with BICIs have spatial acuity that is better than when using their first CI alone as well as better than their peers who use UCI. In addition, longer durations of BICI use tend to result in better performance, although this cannot be generalized to all participants.
Conclusion
This report is consistent with a growing body of evidence that spatial hearing skills can emerge in young children who use BICIs. The observation that these skills are not concomitantly emerging in age- and experience-matched children who use UCIs suggests that BICIs provide cues that are necessary for these spatial hearing skills which UCIs do not provide.
Introduction
There is increasing evidence that bilateral cochlear implants (BICIs) can provide benefits to individuals who are deaf. Although there appears to be widespread variability among recipients in the extent of the benefit, research has shown that, overall, both adults and older children exhibit better performance when using BICIs versus a single implant on studies of either speech understanding in noise (e.g., Litovsky et al. 2006c; Mok et al. 2010), sound localization (e.g., Nopp et al. 2004; Litovsky et al., 2006a; Neuman et al. 2007; Godar & Litovsky 2010; Grieco-Calub & Litovsky 2010), or in studies that evaluated both skill sets (e.g., Tyler et al. 2002; van Hoesel & Tyler 2003; Litovsky et al. 2006b; Litovsky et al. 2009). It also appears that, compared to children who have exposure to unilateral input for longer periods of time, children who are provided with BICIs earlier in life have more mature neural representation at the level of the brainstem for binaural stimuli (Gordon et al. 2007).
In the unilateral research domain, there is evidence showing that better language outcomes and more mature auditory neurophysiology are often associated with earlier ages of implantation (e.g., Kirk et al., 2002; Wang et al., 2008; Nicholas & Geers, 2006; Sharma et al., 2005). These outcomes are most likely due to the fact that early implantation reduces the duration of auditory deprivation and allows stimulation of the auditory system during a time of great neural plasticity. Based on this notion, it might be argued that providing infants with BICIs should result in greater bilateral benefits. To date, however, little is known about whether providing BICIs to young children results in better functional outcomes. Furthermore, it is not clear whether there are benefits to providing the second CI simultaneously with (or near the time of) the first device, versus sequentially (months or years apart).
This uncertainty is in part driven by the unknown extent to which young CI users can access the auditory cues necessary to drive the development of spatial hearing. This is due in large part to the fact that bilateral CIs have independent inputs, and the lack of coordination between the two devices renders binaural cues weak, absent or inconsistent. Previous work has shown that children with sequential BICIs are capable of developing spatial hearing skills that are equivalent to their peers who have normal acoustic hearing, but there is large individual variability in outcomes (Grieco-Calub & Litovsky 2010). There is an open question as to whether providing BICIs at very young ages, with little to no delay between the activation of the two devices, can reduce this variability and improve spatial hearing outcomes for young BICI users. This question will be addressed in the present study. The success of BICIs in post-lingually deafened adults and older children has prompted an increase in the number of infants who are provided with BICIs; thus the present study, which examined spatial hearing skills in 2- to-3-year-old children with BICIs, became feasible.
Infants with normal hearing are born with the rudimentary ability to discriminate between sources presented from the right versus left of midline. Although rather immature, this spatial acuity is present at birth (Muir & Field 1979) and improves with an increase in age. For example, the minimum audible angle (MAA; Mills 1958), the smallest angle at which right-left discrimination performance is better than chance, decreases throughout childhood. With simple stimuli such as brief noise bursts, children exhibit adult-like performance by 5 years of age (Litovsky 1997; for review, see Litovsky & Ashmead 1997; Litovsky 2011). However, under more complex conditions, such as when simulated echoes occur, children’s spatial acuity is worse than that of adults (Litovsky 1997).
On measures of sound location identification using an array of loudspeakers in the horizontal plane, children as young as 4–5 years of age exhibit relatively low error rates, averaging between 10º to 20º root mean square (RMS) error (Grieco-Calub & Litovsky 2010; Litovsky & Godar 2010). Again, when simulated echoes are introduced, children’s performance is significantly worse than in adults (Litovsky & Godar 2010), suggesting that spatial hearing abilities continue to mature during later childhood. The factors that are responsible for these developmental changes are poorly understood.
The auditory experience of young children who are born deaf and who subsequently receive BICIs is quite unique relative to their peers who have normal acoustic hearing. Depending on the etiology and cause of deafness, these children experience severe or complete auditory deprivation prior to, beginning at or shortly after birth, until they receive their first CI. In cases of sequential implantation, children function as unilateral CI users until they receive their second implant. Research to date shows that most children with BICIs have significantly poorer spatial hearing skills than their peers with normal hearing. These children perform significantly better, however, when using both CIs than when a single CI is used. There are bilateral benefits when children are tested on discrimination of sound sources presented to the right versus left hemifields in the frontal horizontal plane (i.e., smaller minimum audible angles; Litovsky et al, 2006a; Grieco-Calub et al. 2008). There is also evidence for bilateral benefits on measures of source location identification (i.e., smaller root-mean-square errors; Grieco-Calub & Litovsky 2010) and speech intelligibility (Litovsky et al. 2006b; Mok et al. 2010).
The children tested to date on right-left discrimination and sound location identification tasks varied in the age at which they received their second implant as well as the amount of time between activation of the first and second implants (e.g., Litovsky et al., 2006a; VanDeun et al. 2010; Grieco-Calub & Litovsky 2010; Godar & Litovsky 2010). Until recently, reports included children who received their first CI at slightly older ages than the children in the present study and who had a number of years of unilateral auditory deprivation prior to the activation of their second implant. Some children in this group were successful users of a hearing aid in the contralateral, non-implanted ear prior to receiving the second implant (i.e., bimodal users, CI+HA). The extent to which pre-implant auditory stimulation in the second ear aids in post-BICI performance is unknown. Studies involving children who are CI+HA users have shown varied outcomes on speech-in-noise and spatial hearing tasks. There is evidence to suggest that bilateral input, regardless of the mode (BICI or CI+HA) can aid in speech perception; however, performance with the second device alone (second CI or HA) is typically better in the BICI users (Mok et al. 2010). There is also evidence, however, suggesting that the addition of a HA can, in some children, impair speech perception relative to using the first CI alone (Litovsky et al., 2006b). Regarding spatial hearing, in a between-subjects design, Litovsky et al. (2006b) found that MAA thresholds were lower (i.e., performance was better) in children who use BICIs compared to children who use CI+HA. In a within subjects design, Godar and Litovsky (2010) tested 10 children who used a single CI with either no other device (UniCI) or a hearing aid (CI+HA) in the opposite ear prior to implantation of a second CI. Upon retesting the children at 3- and 12-months after bilateral activation, the authors reported significant improvement in MAA threshold in the group overall; however, intersubject variability was high. Children who had bilateral input (CI+HA) prior to activation of their second CI did not outperform children who only used their first CI prior to activation of their second CI. Possible reasons for this variability most likely relates to individual factors such as the amount of residual hearing and etiology of the hearing loss in these children.
Periods of bilateral, followed by unilateral auditory deprivation (whether partial or complete), may have rendered spatial hearing mechanisms in these children that are not ideally wired for processing spatial cues with fidelity. In fact, research with nonhuman species has provided some support for this suggestion. For example, bilaterally-implanted cats who experienced long durations of auditory deprivation (from birth) had poorer sensitivity to interaural cues than cats who were deafened after having some experience with sound both within the inferior colliculus (Hancock et al. 2010) as well as in the auditory cortex (Tillein et al. 2010). In addition, unilateral deprivation (in the form of a conductive block) during development disrupted binaural integration of interaural level differences (ILDs) in the auditory cortex of the rat (Popescu & Polley, 2010). Taken together, long periods of bilateral or unilateral deprivation may preclude the development of spatial hearing skills even when BICIs are provided.
There is a growing population of children who received their first implant by one year of age and who have little to no duration between activation of the first and second implants, consistent with the clinical trends of providing BICIs to infants. With this growing trend, the duration of auditory deprivation has been decreased, and the amount of unilateral experience prior to bilateral activation is generally shorter. This study was motivated by the question of whether bilateral stimulation, provided at a time of great neural plasticity, can promote normal or near-normal spatial hearing skills in young implant users. The underlying hypothesis in the current project is that providing BICIs at young ages will improve performance in a spatial hearing task where individuals typically benefit from access to bilateral information.
In an earlier report where preliminary results from this study were presented, the observer-based psychophysical procedure (OPP) was used to determine if 2-to-3-year-old children who receive bilateral CIs before or near 2 years of age perform more similarly to their peers than children who gain access to bilateral hearing at older ages (Grieco-Calub et al. 2008). Results from that preliminary study indicated that early bilateral implantation has mixed results. Only half of the children who used BICIs were able to identify the location of the sound source on a right-left discrimination task in the study at a level that was above chance performance. As a result, discussion about the emergence of spatial hearing skills in these young children was limited since performance could not be quantified for all children.
The current study extends the preliminary findings in 2-to-3-year-old children to 39 implanted children between 26–36 months of age: 27 were BICI users and 12 were UCI users. The primary goal of the current study was to estimate spatial hearing acuity in these young CI users. Like in the Grieco-Calub et al. (2008), children were asked to identify the hemifield containing the sound source on two different versions of a 2-alternative-forced choice right-left task. For the adaptive-tracking method, during which the source angle varied, spatial hearing was quantified by MAA thresholds. For the fixed-angle method, during which the source angle was fixed at ±50º, spatial hearing was quantified by the number of standard deviations above chance. The rationale for using these two methods is discussed in Methods, Procedure. The other goals of this study were to determine if there is a functional difference in performance between the BICI and UCI groups, and to determine the effect of intensity roving, whereby monaural level cues are minimized, on spatial acuity.
Materials and Methods
Subjects
Children with Cochlear Implants (CIs)
Thirty-nine (39) children between 26-to-36 (mean = 31.1) months of age who use CIs participated in this study. All children had a history of severe-to-profound bilateral sensorineural hearing loss, either identified at birth (N=35) or after some experience with acoustic hearing (N=4) per parental report (Table 1). At the time of participation, 12 children were unilaterally implanted (UCI) and 27 children were bilaterally implanted (BICIs). The children were recruited from across the United States through their audiologists, surgeons or self-referrals, and traveled to Madison, WI to participate in the experiments. This type of recruitment tends to result in a biased sample, since those families who enrolled in the study were highly motivated to partake in research, and often traveled long distances to Madison, WI to participate in the studies. In addition, every effort was made to recruit equal numbers of children who use cochlear implants from the three implant manufacturers: Advanced Bionics (16 children), Cochlear Corporation (16 children), Med-El Corporation (7 children).
Table 1.
Demographic information.
| Participant | Sex | Age of ID (yrs;mos) | Etiology | Age at visit (mos) | Age at first CI (mos) | Hearing Age (mos) | Age at second CI (mos) | Duration of BICI (mos) | First CI (Internal device, processor, ear) | First CI Speech processing strategy | Second CI (Internal device, processor, ear) | Second CI Speech processing strategy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
BICI group CIBV |
M | birth | Connexins 26, 30 | 29 | 17 | 12 | 23.5 | 5.5 | HiRes, PSP, R | HiRes-S | HiRes, PSP, L | HiRes-S |
| CIBX | M | birth | Waardenburg Syndrome | 31.5 | 10 | 21.5 | 12 | 19.5 | N24, Sprint, R | ACE | N24, Sprint, L | ACE |
| CICA | M | 2;0 | Unknown | 35 | 29 | 6 | 29 | 6 | Pulsar, Tempo, simultaneous | CIS+ | Pulsar, Tempo, simultaneous | CIS+ |
| CICB | F | birth | Connexin 26 | 31 | 10.5 | 20.5 | 25 | 6 | N24, Sprint, R | ACE | Freedom, Freedom, L | ACE (RE) |
| CICF | F | 2;0 | Meningitis^ | 34.5 | 18 | 16.5 | 28.5 | 6 | Freedom, Freedom, R | ACE | Freedom, Freedom, L | ACE |
| CICG | M | birth | Connexin 26 | 27 | 13 | 14 | 20.5 | 6.5 | C40+, Tempo, R β | CIS+ | Pulsar, Tempo, L β | CIS+ |
| CICH | M | birth | Unknown | 27 | 12 | 15 | 12 | 15 | Pulsar, Tempo, R β | CIS+ | Pulsar, Tempo, L β | CIS+ |
| CICK | M | 0;11 | Connexin 26 | 28.5 | 13 | 15.5 | 15.5 | 13 | HiRes, PSP, R | HiRes P | HiRes, PSP, L | HiRes-P |
| CICO | M | birth | Connexin 26 | 31 | 14 | 17 | 25.5 | 5.5 | Freedom, Freedom, R | ACE (RE) | Freedom, Freedom, L | ACE (RE) |
| CICP | F | birth | Connexin 26 | 26.5 | 13 | 13.5 | 20 | 6.5 | Freedom, Freedom, R | ** | Freedom, Freedom, L | ** |
| CICR | M | birth | Connexin 26 | 26 | 12.5 | 13.5 | 16.5 | 9.5 | HiRes, PSP, L | HiRes-P w/ Fidelity 120 | HiRes, PSP, R | HiRes-P w/ Fidelity 120 |
| CICS | M | 0;10 | Connexin 26 | 34.5 | 18 | 16.5 | 18 | 16.5 | HiRes, Harmony, simultaneous | HiRes-P w/ Fidelity 120 | HiRes, Harmony, simultaneous | HiRes-P w/ Fidelity 120 |
| CICT | M | birth | Connexin 26 | 35 | 11.5 | 23.5 | 22.5 | 12.5 | HiRes, PSP, R | HiRes-P w/ Fidelity 120 | HiRes, PSP, L | HiRes-P w/ Fidelity 120 |
| CICU | F | birth | Unknown^* | 31 | 15 | 16 | 24 | 7 | Freedom, Freedom, L | ACE | Freedom, Freedom, R | ACE |
| CICV | M | birth | Connexin 26 | 33.5 | 12 | 21.5 | 26 | 7.5 | HiRes, Harmony, R (T-mic) | HiRes-P w/ Fidelity 120 | HiRes, Harmony, L (T-mic) | HiRes-P w/ Fidelity 120 |
| CICW | F | 0;1 | Unknown | 29 | 14 | 15 | 15 | 14 | Pulsar, Tempo, R β | CIS+ | Pulsar, Tempo, L β | CIS+ |
| CICX | F | birth | Unknown | 36 | 20.5 | 15.5 | 27.5 | 8.5 | Freedom, Freedom, R | ACE | Freedom, Freedom, L | ACE |
| CIDA | F | birth | Connexin 26 | 34.5 | 11 | 23.5 | 22 | 12.5 | HiRes, PSP, R | HiRes-P w/ Fidelity 120 | HiRes, PSP, L | HiRes-P w/ Fidelity 120 |
| CIDC | M | birth | Unknown^* | 31 | 18 | 13 | 18 | 13 | HiRes, Auria, L | HiRes-S | HiRes, PSP, R | HiRes-S |
| CIDD | F | 1;1 | Unknown | 36 | 21 | 15 | 29 | 7 | Freedom, Freedom, L | ACE | Freedom, Freedom, R | ACE |
| CIDE | M | birth | Unknown | 26.5 | 13 | 13.5 | 13 | 13.5 | HiRes, PSP, simultaneous | HiRes-P | HiRes, PSP, simultaneous | HiRes-P |
| CIDH | M | 0:10 | Unknown | 35.5 | 17 | 18.5 | 26 | 9.5 | Freedom, Freedom, R | ACE | Freedom, Freedom, L | ACE |
| CIDI | M | birth | Connexin 26 | 26 | 8 | 18 | 9 | 17 | Pulsar, Tempo, L β | CIS+ | Pulsar, Tempo, R β | CIS+ |
| CIDK | M | birth | Connexin 26 | 26.5 | 9.5 | 17 | 9.5 | 17 | Freedom, Freedom, simultaneous | ACE | Freedom, Freedom, simultaneous | ACE |
| CIDL | F | birth | Connexin 26 | 27.5 | 14 | 13.5 | 18 | 9.5 | Pulsar, Tempo, R β | CIS+ | Sonata, Tempo, L β | CIS+ |
| CIDM | F | birth | Unknown | 31.5 | 13 | 18.5 | 25 | 6.5 | HiRes, Harmony, L | HiRes-P w/ Fidelity 120 | HiRes, Harmony, R | HiRes-P w/ Fidelity 120 |
| CIEA | M | birth | Connexin 26 | 28 | 8 | 20 | 11 | 17 | Freedom, Freedom, R | ACE (RE) | Freedom, Freedom, L | ACE (RE) |
|
UCI group CICC |
M | birth | Familial (hereditary) | 36 | 15 | 21 | N24, Sprint, L | ACE | ||||
| CICE | M | birth | Connexins 26, 30 | 30 | 10.5 | 19.5 | HiRes, Auria, R | HiRes-P | HA | |||
| CICI | M | birth | Unknown | 36 | 14.5 | 21.5 | Pulsar, Tempo+, R | CIS+ | ||||
| CICJ | M | birth | Connexin 26 | 35 | 10 | 25 | HiRes, R | HiRes-P | ||||
| CICL | M | 1;1 | Connexin 26* | 27 | 16 | 11 | Freedom, Freedom, R | ACE (RE) | ||||
| CICM | M | birth | Connexin 26 | 36 | 13 | 23 | HiRes, PSP, R | ** | ||||
| CICN | F | birth | Connexin 26 | 32.5 | 15.5 | 17 | Freedom, Freedom, R | ACE (RE) | ||||
| CICQ | M | 0;8 | Usher Syndrome Type 1 | 31 | 14.5 | 16.5 | Freedom, Freedom, R | ACE | ||||
| CICZ | F | 0;2 | Unknown | 31 | 7 | 24 | HiRes, PSP, R | HiRes-P w/ Fidelity 120 | HA | |||
| CIDB | F | 0;11 | CMV^ | 35 | 16 | 19 | HiRes, PSP, R | HiRes-P w/ Fidelity 120 | HA | |||
| CIDS | M | 0;3 | Unknown | 25 | 12.5 | 12.5 | Freedom, Freedom, R | ACE | ||||
| CIDU | F | birth | Unknown | 28.5 | 13 | 15.5 | HiRes, Auria, R | HiRes-P |
ID refers to identification. Carets (^) represent children who have a history of acoustic hearing. Asterisks (*) represent children who have a history of progressive hearing loss per parental report. Betas (β) represent children who use implant processors with a body-level microphone. Double asterisks (**) refer to unknown speech processing strategies for respective participants. The abbreviation “HA” reflects children who use a hearing aid in the non-implanted ear either consistently (CICE, CICZ) or intermittently (CIDB). Cochlear implant devices: N24, Freedom (Cochlear Corp.); HiRes (Advanced Bionics); Pulsar, C40+, Sonata (MED-EL Corp.)
Children spent two days being tested in the laboratory, during which time they participated in tasks designed to quantify spatial hearing as well as in tasks designed to quantify spoken word recognition. Participant codes are in the format CIXX, representing the order in which they enrolled in the research program. Results from 18 of the children who participated in this study have been cited in a preliminary report on spatial hearing acuity (Grieco-Calub et al. 2008). Results from 26 of the children have been cited in a previous report that focuses on their spoken word recognition abilities (Grieco-Calub et al. 2009).
Children With Normal Acoustic Hearing (NH)
Eight (8) children who were typically-developing and 26-to-36 (mean = 30.9) months of age participated in the study. These children had no history of hearing loss, middle ear problems, or other developmental delays per parental report.
Experimental Setup
All measures were conducted in a double-walled sound-treated booth [IAC; 2.8 m×3.25 m with reverberation time (RT60) of 250 msec]. Fifteen loudspeakers that were visible to the children were mounted on a custom-made arc spanning 140 degrees and positioned at 10° intervals. The loudspeakers were digitally matched; any subtle differences in frequency response were digitally subtracted prior to stimulus presentation. The purpose of this technique is to ensure consistent spectral representation of the stimuli across speakers. For children in the NH group, and for 5 children in the BICI group (see Procedure below), the fifteen loudspeakers were placed between ±50° (spanning 100 degrees) at the following intervals: 0°, ±2.5°, ±5°, ±10°, ±20°, ±30°, ±40°, ±50°. The rationale for repositioning the loudspeakers was to obtain a more precise estimate of the children’s spatial acuity. Two computer monitors, mounted underneath the loudspeakers located at 45° to the right and left of midline, were used to provide feedback and reinforcement via video presentation. A camera, placed at 0° underneath the center loudspeaker, provided video feed into the observation side of the test booth, which was used by an observer to monitor the children’s behavior. Children sat either on their caregivers’ lap or alone on a chair, always in the center of the loudspeaker array, with the head at an approximate distance of 1.2 m from the loudspeakers. Caregivers and research assistants in the test booth used earphones which provided a diotic presentation of the stimulus on each trial, to mask the source locations, thereby eliminating tester bias and potential for input from caregivers during the experiment. Auditory stimuli were stored as .wav files on a PC host, and played to the loudspeakers via Tucker-Davis System III hardware (Tucker-Davis Technologies, Alachua, FL). Customized software for stimulus presentation and data collection was written in MATLAB programming language.
Stimuli
Stimuli were the spondaic words “baseball” and “birthday”, recorded with a male voice at a sampling rate of 44 kHz and stored as .wav files. On each trial, one word was randomly chosen and repeated 3 times (e.g., “baseball, baseball, baseball”). In some conditions, stimuli were presented at an intensity level that was fixed at 60 dB SPL (i.e., fixed-intensity level conditions). When the intensity is fixed, the listener may have the opportunity to access both interaural difference cues and monaural intensity cues. Monaural intensity cues vary in each ear as sound source angles are varied (Blauert, 1997; Shaw, 1974); therefore, when these cues are available, they might be utilized to locate the sound source location. The extent to which CIs preserve these cues is still an empirical question.
In the remaining conditions, stimuli were presented at varying intensity levels. In these intensity-rove conditions, the stimulus level was randomly varied over an 8-dB range (60±4 dB SPL) across trials to minimize the availability of monaural level cues that are present when sound intensity is fixed. The level rove was selected to maintain stimulation levels within the meaningful dynamic range of CI processors, where compression is minimal or absent. This degree of rove is also consistent with prior studies in this lab (Litovsky et al. 2006a, 2009; Grieco-Calub & Litovsky 2010) and others (Galvin et al. 2008).
Procedure
The children’s CI speech processors were programmed by their audiologist prior to their visit, and testing was conducted under those conditions. Specific details regarding each child’s implant and speech processing strategy can be found in Table 1.
Children participated in a right-left discrimination task. This was a single-interval 2-alternative-forced choice task in which children needed to determine if the target stimulus was presented in the right hemifield or left hemifield of midline. Behavioral responses were measured using the observer-based psychophysical procedure (Olsho et al. 1987). This method is commonly used in infant psychoacoustics and has proven to be accurate in determining auditory sensitivity. For this study, the procedure was modified slightly so that spatial acuity could be assessed. On each trial, an observer, who was located in the observation room and unaware of the stimulus location, observed the children’s behavior via video feed. Each trial was initiated by the observer, who signaled to the computer to randomly present a stimulus on the right or left. This was done only when the children were quiet and looking forward, which was generally achieved by having a research assistant seated in the booth who directed the children’s attention to the front loudspeaker by either waving a toy or engaging the children in a more active task (e.g., holding a block that was dropped into a bucket after the trial). After the stimulus presentation, the observer made a decision regarding the stimulus location (right or left) by watching the children’s responses. Changes in behavior that were considered indicative of a response to the location of the stimulus included a head turn, shift in gaze, or changes in body position toward one side. If the observer chose the correct side of presentation, the children’s response was reinforced by the presentation of a brief video segment from the computer monitor on the same side of stimulus presentation. For some children who appeared distracted during the task, the video segment was shown on the same side of stimulus presentation regardless of whether the observer was correct or incorrect in order to maintain their level of engagement in the task.
All children participated in the right-left discrimination task using their everyday listening mode with two exceptions. First, after completing Experiment 1 and 2 (see below) when using their BICIs, 17/27 children in the BICI group repeated Experiment 2 with their first CI alone. Even fewer of these children (7/27) completed Experiment 1 using their first CI alone, most likely due to the fact that this was the last task of the session. Second, 3/12 children in the UCI group used a hearing aid in their contralateral ear with varied consistency. Attempts at quantifying performance under the bimodal (CI+HA) condition was attempted in two children (CICZ, CIDB), but performance was not greater than chance levels and therefore not reported here.
Performance on the right-left task was quantified in two ways. In Experiment 1, an adaptive-tracking method which varied source angle within the experiment was used to determine the smallest angle, relative to midline, that could be discriminated. In Experiment 2, source angles were fixed at ±50º and a minimum of 10 trials were completed. The objective of Experiment 2 was to determine the level of performance among children who were unable complete Experiment 1 at above chance levels.
Experiment 1: Right-Left Discrimination with Varying Source Angles
All three groups of children participated in this task. For the group of NH children, pairs of loudspeakers were placed at angles ranging from ±2.5° to ±50°. These positions were selected after pilot testing revealed that small angles were necessary for this age group. For children with CIs, initial testing began with loudspeakers placed at angles ranging from ±10° to ±70°. If adaptive tracking revealed that a child was consistently correct at ±10°, the experiment was repeated with the smaller source angles (±2.5° to ±50°). This was required for 5 of the children with BICIs.
On each trial, the angle was pre-determined based on the set of rules outlined below, and the side (right or left) was randomized. Each adaptive track began at the largest angular displacement (±50° or ±70°). Data were collected using a 3-down/1-up adaptive method to vary the source angles from trial to trial, such that three consecutive correct responses resulted in decreased angle, and one incorrect response resulted in increased angle. Decisions regarding the step size leading to increased or decreased angles were based on rules similar to those used in adaptive procedures. Step sizes were doubled if an angle had been used twice and additional increase in angle size was required. Similarly, angle size was halved if an angle was used twice and additional decrease in angle size was required (Litovsky & Macmillan 1994; Litovsky 1997).
Each adaptive track was terminated once 5 reversals were reached or sooner if children became fussy or uncooperative. The a-priori objective of this experiment was to complete one adaptive track for each condition. If, however, children were unable to complete an adaptive track due to fussiness, additional attempts to complete the adaptive track were made. There was a need to repeat testing with adaptive tracks in one or more conditions for 5/12 children in the UCI group and 23/27 children in the BICI group. The number of trials per adaptive track that yielded an MAA averaged 15 trials, and ranged from 8–27 trials. A typical adaptive track had a duration of approximately 5 minutes; however, this duration was influenced by the attention of the child and the amount of time needed between each trial.
The objective of this task was to estimate the MAA threshold, the smallest angle at which listeners can discriminate right versus left sound positions (Mills, 1958). For each adaptive track, MAA threshold was estimated using the Matlab psignifit toolbox, applying the methods described by Wichmann and Hill (2001a, 2001b). A logistic function was fit to all data points from each experimental run for each participant, using a constrained maximum likelihood algorithm. MAA threshold was estimated at the point on the psychometric function intersecting with 80% correct. Because this method requires that listeners achieve performance of 80% correct at one or more angular displacements, MAA threshold could not be determined (CND) for children whose performance was <80% at all angles tested.
Children in the NH group completed the task with sound intensity roving (60 dB SPL ± 4 dB). Based on prior research with young bilaterally implanted children (e.g., Grieco-Calub et al. 2008; Grieco-Calub & Litovsky 2010; Godar & Litovsky 2010), we recognized the fact that the right-left discrimination task may be difficult for some of the children who use CIs, even with the intensity fixed. Therefore, in the BICI and UCI groups, performance was first measured with a fixed intensity level, whereby monaural level cues in addition to interaural difference cues were available to the listener. When possible, regardless of performance in the fixed-intensity level condition, children repeated the task with a roving intensity level. The success of obtaining results in the intensity-rove condition was dependent on the child’s willingness to participate, the time it took to complete the other tasks during their visit, and their fatigue level. As a result, not all children were able to complete this task.
Experiment 2: Right-Left Discrimination, Loudspeakers Fixed (±50°)
The adaptive-tracking version of the right-left discrimination task is an effective method for quickly estimating an individual’s spatial acuity; however, it has some disadvantages. First, if the participant’s performance is less than chance at all angles evaluated, an MAA threshold cannot be estimated and spatial acuity cannot be quantified. Second, the nature of the adaptive measure is to quickly target angles that are near threshold, which means that the number of trials per angle may be small. As a result, performance at larger or smaller angles outside of threshold is difficult to evaluate.
In an attempt to evaluate performance on right-left discrimination for all participants, children in the BICI and UCI groups completed the task with source angles fixed at ±50º relative to midline. The ±50° locations were chosen because these angles were considered large enough to account for most of the variability observed in the adaptive version of the task, yet within the operating range of the directional microphones of CI speech processors. Only children in the CI groups participated in this task since all of the children in the NH group completed the adaptive version of the task, even with intensity roving. Stimuli were presented at ±50° for as many trials as the children would consistently respond to. The number of trials completed ranged from 10–39 (mean = 17).
As with the adaptive-tracking method, children were first tested with a fixed intensity level of 60 dB SPL on each trial. When possible, the task was repeated with the 8 dB rove. Because of inter-subject variability in attention span, and the resulting variability in the number of trials completed by the children, performance was normalized for individual participants. A method using principles of binomial distribution, which takes into account the number of trials completed by each participant, was applied with Equation (1):
Where p=probability of correct answer (.5), n=number of trials, x=proportion of correct responses of the individual child.
Results
Experiment 1: Varying source angles
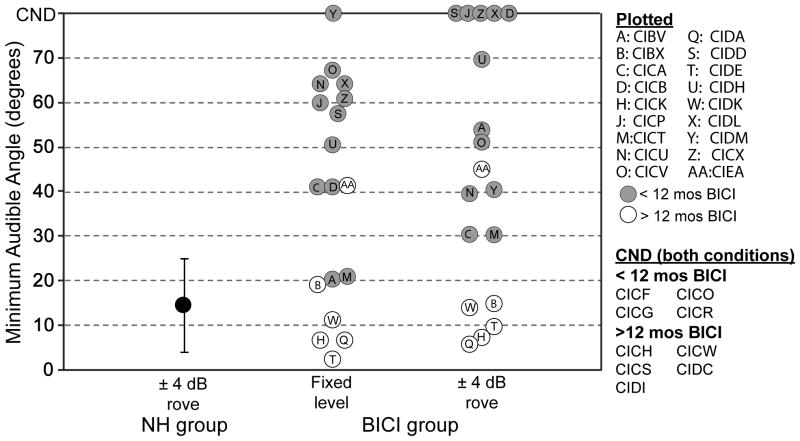
Children in all three groups participated in the right-left discrimination task with varying source angles (adaptive-tracking method). Results were quantified by estimating the MAA threshold (see Figure 1). MAA thresholds for children in the normal-hearing group ranged from 3.3º to 30.2º (mean ± SD = 14.5º ± 10.5º; Figure 1, black circle).
Figure 1.
Results from the adaptive version of the right-left discrimination task as quantified by minimum audible angle (MAA). Average performance (± SD) is shown for children who have normal acoustic hearing (black circles). Individual performance is shown for children in the BICI group with 12 or more months (white circles) or less than 12 months (gray circles) of BICI use. CND: could not determine.
Because of the effect of intensity rove on MAA threshold (Litovsky et al., 2006a), children in the BICI and UCI groups were first tested on the right-left discrimination task with a fixed intensity level of 60 dB SPL. The goal was to test these children with access to all spatial cues that might be naturally available, in order to potentially maximize performance. When using both devices, 17/27 children in the BICI group had a measurable MAA threshold in the fixed-intensity level condition. MAA thresholds of these children were highly variable, ranging from 2.5º to 67º (mean ± SD = 37.4º ± 23.2º; Figure 1, white and gray circles), thus substantially greater than values obtained in the NH group. Due to the large variance in performance within the BICI group, a t-test for independent samples failed normality. Thus, a Wilcoxon Rank-Sum Test for two independent samples was conducted. The analysis revealed a significant difference in MAA threshold between the BICI group with the intensity fixed and NH group with the intensity roved (z = 7.67, p<0.001), suggesting that children in the NH group had significantly better spatial acuity, despite the minimization of monaural level cues.
Also shown in Figure 1 (white and gray circles) are MAA thresholds for the roved-intensity condition. Of the 27 children tested on this task, 13 reached performance of 80% or greater on at least one angle; thus it was possible to estimate MAA thresholds with intensity roving in this subset of children. Similar to the finding in the fixed-intensity level condition, there was large variability in performance. MAA thresholds ranged from 5.7º to 69.6º (mean ± SD= 30.9º ± 21.1º). A paired t-test did not reveal a significant difference in the MAA thresholds obtained in the fixed-intensity and intensity-rove conditions [t(11)=0.347, p=0.74]. There was only one case in which MAA threshold was estimated in the condition with intensity rove but not with fixed intensity (CIDM, data point “Y”). When compared to performance of the NH group with intensity roving, only 5 children in the BICI group performed within one standard deviation of the NH group. It is interesting to note that all 5 of these children had more than 12 months of experience with their BICIs (white circles).
Figure 1 does not contain data from the following groups because the children within each group did not reach 80% correct at any one angle; thus MAA thresholds could not be estimated (see Methods): nine of the 27 children in the BICI group when using their bilateral devices (Figure 1, “CND, both conditions”); the BICI group when they performed the task with their first CI alone; and the UCI group. There was one exception in the UCI group: CICN had an MAA of 69.3º which was near the upper limit of the measurement.
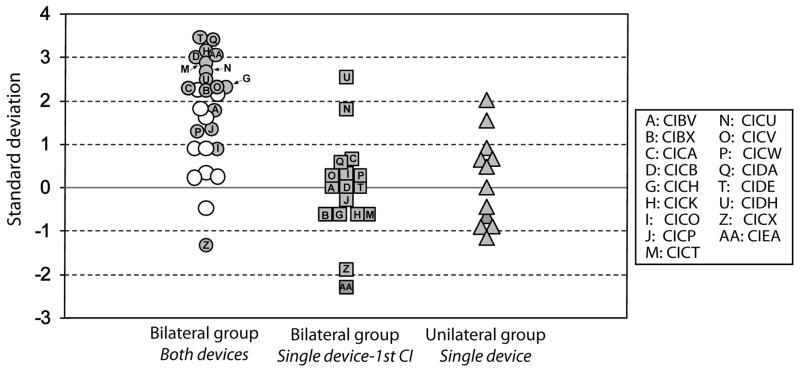
Experiment 2: Fixed source angle, Fixed intensity
Performance on the right-left discrimination task when using a fixed-angle method at a fixed intensity level was quantified for each participant in the BICI and UCI groups by determining the number of standard deviations above chance (see Methods, Equation 1). Using the number of standard deviations above chance as the dependent variable, a one sample t-test revealed that the BICI group performed significantly above chance when using both devices [t(26)=5.3, p<0.001; Figure 2, white and gray circles]. A subset of children in the BICI group (N=17) repeated the task with their first CI alone; however, their performance was at chance levels [t(16)=1.9, p=0.7; Figure 2, squares]. Children in the BICI group who completed both bilateral (Figure 2, gray circles) and unilateral conditions performed significantly better when using both devices than when using their first CI alone [t(16)=6.5, p<0.001].
Figure 2.
Results from the right-left discrimination task with source angles fixed at ±50º with a fixed intensity level of 60 dB SPL. Children within the BICI group were divided based on whether they repeated the task with their first CI alone (gray circles) or did not (white circles).
The performance of the children in the UCI group was also at chance levels for this task [t(11)=1.1, p=0.3; Figure 2, triangles], although two children did score above one standard deviation (CICN=1.5; CICS=2). Unequal N, between-subject analyses revealed that the performance of the UCI group was not significantly different from that of children in the BICI group when tested with their first implant alone [t(27)=0.5, p=0.6]. When using both implants, however, the BICI group performed significantly better than the UCI group [t(37)=3.9, p<0.001].
Experiment 2: Fixed source angle, Intensity roving (± 4 dB)
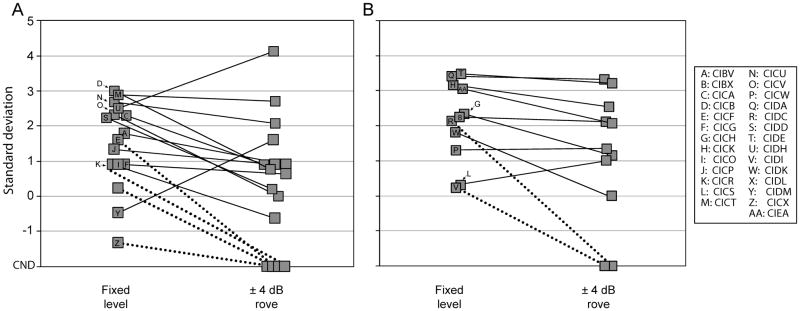
The final experiment was conducted to determine the effect of intensity roving on performance for the right-left discrimination task at fixed angles of ±50º. Although the a-priori objective was to collect data from all children in this condition, only data from the BICI group will be presented. Note that among the children in the BICI group, 6 were unable to complete the task with intensity roving (Figure 3A & 3B, “CND”). Children in the UCI group either did not perform significantly above chance (N=2) or were not tested in this condition due to fatigue (N=10).
Figure 3.
Results from the right-left discrimination task with source angles fixed at ±50º with fixed intensity level of 60 dB SPL and with an intensity rove of ±4 dB from the BICI group. A) Results from children with less than 12 months of BICI experience. B) Results from children with 12 or more months of BICI experience. Solid lines represent data from children who completed the task in the fixed-intensity level and intensity-rove conditions. Dotted lines represent data from children who have a data point for the fixed-intensity level condition only. CND: could not determine.
The intensity roving had wide ranging effects on performance within the BICI group. To better identify trends in performance, results from the BICI group were divided based on whether children had less than or more than 12 months of experience using bilateral devices. The rationale for this division stems from prior work showing significant improvement on the right-left discrimination task following 12 months of listening experience in the BICI mode (Godar & Litovsky 2010).
Figure 3A illustrates data from children in the BICI group who had less than 12 months of bilateral experience. Out of the 10 children whose score was at least one standard deviation above chance in the fixed-intensity level condition, only 3 (30%) demonstrated a similar ability to discriminate right-vs-left when the intensity was roved by ±4 dB. With the exception of one participant, children who scored less than one standard deviation from chance in the fixed-intensity level condition (N=6) either continued to perform at chance levels (N=2) or could not complete the task in the intensity-rove condition (N=3). Contrary to the group trend, participant CIDM (“Y”) performed notably better in the intensity-rove condition compared to the fixed-intensity condition.
Performance among the children who had 12 or more months of bilateral experience was generally better. Nine children’s performance was >1 standard deviation above chance levels in the fixed-intensity level condition (Figure 3B). Of those, seven (78%) had a similar level of performance when the intensity was roved. Taken together, these data are consistent with previous reports in older children showing that longer use of BICIs results in better performance on the right-left discrimination task (Godar & Litovsky 2010).
Relationship to subject variables
Previous reports have suggested that age of bilateral activation (VanDeun et al. 2010) and duration of bilateral CI use (Godar & Litovsky, 2010) have contributed to better spatial hearing skills in older children (ranging 4-to-15 years of age) who use BICIs. To determine if these variables related to the spatial acuity of the 2-to-3-year-old BICI users in the present study, multivariate linear regression analyses were conducted for the following four dependent variables: MAA threshold (fixed-intensity level condition); MAA threshold (intensity-rove condition); performance on the right-left discrimination task fixed at ±50º (fixed-intensity level condition); performance on the right-left discrimination task fixed at ±50º (intensity-rove condition). Initially, seven variables were identified as possible predictors of performance: the children’s age at visit, age at first implant activation, age at second implant activation, duration of hearing experience, duration of bilateral implant use, history of acoustic hearing (present or absent), and cochlear implant device (Cochlear Americas, Advanced Bionics, Med-El). Because a history of acoustic hearing and cochlear implant device were not equally represented across the sample, those two variables were excluded from the models. Results of the four analyses indicated that none of the included variables were found to be significant predictors either of MAA threshold when the intensity was fixed [F(3,13)=2.46, p=0.109] or roved [F(3,9)=1.61, p=0.254] or of performance on the right-left discrimination task fixed at ±50º when the intensity was fixed [F(3,23)=0.49, p=0.694] or roved [F(3,16)=0.92, p=0.454].
Discussion
This study presents novel findings from children with BICIs in that we focused on relatively young bilaterally implanted children. The results presented here add to a growing body of literature showing that, on average, children with BICIs can develop some ability to use spatial cues. Neither these children nor their peers who use UCIs were able to achieve the same level of performance when using their first CI alone.
Spatial acuity in children with normal acoustic hearing and children with bilateral cochlear implants
Results of this study show that 26-to-36 month old children who have normal acoustic hearing are developing the spatial hearing skills necessary to perform the right-left discrimination task even when monaural level cues are minimized with intensity roving. The MAA thresholds obtained in this study, however, were higher than those previously reported for children of this age (e.g., Litovsky, 1997; Morrongiello & Rocca, 1990), most likely due to effects of the level rove. Consistent with these previous reports of MAA thresholds in young children, results of this study suggest that spatial acuity has not matured by 2 years of age. The observation that MAA thresholds are relatively small, however, provides a reasonable anchor against which to compare the data from children who use BICIs.
Consistent with previous work in older children and adults with BICIs (e.g., Van Duen et al. 2010; Grieco-Calub & Litovsky 2010; Litovsky et al. 2009; van Hoesel & Tyler, 2003; Litovsky et al., 2006c), there continues to be a large range of individual variability in performance among children who use BICIs that was not observed in their peers with normal acoustic hearing. The large variability in performance, coupled with a somewhat small sample size, most likely places limits on our ability to identify any participant variables that were related to performance, both when the source angle varied and when it was fixed at ±50º. Larger scale studies with tighter control on participant variables when possible might be able to identify predictors of outcomes. The observation that the field, in general, has not come to a consensus about what variables might or might not contribute to success suggests that there may be more than one factor, or a complex interaction of factors, that will determine outcomes.
Inspection of Figures 1 and 3 would suggest that duration of bilateral experience does influence results, particularly when monaural cues are limited (as in the case of intensity roving). In the intensity-rove condition, five children with BICIs had MAA thresholds within one standard deviation of their peers with normal acoustic hearing. All five of these children had greater than 12 months of BICI experience (Experiment 1). In addition, a larger percentage of children with 12 or more months of BICI experience performed at above chance levels when source angles were fixed at ±50º even when the intensity was roved, compared to a smaller number of children from the group with <12 months of BICI experience who were able to do so (Experiment 2). The caveat to these findings, however, is that out of the children with BICIs who could not perform above chance when source angles were adaptively varied, four had less than 12 months of BICI experience and five had 12 or more months of BICI experience. Taken together, these results would suggest that if children can discriminate right-vs-left, they will tend to get better with more bilateral experience. However, duration of BICI use alone is not predictive of performance.
Unilateral experience
Unlike the variability observed in the BICI group, there was little difference in performance on the right-left discrimination task among the children in the UCI group. Only one (CICN) of the unilaterally-implanted 2-to-3-year-old children was able to perform right-left discrimination using the adaptive-tracking method; however, this was under the fixed-intensity level condition and, even then, MAA threshold was essentially at the limits of our measurement (69.3°). On the fixed-angle task at ±50º, CICN, as well as CICS, were the only two children in the UCI group who scored more than one standard deviation above chance with a fixed intensity level. It is interesting to note that CICS was not able to show this level of performance on the right-left discrimination task with varying source angles. The reasons for why these two children outperformed the other UCI users in this study are unclear. Potential factors that could influence performance would be consistent use of a hearing aid on the contralateral (i.e., non-implanted) ear, longer durations of CI use, or experience with acoustic hearing prior to deafness. None of these factors, however, apply to these two children (see Table 1).
The findings in the current study do not rule out the possibility that all of the children in the UCI group will be able to develop a sense of spatial awareness with additional unilateral experience. It is important to note that the cohort of children in the UCI group had between 11–24 months of auditory experience at the time of testing, which is appreciably smaller than the duration of unilateral experience of adults and older children who exhibit spatial hearing skills in previous studies (Grantham et al. 2008b; Grieco-Calub & Litovsky 2010). The degree to which these children will be able to develop age-appropriate spatial acuity, however, is unknown.
Auditory plasticity
Manipulation of auditory experience during early development can induce abnormal auditory physiology, either at the level of a single neuron (Seidl & Grothe 2005) or in sound localizing behavior (King et al. 2000). These forms of auditory plasticity are observed primarily in juvenile animals, and generally not in adults (but see Linkenhoker & Knudsen 2002), consistent with the idea of a sensitive period for acquiring spatial hearing. In support of this, BICIs tend to promote better spatial hearing in postlingually-deafened individuals than adults and children who were deaf at birth or shortly thereafter (e.g., Litovsky et al. 2004; Litovsky et al. 2006c). In addition, adults with postlingual deafness have better sensitivity to interaural timing differences (ITDs) compared to adults with prelingual deafness (Litovsky et al. 2010). Taken together, early auditory experience appears to be necessary for establishing the appropriate binaural connections involved in the development of spatial hearing.
One of the goals of this study was to determine whether providing bilateral auditory input at a young age, when neural plasticity is great, would influence the emergence of spatial acuity. The observation that a few of the children in the BICI group had spatial acuity skills equivalent to their chronologically-age matched acoustic hearing peers, and that none of the children in the UCI group performed comparably, suggests that earlier bilateral activation will influence the maturation of spatial hearing. This is not to say that the children in the BICI group who performed similarly to their acoustic hearing peers would not have reached the same level of performance if they received their BICIs later (i.e., had longer duration of unilateral experience). However, minimizing the delay of bilateral activation most likely resulted in the observation that their spatial acuity skills were age-appropriate by 2 years of age. As a consequence, the development of more complex spatial hearing skills may also follow a normal developmental trajectory.
Limitations of bilateral cochlear implants
While the benefits from bilateral CI use are being documented, there remains a gap in performance between children who use BICIs and children who have normal hearing. A number of other technical factors are likely candidates in accounting for this gap in performance, including: (1) a lack of fine-structure in the incoming signal, therefore absence of low-frequency ITD cues, (2) the absence of a commercially available binaural processor that can preserve cues such as ILDs or ITDs in the envelopes of the signal and present them to the user with fidelity; (3) the absence of coordinated compression by the microphones at the two ears; (4) a likelihood of mismatched insertion depth in the two ears, leading to a mismatch in auditory nerve fibers stimulated in the two ears for the same range of frequencies; and (5) the fact that children with BICIs undergo periods of auditory deprivation not experienced by normal-hearing children.
Considered together, it is clear that the use of BICIs does not guarantee access to the binaural cues thought to be important for these complex tasks like spatial hearing. Today’s commercially-available cochlear implant devices are designed for one ear and as such are unable to coordinate the auditory input to the two ears. In addition, there are no standard guidelines to fitting independently functioning CIs bilaterally. Such protocols may optimize the availability of bilateral information necessary for spatial hearing.
Despite this, the data presented here support the notion that individuals with BICIs have access to monaural cues in either ear and can exploit at least some of the binaural cues that individuals with normal acoustic hearing rely on for a number of spatial hearing tasks. Most likely, BICI users depend on interaural level cues; the extent to which the BICI users depend on interaural timing cues of the signal envelopes, however, appears minimal (Grantham et al. 2008a; Laback et al. 2004; Seeber & Fastl 2008; van Hoesel 2004).
Microphone placement
Consistent with prior studies is the fact that the CI microphones on the majority of the participants were not placed inside the ear canal but rather behind the ear. Of the cohort of children in the BICI group, however, five were tested when using a speech processor that placed the microphone at body level (CICG, “F”; CICH, “G”; CICW, “P”; CIDI, “V”; CIDL, “X”). On the right-left discrimination task when using the adaptive-tracking method, all children with body-level microphones, except CIDL, were unable to perform above chance; CIDL had an MAA threshold of 64.2º in the fixed-intensity level condition only, which was the second largest MAA threshold among the participants. For the task with source angles fixed at ±50º, only CICH and CICW scored at least one standard deviation above chance in the fixed-intensity level condition. They were able to maintain this level of performance when intensity roving was introduced. It is important to note that both of these children had greater than 12 months of bilateral experience.
There is no evidence that placement of a CI microphone off the head, compared to a behind-the-ear placement, interferes with intended outcomes of CI use such as spoken language development, spoken language comprehension and/or educational outcomes. With regard to interaural cues, microphone placements on or off of the head seem to maintain interaural level cues that arise primarily from the shadowing of the signal by the head, at least for some patients (van Hoesel 2004; Ricketts et al. 2006). The caveat to those findings, however is that front-end compression within the speech processor compromise the ILDs in other patients; this latter finding would potentially impair spatial hearing and possibly other auditory skills that depend on interaural cues in implant users.
A conclusion about the benefits or disadvantages of body-level processor microphones cannot be made from the data presented here. Spatial hearing is, however, an acquired skill that is dependent on the listener’s experience with consistent interaural cues. It is not unreasonable to speculate that providing an inconsistent cue via a body-level microphone, due to varying placement on the child’s body from day-to-day, may impede this development. It is important to note that the five children who used body-level microphones were among the poorest performers on the adaptive-tracking version of the right-left discrimination task. Even though three out of the five children had greater than 12 months of BICI experience, their performance did not reflect this experience. Whether or not microphone placement is a factor to the long-term outcomes with BICIs needs to be investigated further.
Strengths and limitations of the current project
This study provides behavioral spatial hearing data on 2-to-3-year-old children, a population that is often difficult to test due to the lack of age-appropriate methodology. The use of the observer-based psychophysical procedure in this study provides an effective method of determining spatial acuity in young children who either have normal acoustic hearing or who are cochlear implant users. There were, however, some limitations to the data presented here. First, not all children were able to complete every condition. Reasons for this may include task difficulty, fatigue and boredom. These reasons may also have contributed to the variability in performance as well as a potential underestimation of spatial acuity in some children. For example, the lack of response from a child could mean either that he/she could not lateralize the sound source location (inability) or that he/she just didn’t respond (the child had the ability but was unwilling to respond). Possible evidence of this was seen in participants who scored better in more complex tasks (i.e., when intensity roving was present) than easier tasks. One participant (CIDM), however, consistently performed better when intensity roving was implemented. Whether this finding reflects a complex interaction between the discrimination of monaural and interaural level cues or whether it reflects an inconsistent attention component is unclear and will need to be investigated further.
Anecdotally, we made two general observations over the course of this study that may give light to some of the results. First, children who seemed to understand the task at the onset (i.e., who could localize the first stimulus they heard) were better able to stay on task. Second, when the task demands increased (i.e., having the children in the BICI remove their second CI), many children disengaged from the task by misbehaving or not responding. Upon returning to a bilateral listening mode, their willingness to stay on task improved. Taken together, these observations would suggest that the children were better able to stay on task when they understood what was happening and were consistently being reinforced. When the task demands were too high, the children essentially stopped participating. Developing additional age-appropriate methods to use with children under the age of 3 years would be helpful in further elucidating spatial hearing skills in this population of children.
Final Note
The observation that a greater number of children in the BICI group relative to the number of children in the UCI group have spatial acuity that approximates that of their acoustic hearing peers suggests that there appears to be a benefit to providing bilateral input at a young age. There continues, however, to be large variability in overall outcomes; the reasons for this are still unclear and need to be more systematically studied.
Acknowledgments
Sources of support:
NIH NIDCD grant numbers R21DC006642 and 5R01DC8365 to Litovsky and F32DC008452 to Grieco-Calub; Cochlear Corporation, Advanced Bionics, MED-EL Corporation for participant travel and stipend; This study was also supported in part by a core grant to the Waisman Center from the NIH NICHD (P30 HD03352).
The authors would like to thank the children and their families for their time and dedication to this study. The authors would also like to thank the following clinics and cochlear implant manufacturers for their assistance with participant recruitment: Beth Israel/New York Eye & Ear Cochlear Implant Center, Children’s Hospital Boston, Medical College of Wisconsin, Cochlear Corporation, Advanced Bionics, and Med-El Corporation. We would also like to thank Gongqiang Yu for software development and troubleshooting; Shelly Godar and Emily Kishel Cross for help in arranging participant travel and data collection; and numerous undergraduate and graduate students who assisted during data collection. One of the authors (Ruth Y. Litovsky) has consulted and provided written materials for distribution for Cochlear Americas.
Contributor Information
Tina M. Grieco-Calub, Waisman Center, University of Wisconsin-Madison.
Ruth Y. Litovsky, Department of Communicative Disorders and Waisman Center, University of Wisconsin-Madison.
References
- Blauert J. Spatial Hearing: The Psychophysics of Human Sound Localization. Cambridge: MIT Press; 1997. (Revised ed.) [Google Scholar]
- Galvin KL, Mok M, Dowell RC, Briggs RJ. Speech detection and localization results and clinical outcomes for children receiving sequential bilateral cochlear implants before four years of age. Int J Audiol. 2008;47(10):636–646. doi: 10.1080/14992020802203314. [DOI] [PubMed] [Google Scholar]
- Godar SP, Litovsky RY. Experience with bilateral cochlear implants improves sound localization acuity in children. Otol Neurotol. 2010;31(8):1287–1292. doi: 10.1097/MAO.0b013e3181e75784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon KA, Valero J, Papsin BC. Auditory brainstem activity in children with 9–30 months of bilateral cochlear implant use. Hear Res. 2007;233(1–2):97–107. doi: 10.1016/j.heares.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Grantham DW, Ashmead DH, Ricketts TA, Haynes DS, Labadie RF. Interaural time and level difference thresholds for acoustically presented signals in post-lingually deafened adults fitted with bilateral cochlear implants using CIS+ processing. Ear Hear. 2008;29(1):33–44. doi: 10.1097/AUD.0b013e31815d636f. [DOI] [PubMed] [Google Scholar]
- Grantham DW, Ricketts TA, Ashmead DH, Labadie RF, Haynes DS. Localization by postlingually deafened adults fitted with a single cochlear implant. Laryngoscope. 2008;118(1):145–151. doi: 10.1097/MLG.0b013e31815661f9. [DOI] [PubMed] [Google Scholar]
- Grieco-Calub TM, Litovsky RY. Sound localization skills in children who use bilateral cochlear implants and in children with normal acoustic hearing. Ear Hear. 2010;31(5):645–656. doi: 10.1097/AUD.0b013e3181e50a1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco-Calub TM, Litovsky RY, Werner LA. Using the observer-based psychophysical procedure to assess localization acuity in toddlers who use bilateral cochlear implants. Otol Neurotol. 2008;29(2):235–239. doi: 10.1097/mao.0b013e31816250fe. [DOI] [PubMed] [Google Scholar]
- Grieco-Calub TM, Saffran JR, Litovsky RY. Spoken word recognition in toddlers who use cochlear implants. J Speech Lang Hear Res. 2009;52(6):1390–1400. doi: 10.1044/1092-4388(2009/08-0154). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock KE, Noel V, Ryugo DK, Delgutte B. Neural coding of interaural time differences with bilateral cochlear implants: Effects of congenital deafness. J Neurosci. 2010;30(42):14068–14079. doi: 10.1523/JNEUROSCI.3213-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AJ, Parsons CH, Moore DR. Plasticity in the neural coding of auditory space in the mammalian brain. Proc Natl Acad Sci U S A. 2000;97(22):11821–11828. doi: 10.1073/pnas.97.22.11821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk KI, Miyamoto RT, Lento CL, Ying E, O'Neill T, Fears B. Effects of age at implantation in young children. Ann Otol Rhinol Laryngol Suppl. 2002;189:69–73. doi: 10.1177/00034894021110s515. [DOI] [PubMed] [Google Scholar]
- Laback B, Pok SM, Baumgartner WD, Deutsch WA, Schmid K. Sensitivity to interaural level and envelope time differences of two bilateral cochlear implant listeners using clinical sound processors. Ear Hear. 2004;25(5):488–500. doi: 10.1097/01.aud.0000145124.85517.e8. [DOI] [PubMed] [Google Scholar]
- Linkenhoker BA, Knudsen EI. Incremental training increases the plasticity of the auditory space map in adult barn owls. Nature. 2002;419(6904):293–296. doi: 10.1038/nature01002. [DOI] [PubMed] [Google Scholar]
- Litovsky RY. Developmental changes in the precedence effect: Estimates of minimum audible angle. J Acoust Soc Am. 1997;102(3):1739–1745. doi: 10.1121/1.420106. [DOI] [PubMed] [Google Scholar]
- Litovsky RY. Development of Binaural and Spatial Hearing. In: Werner LA, Popper A, Fay R, editors. Human Auditory Development. Springer; 2011. in press. [Google Scholar]
- Litovsky R, Ashmead D. Development of binaural and spatial hearing in infants and children. In: Gilkey RH, Anderson TR, editors. Binaural and Spatial Hearing. Hillsdale, NJ: Lawrence Earlbaum Associates; 1997. pp. 571–592. [Google Scholar]
- Litovsky RY, Godar SP. Difference in precedence effect between children and adults signifies development of sound localization abilities in complex listening tasks. J Acoust Soc Am. 2010;128(4):1979–1991. doi: 10.1121/1.3478849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky RY, Johnstone PM, Godar S, Agrawal S, Parkinson A, Peters R, et al. Bilateral cochlear implants in children: Localization acuity measured with minimum audible angle. Ear Hear. 2006a;27(1):43–59. doi: 10.1097/01.aud.0000194515.28023.4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky RY, Johnstone PM, Godar SP. Benefits of bilateral cochlear implants and/or hearing aids in children. Int J Audiol. 2006b;45(Suppl 1):S78–91. doi: 10.1080/14992020600782956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky RY, Jones GL, Agrawal S, van Hoesel R. Effect of age at onset of deafness on binaural sensitivity in electric hearing in humans. J Acoust Soc Am. 2010;127(1):400–414. doi: 10.1121/1.3257546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky RY, Parkinson A, Arcaroli J, Peters R, Lake J, Johnstone P, Yu G. Bilateral cochlear implants in adults and children. Arch Otolaryngol Head Neck Surg. 2004;130(5):648–55. doi: 10.1001/archotol.130.5.648. [DOI] [PubMed] [Google Scholar]
- Litovsky R, Parkinson A, Arcaroli J, Sammeth C. Simultaneous bilateral cochlear implantation in adults: a multicenter clinical study. Ear Hear. 2006c;27(6):714–731. doi: 10.1097/01.aud.0000246816.50820.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky R, Parkinson A, Arcaroli J. Spatial hearing and speech intelligibility in bilateral cochlear implant users. Ear Hear. 2009;30(4):419–431. doi: 10.1097/AUD.0b013e3181a165be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky RY, Macmillan NA. Sound localization precision under conditions of the precedence effect: Effects of azimuth and standard stimuli. J Acoust Soc Am. 1994;96(2 Pt 1):752–758. doi: 10.1121/1.411390. [DOI] [PubMed] [Google Scholar]
- Litovsky RY, Parkinson A, Arcaroli J. Spatial hearing and speech intelligibility in bilateral cochlear implant users. Ear Hear. 2009;30(4):419–431. doi: 10.1097/AUD.0b013e3181a165be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills AW. On the minimum audible angle. J Acoust Soc Am. 1958;30:237–246. [Google Scholar]
- Mok M, Galvin KL, Dowell RC, McKay CM. Speech perception benefit for children with a cochlear implant and a hearing aid in opposite ears and children with bilateral cochlear implants. Audiol Neurootol. 2010;15(1):44–56. doi: 10.1159/000219487. [DOI] [PubMed] [Google Scholar]
- Morrongiello BA, Rocca PT. Infants' localization of sounds within hemifields: Estimates of minimum audible angle. Child Dev. 1990;61(4):1258–1270. [PubMed] [Google Scholar]
- Muir D, Field J. Newborn infants orient to sounds. Child Dev. 1979;50(2):431–436. [PubMed] [Google Scholar]
- Neuman AC, Haravon A, Sislian N, Waltzman SB. Sound-direction identification with bilateral cochlear implants. Ear Hear. 2007;28(1):73–82. doi: 10.1097/01.aud.0000249910.80803.b9. [DOI] [PubMed] [Google Scholar]
- Nicholas JG, Geers AE. Effects of early auditory experience on the spoken language of deaf children at 3 years of age. Ear Hear. 2006;27(3):286–298. doi: 10.1097/01.aud.0000215973.76912.c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopp P, Schleich P, D'Haese P. Sound localization in bilateral users of MED-EL COMBI 40/40+ cochlear implants. Ear Hear. 2004;25(3):205–214. doi: 10.1097/01.aud.0000130793.20444.50. [DOI] [PubMed] [Google Scholar]
- Olsho LW, Koch EG, Haplin CF, Carter EA. An observer-based psychoacoustic procedure for use with young infants. Dev Psychol. 1987;23:627–40. [Google Scholar]
- Popescu MV, Polley DB. Monaural deprivation disrupts development of binaural selectivity in auditory midbrain and cortex. Neuron. 2010;65(5):718–731. doi: 10.1016/j.neuron.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricketts T, Grantham DW, D'Haese P, Edwards J, Barco A. Cochlear implant speech processor placement and compression effects on sound sensitivity and interaural level difference. J Am Acad Audiol. 2006;17(2):133–140. doi: 10.3766/jaaa.17.2.5. [DOI] [PubMed] [Google Scholar]
- Seeber BU, Fastl H. Localization cues with bilateral cochlear implants. J Acoust Soc Am. 2008;123(2):1030–1042. doi: 10.1121/1.2821965. [DOI] [PubMed] [Google Scholar]
- Seidl AH, Grothe B. Development of sound localization mechanisms in the mongolian gerbil is shaped by early acoustic experience. J Neurophysiol. 2005;94(2):1028–1036. doi: 10.1152/jn.01143.2004. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman MF, Kral A. The influence of a sensitive period on central auditory development in children with unilateral and bilateral cochlear implants. Hear Res. 2005;203(1–2):134–143. doi: 10.1016/j.heares.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Shaw EAG. The external ear. In: Keidel WD, Neff WD, editors. Handbook of Sensory Physiology. New York: Springer-Verlag; 1974. pp. 455–490. [Google Scholar]
- Tillein J, Hubka P, Syed E, Hartmann R, Engel AK, Kral A. Cortical representation of interaural time difference in congenital deafness. Cereb Cortex. 2010;20(2):492–506. doi: 10.1093/cercor/bhp222. [DOI] [PubMed] [Google Scholar]
- Tyler RS, Gantz BJ, Rubinstein JT, Wilson BS, Parkinson AJ, Wolaver A, et al. Three-month results with bilateral cochlear implants. Ear Hear. 2002;23(1 Suppl):80S–89S. doi: 10.1097/00003446-200202001-00010. [DOI] [PubMed] [Google Scholar]
- Van Deun L, van Wieringen A, Scherf F, Deggouj N, Desloovere C, Offeciers FE, et al. Earlier intervention leads to better sound localization in children with bilateral cochlear implants. Audiol Neurootol. 2010;15(1):7–17. doi: 10.1159/000218358. [DOI] [PubMed] [Google Scholar]
- van Hoesel RJ. Exploring the benefits of bilateral cochlear implants. Audiol Neurootol. 2004;9(4):234–246. doi: 10.1159/000078393. [DOI] [PubMed] [Google Scholar]
- van Hoesel RJ, Tyler RS. Speech perception, localization, and lateralization with bilateral cochlear implants. J Acoust Soc Am. 2003;113(3):1617–1630. doi: 10.1121/1.1539520. [DOI] [PubMed] [Google Scholar]
- Wang NY, Eisenberg LS, Johnson KC, Fink NE, Tobey EA, Quittner AL, et al. Tracking development of speech recognition: Longitudinal data from hierarchical assessments in the childhood development after cochlear implantation study. Otol Neurotol. 2008;29(2):240–245. doi: 10.1097/MAO.0b013e3181627a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: I. fitting, sampling, and goodness of fit. Percept Psychophys. 2001a;63(8):1293–1313. doi: 10.3758/bf03194544. [DOI] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: II. bootstrap-based confidence intervals and sampling. Percept Psychophys. 2001b;63(8):1314–1329. doi: 10.3758/bf03194545. [DOI] [PubMed] [Google Scholar]