Abstract
Sphingosine 1-phosphate, a bioactive signaling molecule with diverse cellular functions, is irreversibly degraded by the endoplasmic reticulum enzyme sphingosine 1-phosphate lyase, generating trans-2-hexadecenal and phosphoethanolamine. We recently demonstrated that trans-2-hexadecenal causes cytoskeletal reorganization, detachment, and apoptosis in multiple cell types via a JNK-dependent pathway. These findings and the known chemistry of related α,β-unsaturated aldehydes raise the possibility that trans-2-hexadecenal may interact with additional cellular components. In this study, we show that it reacts readily with deoxyguanosine and DNA to produce the diastereomeric cyclic 1,N2-deoxyguanosine adducts 3-(2-deoxy-β-D-erythro-pentofuranosyl)-5,6,7,8-tetrahydro-8R-hydroxy-6R-tridecylpyrimido[1,2-a]purine-10(3H)one and 3-(2-deoxy-β-D-erythro-pentofuranosyl)-5,6,7,8-tetrahydro-8S-hydroxy-6S-tridecylpyrimido[1,2-a]purine-10(3H)one. Thus, our findings suggest that trans-2-hexadecenal produced endogenously by sphingosine 1-phosphate lyase can react directly with DNA forming aldehyde-derived DNA adducts with potentially mutagenic consequences.
Keywords: trans-2-hexadecenal; sphingosine 1-phosphate; sphingosine 1-phosphate lyase; 1,N2-propanodeoxyguanosine adducts; DNA adducts
1. Introduction
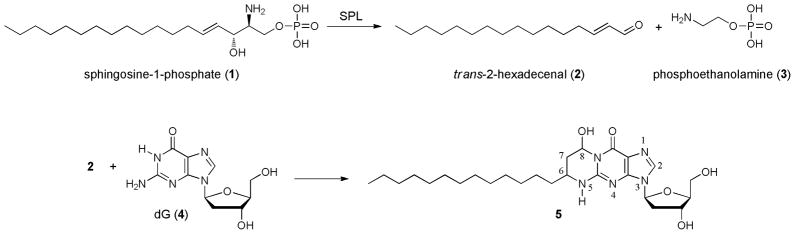
Sphingosine 1-phosphate lyase (SPL) catalyzes the conversion of sphingosine 1-phosphate (1) to trans-2-hexadecenal (2) and phosphoethanolamine (3) (Scheme 1) [1]. The roles of 1 in cell and animal physiology have been well described [2], but the biological consequences and fate of metabolic products of 1 such as 2 are not well understood. We have previously shown that 2 causes cytoskeletal reorganization, detachment, and apoptosis in multiple cell types via a JNK-dependent pathway, indicating that it may interact with multiple cellular components including DNA [3]. Previous studies have shown that α,β-unsaturated aldehydes such as acrolein, crotonaldehyde (2-butenal), and 4-hydroxy-2-nonenal react readily with deoxyguanosine (dG, 4) and DNA to produce cyclic 1,N2-propanodeoxyguanosine adducts with a variety of mutagenic properties [4–10]. Therefore, we hypothesized that 2 would undergo a similar reaction (Scheme 1), potentially producing an adduct such as 5, which could be central to some of the physiologic effects of 2. We tested this hypothesis in the study reported here.
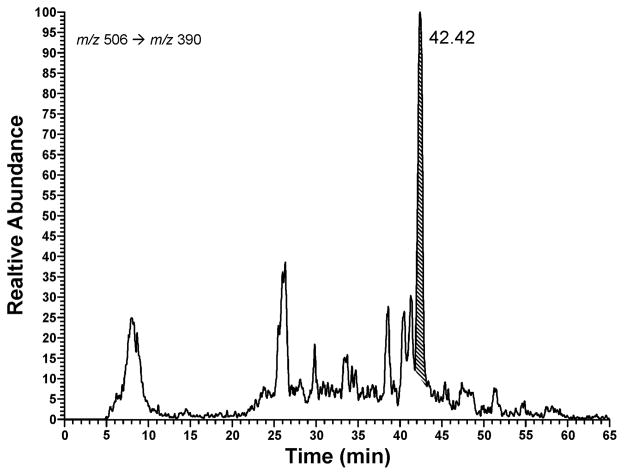
Fig. 2.
LC-ESI-MS/MS-SRM analysis of an enzymatic hydrolysate of calf thymus DNA that had been reacted with 2. The shaded peak is adduct 5.
2. Materials and methods
2.1. HPLC systems
System 1 consisted of a Waters Corp. model 600 system controller, two model 501 pumps, a model 440 UV detector (254 nm), and an Agilent Technologies 1100 series auto-sampler. A Luna C18(2) reversed-phase column (250 x 4.6 mm, 5 μm; Phenomenex) was used for the separation. Elution was performed with a linear gradient from 85% 15 mM NH4OAc in CH3OH to 100% CH3OH over 30 min and held for 20 min. The flow rate was 0.7 ml/min.
System 2 used an Agilent Technologies 1100 capillary HPLC system equipped with a 5 μm, 150 x 0.5 mm ZorbaxSB-C18 column eluted at 8 μl/min with 70% 15 mM NH4OAc in CH3OH to 99% CH3OH in 30 min and held for 15 min.
System 3 was the same as System 2 except that elution was with 85% 15 mM NH4OAc in CH3OH to 95% CH3OH in 30 min and held for an additional 25 min.
2.2. NMR spectra
These were recorded in DMSO-d6 using a Bruker Biospin (Billerica, MA) spectrometer operating at 850 MHz. Resonance assignments were made based on 1H-1H COSY and 1H -13C- HMBC and HSQC experiments acquired at 850 MHz.
2.3. trans-2-Hexadecenal (2)
This compound was prepared as described previously [11].
2.4. Reaction of 2 with dG
trans-2-Hexadecenal (2, 4.7 mg, 20 μmol) was allowed to react with dG (4, 20 mg, 0.074 mmol) in 5 ml of 0.10 M phosphate buffer, pH 7.0, at 50 °C for 72 h. Adduct 5, retention time 34 min, was purified using HPLC system 1 and analyzed by liquid chromatography-electrospray ionization-tandem mass spectrometry-selected reaction monitoring (LC-ESI-MS/MS-SRM) using system 2 and a Thermo TSQ Quantum Discovery MAX instrument, in the positive ion mode with N2 as the nebulizing and drying gas. MS parameters were set as follows: spray voltage, 4Kv; scan width, 0.4 amu; sheath gas pressure, 20; capillary temperature 250 °C; collision energy 20 V (for m/z 390 to 372 and m/z 390 to 346) and 28V for other ions; and tube lens offset, 140 V. Data were acquired and processed by Xcalibur software version 1.4. The mass transitions monitored were m/z 506 → 390, m/z 390 → 346, m/z 390 → 372, m/z 390 → 190, and m/z 390 → 152.
To enable collection of sufficient material for NMR analysis, a larger scale reaction with 56.4 mg (0.24 mmol) of 2 and 240 mg (0.89 mmol) of 4 was performed for 72 h at 50 °C. The reaction was carried out in 25 ml of 25 mM potassium phosphate buffer, pH 9.0, since Michael addition of dG to α,β-unsaturated aldehydes appears to proceed at higher rates at alkaline pH [9]. Adduct 5 was collected using system 1 (less than 0.5% yield). 1H NMR (850 MHz, DMSO-d6) δ 7.912 and 7.913 (2s, 1H, C2-H), 7.82 (d, 1H, N5-H), 6.65 (m, 1H, C8-OH), 6.2 (s, 1H, C8- H), 6.1 (1H, t, J = 6.3 Hz, 1′-H), 5.28 (m, 1H, 3′-OH), 4.92 (m, 1H, 5′-OH), 4.36 (br s, 1H, 3′-H), 3.79 (m, 1H, 4′-H), 3.54 (m, 1H, C6-H), 3.5-3.49 (m, 2H, 5′-H2), 2.5 (m, 1H, 2′-H), 2.18 (m, 1H, 2′-H), 2.06 (1H, d, J = 14Hz, C7H), 1.4 (m, 3H, C7-H + C9-H2), 1.2 (br s, 20H, alkyl chain CH2), 0.87 (t, 3H, CH3); 13C NMR (850 MHz, DMSO-d6) exocyclic carbons 44.81(C6), 34.56 (C7), 69.77 (C8); guanine carbons 135.75 (C2), 156.08 (C=O); alkyl chain 31.77 (CH2-Gua), 14.45 (CH3); deoxyribose 62.14 (C-5′), 87.92 ( C-4′), 71.18 (C-3′), and 82.71 (C-1′); UV (90% CH3OH/10% 15 mM NH4OAc) λmax 204, 254, 275 (sh) nm: ESI-HRMS (m/z) [M + H]+ calcd for C26H43N5O5Na, 528.3156; found 528.3169.
2.5. Acid hydrolysis of 5
In brief, 50 μg of 5 was dissolved in 1 ml of HCl (0.10 N) and the mixture was heated at 90 °C for 60 min. The mixture was cooled, neutralized with 1 N NaOH, and applied to a Strata-X polymeric sorbent solid-phase extraction cartridge (33 μm, 30 mg/1 ml, Phenomenex). The cartridge was sequentially eluted with 1 ml of H2O, 1 ml of 10% methanol, and 2 ml of methanol, and then the product was worked up as described (9). The resulting G adduct was analyzed by LC-ESI-MS-MS-SRM using system 2, positive ion mode m/z 390 → m/z 346, 390→ m/z 372, m/z 390 → m/z 190, and m/z 390 → m/z 152.
2.6. Reaction of 2 with DNA
trans-2-Hexadecenal (2, 2.4 mg, 10 μmol) was allowed to react with calf thymus DNA (10 mg) in 5 ml of 0.10 M phosphate buffer, pH 7.0, for 24 h at 37 °C. The DNA was precipitated by addition of ethanol and washed with 70% and 95% ethanol. After the DNA (0.5 mg) was dissolved in 1 ml of Tris buffer, pH 7.0, enzyme hydrolysis was carried out as described [12]. The hydrolysate was loaded onto a Strata-X polymeric sorbent solid-phase extraction cartridge (33 μm, 30 mg/1 ml) that was previously activated with 1 ml of CH3OH and 1 ml of H2O. The cartridge was washed with 2 ml of H2O, and the analyte was eluted with 2 ml of 100% CH3OH. The eluant was collected in a 2-ml silanized vial, and the solvents were removed on a Speedvac. The residue was taken up in 300 μl of CH3OH, transferred to an insert vial, and concentrated to dryness. The residue was dissolved in 20 μl of 70% aqueous NH4OAc in CH3OH, and 8 μl was analyzed by LC-ESI-MS/MS-SRM using system 3, positive ion mode, m/z 506 → 390. Incubation of 1 with calf thymus DNA for 72 h gave a higher yield of 5.
2.7. Acid hydrolysis of the calf thymus DNA adduct
In brief, 0.5 mg of DNA from reaction of 2 and calf thymus DNA was dissolved in 1 ml of HCl (0.10 N), heated at 90 °C for 60 min, and then was worked up as described under “Acid hydrolysis of 5” (section 2.5). The resulting G adduct was analyzed by LC-ESI-MS-MS-SRM using system 2 (m/z 390 → 346, m/z 390 → 372, m/z 390 → 190, and m/z 390 → 152).
3. Results and discussion
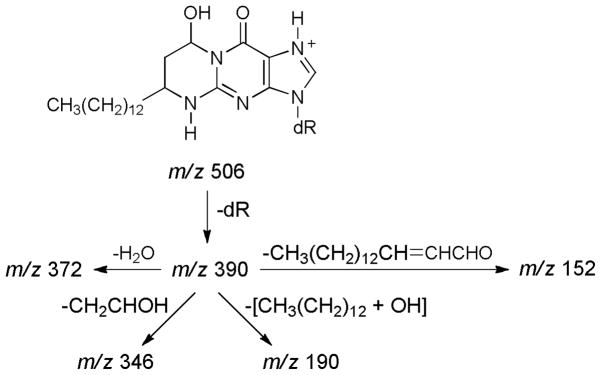
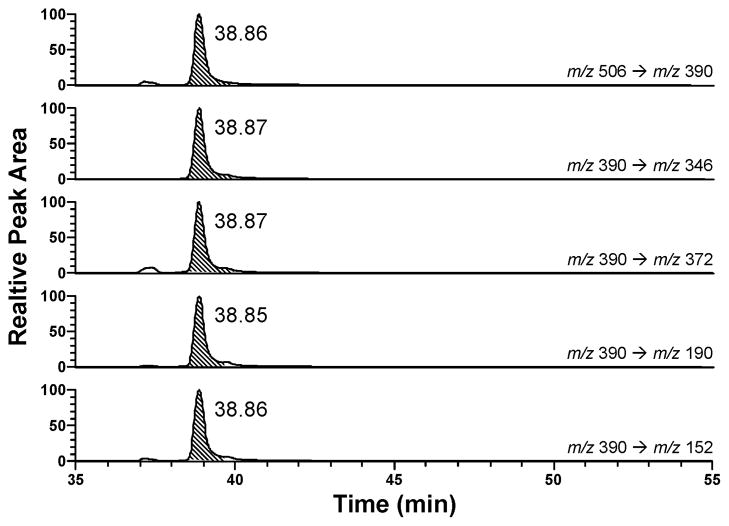
trans-2-Hexadecenal (2) was allowed to react with dG (4), and the products were analyzed by LC-ESI-MS/MS-SRM. The transitions monitored were m/z 506→390 [BH]+, m/z 390→346 [BH - CH2CHOH]+, m/z 390 → 372 [BH - H2O]+, m/z 390 → 190 [BH − (CH3(CH2)12 + OH)]+, and m/z 390 → 152 [GH]+ (Scheme 2). As shown in Figure 1, all of these transitions were observed and are consistent with the expected product, 5.
Scheme 2.
Rationalization of the MS fragmentation of 5, as seen in Figure 1. dR = deoxyribose
Fig. 1.
LC-ESI-MS/MS-SRM analysis of 5 formed in the reaction of trans-2-hexadecenal (2) with dG (4). See Scheme 1 for structures and Scheme 2 for MS fragmentation.
The UV spectrum and the 850 MHz 1H-NMR and 13C-NMR spectra of 5 are completely consistent with those of other exocyclic 1,N2-propano-dG adducts produced by the reaction of α,β-unsaturated aldehydes with dG [9;13;14], and all of the assignments were confirmed by 1H-1H COSY and 1H -13C HMBC and HSQC experiments (data not shown).
Previous studies demonstrated that adducts such as 5 are mixtures of 6S, 8S- and 6R, 8R-diastereomers in which the alkyl and hydroxy groups are trans- to each other [4–10]. Importantly, the 850 MHz 1H-NMR data obtained in this study allowed us to observe 2 singlets at 7.912 and 7.913 ppm, corresponding to the protons at the 2-position of each of the two diastereomers. Observation of these distinct signals has not been reported previously in studies using lower field strength NMR instruments.
Acid hydrolysis of adduct 5 provided the corresponding G adduct, as determined by LC-ESI-MS/MS-SRM analysis for the transitions m/z 390 → 346, m/z 390 → 372, m/z 390 → 190, and m/z 390 → 152. The results are virtually identical to those shown in Figure 1, except that the retention time was 39.6 min. All fragments are consistent with Scheme 2, starting with the G adduct.
Reaction of 2 with calf thymus DNA, followed by enzymatic hydrolysis and LC-ESI-MS/MS-SRM analysis, produced the chromatogram illustrated in Figure 2, which shows the presence of 5 in the hydrolysate. Acid hydrolysis of this DNA produced the G adduct, which was identified by LC-ESI-MS/MS-SRM analysis and co-injection with the standard G adduct.
Collectively, these results establish the structures of the products of the reaction of 2 with dG or DNA as a mixture of 3-(2-deoxy-β-D-erythro-pentofuranosyl)-5,6,7,8-tetrahydro-8R-hydroxy-6R-tridecylpyrimido[1,2-a]purine-10(3H)one and 3-(2-deoxy-β-D-erythro-pentofuranosyl)-5,6,7,8-tetrahydro-8S-hydroxy-6S-tridecylpyrimido[1,2-a]purine-10(3H)one (5). While these are the expected products based on literature precedent [4–10], we note that there are seven more carbons in the alkyl side chain than any previously reported structures of this type. These amphilic adducts may have some special properties because of the combination of lipophilic and hydrophilic residues in the same molecule.
The results of this study support our hypothesis that 2, an endogenous α,β-unsaturated aldehyde produced by the action of SPL on 1, as shown in Scheme 1, reacts with DNA and may lead to potentially mutagenic consequences or perhaps trigger a previously unrecognized DNA damage response. A variety of mutagenic properties of structurally related dG adducts derived from acrolein, crotonaldehyde, and 4-hydroxynonenal have been observed, and the ultimate biological properties of these adducts are influenced by DNA sequence context effects, chromatin condensation, DNA repair mechanisms, cross-linking, and other factors [10]. It will be important to determine the biological properties of adduct 5 and analyze human DNA samples for its occurrence, as many previous studies have shown the presence of related endogenous DNA adducts such as those formed from acrolein and crotonaldehyde in DNA from human liver, lung, leukocytes, and other tissues [7;15–19].
Scheme 1.
Conversion of sphingosine 1-phosphate (1) to trans-2-hexadecenal (2) and reaction of 2 with dG (4) to form adduct 5.
Highlights.
trans-2-Hexadecenal reacts with DNA to produce adducts.
Structurally unusual lipophilic DNA adducts are formed.
The adducts were characterized by NMR, MS, and UV.
Acknowledgments
This study was supported by NIH grants CA-77528 and CA-129438 (JDS), CA-81301 (SSH), and HL-083187 (RB). Mass spectrometry was carried out in the Analytical Biochemistry Shared Resource of the Masonic Cancer Center, supported in part by NIH grant CA-77598. We thank Todd Rappe, University of Minnesota NMR Center for acquiring the NMR spectra.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oskouian B, Saba J. Sphingosine-1-phosphate metabolism and intestinal tumorigenesis: lipid signaling strikes again. Cell Cycle. 2007;6:522–527. doi: 10.4161/cc.6.5.3903. [DOI] [PubMed] [Google Scholar]
- 2.Kumar A, Saba JD. Lyase to live by: sphingosine phosphate lyase as a therapeutic target. Expert Opin Ther Targets. 2009;13:1013–1025. doi: 10.1517/14728220903039722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar A, Byun HS, Bittman R, Saba JD. The sphingolipid degradation product trans-2-hexadecenal induces cytoskeletal reorganization and apoptosis in a JNK-dependent manner. Cell Signal. 2011;23:1144–1152. doi: 10.1016/j.cellsig.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung FL, Young R, Hecht SS. Formation of cyclic 1,N2-propanodeoxyguanosine adducts in DNA upon reaction with acrolein or crotonaldehyde. Cancer Res. 1984;44:990–995. [PubMed] [Google Scholar]
- 5.Chung FL, Roy KR, Hecht SS. A study of reactions of α,β-unsaturated carbonyl compounds with deoxyguanosine. J Org Chem. 1988;53:14–17. [Google Scholar]
- 6.Nath RG, Ocando JE, Chung FL. Detection of 1,N2-propanodeoxyguanosine adducts as potential endogenous DNA lesions in rodent and human tissues. Cancer Res. 1996;56:452–456. [PubMed] [Google Scholar]
- 7.Chung FL, Zhang L, Ocando JE, Nath RG. Role of 1,N2-propanodeoxyguanosine adducts as endogenous DNA lesions in rodents and humans. In: Singer B, Bartsch H, editors. Exocyclic DNA Adducts in Mutagenesis and Carcinogenesis. International Agency for Research on Cancer; Lyon, France: 1999. pp. 45–54. [Google Scholar]
- 8.Chung FL, Nath RG, Ocando J, Nishikawa A, Zhang L. Deoxyguanosine adducts of t-4-hydroxy-2-nonenal are endogenous DNA lesions in rodents and humans: detection and potential sources. Cancer Res. 2000;60:1507–1511. [PubMed] [Google Scholar]
- 9.Winter CK, Segall HJ, Haddon WF. Formation of cyclic adducts of deoxyguanosine with the aldehydes trans-4-hydryoxy-2-hexenal and trans-4-hydroxy-2-nonenal in vitro. Cancer Res. 1986;46:5682–5686. [PubMed] [Google Scholar]
- 10.Minko IG, Kozekov ID, Harris TM, Rizzo CJ, Lloyd RS, Stone MP. Chemistry and biology of DNA containing 1,N(2)-deoxyguanosine adducts of the alpha,beta-unsaturated aldehydes acrolein, crotonaldehyde, and 4-hydroxynonenal. Chem Res Toxicol. 2009;22:759–778. doi: 10.1021/tx9000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baumann WJ, Schmid HH, Mangold HK. Oxidative cleavage of lipids with sodium metaperiodate in pyridine. J Lipid Res. 1969;10:132–133. [PubMed] [Google Scholar]
- 12.Zhang S, Wang M, Villalta PW, Lindgren BR, Upadhyaya P, Lao Y, Hecht SS. Analysis of pyridyloxobutyl and pyridylhydroxybutyl DNA adducts in extrahepatic tissues of F344 rats treated chronically with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and enantiomers of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Chem Res Toxicol. 2009;22:926–936. doi: 10.1021/tx900015d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung FL, Hecht SS. Formation of cyclic 1,N2-adducts by reaction of deoxyguanosine with α-acetoxy-N-nitrosopyrrolidine, 4-(carbethoxynitrosamino)butanal, or crotonaldehyde. Cancer Res. 1983;43:1230–1235. [PubMed] [Google Scholar]
- 14.Eder E, Hoffman C. Identification and characterization of deoxyguanosine adducts of mutagenic β-alkyl-substituted acrolein congeners. Chem Res Toxicol. 1993;6:486–494. doi: 10.1021/tx00034a015. [DOI] [PubMed] [Google Scholar]
- 15.Chung FL, Chen HJC, Nath RG. Lipid peroxidation as a potential source for the formation of exocyclic DNA adducts. Carcinogenesis. 1996;17:2105–2111. doi: 10.1093/carcin/17.10.2105. [DOI] [PubMed] [Google Scholar]
- 16.Emami A, Dyba M, Cheema AK, Pan J, Nath RG, Chung FL. Detection of the acrolein-derived cyclic DNA adduct by a quantitative 32P-postlabeling/solid-phase extraction/HPLC method: blocking its artifact formation with glutathione. Anal Biochem. 2008;374:163–172. doi: 10.1016/j.ab.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang S, Villalta PW, Wang M, Hecht SS. Analysis of crotonaldehyde- and acetaldehyde-derived 1,N2-propanodeoxyguanosine adducts in DNA from human tissues using liquid chromatography-electrsopray ionization-tandem mass spectrometry. Chem Res Toxicol. 2006;19:1386–1392. doi: 10.1021/tx060154d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang S, Villalta PW, Wang M, Hecht SS. Detection and quantitation of acrolein-derived 1,N2-propanodeoxyguanosine adducts in human lung by liquid chromatography-electrospray ionization-tandem mass spectrometry. Chem Res Toxicol. 2007;20:565–571. doi: 10.1021/tx700023z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang S, Balbo S, Wang M, Hecht SS. Analysis of acrolein-derived 1,N2-propanodeoxyguanosine adducts in human leukocyte DNA from smokers and nonsmokers. Chem Res Toxicol. 2011;24:119–124. doi: 10.1021/tx100321y. [DOI] [PMC free article] [PubMed] [Google Scholar]