Abstract
Background
Stroke risk and outcome are different in men and women. We hypothesized that this is partly due to an inherent difference in susceptibility to ischemia between neurons from male vs. female brains. We tested whether neurons from male rodents are more susceptible to in-vitro ischemia than cells from females, and if this is related to increased expression of soluble epoxide hydrolase (sEH). SEH contributes to neuronal cell death by inactivating neuroprotective epoxyeicosatrienoic acids (EETs).
Methods
Rodent cortical neurons were cultured, and exposed to oxygen-glucose deprivation (OGD); then cell death was measured. EETs levels were determined by LC-MS/MS. Expression of sEH-encoding ephx2 was determined by qRT-PCR. Western blotting, immunocytochemistry, and hydrolase activity assay assessed protein expression and activity.
Results
Cell death after OGD was higher in neurons from males vs. females, which correlated with higher ephx2 mRNA and stronger sEH immunoreactivity. However, EETs levels were similar in both sexes and pharmacological inhibition of the hydrolase domain of sEH did not abolish the sex difference in cell death. Genetic knockout of sEH in mice abolished the sex difference observed in neurons isolated from these mice after OGD.
Conclusions
Cultured cortical neurons from females are more resistant to ischemia than neurons from males. Neurons from females have less sEH activity compared to neurons from males at baseline, although sEH levels were not measured after OGD. While pharmacological inhibition of the hydrolase domain of sEH does not affect cell death, knockout of the gene encoding sEH eradicates the sex difference seen in wild-type neurons, suggesting a role for further study of the lesser-known phosphatase domain of sEH and its role in sexual dimorphism in neuronal sensitivity to ischemia.
Keywords: Acute Stroke, EETs, Brain Ischemia, Gender, Soluble epoxide hydrolase
Introduction
Important differences in stroke risk and outcome exist between men and women. In general, stroke incidence and mortality rates are lower in premenopausal women relative to men of the same age (Reeves et al. 2008). The female advantage in relation to stroke risk is present in childhood (Golomb et al. 2008) and persists after menopause (Lloyd-Jones et al. 2009), suggesting that some of the sex differences are unrelated to sex hormones and are, in fact, inherent gender differences at the cellular level. Evidence for inherent genetic differences between males and females have been documented recently in cellular sub-populations in the brain, in neurons in particular. For example, studies conducted using cultured neurons in the absence of sex hormones have shown that cell viability is, in part, dependent on whether cells are derived from the male or female brain (Lei Zhang et al. 2003). In addition, sex differences in neuronal survival have been observed in response to cytotoxic and apoptotic stimuli (Lieb et al. 1995) (Du et al. 2004). These observations suggest that male and female neurons may have inherently different susceptibility to ischemia, and that this difference is triggered by innate variations between the sexes in gene regulation and protein expression that are independent of post-natal exposure to sex hormones.
A potential gene involved in the sexual dimorphism of neuronal survival is EPHX2, the gene coding for the protein soluble epoxide hydrolase. Soluble epoxide hydrolase (sEH) is a protein known to be sexually dimorphic in the whole brain, and in liver and kidney, but it is not known if it is sexually dimorphic in neurons, and if it mediates the sex difference in neuronal ischemic sensitivity. sEH is a heterodimer that possesses a C-terminal hydrolase domain as well as an N-terminal phosphatase domain. The C-terminal hydrolase metabolizes and inactivates a lipid signaling molecule called epoxyeicosatrienoic acids (EETs) via hydrolysis. EETs have been shown to protect neurons from ischemic injury both in vivo and in vitro(Iliff and Nabil J Alkayed 2009), and sEH inhibition and gene deletion have also been shown to be protective against ischemic injury(Wenri Zhang et al. 2009)(W Zhang et al. 2008). The N-terminal phosphatase domain of sEH is less studied but may be involved in fatty acid metabolism (Newman et al. 2003) and may participate in the regulation of eNOS activity in vivo (Hou et al. 2011).
In the current study, we sought to explore the mechanism of sexual dimorphism in neuronal ischemic sensitivity at the cellular level. Using an in vitro model of ischemia, oxygen-glucose deprivation (OGD), we examined cell death in relation to sEH expression and activity in neurons derived from male versus female murine fetus. Additionally, we measured total intracellular EETs levels in these cultures using LC-MS/MS. Finally, we examined the effect of pharmacological and genetic ablation of sEH on cell death.
Materials and Methods
This study was conducted in accordance with the National Institutes of Health guidelines for the care and use of animals in research and the protocols were approved by the Animal Care and Use Committee of Oregon Health & Science University.
Neuronal Cell Culture
All cell culture reagents were purchased from Invitrogen (Carlsbad, CA) except as specified. Highly-enriched neuronal cultures were prepared from embryonic day 18 Sprague-Dawley rat fetuses (Charles River, Wilmington, MA) as previously described (I P Koerner et al. 2007) or embryonic day 16 mouse fetuses from C57BL/6 (Charles River) or sEH knockout (sEHKO) mice as previously described (Jia et al. 2011). Rat and mouse fetuses were separated by sex after laparotomy and visual inspection of internal sex organs.
Rat Neuronal Culture
Briefly, brains were removed; cortices were dissected in HEPES-buffered HBSS and dissociated by digestion with trypsin and trituration. Neurons from male (NM) and female (NF) littermates were cultured side-by-side and were seeded at a density of 1.5 × 105 cells/cm2 onto poly-D-lysine coated plates. Neurons were grown in Neurobasal medium without phenol red supplemented with 2% B27, 1% Glutamax, and 1% penicillin/streptomycin. Cytosine-1-β-D-arabino furanoside (Ara-C, 1 µM) was added to the culture medium on DIV 3 to suppress growth of glial cells. Cultures consisted of >98% microtubule-associated protein 2 (MAP2)-positive neurons and <2% glial fibrillary acidic protein (GFAP)-positive astrocytes.
Mouse Neuronal Culture
After determining sex, brains were removed; cortices were dissected in HEPES-buffered HBSS. Tissue was digested with a 0.5 mg/mL papain solution (Worthington Biochemical Corporation, Lakewood, NJ) at 37°C for 8 minutes. Papain was removed and tissue was washed twice with Trypsin Inhibitor (1 mg/mL, Trypsin Inhibitor, soybean, Sigma-Aldrich, St. Louis, MO) for 2 minutes. Cells were rinsed once with neurobasal medium and then dissociated into a total of 10 mL neurobasal medium via tituration with a 5 mL pipette. Cells were then spun at 1,000 rpm for 5 minutes, supernatant was removed; cells were resuspended in fresh neurobasal medium and filtered with a cell strainer. Cells were then counted and plated at equal densities as described above.
Soluble epoxide hydrolase knockout (sEHKO) mice
Mice with targeted deletion of sEH (sEH knockout/sEHKO) were used only for the sEHKO cell death experiments. The mice originated on a B6;129X1 background and have been backcrossed to C57BL/6 for more than 7 generations, as previously described (W Zhang et al. 2008). Homozygous sEHKO mice are viable, fertile, normal in size, and phenotypically identical to C57BL/6 mice. Mice were genotyped by PCR as previously described (W Zhang et al. 2008)(Sinal et al. 2000).
Oxygen-Glucose Deprivation
To simulate ischemia, cells were subjected to oxygen-glucose deprivation (OGD) on DIV 10. OGD was performed as published in Koerner, et al (2007) (I P Koerner et al. 2007). Briefly, culture plates were placed in an anaerobic chamber (COY Laboratory Products, Grass Lake, MI) filled with anoxic gas mixture (5% CO2, 5% H2, 90% N2). Oxygen concentration was maintained at 0 parts per million (ppm) using a palladium catalyst. Culture medium was then replaced with Dulbecco’s Modified Eagle Medium (DMEM) without glucose, and cells were maintained in the OGD chamber for 2 hours. After 2 hours of OGD, DMEM was exchanged with prewarmed culture growth medium, and cells were returned to the normoxic incubator for 24 hours. After 24 hours of reoxygenation, neuronal cell death was assessed as described below.
Drug Treatment
Primary cultured cortical neurons were treated with 2 µM 4-phenylchalcone oxide (4-PCO, Biomol, Plymouth Meeting, PA). Treatment was started one hour before OGD and continued throughout the OGD/reperfusion period. 4-PCO was dissolved in Dimethylsulfoxide (DMSO), and DMSO was used as vehicle control. The drug’s concentration and treatment duration were the same as previously used in Koerner et al (Ines P Koerner et al. 2008).
Assessment of Cell Death
Cell death was determined by the release of lactate dehydrogenase into the media (LDH Cytotoxicity Detection Kit; Roche Diagnostics, Basel, Switzerland). Data from 3 to 5 wells per condition per experiment were averaged to n=1. Each experiment represents an independent culture from a separate litter. Cell death was confirmed by the reduction of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), which is converted by viable cells to a formazan that can be measured spectrophotometrically at 540 nm as an indicator of cell viability
Hydrolase Activity Assay
Soluble epoxide hydrolase activity was determined using Epoxyfluor 7 (EP7), (Cayman Chemical Company) as previously described (Jones et al. 2005). Cells were lysed in PBS on ice before immediate quantification of hydrolase activity. Reactions were carried out in 200 µL of 25 mM BisTris-HCl containing 1 mg/mL bovine serum albumin (BSA) and the substrate EP7 (5 µM). The resulting solution was incubated at 37°C for 60 minutes in a black 96-well flat bottom plate (Corning, Corning, NY). Fluorescence of hydrolyzed EP7 was determined using an excitation wavelength of 330 nm (bandwidth=20 nm) and an emission wavelength of 465 nm (bandwidth=20 nm) on a plate reader (VICTOR, Wallac / Perkin Elmer, Waltham, MA). Activity was normalized to sample protein concentration and expressed as relative fluorescence units (RFU). All determinations were performed with at least three replicates.
Immunocytochemistry
To localize sEH, a primary rabbit polyclonal antibody against sEH (1:1000, a gift from Dr. Bruce Hammock) was used. A cy-2 labeled goat secondary antibody was used for labeling (1:2500, Jackson ImmunoResearch).
Rat cortical neurons were plated onto 18 mm poly-D-lysine coated glass coverslips. On DIV 7, coverslips were washed, fixed with 4% paraformaldehyde in PBS for 10 min, washed and blocked with blocking buffer consisting of 5% normal goat serum (Jackson ImmunoResearch, West Grove, PA), 1% bovine serum albumin (BSA, Sigma), 0.2% Fish Gelatin, and 0.1% Triton-X (Sigma) in PBS. Coverslips were incubated with primary antibodies diluted in blocking buffer at 4°C overnight, washed with 0.1% Tween-20 in PBS (PBS-T), and incubated with secondary antibody for 2 hrs at room temperature. Finally, Hoechst 33342 (Invitrogen) stain was applied to the coverslips then rinsed once with PBS-T. Coverslips were then mounted with Southern Biotech Fluoromount-G mounting media (InterScience, Markham, ON, Canada).
Slides were photographed using a Zeiss 710 laser confocal microscope. Identical conditions were applied for each photograph. sEH immunofluorescence was quantified using Imaris software (BitPlane Scientific Software, Zurich, Switzerland). The sum of fluorescent pixels was obtained for three fields of view and averaged per slide and the sum of the background fluorescence was subtracted. Three slides each from at least four independent cell culture experiments were used for analysis (n=4 biological replicates).
Western Blot
Cells were lysed in a solution containing sucrose (250mM), potassium chloride (60 mM), tris(hydroxymethyl)aminomethane hydrochlorate (15mM), sodium chloride (15 mM), ethylenediaminetetraaacetate (5mM), ethylene glycol tetraacetic acid (1 mM), phenylmethanesulfonylfluoride (0.5mM) and dithiothreitol (10 mM). Cell lysates were then centrifuged at 2000× g for 10 minutes at 4°C. Protein samples (50 µg) were separated by gel electrophoresis and then transferred to Polyvinylidene Difluoride (PVDF) membranes. Blots were blocked in 5% dry milk, and incubated at 4°C overnight with a primary rabbit polyclonal antibody against murine sEH (1:100, Cayman Chemical). The signal was visualized using a horseradish peroxidase-linked (HRP) rabbit secondary antibody (1:500, GE Healthcare, Piscataway, NJ) followed by detection using Supersignal chemiluminescent reagents (Pierce). Images were obtained on a Fluorchem FC2 MultiImage II (Alpha Innotech, St. Leandro, CA) and band optical densities were quantified and expressed relative to GAPDH.
TaqMan Real-Time Quantitative RT-PCR
To quantify ephx2 mRNA expression in cultured cells, total RNA was extracted using a RNAqueous-Micro kit (Ambion, Foster City, CA). First-strand cDNA was prepared using the High Capacity cDNA Reverse Transcriptase kit (Applied Biosystems, Foster City, CA). TaqMan® quantitative PCR reactions were performed in a 96-well plate on an ABI Prism 7000 DNA Detection System. The following specific probe and primer sets for ephx2 were designed with Primer Express software (Applied Biosystems): 5’-FAM-cca gcc cag tca tgg cca at-TAMRA-3 (Probe); 5’-act ggg aat ccc tca agca-3’ (Forward Primer); 5’-aga gag cca tat tcc aca ccag-3’ (Reverse Primer). Each sample was run in triplicate. Levels of ephx2 were normalized to 18S RNA (18s rRNA Control Kit, Eurogentec. Fremont, CA).
Liquid Chromatography-Tandem Mass Spectrometry
EETs quantification in primary cultured cortical neurons was determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Primary cultured cortical neurons were grown in 6-well cell culture dishes for 10 days. The cells were exposed to OGD on DIV 10 and compared to sham controls. After 24 hours of reoxygenation, cells were washed with PBS, then scraped and collected. Methods are identical to previous published methods (Iliff et al. 2010), except that protein was quantified for each sample; therefore, absolute EETs concentrations are available.
Statistics
All values are expressed as the mean ± standard error of the mean (SEM). Statistically significant differences between groups were determined by a t-test for two groups or analysis of variance (ANOVA) for multiple groups using the Sigmastat software (Systat Software, Inc., Chicago, IL). When using ANOVA, two-way ANOVA of the difference of paired means (i.e. repeated measures) was used to allow for a paired littermate analysis (males vs. females of the same litter), comparing the factors of treatment group and sex. This analysis controlled for variability in absolute cell death values among cultures. The post-hoc Student Newman-Keuls tested for differences between treatment group and sex. P<0.05 was considered significant.
Results
Neurons from Female Rodents were Less Susceptible to Oxygen-Glucose Deprivation
When cell death was compared between primary cultured cortical neurons from male (NM) vs. female (NF) embryos of the same litter, NF were consistently more resistant to OGD than NM. This was true in both rat and mouse species. On average, as a percentage of cell death in corresponding NM, NF sustained 16.3±4.5% less death. These results are summarized in Figure 1, which compares cell death data from NM and NF cultured from the rat brain, showing that cell death in NF was significantly less than cell death in NM (n=9, p<0.05).
Figure 1. Cell death in primary cultured cortical neurons from male and female rat brains.
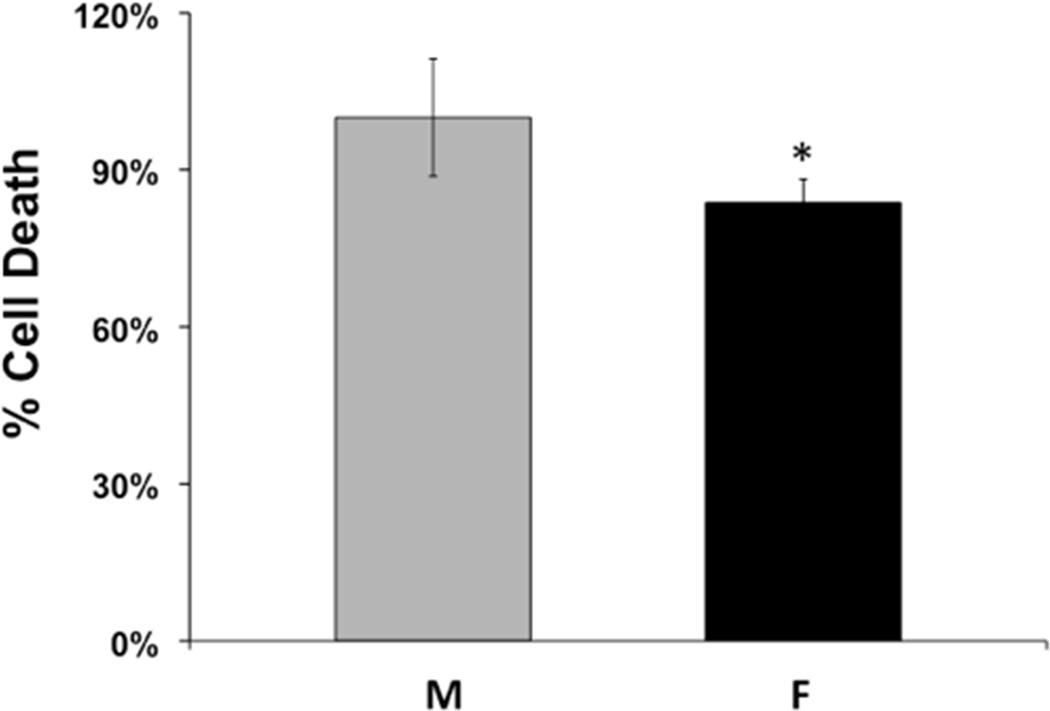
Cortical neurons were cultured from embryonic day 18 male (M) and female (F) rat embryos and were subjected to OGD on day 10 in vitro. Cell death was measured by LDH release in neurons from male and female rats 24 hrs after reoxygenation. The graph depicts average cell death in neurons from male and female embryos expressed relative to the mean of cell death in males. (n=9, *p=0.0019).
Sex Differences in Quantity and Activity of Soluble Epoxide Hydrolase in Neurons
In order to investigate a potential contributing mechanism for the sex differences exhibited in cell death after in vitro ischemia, we used several techniques to measure the expression and activity of sEH and EETs, which are known to be involved in neuroprotection(I P Koerner et al. 2007)(Wenri Zhang et al. 2009). We measured sEH C-terminal hydrolase activity because robust activity of the hydrolase would suggest that neuroprotective EETs levels would be reduced. Using the epoxyfluor 7 (EP7) assay to measure hydrolase activity, we found that it was indeed higher in primary cultured rat cortical NM when compared to NF (Figure 2, n=6, p=0.01). After determining that NM exhibit significantly more hydrolase activity than NF, we hypothesized that sEH protein expression would be higher in NM compared to NF. We investigated this using both immunocytochemistry and Western blotting with anti-sEH antibody. sEH immunoreactivity was detected in both rat NM and NF. Figure 3A represents confocal microscopy images showing increased expression of sEH. Figure 3B is a graphical representation of sEH fluorescence, demonstrating the increased expression of sEH (by 53%) in NM compared with NF (n=4, p=0.011). Additionally, Western blotting demonstrates that NM had consistently more sEH compared to NF littermates (Figure 4, n=4, p=0.004).
Figure 2. Hydrolase activity assay in cultured neurons from male and female rat brains.
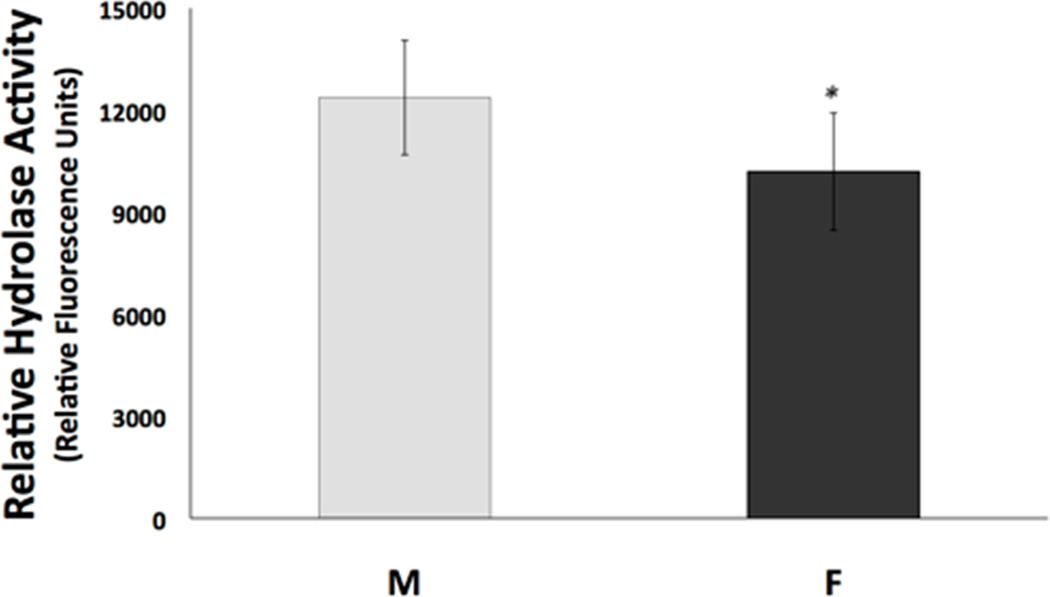
On day 10 in vitro, neuronal cultures from male (M) and female (F) rats were homogenized and incubated with epoxyfluor 7, which, when hydrolyzed by sEH, becomes a fluorescent compound. Fluorescence was measured using a fluorescent plate reader, and activity was normalized to reaction time and amount of protein. (n=6, *p=0.01)
Figure 3. Immunocytochemistry for sEH in neuronal cultures.
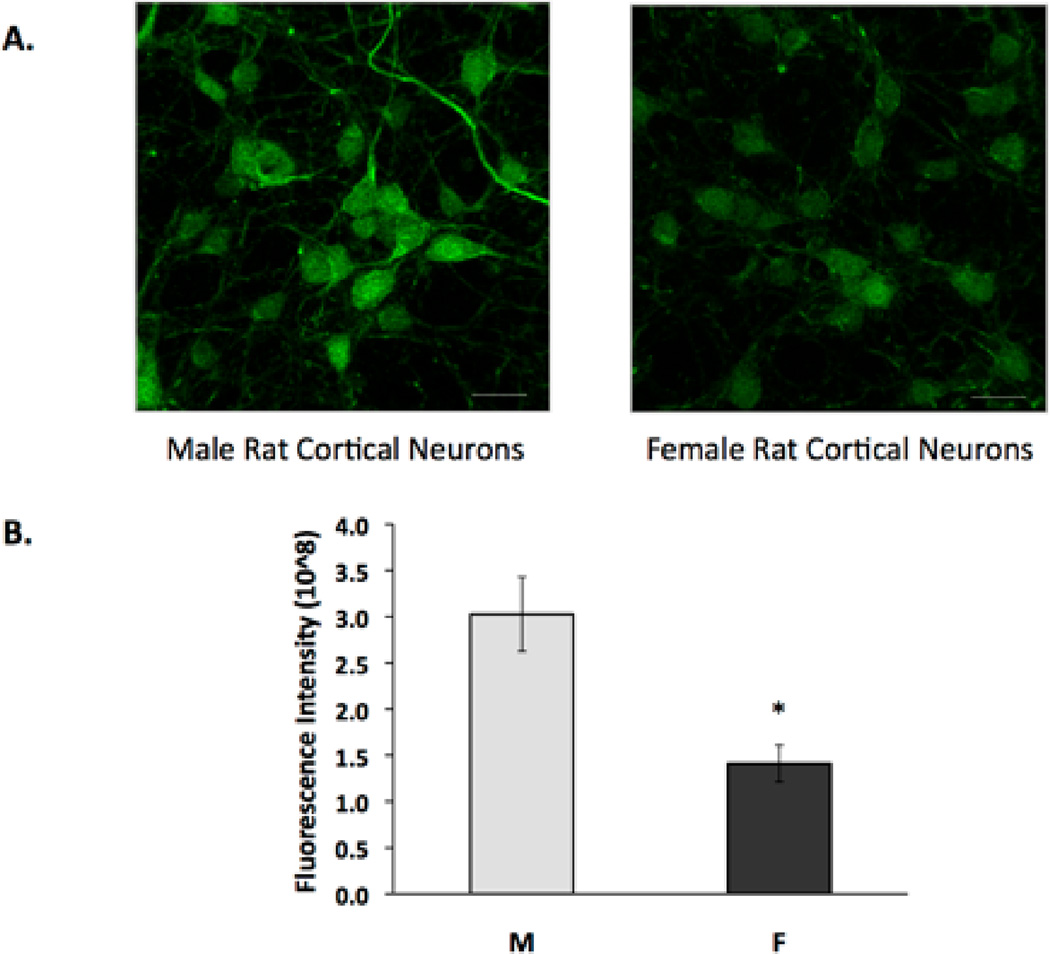
Cultured cortical neurons were immunolabeled for sEH, and images were obtained using confocal microscopy. All images were captured at identical time points with neurons cultured from the same litter and plated at equal densities. Microscope settings were identical for all imaged cells. (A) sEH is broadly expressed in cortical neurons as seen in representative confocal images. Scale bar, 20 µm. (B) The bar graph represents average total fluorescent intensity minus background intensity in neurons from males (M) and females (F). (n=4, *p=0.011) Images are representative of at least four biological replicates.
Figure 4. Western blot analysis of soluble epoxide hydrolase in cortical neurons from males and females.
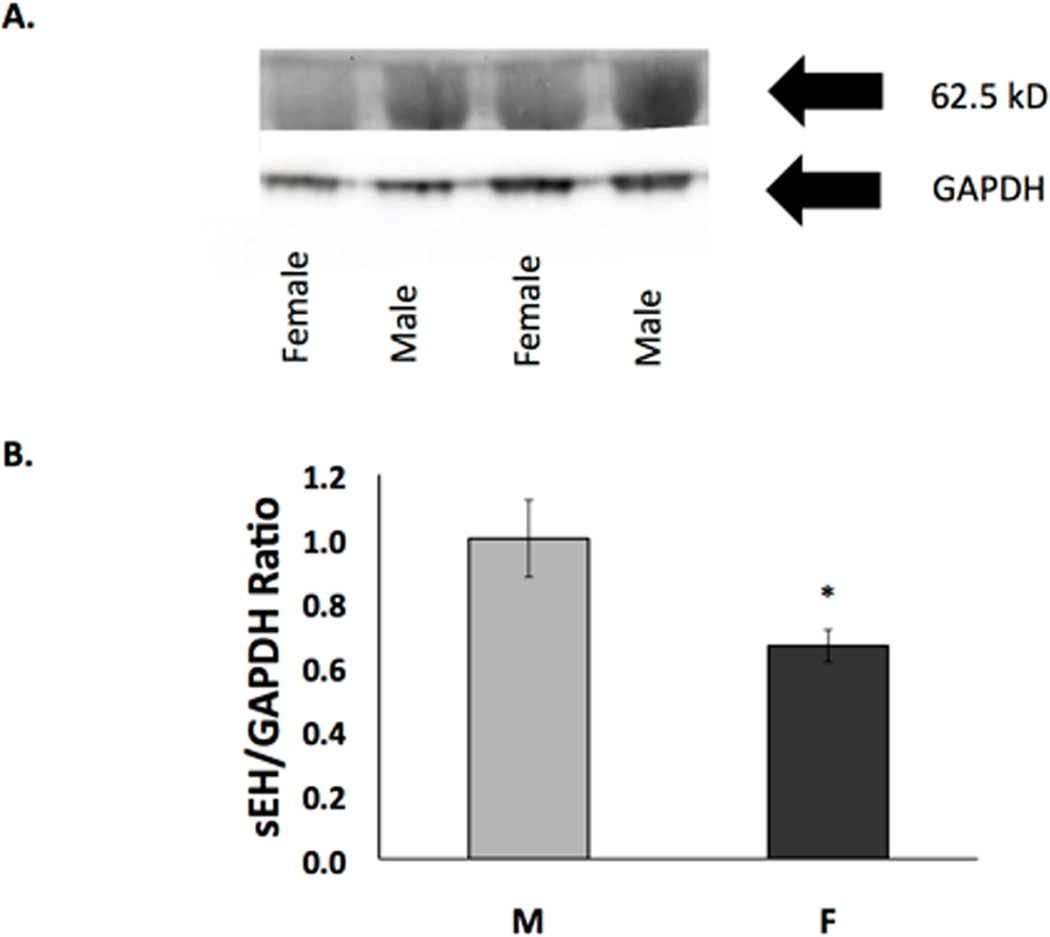
Western blot of protein extracts of neurons from males and females harvested after 10 days in vitro. sEH expression in cultured cortical neurons from males is significantly higher than in neurons from females. (A) Representative image of Western blot. Top panel is probed with anti-sEH antibody and bottom panel is probed with anti-GAPDH. (B) Quantification of Western blots of sEH normalized against GAPDH. Cells from males and females were paired within each experiment (n=4, *p=0.004)
Sex Differences in the Level of EPHX2 mRNA Before and After OGD
We used quantitative real-time RT-PCR (qPCR) to determine the sex difference and effect of ischemia on EPHX2, the gene encoding sEH. At baseline and after 2 hrs OGD, rat NM had significantly more EPHX2 mRNA when compared to NF (Figure 5, n=6, p<0.05). Levels of EPHX2 were much more variable in both NM and NF after OGD. Further, OGD did not affect EPHX2 mRNA, since there was no significant difference between baseline and post-OGD levels of EPHX2 mRNA in either group.
Figure 5. Real-time quantitative PCR for EPHX2 in cultured cortical neurons.
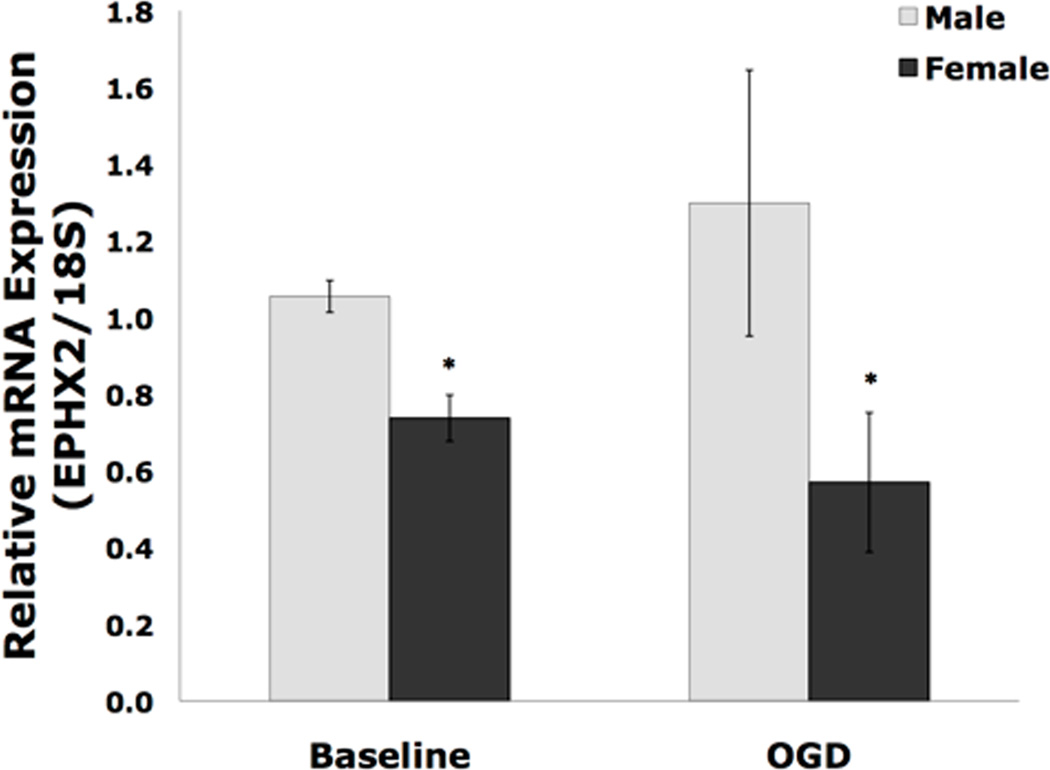
Real-time quantitative PCR was performed on the 11th day in vitro on cortical cultured neurons from males (M) and females (F). Baseline represents untreated cells, while OGD represents cells that have undergone 2 hours OGD and then 24 hours reoxygenation. (n=6, *p<0.05) There were no differences between baseline and post-OGD values in either group.
14,15-EET was Present in Male and Female Cells at Baseline and After OGD
Because sex differences exist in both quantity and activity of sEH, we determined whether sex differences also exist in the quantity of 14,15-EET, which is synthesized from arachidonic acid by cytochrome P450 epoxygenase enzymes and hydrolyzed by sEH. Figure 6A depicts representative peaks used in the quantification of 14,15-EET in cells using LC-MS/MS. Interestingly, there was no significant difference in total 14,15-EET concentrations between rat NM and NF at baseline (Figure 6B, n=5, p=0.07). After OGD 14,15-EET significantly decreased in NF, but not NM (n=5, p=0.015). Other EETs regioisomers were also present, albeit at lower concentrations, and similar to 14,15-EET, there were no sex differences in other EETs (data not shown).
Figure 6. 14,15-EET was present in cells from males and females at baseline and after OGD.
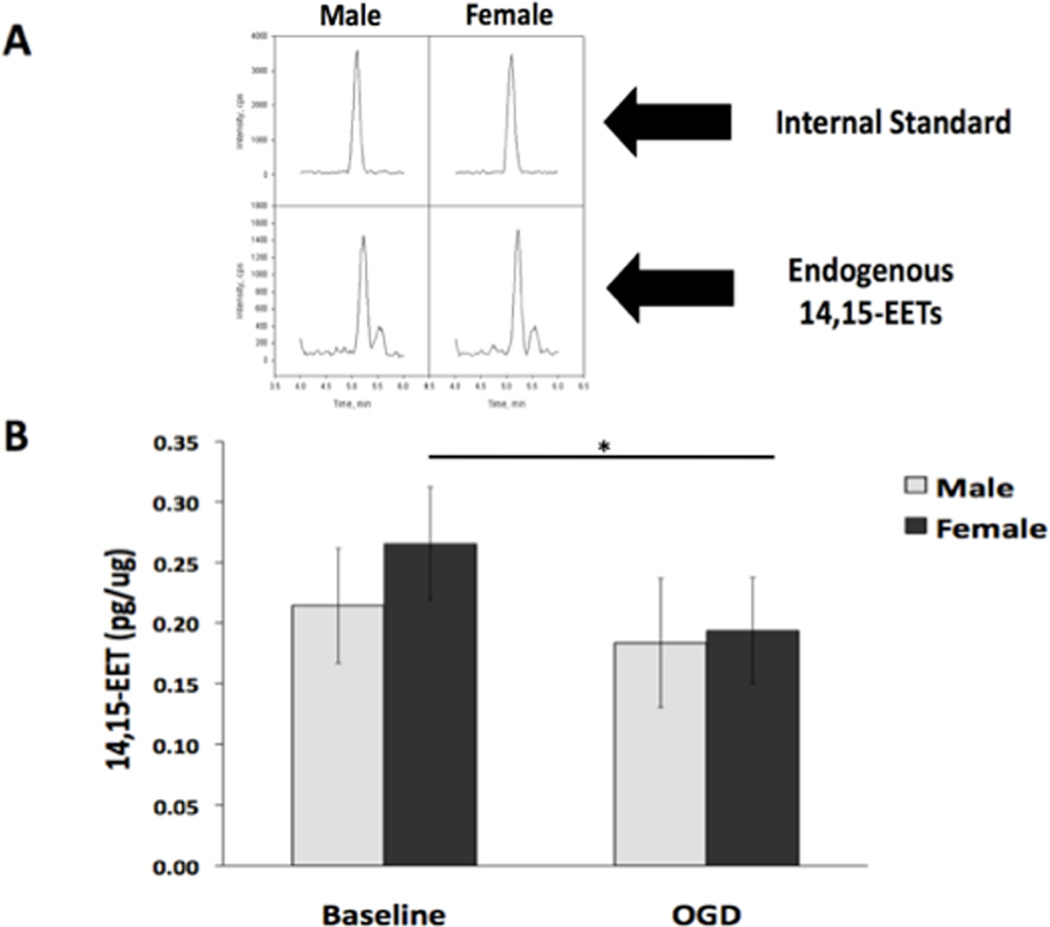
The concentration of EETs in neurons from males and females was evaluated using liquid chromatography-tandem mass spectrometry (LC-MS/MS) in untreated (baseline) cells and cells subjected to 2 hours of OGD followed by 24 hours reoxygenation. (A) Representative 14,15-EET peaks obtained via LC-MS/MS in both male (M) and female (F) cells. The top panel represents the d8 14,15-EET internal standard. The bottom panel shows the endogenous 14, 15-EET peaks from the cells. The left two are from male cells and the right two are from female cells. The SRM transitions monitored were m/z 327.2 to 182.2 for the internal standard and the m/z for the 14,15 EET is 319.2 to 175. (B) The concentration of total 14,15-EET is not significantly different in cells from males and females at baseline (n=5, p=0.07). After OGD, 14,15-EET significantly decreased in cells from females, but not males. * Indicates a significant decrease in 14,15-EET after OGD in cells from females (n=5, *p=0.015).
We also measured 14,15-EET concentration in media, to account for the possibility that EETs are released from the cells in response to OGD; however, the media EETs concentrations were highly variable and no conclusions could be made.
Inhibition of sEH Using 4-PCO
To determine if the sex difference in sEH contributes to the sex difference in ischemic cell death, we tested whether treatment with an sEH inhibitor would abolish the sexual dimorphism seen in neuronal ischemic cell death. We used 4-Phenylchalcone Oxide (4-PCO), a known inhibitor of sEH (Koerner et al, 2007). 4-PCO inhibits the hydrolase domain of sEH without inhibiting the lesser-known phosphatase domain(Newman et al. 2003). There is no known inhibitor currently available that specifically inhibits the phosphatase domain of sEH. We found that at a dose of 2 µM, 4-PCO inhibited 97% of the hydrolase activity of recombinant sEH when tested with the Epoxyfluor 7 hydrolase activity assay (data not shown). Accordingly, on DIV 10, primary neuronal cultures were treated with 2 µM 4-PCO or DMSO vehicle one hour prior to OGD, and treatment continued throughout OGD and 24 hours of reoxygenation. Cell death was lower in NF vs. NM neurons regardless of treatment, but 4-PCO did not eliminate the sex difference in cell death (Figure 7, n=7).
Figure 7. Inhibition of sEH using 4-PCO does not alter the difference in sensitivity to OGD between cells from males vs. females.
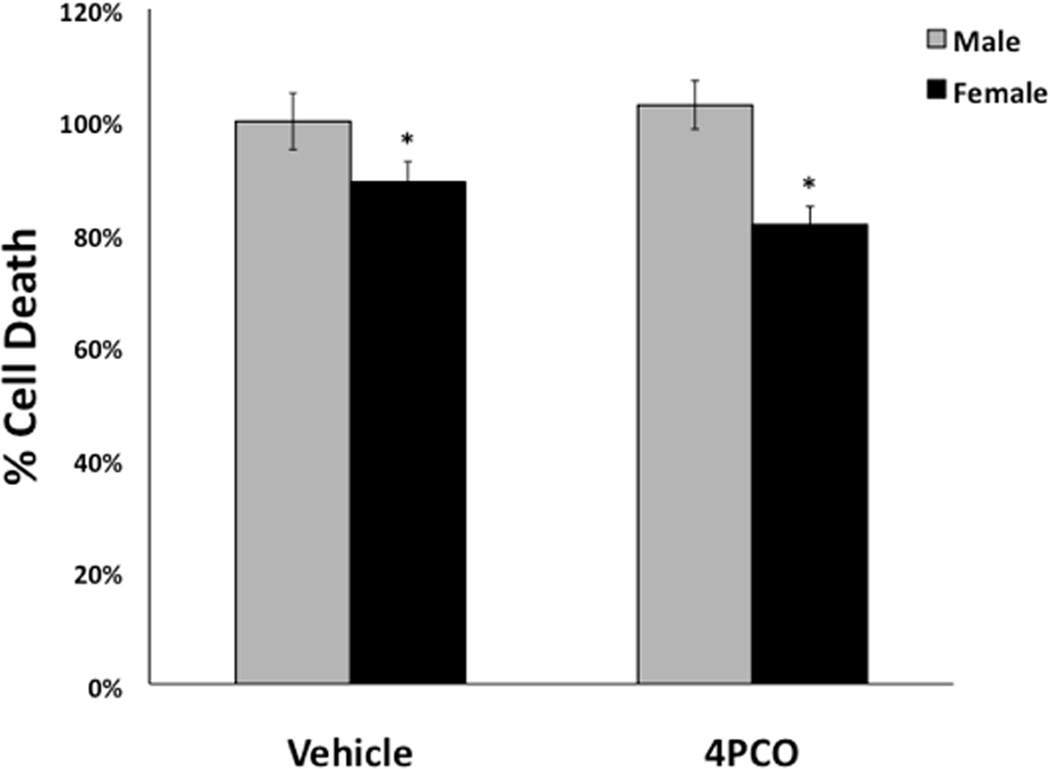
On day 10 in vitro, cultured cortical neurons from males and females were treated with 4-Phenylchalcone oxide (4-PCO, 2 µM), an inhibitor of the hydrolase activity of soluble epoxide hydrolase, or vehicle (DMSO). Treatment was initiated 1 hour prior to OGD and continued throughout OGD and 24 hours of reoxygenation. Cells from females were more resistant to cell death in both vehicle and 4-PCO treated cells as expressed relative to the mean in cells from males (n=5, *p<0.05).
Neurons Cultured from Male and Female sEH Knockout Animals were not Differentially Susceptible to Ischemia
In order to further test whether sEH may be involved in the sexual dimorphism in neuronal cell death, we cultured neurons from sEH knockout (sEHKO) mice and exposed them to OGD. Contrary to our findings using the sEH hydrolase inhibitor 4-PCO, we found that total deletion of sEH completely abolished the sex difference in ischemic neuronal cell death. As shown in Figure 8, whereas WT neurons retain the sex difference in ischemic sensitivity (n=6–8, p=0.026), neurons derived from sEHKO mice did not demonstrate a sex difference in cell death after OGD.
Figure 8. Knockout of sEH eliminates the difference in sensitivity to OGD between cells from males vs. females.
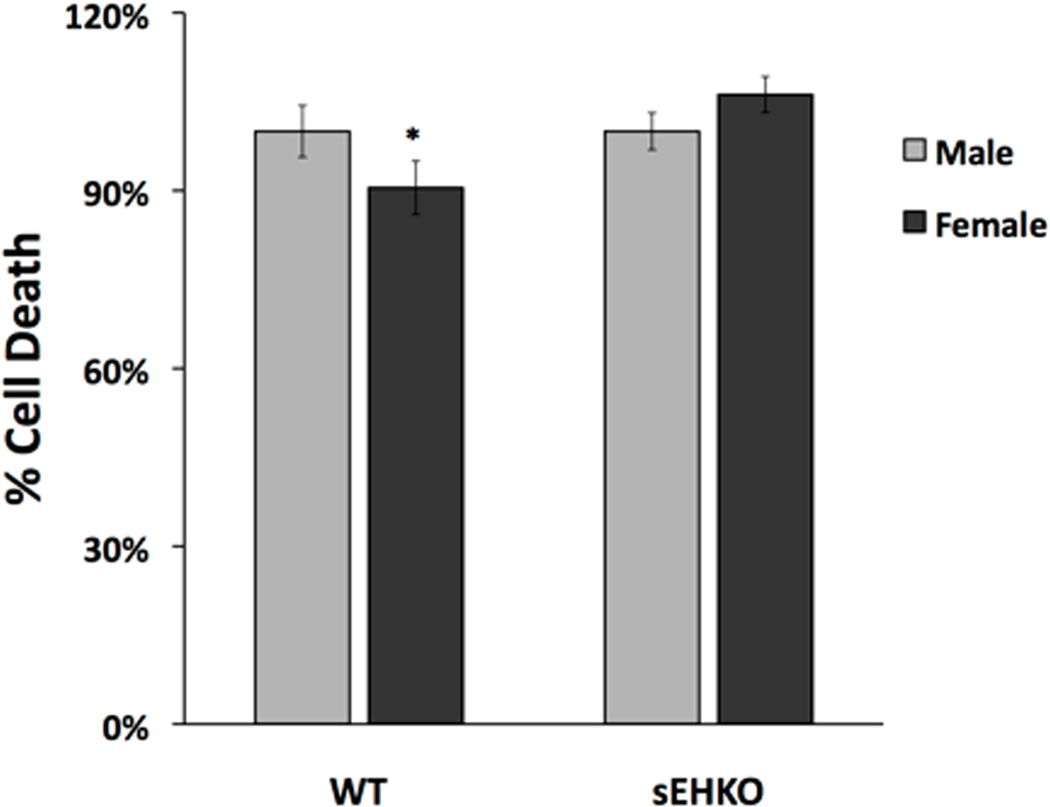
Cortical neurons from wild-type (WT) and sEH knockout (sEHKO) mice were cultured and exposed to OGD on the 10th day in vitro. WT male mice had significantly more cell death after OGD compared with corresponding WT female mice, but this sex difference was not significant in the knockout mice. Average cell death in neurons from male and female embryos was expressed relative to the mean of cell death in males (n=6–8, *p<0.05).
Discussion
This study demonstrates four important findings. First, neurons derived from male and female murine cortices responded differently when exposed to OGD. Second, we, for the first time were able to detect sEH and endogenous 14,15-EET in primary cultured cortical neuronal cells. Third, the sex difference in susceptibility to OGD was associated with baseline differences in activity and expression of sEH, although sEH levels were not measured after OGD. Lastly, inhibition of the hydrolase activity of sEH did not eliminate the sex difference in cell death after in vitro ischemia, but cells from genetic knockout of sEH did eliminate the sex difference. Based on these findings, we conclude that ischemic neuronal cell death is sexually dimorphic, and the sex difference is linked to differences in sEH, which could not be explained by differences in the enzyme’s hydrolase activity and levels of EETs.
Sex differences in stroke have been observed and reported using both in vitro and in vivo models of ischemic neuronal injury. However, this difference is usually attributed to the exposure to, and protection by, estrogen. For example, we previously demonstrated that adult female rats sustain smaller infarcts after experimental stroke induced by middle cerebral artery occlusion (MCAO) compared to age-matched males (N J Alkayed et al. 1998). The sex difference in infarct size is partly due to the protective effect of the estrogen, because absence of estrogen due to ovariectomy increases ischemic brain damage in female rats, and estrogen replacement is protective against cerebral ischemia in ovariectomized and reproductively senescent male and female rats (Rusa et al. 1999)(N J Alkayed et al. 2000). In our experiments, neuronal cells were grown in steroid-free media in the absence of sex hormones; therefore, the sex differences in neuronal cell death cannot be explained by differences in sex hormone exposure. Our experiments focused on the absence of sex hormones in culture, so organizational effects of the sex hormones on the developing embryo in utero until the day of culture cannot be completely discounted.
Soluble epoxide hydrolase is involved in the metabolism and terminal inactivation of EETs, which are lipid signaling molecules. We previously demonstrated that sEHKO male mice were protected from ischemic brain injury and that protection was associated with higher blood flow during MCAO compared with WT male mice (W Zhang et al. 2008). Soluble epoxide hydrolase is known to be sexually dimorphic in multiple tissues, including whole brain (Wenri Zhang et al. 2009), but whether it is sexually dimorphic specifically in pure neuronal cultures had not been investigated. Here we tested the hypothesis that NF have lower sEH expression compared to NM. Indeed, we were able to demonstrate that NF have lower baseline sEH expression, lower hydrolase activity, and decreased levels of EPHX2 mRNA, although sEH levels were not measured after OGD. The physiologic role of sEH in neurons is not completely understood. It is known that the C-terminal hydrolase domain of sEH is involved in metabolizing EETs. EETs protect against ischemic brain injury in vivo(Wenri Zhang et al. 2009) and against neuronal cell death induced in vitro by OGD (I P Koerner et al. 2007). This protection is by multiple mechanisms, including vasodilation, cytoprotection and suppression of post-ischemic inflammation (Iliff and Nabil J Alkayed 2009).
Because we found that NM have increased sEH expression and activity compared with NF, we hypothesized that the increase in sEH in NM leads to decreased protective EETs levels, which, in turn would lead to increased susceptibility to ischemic cell death. Surprisingly, we did not see a corresponding difference in baseline levels of EETs between NM and NF. We did, however, find a difference in how male- and female-derived neurons respond to OGD. Specifically, NF had significantly less total cellular EETs levels after OGD compared to baseline, whereas there is no change in NM following OGD. EETs are incorporated into membrane phospholipids and released in response to phospholipase A2 (PLA2) activation (Bernstrom et al. 1992). Therefore, the decrease observed in NF possibly represents a stronger PLA2 response to OGD. We could not confirm this hypothesis by measuring EET levels in media due to high variability, although the average concentration of 14,15-EET in media indeed tended to increase in NF after OGD, while in NM, no such trend was observed.
A possible explanation for the lack of difference in EETs levels between male- and female-derived neurons is that the sex difference may be linked to a different, non-eicosanoid substrate for the hydrolase activity of sEH. Contrary to what we had predicted, pharmacologic inhibition of sEH using 4-PCO did not abolish the sex difference in ischemic cell death in the neurons, despite 4-PCO being an effective inhibitor of the hydrolase activity in vitro. Interestingly, the sex difference in ischemic cell death was abolished when we used neurons cultured from sEH null mice (sEHKO). This finding suggests that, while the sex difference in hydrolase activity may not contribute to the sex difference in cell death, the secondary function of sEH, i.e., its phosphatase activity, may contribute to the difference in neuronal cell death. In support of this notion, in vivo studies have also observed a discrepancy between the effects of pharmacological sEH inhibition (which inhibits only hydrolase) versus total sEH gene deletion (which removes both hydrolase and phosphatase). For example, sEH gene deletion in the sEHKO mouse reduces inflammatory cytokine expression in brain after stroke, whereas pharmacological inhibition did not have any effect on cerebral inflammation after stroke (Ines P Koerner et al. 2008). Similarly, sEH gene deletion was associated with increased blood flow after MCAO in the mouse (W Zhang et al. 2008), while pharmacological inhibition was not (W Zhang et al. 2008). These observations suggest that the enzymes hydrolase activity does not fully account for all phenotypes observed by complete sEH deletion. This may be related to unrecognized roles for the phosphatase domain, a domain that remains largely unstudied due to lack of specific tools to evaluate it and because its endogenous substrate, if any, remains unidentified. Limitations to these observations include the validity of comparing data from a pharmacological inhibitor with data from a genetic knockout. The sEHKO mice are completely devoid of sEH throughout the animal’s entire life, allowing for possible developmental compensations that may influence study outcomes. Use of conditional knockouts in future experiments would help to further clarify this issue.
In summary, primary cultured cortical neurons exhibit an innate sex difference in response to ischemic cell death, with cells from female murine brain exhibiting less death when compared to male-derived neurons. The mechanism supporting this cell difference is yet to be determined, but may involve the phosphatase domain of sEH. Elucidating innate sex-specific mechanisms of brain injury after stroke may allow the development of sex-tailored and more effective therapies against stroke injury in both men and women.
Highlights.
We examine the effects of in vitro ischemia on cultured neurons.
Cultured neurons from female brain are more resistant to in vitro ischemia than neurons from males.
Neurons from females have less sEH expression than those from males at baseline.
Levels of sEH were not measured after OGD.
Knockout of the gene encoding sEH eradicates the sex difference in wild-type neurons.
Acknowledgements
Funding sources: Foundation for Anesthesia Education and Research (FAER) Research Fellowship Grant (Rochester, MN); NIH T-32 Training Grant #T32 GM082770-03; NIH R01 NS044313 and NS070837.
The authors thank Dr. Dennis Koop (PhD, Professor, Department of Physiology & Pharmacology, OHSU, Portland, OR, USA) and the Oregon Health & Science University Bioanalytical Shared Resource/Pharmacokinetics Core for advice on experimental design and for performance of LC/MS-MS analysis; Dr. Bruce D. Hammock, (PhD, Distinguished Professor of Entomology & Cancer Research Center, University of California at Davis, Davis, CA, USA) for the generous contribution of anti-sEH antibody. We also thank Dr. Rachel Dresbeck (PhD) for assistance with editing and language. We also thank the Foundation for Anesthesia Education and Research (Rochester, MN, USA) for the primary source of funding for this project via the FAER Research Fellowship Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998 Jan;29(1):159–165. doi: 10.1161/01.str.29.1.159. discussion 166. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD, Miller VM. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke. 2000 Jan;31(1):161–168. doi: 10.1161/01.str.31.1.161. [DOI] [PubMed] [Google Scholar]
- Bernstrom K, Kayganich K, Murphy RC, Fitzpatrick FA. Incorporation and distribution of epoxyeicosatrienoic acids into cellular phospholipids. The Journal of biological chemistry. 1992 Feb 25;267(6):3686–3690. [PubMed] [Google Scholar]
- Du Lina, Bayir Hülya, Lai Yichen, Zhang Xiaopeng, Kochanek Patrick M, Watkins Simon C, Graham Steven H, Clark Robert S B. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. The Journal of biological chemistry. 2004 Sep 10;279(37):38563–38570. doi: 10.1074/jbc.M405461200. [DOI] [PubMed] [Google Scholar]
- Golomb MR, Fullerton HJ, Nowak-Gottl U, deVeber G for the International Pediatric Stroke Study Group. Male Predominance in Childhood Ischemic Stroke: Findings From the International Pediatric Stroke Study. Stroke. 2008 Dec 29;40(1):52–57. doi: 10.1161/STROKEAHA.108.521203. [DOI] [PubMed] [Google Scholar]
- Hou Hsin-Han, Hammock Bruce D, Su Kou-Hui, Morisseau Christophe, Kou Yu Ru, Imaoka Susumu, Oguro Ami, Shyue Song-Kun, Zhao Jin-Feng, Lee Tzong-Shyuan. N-terminal domain of soluble epoxide hydrolase negatively regulates the VEGF-mediated activation of endothelial nitric oxide synthase. Cardiovascular research. 2011 Nov 8; doi: 10.1093/cvr/cvr267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff Jeffrey J, Fairbanks Stacy L, Balkowiec Agnieszka, Alkayed Nabil J. Epoxyeicosatrienoic acids are endogenous regulators of vasoactive neuropeptide release from trigeminal ganglion neurons. Journal of neurochemistry. 2010 Dec;115(6):1530–1542. doi: 10.1111/j.1471-4159.2010.07059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff Jeffrey J, Alkayed Nabil J. Soluble Epoxide Hydrolase Inhibition: Targeting Multiple Mechanisms of Ischemic Brain Injury with a Single Agent. Future neurology. 2009 Mar 1;4(2):179–199. doi: 10.2217/14796708.4.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Jia, Verma Saurabh, Nakayama Shin, Quillinan Nidia, Grafe Marjorie R, Hurn Patricia D, Herson Paco S. Sex differences in neuroprotection provided by inhibition of TRPM2 channels following experimental stroke. Journal of Cerebral Blood Flow & Metabolism. 2011 Nov;31(11):2160–2168. doi: 10.1038/jcbfm.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones Paul D, Wolf Nicola M, Morisseau Christophe, Whetstone Paul, Hock Bertold, Hammock Bruce D. Fluorescent substrates for soluble epoxide hydrolase and application to inhibition studies. Analytical biochemistry. 2005 Aug 1;343(1):66–75. doi: 10.1016/j.ab.2005.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerner IP, Jacks R, DeBarber AE, Koop D, Mao P, Grant DF, Alkayed NJ. Polymorphisms in the Human Soluble Epoxide Hydrolase Gene EPHX2 Linked to Neuronal Survival after Ischemic Injury. Journal of Neuroscience. 2007 Apr 25;27(17):4642–4649. doi: 10.1523/JNEUROSCI.0056-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerner Ines P, Zhang Wenri, Cheng Jian, Parker Susan, Hurn Patricia D, Alkayed Nabil J. Soluble epoxide hydrolase: regulation by estrogen and role in the inflammatory response to cerebral ischemia. Frontiers in bioscience : a journal and virtual library. 2008;13:2833–2841. doi: 10.2741/2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb K, Andrae J, Reisert I, Pilgrim C. Neurotoxicity of dopamine and protective effects of the NMDA receptor antagonist AP-5 differ between male and female dopaminergic neurons. Experimental neurology. 1995 Aug;134(2):222–229. doi: 10.1006/exnr.1995.1052. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones Donald, Adams Robert, Carnethon Mercedes, de Simone Giovanni, Ferguson T Bruce, Flegal Katherine, Ford Earl, et al. Heart disease and stroke statistics-2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009 Jan 27;119(3):480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- Newman John W, Morisseau Christophe, Harris Todd R, Hammock Bruce D. The soluble epoxide hydrolase encoded by EPXH2 is a bifunctional enzyme with novel lipid phosphate phosphatase activity. Proceedings of the National Academy of Sciences of the United States of America. 2003 Feb 18;100(4):1558–1563. doi: 10.1073/pnas.0437724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves Mathew J, Bushnell Cheryl D, Howard George, Gargano Julia Warner, Duncan Pamela W, Lynch Gwen, Khatiwoda Arya, Lisabeth Lynda. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet neurology. 2008 Oct;7(10):915–926. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusa R, Alkayed NJ, Crain BJ, Traystman RJ, Kimes AS, London ED, Klaus JA, Hurn PD. 17beta-estradiol reduces stroke injury in estrogen-deficient female animals. Stroke. 1999 Aug;30(8):1665–1670. doi: 10.1161/01.str.30.8.1665. [DOI] [PubMed] [Google Scholar]
- Sinal CJ, Miyata M, Tohkin M, Nagata K, Bend JR, Gonzalez FJ. Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation. The Journal of biological chemistry. 2000 Dec 22;275(51):40504–40510. doi: 10.1074/jbc.M008106200. [DOI] [PubMed] [Google Scholar]
- Zhang Lei, Li Pi-Peng, Feng Xu, Barker Jeffery L, Smith Susan V, Rubinow David R. Sex-related differences in neuronal cell survival and signaling in rats. Neuroscience letters. 2003 Feb 6;337(2):65–68. doi: 10.1016/s0304-3940(02)01179-5. [DOI] [PubMed] [Google Scholar]
- Zhang W, Otsuka T, Sugo N, Ardeshiri A, Alhadid YK, Iliff JJ, DeBarber AE, Koop DR, Alkayed NJ. Soluble Epoxide Hydrolase Gene Deletion Is Protective Against Experimental Cerebral Ischemia. Stroke. 2008 Jun 23;39(7):2073–2078. doi: 10.1161/STROKEAHA.107.508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Wenri, Iliff Jeffrey J, Campbell Caitlyn J, Wang Ruikang K, Hurn Patricia D, Alkayed Nabil J. Role of soluble epoxide hydrolase in the sex-specific vascular response to cerebral ischemia. Journal of Cerebral Blood Flow & Metabolism. 2009 May 27;29(8):1475–1481. doi: 10.1038/jcbfm.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]


