Abstract
Humans who exercise are less likely to suffer from stress-related mood disorders. Similarly, rats allowed voluntary access to running wheels have constrained corticosterone responses to mild stressors and are protected against several behavioral consequences of uncontrollable stress which resemble symptoms of human anxiety and depression, including exaggerated fear and deficits in shuttle box escape learning. Although exercise conveys clear stress resistance, the duration of time the protective effects of exercise against the behavioral consequences of uncontrollable stress persist following exercise cessation is unknown. The current studies investigated 1) whether exercise-induced stress resistance extends to social avoidance, another anxiety-like behavior elicited by uncontrollable stressor exposure, and 2) the duration of time the protective effects of exercise persist following forced cessation of exercise. Six weeks of wheel running constrained the increase in corticosterone elicited by social exploration testing, and prevented the reduction in social exploration, exaggerated shock-elicited fear, and deficits in escape learning produced by uncontrollable stress. The protective effect of voluntary exercise against stress-induced interference with escape learning persisted for 15 days, but was lost by 25 days, following cessation of exercise. An anxiogenic effect, as revealed by a reduction in social exploration and an increase in fear behavior immerged as a function of time following cessation of exercise. Results demonstrate that the protective effect of voluntary exercise against the behavioral consequences of uncontrollable stress extends to include social avoidance, and can persist for several days following exercise cessation despite an increase in anxiety produced by forced cessation of exercise.
Keywords: wheel running, depression, anxiety, learned helplessness, serotonin, social exploration
1.0 Introduction
Physical activity is one of the few environmental manipulations known to increase stress resistance. The stress resistance conferred by physical activity can be observed on many levels, from neuroendocrine [5,11,16,41] and immunological [15,17,37], to behavioral [4,9,12,20]. Laboratory rats allowed voluntary access to running wheels, for example, are protected against the development of anxiety- and depression- like behavioral consequences of exposure to a variety of stressors. Investigation of the powerful stress resistance produced by exercise could provide insight into novel therapeutic or preventative strategies, including the design of exercise programs optimal for mental health.
Exposure to uncontrollable stress produces a sequelae of behaviors in rodents which resemble symptoms of human stress-related psychiatric disorders. We have observed that rats allowed 6 weeks of voluntary access to running wheels are protected against many of these behavioral consequences of uncontrollable stress, including exaggerated fear conditioning [24], interference with shuttle box escape [9,13,24], and potentiation of the rewarding effects of morphine [39]. In addition to these behaviors, uncontrollable stress reduces social exploratory behavior [6], an effect argued to resemble anxiety [7]. Social avoidance produced by uncontrollable stress is dependent on hyperactivation of serotonin (5-HT) neurons in the dorsal raphe nucleus (DRN) [6]. Six weeks of wheel running constrains activity of DRN 5-HT neurons during uncontrollable stress [24]; therefore we might expect that 6 weeks of wheel running would also prevent the stress-induced reduction in social exploration. One goal of the current studies is to test this hypothesis.
To date, every animal study investigating the behavioral effects of exercise on anxiety- or depression-like behaviors of which we are aware have exposed the animals to stress or behavioral testing within 24 hours following the last exercise bout. In our own experiments, rats are typically allowed access to their wheels before and after stressor exposure, and are tested for stress-induced behaviors the morning following a night of voluntary activity. It is unlikely that the protective effect of exercise against the behavioral consequences of stress is permanent. The second goal of the current studies, therefore, is to identify how long the protective effect of voluntary exercise persists following forced cessation of exercise. This knowledge could have important implications for the effects of human exercise participation, which is often discontinuous [34].
2.0 Materials and methods
2.1 Animals
Male, Fischer F344 rats (total N = 161) were housed in a temperature (22°C) and humidity-controlled environment and were maintained on a 12:12 h light/dark cycle (lights on 0600–1800). Experimental rats were 6–7 weeks old upon arrival to the animal colony. Juvenile rats used for the social exploration tests were housed in groups of 6 and were 28–32 days old (90 – 100 g) at the time of testing. Animals acclimatized to these housing conditions for 1 week prior to any experimental manipulation. Experimental animals were individually housed in Nalgene Plexiglas cages (45 × 25.2 × 14.7 cm). Care was taken to minimize animal discomfort during all procedures. All experimental protocols were approved by the University of Colorado Animal Care and Use Committee.
2.2 Voluntary wheel running
Animals were randomly assigned to either remain sedentary with no wheels (Sedentary condition) or were housed in cages with attached running wheels (Mini Mitter, Bend, OR; Run condition) that were rendered immobile with a metal stake during the 1 week acclimatization period. Prior studies indicate that sedentary rats housed without a wheel or with a locked wheel behave similarly following uncontrollable stress [20]. Following the acclimatization period, all wheels were unlocked and rats in the run condition were allowed voluntary access to their wheels. Daily wheel revolutions were recorded digitally using Vital View software (Mini Mitter) and distance was calculated by multiplying number of revolutions by wheel circumference (1.081 m).
2.3 Uncontrollable stress
Rats were randomly assigned to be left undisturbed in their home cages (No Stress) or were restrained in Plexiglass tubes (23.4 cm long and 7.0 cm in diameter) and exposed to 100, 5 s, 1.5 mA (average ITI of 1 min) uncontrollable tail shocks (Stress) following our previously published protocols [22,24,27]. All rats were stressed during their inactive (light) cycle, between 0800 and 1000. This tail shock protocol was used because tail shock is a consistent, quantifiable stressor that is known to produce behaviors in rats that resemble symptoms of stress-related mood disorders, including reductions in social exploratory behavior, exaggerated fear conditioning, and deficits in instrumental escape learning [7,31].
2.4 Behavioral testing
2.4.1 Social exploration testing
The juvenile social exploration test described by Christianson et al. [7] was used here. Testing for baseline juvenile social exploration occurred 1 week prior to uncontrollable stress. During social exploration testing, each adult experimental subject was placed into separate, plastic cages identical to their home cages with bedding and a plastic, filter-top lid between 0700 – 0800 h. After 1 h, a 28–32-day-old male juvenile was introduced to the cage for 3 min and exploratory behaviors (sniffing, pinning and allogrooming) were timed by an observer blind to treatment. After the test the juvenile was removed and the experimental rat was returned to the home cage. Baseline testing was used to reduce neophobia to the social exploration procedure. Two rats (one sedentary and one physically active) displayed less than 20 seconds of social exploration during the baseline testing and were excluded from the study.
One week after baseline testing, and 24 h following uncontrollable stress or no stress, rats were again tested for social exploratory behaviors as described for the baseline test. Different juvenile rats were used for the two social exploration tests, so that experimental rats were not exposed repeatedly to the same juvenile. Following the completion of social exploration testing, rats that were tested for freezing and escape behaviors (Experiment 2) were transferred to brightly lit shuttle boxes. Social exploration occurred prior to fear and escape behavioral testing so that shock administration during fear and escape testing wouldn’t interfere with social exploration behavior.
2.4.2 Shock-elicited freezing and escape behavior
Shock-elicited freezing and escape behavior were assessed as previously described [22,24]. Briefly, freezing behavior, defined as the absence of all movement except that required for respiration, was observed for 10 min immediately after placement of the rats into the shuttle boxes. Freezing was scored using a random sampling procedure whereby rats were either scored as freezing or not freezing every 10 seconds by an observer blind to the treatment condition of the animals. Rats then received 2 fixed ratio-1 (FR-1) foot shocks (0.7 mA, 1 min ITI), escape from which was possible by crossing through the shuttle box door to the opposite side of the shuttle box. The 2 FR-1 trials were followed by a 20 min, post-shock freezing observation period. Freezing immediately following shock presentation is a measure of fear conditioned to cues present in the shuttle box [14]. Following the post-shock freezing period, rats received 25 FR-2 foot shocks (0.6 mA, 1 min ITI). If rats failed to perform the FR-2 contingency within 30 s, the shock was terminated and a 30 s escape latency was recorded for that trial.
2.5 Assessment of corticosterone
Trunk blood was collected from non-stressed, sedentary and exercised rats that were either naïve to behavioral testing, or immediately after a juvenile social exploration test (Experiment 1). Plasma corticosterone levels were assessed using the Corticosterone Enzyme Immunoassay Kit (Assay Designs; Ann Arbor, Michigan) following the manufacturer’s instructions. Samples were diluted 1:50 in Steroid Displacement Reagent made by adding 5.0 μl of the concentrated Steroid Displacement Reagent to 10.0 ml of Assay Buffer 15.
2.6 Procedures
The first experiment tested the hypothesis that 6 weeks of wheel running would 1) prevent the reduction of social exploratory behavior produced by uncontrollable stress and 2) attenuate the slight increase in corticosterone elicited by the mild stress of the social exploration testing procedure. Baseline social exploration tests were conducted following 5 weeks of the sedentary or exercise conditions. One week later, during which exercised rats were allowed access to their running wheels, rats were exposed to no stress or uncontrollable tail shock. Social exploration testing occurred 24 h later. Rather than being tested for conditioned fear and shuttle box escape behaviors, non-stressed rats in Experiment 1 were sacrificed immediately following social exploration for assessment of plasma corticosterone. In order to determine the effect of the social exploration testing procedure and prior wheel running on corticosterone, corticosterone values from rats exposed to social exploration were compared to sedentary and exercised rats naïve to social exploration behavioral testing.
The goal of the second experiment was to determine if the protective effects of voluntary exercise against the behavioral consequences of uncontrollable stress persist for up to 25 days following forced cessation of exercise. At 7–8 weeks of age, wheels in the cages of rats assigned to the voluntary exercise condition were unlocked and remained unlocked for 6 weeks. All rats started exercising at the same time to avoid potential confounds associated with varying the age of onset of exercise. Following 6 weeks of the sedentary or exercise conditions, wheels in the cages of the exercised rats either remained unlocked and mobile (Run 0), or were rendered immobile with a metal stake. Rats were then exposed to no stress or uncontrollable stress the next day, 4, 14, or 24 days later. Behavioral testing for social exploration, shock-elicited fear, and shuttle box escape occurred 24 h following no stress or uncontrollable stress, so that exercised rats were forced to remain sedentary for either 0 days (Run 0), 5 days (Run 5), 15 days (Run 15), or 25 days (Run 25) prior to behavioral testing. Cohorts of non-stressed and stressed sedentary rats were tested at each time point. In experiment 2, cage crossings during the 3 min exploration test were also recorded as a measure of locomotor activity. A cage crossing was counted whenever all 4 paws belonging to the experimental rat crossed over line dividing the center of the cage (perpendicular to the long edge of the cage).
2.7 Statistical analysis
Body weights and running data were analyzed using repeated measures ANOVA. Plasma corticosterone and total time spent exploring during the 3 minute social exploration test (during experiment 1) was compared using a 2 (sedentary vs. run) by 2 (no stress vs. stress) ANOVA. Post-shock freezing data were averaged into 10, 2 minute blocks and analyzed with 2 (no stress vs. stress) by 5 (sedentary vs. Run 0 vs. Run 5 vs. Run 15 vs. Run 25) repeated measures ANOVA. Number of cage crossings and time spent exploring during experiment 2, average % freezing during the pre-shock freezing period and over the entire 20 minute post-shock freezing period, as well as average FR-1 and FR-2 escape latencies were compared using 2 (no stress vs. stress) by 5 (sedentary vs. Run 0 vs. Run 5 vs. Run 15 vs. Run 25) ANOVAs. Post-hoc within group comparisons using Fisher PLSD tests were performed when required. Significance was set at p < 0.05.
3.0 Results
3.1 Experiment 1
3.1.1 Body weight and running behavior
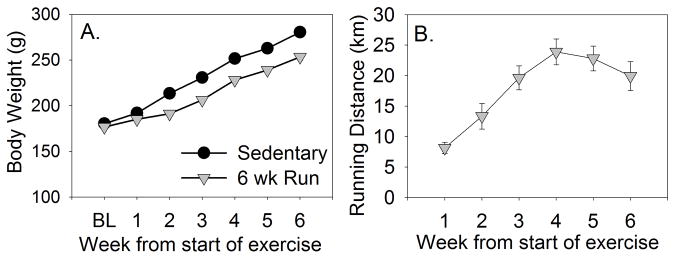
Average body weight gain over the course of the experiment is shown in Figure 1A. Rats in the Sedentary and Run groups weighed similar amounts prior to the start of voluntary running. All rats gained weight over time (F (6, 168) = 812.7; p < 0.0001), but exercised rats gained less weight than sedentary rats (F (6, 168) = 16.6; p < 0.0001). Average weekly distance run is shown in Figure 1B. Weekly running distance increased steadily over the course of the experiment (F (5, 70) = 15.3; p < 0.0001) from 8.1 ± 0.92 km during week 1 to a peak of 23.8 ± 2.12 km during week 4. Similar to our prior observations in F344 rats [22,23], running distance plateaued during the final 3 weeks of wheel running.
Figure 1.
Male Fischer 344 rats used in experiment 1 remained sedentary or were allowed voluntary access to running wheels for 6 weeks. A. Mean weekly body weight change (grams) of sedentary and physically active (6 wk Run) rats. B. The mean distance (kilometers) ran each week by the physically active rats. Values represent group means ± SEM. BL, Baseline.
3.1.2 Social exploration
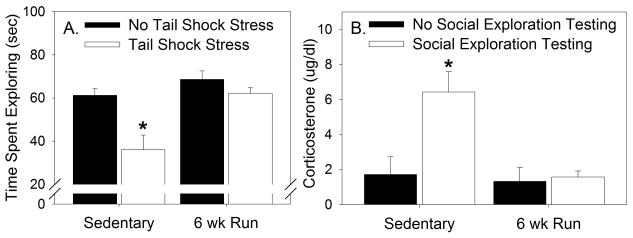
Group sizes for experiment 1 were as follows: Sedentary, No Stress (n =7), Sedentary, Stress (n = 8), Run, No Stress (n =8), Run, Stress (n =7). Exposure to stress reduced social exploration in sedentary rats and 6 weeks of prior wheel running blocked the stress-induced reduction of social exploration (Figure 2A). The main effects of exercise (F (1, 26) = 13.01; p < 0.01), stress (F (1, 26) = 11.58; p < 0.05), and the interaction between exercise and stress (F (1, 26) = 4.2; p < 0.05) were all significant. Post-hoc comparisons revealed that rats in the Sedentary, Stress group explored the juvenile less than rats in all other groups (Figure 2A).
Figure 2.
Male Fischer 344 rats remained sedentary or were allowed voluntary access to running wheels for 6 weeks (6 wk Run). A. Rats were exposed to 100 uncontrollable tail shocks (Stressed) or remained in their home cages (No Stress). Time spent exploring (seconds) was assessed in a juvenile social exploration test 24 hours later. B. Rats either remained in their home cages (No Social Exploration Testing) or were exposed to the social exploration testing procedure (Social Exploration Testing). Trunk blood was collected immediately after social exploration testing and corticosterone was assessed. None of the rats used in Figure 2B were exposed to uncontrollable tail shock stress. Values represent group means ± SEM. * p < 0.05 relative to all other groups.
3.1.3 Corticosterone
Group sizes were as follows: Sedentary, No Social Exploration Testing (n = 6), Sedentary, Social Exploration Testing (n = 7), Run, No Social Exploration Testing (n = 6), Run, Social Exploration Testing (n = 7). Exposure to the social exploration procedure increased corticosterone in sedentary, but not physically active, rats (Figure 2B). The main effects of exercise (F (1, 22) = 8.79; p < 0.01) and social exploration testing (F (1, 22) = 7.93; p < 0.05), as well as the interaction between exercise and testing (F (1, 22) = 6.37; p < 0.05) were all significant. Post-hoc comparisons revealed that corticosterone levels in rats belonging to the Sedentary, Social Exploration Testing group were higher than all other groups.
3.2 Experiment 2
3.2.1 Body weight and running behavior
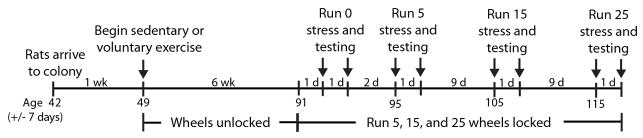
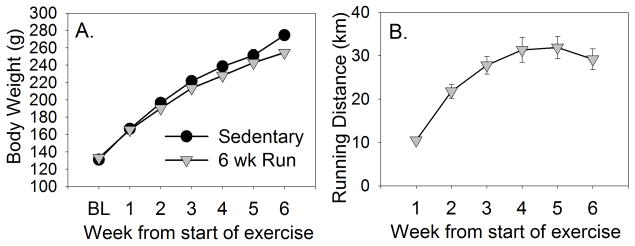
Figure 3 depicts the design of experiment 2. Average body weight gain over the course of the experiment is shown in Figure 4A. As in the first experiment, rats in the Sedentary and Run groups weighed similar amounts prior to the start of voluntary running. All rats gained weight over time (F (6, 612) = 1234.3; p < 0.0001), but physically active rats gained less weight than sedentary rats (F (6, 612) = 7.16; p < 0.0001). Average weekly distance run is shown in Figure 4B. Weekly running distance increased steadily over the course of the experiment from 10.46 ± 0.6 km during week 1 to a peak of 31.9 ± 2.56 km during week 5 (F (5, 350) = 51.04; p < 0.0001).
Figure 3.
Timeline for experiment 2. Following 1 week of habituation to the colony following arrival, male Fischer 344 rats remained sedentary or were allowed voluntary access to running wheels for 6 weeks. Following 6 weeks of the sedentary or exercise conditions, wheels in the cages of the exercised rats either remained unlocked and mobile (Run 0), or were rendered immobile with a metal stake. Rats were then exposed to no stress or uncontrollable tail shock stress the next day, or 4, 14, or 24 days later. Behavioral testing occurred 24 h later, so that exercised rats were forced to remain sedentary for either 0 days (Run 0), 5 days (Run 5), 15 days (Run 15), or 25 days (Run 25) prior to behavioral testing. Groups of non-stressed and stressed sedentary rats were tested at each time point.
Figure 4.
Male Fischer 344 rats used in experiment 2 remained sedentary or were allowed voluntary access to running wheels for 6 weeks prior to wheel lock. A. Mean weekly body weight change (grams) of sedentary and physically active (6 wk Run) rats. B. The mean distance (kilometers) ran each week by the physically active rats. Values represent group means ± SEM. BL, Baseline.
3.2.2 Behavior
Behavior of sedentary rats tested at the different time points was indistinguishable, and sedentary rats were similarly impacted by stress regardless of age; thus rats in the Sedentary, No Stress and Sedentary, Stress groups tested at the various time points (n = 4–5/group) were pooled to yield one Sedentary, No Stress group (n = 17) and one Sedentary, Stress group (n = 16). Non-stressed and stressed physically active rats were tested at each time point following wheel lock, resulting in the following group sizes: Run 0, No Stress (n = 4); Run 0, Stress (n = 8); Run 5, No Stress (n = 8); Run 5, Stress (n = 13); Run 15, No Stress (n = 8); Run 15, Stress (n = 13); Run 25, No Stress (n = 8); Run 25, Stress (n = 9). Escape latency data from 1 rat in the Run 15, No Stress were not included in the analyses because the data were lost due to a hardware malfunction.
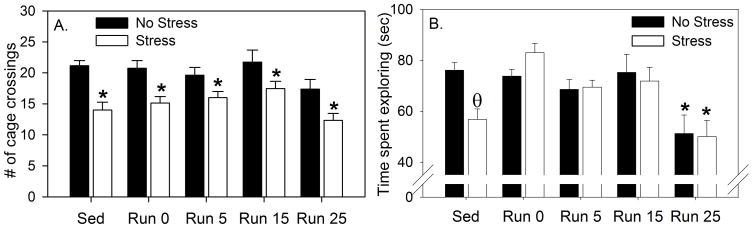
Cage crossings during the 3 min social exploration test are shown in Figure 5A and social exploration behavior is shown in Figure 5B. Rats with a history of wheel running performed fewer cage crossings during the social exploration test compared to sedentary rats (F (4, 94) = 3.09; p = 0.01), and stressor exposure reduced the number of cage crossings (F (1, 94) = 34.5; p < 0.0001). Stress reduced cage crossings regardless of history of prior physical activity (F (4, 94) = 0.71; p > 0.05). Stress reduced social exploration in sedentary rats, and 6 weeks of wheel running again prevented the stress-induced reduction in social exploration behavior. ANOVA revealed that both the main effect of exercise (F (4, 94) = 7.08; p < 0.0001) and the interaction between exercise and stress (F (4, 94) = 2.5; p = 0.04) on social exploration were significant. Post-hoc analyses revealed that stress reduced social exploration in sedentary rats only (p < 0.001, mean difference = 19.27). At no point following exercise cessation did stressor exposure reduce social exploration if rats had prior access to a running wheel. Interestingly, however, previously physically active rats whose wheels were locked 25 days prior to behavioral testing displayed a reduction in social exploration in the absence of stress. The Run 25, No Stress and Stress groups differed from all other groups, except the Sedentary, Stress group.
Figure 5.
Male Fischer 344 rats remained sedentary (Sed) or were allowed voluntary access to running wheels for 6 weeks. Following 6 weeks of the sedentary or exercise conditions, wheels in the cages of the exercised rats either remained unlocked and mobile (Run 0), or were rendered immobile with a metal stake. Rats were then exposed to no stress (No Stress) or uncontrollable stress (Stress) the next day, or 4, 14, or 24 days later. Behavioral testing occurred 24 h later, so that exercised rats were forced to remain sedentary for either 0 days (Run 0), 5 days (Run 5), 15 days (Run 15), or 25 days (Run 25) prior to behavioral testing. A. Number of spontaneous cage crossings performed by experimental rats during the 3 minute juvenile exploration test. * p < 0.05 relative to respective No Stress groups. B. Time spent exploring (seconds) in a juvenile social exploration test. Values represent group means ± SEM. θ p < 0.05 relative to all other groups except Run 25 No Stress and Run 25 Stress groups. * p < 0.05 relative to all other groups except Sed Stress group.
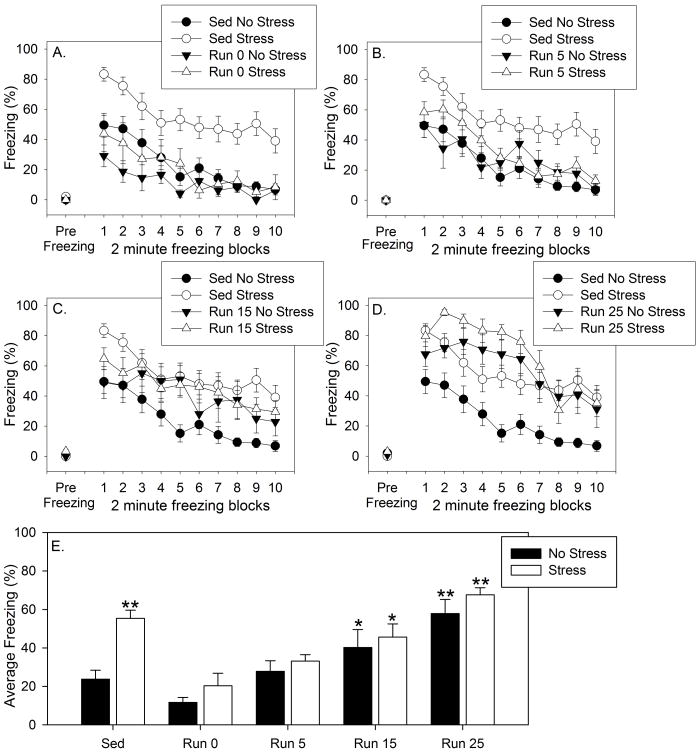
Freezing behavior across 2 minute blocks prior to and following the 2 FR-1 trials is shown in Figure 6A–D. Pre-freezing was minimal and did not differ between groups. Exposure to stress potentiated shock-elicited freezing in sedentary rats and, similar to our previous reports [22,24], 6 weeks of wheel running prevented the effect of stress on shock-elicited freezing. Repeated measures ANOVA revealed significant main effects of exercise (F (4, 94) = 12.3; p < 0.0001), stress (F (1, 94) = 9.47; p < 0.01), and time (9, 846) = 34.6; p < 0.0001) on shock-elicited freezing behavior. The interactions between exercise and stress (F (4, 94) = 2.47; p = 0.04) and exercise and time (F (36, 846) = 1.78; p < 0.01) were also significant. Average shock-elicited freezing behavior is shown in Figure 6E. Post-hoc comparisons revealed that stress potentiated shock elicited freezing behavior in sedentary rats only (p < 0.0001, mean difference = −31.53). At no time point following exercise cessation did stress potentiate freezing behavior in rats with a history of wheel running. Exercise cessation by itself, however, increased shock-elicited freezing as a function of time following wheel lock. Rats in both the Run 15, No Stress and Stress groups displayed higher shock-elicited freezing than rats in the Sedentary, No Stress (p = 0.04, mean difference = 16.43; p = 0.001, mean difference = 21.86, respectively), Run 0, No Stress (p = 0.01, mean difference = −28.54; p = 0.002, mean difference = −33.97, respectively), and Run 0, Stress (p = 0.03, mean difference = −19.88; p = 0.003, mean difference = −25.31, respectively) groups. Similarly, rats in the Run 25, No Stress and Stress groups displayed higher shock-elicited freezing compared to rats in all other groups except the Sedentary, Stress group and both Run 15 groups.
Figure 6.
Male Fischer 344 rats remained sedentary (Sed) or were allowed voluntary access to running wheels for 6 weeks. Following 6 weeks of the sedentary or exercise conditions, wheels in the cages of the exercised rats either remained unlocked and mobile (Run 0), or were rendered immobile with a metal stake. Rats were then exposed to no stress (No Stress) or uncontrollable stress (Stress) the next day, or 4, 14, or 24 days later. Behavioral testing occurred 24 h later, so that exercised rats were forced to remain sedentary for either 0 days (Run 0), 5 days (Run 5), 15 days (Run 15), or 25 days (Run 25) prior to behavioral testing. A. – D. Freezing behavior prior to (Pre Freezing) and immediately following 2 foot shocks in a shuttle box expressed by non-stressed and stressed rats immediately following 6 weeks of wheel running or sedentary conditions (A), or 5 (B), 15 (C), or 25 (D) days following forced exercise cessation. The sedentary data are repeated in each graph for ease of comparison. E. The average % time spent freezing during the 20 minute post-shock freezing period. Values represent group means ± SEM. * p < 0.05 relative to the Sed No stress, Run 0 No Stress, and Run 0 Stress groups. ** p < 0.001 relative to the Sed No stress, Run 0 No Stress, and Run 0 Stress, Run 5 No Stress, and Run 5 Stress groups.
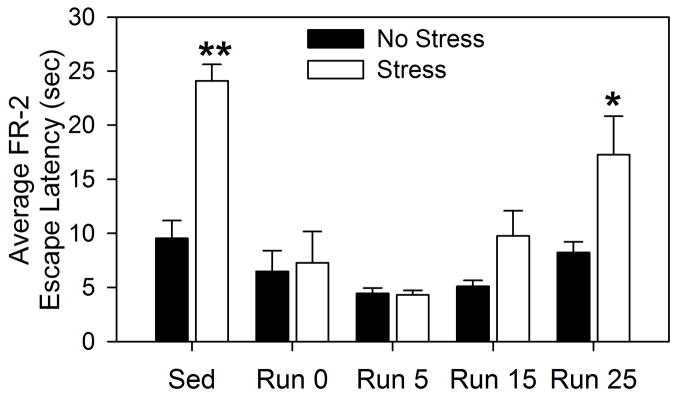
Six weeks of wheel running prevented the shuttle box escape deficit produced by uncontrollable stress and this effect persisted at least 15 days following forced cessation of exercise (Figure 7). The protective effect of exercise against the escape deficit, however, was no longer present 25 days following forced cessation of exercise. The mean FR-1 escape time was 1.08 ± 0.55 seconds and neither exercise (F (4, 94) = 0.72; p > 0.05) nor stress (F (1, 94) = 1.22; p > 0.05) altered FR-1 escape latency (data not shown). The main effects of exercise (F (4, 93) = 14.09; p < 0.0001) and stress (F (1, 93) = 17.5; p < 0.0001), as well as the interaction between exercise and stress (F (4, 93) = 4.7; p < 0.01) on FR-2 escape latency were all significant. Post-hoc comparisons indicated that sedentary rats exposed to stress escaped slower than all other groups including the Sedentary, No Stress (p < 0.0001, mean difference = −14.56) and Run 25, Stress (p = 0.01, mean difference = −6.8) groups. At no time point did FR-2 escape latencies of non-stressed physically active rats differ from that of non-stressed sedentary rats.
Figure 7.
Mean escape latencies (seconds) for 25, FR-2 escape trials measured in a shuttle box. Male Fischer 344 rats remained sedentary (Sed) or were allowed voluntary access to running wheels for 6 weeks. Following 6 weeks of the sedentary or exercise conditions, wheels in the cages of the exercised rats either remained unlocked and mobile (Run 0), or were rendered immobile with a metal stake. Rats were then exposed to no stress (No Stress) or uncontrollable stress (Stress) the next day, or 4, 14, or 24 days later. Behavioral testing occurred 24 h later, so that exercised rats were forced to remain sedentary for either 0 days (Run 0), 5 days (Run 5), 15 days (Run 15), or 25 days (Run 25) prior to behavioral testing. Values represent group means ± SEM. * p < 0.05 relative to all other groups except Sed Stress group; ** p < 0.0001 relative to all other groups except Run 25 Stress group.
4.0 Discussion
Here we report the novel finding that the protective effect of voluntary exercise against the behavioral consequences of uncontrollable stress extends to include the anxiety-like reduction in social exploratory behavior. Moreover, exercise-induced resistance against the behavioral consequences of uncontrollable stress, including the reduction in social exploration, exaggerated fear, and the shuttle box escape deficit, persists between 15 and 25 days following forced exercise cessation despite the emergence of anxiety-like behaviors (social avoidance and exaggerated shock-elicited fear) in regularly-exercising rats denied access to their wheels. Similar to the attenuation of hypothalamic-pituitary-adrenal axis responses to other mild stressors such as noise, saline injection, and exposure to a novel environment following voluntary exercise [5,10,11], we report that 6 weeks of wheel running also attenuates the corticosterone response to the social exploration procedure, which includes both exposure to a novel cage and a juvenile. If our findings extend to humans, the current results imply that although forced cessation of exercise is potentially anxiogenic, people with a history of regular physical activity need not exercise every day in order to maintain exercise-induced stress resistance.
Despite the clear anxiolytic effects of physical activity in humans [33,38], the effects of exercise on anxiety in rodent models of baseline and conflict anxiety remain equivocal (e.g. [3,4,12,19]). What is becoming clear, however, is that anxiolytic effects of exercise in rodents seem to be most robust following exposure to manipulations, be they environmental or pharmacological, which can increase anxiety beyond that elicited by the tests alone (for a review, see [21]). Using a variety of anxiety tests including open field, light-dark box, the hole-board test, fear conditioning, and acoustic startle, for example, exercise has been observed to prevent anxiety elicited by 24 hours of sleep deprivation [46], oxidative stress [40], experimentally-induced colitis [29], uncontrollable stress [21,24], morphine withdrawal [35], acute administration of a selective 5-HT reuptake inhibitor [25], and the non-selective serotonin (5-HT) 2 receptor agonist mCPP [18]. The current results indicate that the anxiolytic effect of voluntary exercise also includes protection against uncontrollable stress-induced social avoidance. Because stressor exposure reduced spontaneous motor activity in all groups regardless of history of wheel running, the protective effect of wheel running against the behavioral consequences of uncontrollable stress observed in the current study are likely independent of a potential effect of exercise on motor activity. In fact, rats with a prior history of wheel running performed even fewer cage crossings during the social exploration procedure than sedentary rats; despite spending more time actively exploring the juvenile. The protective effect of exercise against stress-induced anxiety thus appears to be a relatively robust, consistent observation that extends across many stressors and rodent anxiety tests. This is interesting considering the human data suggesting the anxiolytic effects of exercise are most robust in individuals who are most susceptible to anxiety [43].
We have previously reported that the protective and therapeutic effects of voluntary exercise against the behavioral consequences of uncontrollable stress take time to develop. Whereas 2–3 weeks of wheel running fails to prevent [22] or reverse [26] the behavioral consequences of stress, 6 weeks of wheel running does both [22,26 ]. The current study investigated a similar question: if, and for how long, the protective effect of wheel running would persist following termination of exercise. Following 6 weeks of voluntary exercise, rats’ access to wheel running was denied by locking the wheels to prevent rotation. Stressor exposure and behavioral testing then occurred at various time points later. Inclusion of both non-stressed and stressed rats at each time point following wheel lock allowed the investigation of not only the duration of the persistence of the protective effect of wheel running against the behavioral consequences of uncontrollable stress, it also allowed us to determine the effect of forced cessation of exercise on anxiety-like behavior. Interestingly, anxiety-like behavior emerged in previously physically active rats following forced exercise cessation. Exaggerated shock-elicited fear emerged in non-stressed, previously physically active rats 15 days following wheel lock, and this exaggerated fear was even more robust in the group of previously physically active rats tested 25 days after wheel lock. Similarly, a reduction in social exploratory behavior emerged in previously physically active rats 25 days following forced exercise cessation. The impact of forced exercise cessation was behaviorally specific, as locking the wheel did not by itself impair escape behavior. This divergence suggests that forced exercise withdrawal might selectively impact the neurocircuitry underlying social and fear behaviors, but not escape learning. The basolateral amygdala has been implicated in social avoidance and exaggerated fear behavior [7,44], whereas the dorsal striatum is a region critical for the deficit in escape learning following stressor exposure [44]. Forced cessation of exercise could therefore most readily impact anxiety-like behaviors involving the amygdala. These results suggest that forced cessation of exercise could be stressful and anxiogenic to rats with a history of habitual exercise. Indeed, this interpretation is consistent with prior data reporting forced cessation from voluntary exercise produces signs of stress in rats, such as increased aggression [28], and clinical observations indicating withdrawal from regular exercise can promote negative mood, including anxiety [2,36]. Similar to the anxiogenic effect of forced exercise cessation observed in the current study, withdrawal from drugs of abuse is also associated with increases in anxiety in rodents [8,42]. This similarity suggests that exercise and abusive drugs could produce some similar plastic changes in neural circuitries underlying anxiety elicited by withdrawal from habitual rewarding stimuli.
The protective effect of exercise against the social avoidance and impaired escape behavior produced by stressor exposure clearly remained intact for as long as 15 days following forced exercise cessation. Although levels of freezing started to increase in rats forced to stop exercising 15 days prior to behavioral testing, stressor exposure did not increase freezing at this time point any more than exercise cessation alone; despite the freezing level being far from maximal. The protective effect of exercise against stress-induced exaggerated fear, therefore, also appears to remain intact for at least 15 days following forced withdrawal from exercise. Resistance against the behavioral consequences of uncontrollable stress produced by voluntary exercise thus appears to be an enduring effect. Rats in this study began running at a relatively young age of 6–7 weeks. It would thus be interesting to determine if similar, lasting stress resistance can be conferred by exercise initiated at an older age, or even more permanent protection can be conferred if rats begin exercising at an even younger age.
Rats tested 25 days after forced exercise withdrawal were clearly no longer protected against the deficit in escape learning produced by uncontrollable stress. The protective effect of voluntary exercise against at least some of the behavioral consequences of uncontrollable stress thus appears to dissipate by 25 days following exercise cessation. Because of the dramatic increase in anxiety behaviors expressed by previously physically active rats 25 days following wheel lock, it is difficult to determine whether the protective effect of wheel running against social avoidance and exaggerated fear are similarly dissipated by this point.
It is interesting that the anxiety-like behaviors produced by exercise cessation weren’t apparent immediately after wheel lock (e.g. at the 5 day time point), but instead took between 15 to 25 days to be revealed. Perhaps the exercise-induced neuronal plasticity that protects physically active rats from the behavioral consequences of uncontrollable stressor exposure also protects the animals from the potential stress of forced exercise withdrawal. Indeed, the protective effect of wheel running against the behavioral consequences of uncontrollable stress take at least 3 weeks to develop [22]. Here we show that a similar time period of approximately 25 days is required for this protection to dissipate. The enduring anxiety-like behavioral impact of forced withdrawal from exercise may thus only become apparent as these plastic changes fade. Additional studies will be required to determine the duration of time the anxiety-like effects of forced exercise withdrawal persist.
The current data could help provide insight into the mechanisms by which exercise increases stress resistance. Because the protective effects of wheel running against the behavioral consequences of uncontrollable stress persist at least 15 days following exercise cessation, there should be some physiological change elicited by wheel running that is also long-lasting. The increase in brain-derived neurotrophic factor (BDNF) in the hippocampus elicited by exercise has been suggested to be important for some behavioral effects of exercise [45]. The persistence of the increase in BDNF following termination of voluntary exercise remains equivocal, however; with one study reporting a rapid decrease in BDNF mRNA in the hippocampus of spontaneous hypertensive rats following termination of long-term voluntary exercise [47], and another reporting an increase in BDNF protein in the hippocampus of C57BL/6 mice that persisted for 2 weeks following exercise cessation [1]. These conflicting data, along with our prior report that the protective effects of wheel running against stress-induced exaggerated fear and interference with shuttle box escape are independent of hippocampal BDNF [27], suggest that other factors might be involved. Given the important role of 5-HT in stress, anxiety, and the behavioral consequences of uncontrollable stress [30,32]; exercise-induced plasticity in the 5-HT system [20,23] might linger following exercise cessation and could contribute to the maintenance of exercise-induced stress resistance.
Research Highlights.
Voluntary exercise prevents stress-induced social avoidance
Voluntary exercise attenuates mild stress-evoked increases in corticosterone
Forced cessation of exercise increases anxiety-like behaviors in rodents
Exercise-induced stress resistance endures following forced cessation of habitual exercise
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berchtold NC, Castello N, Cotman CW. Exercise and time-dependent benefits to learning and memory. Neuroscience. 2010;167:588–97. doi: 10.1016/j.neuroscience.2010.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berlin AA, Kop WJ, Deuster PA. Depressive mood symptoms and fatigue after exercise withdrawal: the potential role of decreased fitness. Psychosomatic medicine. 2006;68:224–30. doi: 10.1097/01.psy.0000204628.73273.23. [DOI] [PubMed] [Google Scholar]
- 3.Binder E, Droste SK, Ohl F, Reul JM. Regular voluntary exercise reduces anxiety-related behaviour and impulsiveness in mice. Behav Brain Res. 2004;155:197–206. doi: 10.1016/j.bbr.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Burghardt PR, Fulk LJ, Hand GA, Wilson MA. The effects of chronic treadmill and wheel running on behavior in rats. Brain Res. 2004;1019:84–96. doi: 10.1016/j.brainres.2004.05.086. [DOI] [PubMed] [Google Scholar]
- 5.Campeau S, Nyhuis TJ, Sasse SK, Kryskow EM, Herlihy L, Masini CV, Babb JA, Greenwood BN, Fleshner M, Day HE. Hypothalamic pituitary adrenal axis responses to low-intensity stressors are reduced after voluntary wheel running in rats. Journal of Neuroendocrinology. 2010;22:872–88. doi: 10.1111/j.1365-2826.2010.02007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christianson JP, Paul ED, Irani M, Thompson BM, Kubala KH, Yirmiya R, Watkins LR, Maier SF. The role of prior stressor controllability and the dorsal raphe nucleus in sucrose preference and social exploration. Behav Brain Res. 2008;193:87–93. doi: 10.1016/j.bbr.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christianson JP, Ragole T, Amat J, Greenwood BN, Strong PV, Paul ED, Fleshner M, Watkins LR, Maier SF. 5-hydroxytryptamine 2C receptors in the basolateral amygdala are involved in the expression of anxiety after uncontrollable traumatic stress. Biological Psychiatry. 2010;67:339–45. doi: 10.1016/j.biopsych.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cito M, do C, da Silva FC, Silva MI, Moura BA, Macedo DS, Woods DJ, Fonteles MM, Vasconcelos SM, Sousa FC. Reversal of cocaine withdrawal-induced anxiety by ondansetron, buspirone and propranolol. Behavioural brain research. 2012;231:116–23. doi: 10.1016/j.bbr.2012.01.056. [DOI] [PubMed] [Google Scholar]
- 9.Dishman RK. Brain monoamines, exercise, and behavioral stress: animal models. Medicine and science in sports and exercise. 1997;29:63–74. doi: 10.1097/00005768-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Dishman RK, Bunnell BN, Youngstedt SD, Yoo HS, Mougey EH, Meyerhoff JL. Activity wheel running blunts increased plasma adrenocorticotrophin (ACTH) after footshock and cage-switch stress. Physiol Behav. 1998;63:911–7. doi: 10.1016/s0031-9384(98)00017-1. [DOI] [PubMed] [Google Scholar]
- 11.Droste SK, Gesing A, Ulbricht S, Muller MB, Linthorst AC, Reul JM. Effects of long-term voluntary exercise on the mouse hypothalamic-pituitary-adrenocortical axis. Endocrinology. 2003;144:3012–23. doi: 10.1210/en.2003-0097. [DOI] [PubMed] [Google Scholar]
- 12.Duman CH, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008 doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duman CH, Schlesinger L, Taylor JR, Duman RS. Wheel running produces an antidepressant effect in the learned helplessness model of depression. Society for Neuroscience Abstracts, 2002, CD-ROM. 2002 Program Number 102.10. [Google Scholar]
- 14.Fanselow M, Lester L. Evolution and Learning. Erlbaum; Hillsdale, NJ: 1988. A functional behavioristic approach to aversively motivated behavior: predatory imminence as a determinant of the topography of defensive behavior; pp. 185–212. [Google Scholar]
- 15.Fleshner M. Exercise and neuroendocrine regulation of antibody production: protective effect of physical activity on stress-induced suppression of the specific antibody response. International Journal of Sports Medicine. 2000;21:S14–S19. doi: 10.1055/s-2000-1454. [DOI] [PubMed] [Google Scholar]
- 16.Fleshner M. Physical activity and stress resistance: sympathetic nervous system adaptations prevent stress-induced immunosuppression. Exerc Sport Sci Rev. 2005;33:120–6. doi: 10.1097/00003677-200507000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Fleshner M, Kennedy SL, Johnson JD, Day HEW, Greenwood BN. Exercise and Stress Resistance: Neural-Immune Mechanisms. Springer Publishing; New York: 2009. pp. 87–107. [Google Scholar]
- 18.Fox JH, Hammack SE, Falls WA. Exercise is associated with reduction in the anxiogenic effect of mCPP on acoustic startle. Behav Neurosci. 2008;122:943–8. doi: 10.1037/0735-7044.122.4.943. [DOI] [PubMed] [Google Scholar]
- 19.Fuss J, Ben Abdallah NM, Hensley FW, Weber KJ, Hellweg R, Gass P. Deletion of running-induced hippocampal neurogenesis by irradiation prevents development of an anxious phenotype in mice. PloS one. 2010;5 doi: 10.1371/journal.pone.0012769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenwood BN, Fleshner M. Exercise, stress resistance, and central serotonergic systems. Exercise and sport sciences reviews. 2011;39:140–9. doi: 10.1097/JES.0b013e31821f7e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenwood BN, Fleshner M. Mechanisms Underlying the Relationship Between Physical Activity and Anxiety: Animal data. Routledge; New York, NY: In Press. [Google Scholar]
- 22.Greenwood BN, Foley TE, Burhans D, Maier SF, Fleshner M. The consequences of uncontrollable stress are sensitive to duration of prior wheel running. Brain Res. 2005;1033:164–78. doi: 10.1016/j.brainres.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 23.Greenwood BN, Foley TE, Day HE, Burhans D, Brooks L, Campeau S, Fleshner M. Wheel running alters serotonin (5-HT) transporter, 5-HT(1A), 5-HT(1B), and alpha(1b)-adrenergic receptor mRNA in the rat raphe nuclei. Biol Psychiatry. 2005;57:559–68. doi: 10.1016/j.biopsych.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 24.Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 2003;23:2889–98. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenwood BN, Strong PV, Brooks L, Fleshner M. Anxiety-like behaviors produced by acute fluoxetine administration in male Fischer 344 rats are prevented by prior exercise. Psychopharmacology (Berl) 2008;199:209–22. doi: 10.1007/s00213-008-1167-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenwood BN, Strong PV, Dorey AA, Fleshner M. Therapeutic effects of exercise: Wheel running reverses stress-induced interference with shuttle box escape. Behav Neurosci. 2007;121:992–1000. doi: 10.1037/0735-7044.121.5.992. [DOI] [PubMed] [Google Scholar]
- 27.Greenwood BN, Strong PV, Foley TE, Thompson RS, Fleshner M. Learned helplessness is independent of levels of brain-derived neurotrophic factor in the hippocampus. Neuroscience. 2007;144:1193–208. doi: 10.1016/j.neuroscience.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffmann P, Thoren P, Ely D. Effect of voluntary exercise on open-field behavior and on aggression in the spontaneously hypertensive rat (SHR) Behav Neural Biol. 1987;47:346–55. doi: 10.1016/s0163-1047(87)90461-4. [DOI] [PubMed] [Google Scholar]
- 29.Kasimay O, Guzel E, Gemici A, Abdyli A, Sulovari A, Ercan F, Yegen BC. Colitis-induced oxidative damage of the colon and skeletal muscle is ameliorated by regular exercise in rats: the anxiolytic role of exercise. Experimental physiology. 2006;91:897–906. doi: 10.1113/expphysiol.2006.034439. [DOI] [PubMed] [Google Scholar]
- 30.Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8:233–46. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- 31.Maier SF. Role of fear in mediating shuttle escape learning deficit produced by inescapable shock. J Exp Psychol Anim Behav Process. 1990;16:137–49. [PubMed] [Google Scholar]
- 32.Maier SF, Watkins LR. Stressor controllability and learned helplessness: The roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–41. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 33.Manger TA, Motta RW. The impact of an exercise program on posttraumatic stress disorder, anxiety, and depression. Int J Emerg Ment Health. 2005;7:49–57. [PubMed] [Google Scholar]
- 34.Marcus BH, Dubbert PM, Forsyth LH, McKenzie TL, Stone EJ, Dunn AL, Blair SN. Physical activity behavior change: issues in adoption and maintenance. Health psychology: official journal of the Division of Health Psychology, American Psychological Association. 2000;19:32–41. doi: 10.1037/0278-6133.19.suppl1.32. [DOI] [PubMed] [Google Scholar]
- 35.Miladi-Gorji H, Rashidy-Pour A, Fathollahi Y. Anxiety profile in morphine-dependent and withdrawn rats: effect of voluntary exercise. Physiology & behavior. 2012;105:195–202. doi: 10.1016/j.physbeh.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Mondin GW, Morgan WP, Piering PN, Stegner AJ, Stotesbery CL, Trine MR, Wu MY. Psychological consequences of exercise deprivation in habitual exercisers. Medicine and science in sports and exercise. 1996;28:1199–203. doi: 10.1097/00005768-199609000-00018. [DOI] [PubMed] [Google Scholar]
- 37.Moraska A, Fleshner M. Voluntary physical activity prevents stress-induced behavioral depression and anti-KLH antibody suppression. Am J Physiol Regul Integr Comp Physiol. 2001;281:R484–9. doi: 10.1152/ajpregu.2001.281.2.R484. [DOI] [PubMed] [Google Scholar]
- 38.Newman CL, Motta RW. The effects of aerobic exercise on childhood PTSD, anxiety, and depression. Int J Emerg Ment Health. 2007;9:133–58. [PubMed] [Google Scholar]
- 39.Rozeske RR, Greenwood BN, Fleshner M, Watkins LR, Maier SF. Voluntary wheel running produces resistance to inescapable stress-induced potentiation of morphine conditioned place preference. Behavioural brain research. 2011;219:378–81. doi: 10.1016/j.bbr.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salim S, Sarraj N, Taneja M, Saha K, Tejada-Simon MV, Chugh G. Moderate treadmill exercise prevents oxidative stress-induced anxiety-like behavior in rats. Behavioural brain research. 2010;208:545–52. doi: 10.1016/j.bbr.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 41.Sasse SK, Masini C, Nythuis T, Day E, Campeau S. Neuroscience Meeting Planner. Atlanta, GA: Society for Neuroscience Online; 2006. Voluntary physical exercise facilitates HPA axis adaptation to audiogenic stress: Potential neurochemical mediators. Program No 563.21. [Google Scholar]
- 42.Schulteis G, Yackey M, Risbrough V, Koob GF. Anxiogenic-like effects of spontaneous and naloxone-precipitated opiate withdrawal in the elevated plus-maze. Pharmacology, biochemistry, and behavior. 1998;60:727–31. doi: 10.1016/s0091-3057(98)00034-3. [DOI] [PubMed] [Google Scholar]
- 43.Smits JA, Tart CD, Rosenfield D, Zvolensky MJ. The interplay between physical activity and anxiety sensitivity in fearful responding to carbon dioxide challenge. Psychosomatic medicine. 2011;73:498–503. doi: 10.1097/PSY.0b013e3182223b28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strong PV, Christianson JP, Loughridge AB, Amat J, Maier SF, Fleshner M, Greenwood BN. 5-hydroxytryptamine 2C receptors in the dorsal striatum mediate stress-induced interference with negatively reinforced instrumental escape behavior. Neuroscience. 2011;197:132–44. doi: 10.1016/j.neuroscience.2011.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–90. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 46.Vollert C, Zagaar M, Hovatta I, Taneja M, Vu A, Dao A, Levine A, Alkadhi K, Salim S. Exercise prevents sleep deprivation-associated anxiety-like behavior in rats: Potential role of oxidative stress mechanisms. Behavioural brain research. 2011;224:233–40. doi: 10.1016/j.bbr.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 47.Widenfalk J, Olson L, Thoren P. Deprived of habitual running, rats downregulate BDNF and TrkB messages in the brain. Neurosci Res. 1999;34:125–32. doi: 10.1016/s0168-0102(99)00051-6. [DOI] [PubMed] [Google Scholar]