Abstract
Background
The most used prognostic scheme for malignant gliomas only included patients between ages 18 to 70 years. The purpose of this study was to develop a prognostic model for patients ≥70 years of age with newly diagnosed glioblastoma.
Methods
Four hundred and thirty-seven patients ≥70 years of age with newly diagnosed glioblastoma, pooled from two tertiary academic institutions, were identified for recursive partitioning analysis (RPA). A resulting prognostic model, based on the final pruned RPA tree, was validated using two hundred and sixty-five glioblastoma patients ≥70 years of age from a dataset independently compiled by a French consortium.
Results
RPA produced nine terminal nodes, which were pruned to four prognostic subgroups with markedly different median survivals: I – patients <75.5 years of age who underwent surgical resection (9.3 mos); II – patients ≥75.5 years of age who underwent surgical resection (6.4 mos); III – patients with KPS of 70–100 who underwent biopsy only (4.6 mos); and IV – patients with KPS <70 who underwent biopsy only (2.3 mos). Application of this prognostic model to the French cohort also resulted in significantly different (P<0.0001) median survivals for subgroups I (8.5 mos), II (7.7 mos), III (4.3 mos), and IV (3.1 mos).
Conclusion
This model divides elderly glioblastoma patients into prognostic subgroups that can be easily implemented in both the patient care and the clinical trial settings. This purely clinical prognostic model serves as a backbone for the future incorporation of the increasing number of potential molecular prognostic markers.
INTRODUCTION
Malignant gliomas represent approximately 70% of the 22,500 cases of malignant primary brain tumors diagnosed in adults in the United States each year.1 Given the median age of 65 years at glioblastoma (World Health Organization grade IV glioma) diagnosis, a sizeable proportion of cases occurs in the elderly population.2 Advancing age is one of the strongest negative prognostic factors in glioblastoma,3 which may be attributable to age-related molecular differences4 – including the significantly smaller percentage of patients in this age group with mutations in the IDH1 gene, which seems to confer a survival advantage.5 Unfortunately, previous studies examining the effects of multiple prognostic factors in the glioblastoma population have excluded patients aged 70 years or older6 or have included relatively small numbers of patients in this age range.7 For example, the most used prognostic scheme for malignant gliomas, derived from 4 trials conducted by the Radiation Therapy Oncology Group (RTOG), only included patients between ages 18 to 70 years.6 Consequently, clinicians have struggled with management decisions for elderly glioblastoma patients.
In light of this uncertainty, we sought to generate a prognostic model for glioblastoma patients aged 70 years and older based on both pretreatment factors and extent of surgery. Recursive partitioning analysis (RPA) enables classification of patients into successively more homogeneous prognostic groups based on multiple input variables.8 This statistical tool has been utilized successfully in several retrospective studies of the glioblastoma patient population.6, 7, 9 In this study, we employed RPA to divide glioblastoma patients aged 70 years or older into clinically useful prognostic groups, using data pooled from two previous retrospective studies conducted by Memorial Sloan-Kettering Cancer Center (MSKCC)10 and the Cleveland Clinic Foundation (CCF)11; additionally, we use data published by a French consortium12 to validate the resulting prognostic model.
PATIENTS AND METHODS
Study design
We identified patients ≥70 years of age with pathologically confirmed, newly diagnosed glioblastoma by acquiring datasets from clinical studies previously published by authors from MSKCC10 (394 patients), CCF11 (206 patients) and a French consortium12 (952 patients). We excluded 10 patients from the CCF dataset with glioblastoma diagnoses prior to 1990, for a total of 196 patients. We also excluded 687 patients from the French dataset and 153 patients from the MSKCC dataset who had their initial diagnosis of glioblastoma diagnoses made prior to 70 years of age, for totals of 265 patients and 241 patients, respectively. No other inclusion or exclusion criteria were used, beyond those already described for these published datasets.10–12
Detailed descriptions of the acquisition methods for the three constituent studies included in this work have already been published.10–12 From these datasets, we collected various patient, tumor, and treatment characteristics (Table 1) for all patients meeting the above-described criteria. The three individual studies represented in our present work were approved by the Institutional Review Boards (IRBs) of the Memorial Sloan-Kettering Cancer Center (MSKCC) and the Cleveland Clinic Foundation (CCF), as well as the French government (INCa), Association des Neuro-Oncologues d’Expression Française (ANOCEF), Société Française de NeuroChirurgie (SFNC), and Société Française de Neuropathologie (SFNP), and an official data sharing agreement was signed between the institutions’ IRBs and the data were de-identified before final processing.
Table 1.
Patient, tumor, and treatment characteristics.
| Characteristic | Percent of assessable patients: all cohorts (absolute number) | Percent of assessable patients: MSKCC + CCF only (absolute number) | Percent of assessable patients: French only (absolute number) |
|---|---|---|---|
|
| |||
| Site | |||
| MSKCC | 34 (241) | 55 (241) | 0.0 (0) |
| CCF | 28 (196) | 45 (196) | 0.0 (0) |
| French | 38 (265) | 0.0 (0) | 100.0 (265) |
|
| |||
| Gender | |||
| Men | 58 (404) | 60 (246) | 56 (158) |
| Women | 42 (298) | 40 (191) | 44 (107) |
|
| |||
| Age (yrs) | |||
| ≤73.5 | 38 (265) | 36 (157) | 41 (108) |
| 73.6–75.5 | 17 (122) | 16 (72) | 19 (50) |
| 75.6–76.5 | 10 (68) | 10 (44) | 9 (24) |
| 76.6–78.5 | 13 (89) | 11 (50) | 15 (39) |
| 78.6–83.5 | 18 (130) | 21 (90) | 15 (40) |
| ≥83.6 | 4 (28) | 6 (24) | 1 (4) |
|
| |||
| KPS | |||
| <70 | 31 (175) | 33(135) | 27 (40) |
| 70–100 | 69 (387) | 67 (279) | 73 (108) |
|
| |||
| Symptoms | |||
| Headache | 18 (125) | 19 (80) | 17 (45) |
| Seizure | 19 (130) | 17 (73) | 22 (57) |
| Hemiparesis | 17 (117) | 26 (112) | 2 (5) |
| Language | 18 (123) | 27 (117) | 2 (6) |
| Mental status | 47 (326) | 46 (199) | 48 (127) |
| Visual | 7 (51) | 11 (45) | 2 (6) |
| General sensory | 3 (23) | 5 (23) | 0 (0) |
| Cranial nerves | 3 (19) | 4 (17) | <1 (2) |
| Increased ICP | 3 (24) | 1 (5) | 7 (19) |
| Gait | 12 (82) | 18 (79) | 1 (3) |
|
| |||
| Lesion number | |||
| Single | 88 (381) | 88 (381) | Not recorded |
| Multiple | 12 (54) | 12 (54) | Not recorded |
|
| |||
| Lesion location | |||
| Frontal | 38 (165) | 38 (165) | Not detailed* |
| Temporal | 38 (166) | 38 (166) | |
| Parietal | 36 (154) | 36 (154) | |
| Occipital | 11 (48) | 11 (48) | |
| Corpus callosum | 6 (24) | 6 (24) | |
| Cerebellum | <1 (1) | <1 (1) | |
| Brainstem | <1 (2) | <1 (2) | |
| Gliomatosis | <1 (2) | <1 (2) | |
| Other | 5 (22) | 5 (22) | |
|
| |||
| Surgery | |||
| Biopsy | 47 (324) | 36 (154) | 64 (170) |
| PR | 33 (231) | 43 (186) | 17 (45) |
| GTR | 20 (141) | 21 (91) | 19 (50) |
|
| |||
| RT | |||
| Yes | 78 (419) | 72 (304) | 45 (119) |
| No | 22 (118) | 28 (118) | 55 (146) |
|
| |||
| RT dose (cGy) | |||
| <6000 | 54 (202) | 60 (156) | 40 (48) |
| ≥6000 | 46 (172) | 40 (104) | 60 (71) |
|
| |||
| Chemotherapy | |||
| Yes | 35 (234) | 35 (143) | 34 (91) |
| No | 65 (442) | 65 (268) | 66 (174) |
MSKCC=Memorial Sloan-Kettering Cancer Center; CCF=Cleveland Clinic Foundation; KPS=Karnofsky performance status; PR=partial resection; GTR=gross total resection; RT=radiation therapy. .
Tumor location were noted but rarely detailed in the French study. Globally, 144 patients (54%) presented with neurologic deficits in the French cohort.
Statistical analysis
The date of initial diagnosis was considered the date of the surgical procedure that established the histopathologic diagnosis. Overall survival was defined as the interval between the initial diagnosis and the date of death; patients with unknown survival status were censored at the date of last follow-up. Survival curves were generated using Kaplan-Meier methods, and survival differences were evaluated via the log-rank test. RPA divides patients into successively more homogeneous groups based on chosen input variables, with respect to a predetermined outcome parameter. We employed rpart routines in R (http://cran.r-project.org/) to generate divisions, with respect to overall survival, based on patient and tumor characteristics (Table 1), as well as the extent of surgery leading to a histopathologic diagnosis. All patient and tumor characteristics were included as input elements to the RPA including age as a continuous variable. Beyond the extent of surgery, however, we excluded all treatment characteristics (e.g., radiation therapy, radiation therapy dose, and chemotherapy) when selecting input variables for the RPA, thereby ensuring that any prognostic model generated would be used for all glioblastoma patients ≥70 years of age at the time of diagnosis.
Any of the included input variables were candidates for defining each successive split in the RPA tree, and only patients with recorded entries for a given input variable were used in defining the corresponding split. This process continued until all subgroups generated could no longer be made more homogeneous via additional splits. In order to reduce overfitting, we used rpart to implement tree pruning with a 10-fold cross-validation, thereby estimating the error for each RPA tree size and allowing a tree with minimal error to be selected. Because the estimated error from pruning also involves a degree of randomness, the pruning process was repeated 100 times. A final RPA tree was selected based on the most common number of terminal nodes generated by pruning. The MSKCC + CCF was considered the ‘training’ dataset and we used the French dataset for external validation of the prognostic model.
RESULTS
Patient, tumor, and treatment characteristics
A complete listing of patient, tumor, and treatment characteristics for all 702 patients ≥70 years of age diagnosed with glioblastoma can be found in Table 1. Characteristics are also reported according to MSKCC + CCF (437 patients) versus French (265 patients) cohorts. Percentages displayed reflect only those patients for whom information about a given parameter was available. For the entire cohort, the median age at diagnosis was 75.0 years, and 58% of patients were men. Median Karnofsky Performance Status (KPS) at diagnosis was 70. For the MSKCC + CCF cohort, 36%, 43%, and 21% of histopathologic diagnoses were made by biopsy, partial resection (PR), and gross total resection (GTR), respectively; for the French cohort, the corresponding proportions were 64%, 17%, and 19%, respectively. Overall, the most common symptom at diagnosis was mental status changes (47%). For the MSKCC + CCF dataset, lesion number was most commonly single (88%); lesion location was most commonly temporal (38%) or frontal (38%), followed closely by parietal (36%). The French dataset did not include information pertaining to lesion location and number.
Recursive partitioning analysis (RPA)
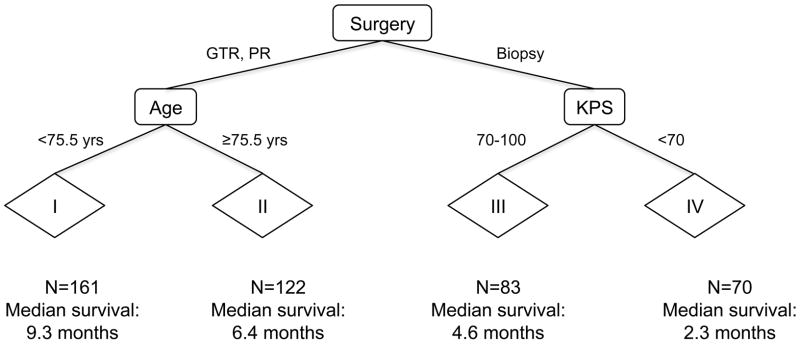
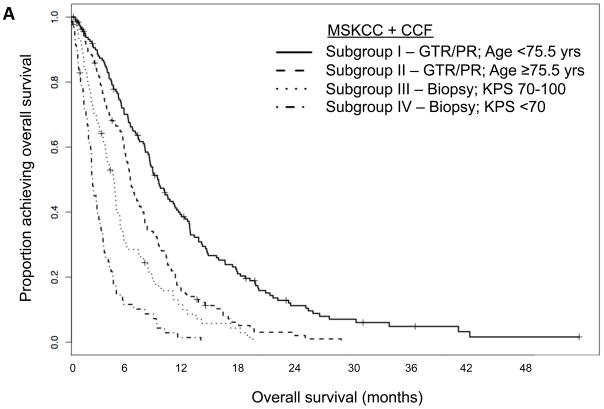
For the MSKCC + CCF cohort, RPA resulted in a tree with nine terminal nodes (Figure 1). To minimize over-fitting, we repeated 10-fold cross-validated pruning 100 times; we identified a primary split corresponding to extent of surgery and secondary splits corresponding to age and KPS. Median survival was markedly different for these four prognostic subgroups (Table 2), as the corresponding survival curves indicate (Figure 2A).
Figure 1.
Recursive partitioning analysis (RPA) trees for the 437 patients in the Memorial Sloan-Kettering Cancer Center (MSKCC) + Cleveland Clinic Foundation (CCF) data set (All patient and tumor characteristics, as well as extent of surgery, (Table 1) were evaluated as potential split points. Nine terminal nodes were pruned to generate four prognostic subgroups using the endpoint of overall survival. Abbreviations: KPS=Karnofsky performance status; PR=partial resection; GTR=gross total resection; N=number of patients in subgroup.
Table 2.
Survival by RPA-derived subgroups for MSKCC + CCF data set, and French data set split according to RPA subgroups.
| Site | Subgroup | N | Median survival (mos) | 95% CI |
|---|---|---|---|---|
| MSKCC + CCF | I – GTR/PR; Age <75.5 yrs | 161 | 9.3 | 8.4 – 11.2 |
| II – GTR/PR; Age ≥75.5 yrs | 122 | 6.4 | 5.8 – 7.6 | |
| III – Biopsy; KPS 70–100 | 83 | 4.6 | 3.7 – 5.3 | |
| IV – Biopsy; KPS <70 | 70 | 2.3 | 2.1 – 3.1 | |
| French | I – GTR/PR; Age <75.5 yrs | 68 | 8.5 | 7.1 – 10.5 |
| II – GTR/PR; Age ≥75.5 yrs | 27 | 7.7 | 4.3 – 15.1 | |
| III – Biopsy; KPS 70–100 | 68 | 4.3 | 3.2 – 6.3 | |
| IV – Biopsy; KPS <70 | 32 | 3.1 | 1.4 – 4.6 |
MSKCC=Memorial Sloan-Kettering Cancer Center; CCF=Cleveland Clinic Foundation; KPS=Karnofsky performance status; PR=partial resection; GTR=gross total resection; CI=confidence interval; N=number of patients in subgroup
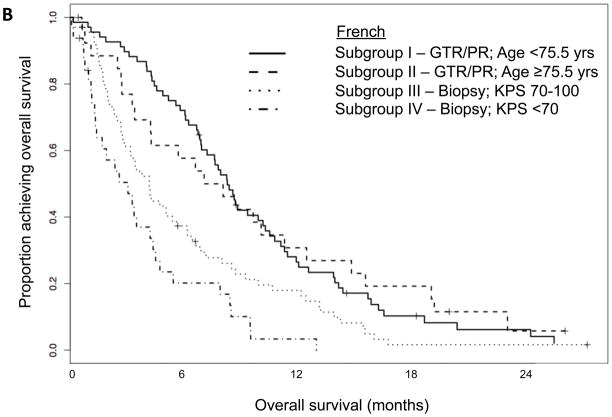
Figure 2.
Kaplan-Meier curves showing overall survival for (A) the Memorial Sloan-Kettering Cancer Center (MSKCC) + Cleveland Clinic Foundation (CCF) data set split according to subgroups derived from its RPA; (B) the French data set split according to subgroups derived from MSKCC + CCF RPA. Abbreviations: KPS=Karnofsky performance status; PR=partial resection; GTR=gross total resection
We applied the four-subgroup model to the French cohort to validate its generalizability. The resulting French subgroups had median survivals similar to those observed for the MSKCC + CCF subgroups (Table 2). Additionally, there were significant differences (P<0.0001) in the survival curves for the French cohort when they were divided according to the MSKCC + CCF subgroups (Figure 2B).
DISCUSSION
Our retrospective RPA study identified four prognostic subgroups with markedly different median survivals: I – patients <75.5 years of age who underwent GTR/PR; II – patients ≥75.5 years of age who underwent GTR/PR; III – patients with KPS of 70–100 who underwent biopsy only; and IV – patients with KPS <70 who underwent biopsy only. The 95% confidence intervals for the median survivals of these four subgroups were entirely non-overlapping for the MSKCC + CCF cohort from which they were derived, indicating the magnitude of these prognostic differences. When this four-subgroup model was applied to an independent cohort of glioblastoma patients 70 years and older, we observed significant differences (P<0.0001) in median survival for these subgroups. The number of patients in the French cohort was smaller, especially with respect to the subgroups that underwent GTR/PR (95 patients for French versus 283 patients for MSKCC + CCF). Consequently, when the MSKCC + CCF model was applied to the French cohort, subgroups I and II (i.e., those derived from splitting the 95 patients who underwent GTR/PR by age) were not significantly different, which may be attributable to this small sample size.
Previously published reports pertaining to our study’s constituent datasets also demonstrate a survival benefit for GTR/PR versus biopsy only; more specifically, for glioblastoma patients aged 65 years or older,10 aged 70 years or older,11 and aged 10 years or older.12 Another small retrospective study, which compared 88 patients aged 65 years or older who had undergone biopsy to 40 patients who had undergone GTR/PR instead, similarly demonstrated a moderate improvement in survival for surgical resection.13 While all these studies were retrospective, a single prospective study of 23 patients aged 65 years or older, in which subjects were randomized to undergo either GTR/PR or biopsy, also found a survival advantage for surgical resection.14
Despite the common belief that elderly patients require longer recoveries after extensive neurosurgical procedures and exhibit higher postoperative complication rates,15 the survival benefit of GTR/PR versus biopsy seems to hold regardless of age. Thus, maximal surgical resection preceding RT and chemotherapy, which has become standard of care for younger glioblastoma patients,16 should also be considered a viable therapeutic course for appropriate elderly glioblastoma patients.
In addition, we also found age and KPS to be important prognostic factors in the GTR/PR and biopsy only subgroups. Age at diagnosis is one of the most important prognostic factors within the general adult glioblastoma population, and3, 7 based on the results of our study and others,10, 11 it continues to exert a significant effect within the elderly glioblastoma population. The same holds true for KPS.
Patient and tumor characteristics can influence clinical management and thus survival, so our study did not include treatment variables other than the extent of surgery in the RPA. Our goal was to derive a prognostic model that could apply to all glioblastoma patients aged 70 years or older at diagnosis. Thus, we included extent of surgery as an RPA variable, because all patients must undergo some surgical procedure to establish a glioblastoma diagnosis. In contrast, we excluded variables related to RT or chemotherapy from the RPA, because these treatments are not initiated until later in the disease course and are employed much less consistently in this specific population. Elderly glioblastoma patients are often less likely to receive RT or chemotherapy than their younger counterparts17, 18 particularly through the earlier years included in this study as evidence that these modalities improve OS in this population is fairly recent.19,20 Because other RPA studies of glioblastoma have included post-surgical treatment as input variables,6, 7 the resulting models are less useful at glioblastoma diagnosis than the current study. In addition, because radiation and chemotherapy are usually given sequentially after diagnosis and older glioblastoma patients have a short survival, inclusion of such variables would require multiple landmark survival analyses to exclude patients who died before the time they were eligible to receive such treatments.
Our current study has several limitations, most notably its retrospective design and its inherent associated biases. While patients in the French cohort were identified via the French Brain Tumor Database, which draws records of glioblastoma patients from multiple medical settings across the country, the patients in the MSKCC and CCF cohorts may differ from the larger glioblastoma population in the United States due to referral and practice biases at these two tertiary medical centers. Additionally, our study lacked several potentially important input variables, including quality-of-life data and tumor molecular studies such as MGMT methylation status, and potential imaging biomarkers.21 Finally, therapy was not standardized in any of the 3 data sets. Nonetheless, our study represents a large-scale RPA in the elderly glioblastoma population and complements data from previous RPA studies that either included the entire adult glioblastoma population7 or excluded patients aged 70 years or older.6
Overall, our study provides an RPA-derived prognostic model specifically tailored to glioblastoma patients aged 70 years or older, which we have validated using separate American (MSKCC + CCF) and French datasets. Hopefully, the four subgroups identified by the present study will be validated by prospective studies in elderly glioblastoma patients. This prognostic scheme can be implemented easily in both the patient care and the clinical trial settings. This clinical prognostic model serves as the backbone for the future incorporation of developing molecular prognostic markers.
Acknowledgments
Study Support: NIH Intramural Research Program (1ZIDBC011098-02). T.J.F. is a Fellow in the Clinical Research Training Program, a public-private partnership supported jointly by the NIH and Pfizer Inc (via a grant to the Foundation for NIH from Pfizer Inc). F.M.I. is supported by the National Cancer Institute’s Clinical Investigator Development Program and the NIH Intramural Program (1ZIABC011347-01 and 1ZIABC011348-01). S.Z., H.M., P.F., V.R., L.T., L.B. acknowledge the support of the French government (INCa), Association des Neuro-Oncologues d’Expression Française (ANOCEF), Société Française de NeuroChirurgie (SFNC), and Société Française de Neuropathologie (SFNP).
Footnotes
Disclaimers: This study was presented in part at the 2010 Annual Meeting of the American Society of Clinical Oncology, Chicago, IL.
Disclosures: The authors report no financial disclosures.
References
- 1.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Fisher JL, Schwartzbaum JA, Wrensch M, Wiemels JL. Epidemiology of brain tumors. Neurol Clin. 2007;25(4):867–890. vii. doi: 10.1016/j.ncl.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Wrensch M, Minn Y, Chew T, Bondy M, Berger MS. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro Oncol. 2002;4(4):278–299. doi: 10.1093/neuonc/4.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batchelor TT, Betensky RA, Esposito JM, et al. Age-dependent prognostic effects of genetic alterations in glioblastoma. Clin Cancer Res. 2004;10(1 Pt 1):228–233. doi: 10.1158/1078-0432.ccr-0841-3. [DOI] [PubMed] [Google Scholar]
- 5.Nobusawa S, Watanabe T, Kleihues P, Ohgaki H. IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res. 2009;15(19):6002–6007. doi: 10.1158/1078-0432.CCR-09-0715. [DOI] [PubMed] [Google Scholar]
- 6.Curran WJ, Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85(9):704–710. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 7.Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro Oncol. 2004;6(3):227–235. doi: 10.1215/S1152851703000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciampi A, Lawless JF, McKinney SM, Singhal K. Regression and recursive partition strategies in the analysis of medical survival data. J Clin Epidemiol. 1988;41(8):737–748. doi: 10.1016/0895-4356(88)90160-6. [DOI] [PubMed] [Google Scholar]
- 9.Paravati AJ, Heron DE, Landsittel D, et al. Radiotherapy and temozolomide for newly diagnosed glioblastoma and anaplastic astrocytoma: validation of Radiation Therapy Oncology Group-Recursive Partitioning Analysis in the IMRT and temozolomide era. J Neurooncol. 2010 doi: 10.1007/s11060-010-0499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwamoto FM, Cooper AR, Reiner AS, Nayak L, Abrey LE. Glioblastoma in the elderly: the Memorial Sloan-Kettering Cancer Center Experience (1997–2007) Cancer. 2009;115(16):3758–3766. doi: 10.1002/cncr.24413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott JG, Suh JH, Elson P, et al. Aggressive treatment is appropriate for glioblastoma multiforme patients 70 years old or older: a retrospective review of 206 cases. Neuro Oncol. 2011;13(4):428–436. doi: 10.1093/neuonc/nor005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauchet L, Mathieu-Daude H, Fabbro-Peray P, et al. Oncological patterns of care and outcome for 952 patients with newly diagnosed glioblastoma in 2004. Neuro Oncol. 2010;12(7):725–735. doi: 10.1093/neuonc/noq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly PJ, Hunt C. The limited value of cytoreductive surgery in elderly patients with malignant gliomas. Neurosurgery. 1994;34(1):62–66. discussion 66–67. [PubMed] [Google Scholar]
- 14.Vuorinen V, Hinkka S, Farkkila M, Jaaskelainen J. Debulking or biopsy of malignant glioma in elderly people - a randomised study. Acta Neurochir (Wien) 2003;145(1):5–10. doi: 10.1007/s00701-002-1030-6. [DOI] [PubMed] [Google Scholar]
- 15.Laigle-Donadey F, Delattre JY. Glioma in the elderly. Curr Opin Oncol. 2006;18(6):644–647. doi: 10.1097/01.cco.0000245324.19411.19. [DOI] [PubMed] [Google Scholar]
- 16.Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62(4):753–764. doi: 10.1227/01.neu.0000318159.21731.cf. discussion 264–756. [DOI] [PubMed] [Google Scholar]
- 17.Iwamoto FM, Reiner AS, Panageas KS, Elkin EB, Abrey LE. Patterns of care in elderly glioblastoma patients. Ann Neurol. 2008;64(6):628–634. doi: 10.1002/ana.21521. [DOI] [PubMed] [Google Scholar]
- 18.Barnholtz-Sloan JS, Williams VL, Maldonado JL, et al. Patterns of care and outcomes among elderly individuals with primary malignant astrocytoma. J Neurosurg. 2008;108(4):642–648. doi: 10.3171/JNS/2008/108/4/0642. [DOI] [PubMed] [Google Scholar]
- 19.Keime-Guibert F, Chinot O, Taillandier L, et al. Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356(15):1527–1535. doi: 10.1056/NEJMoa065901. [DOI] [PubMed] [Google Scholar]
- 20.Scott J, Tsai YY, Chinnaiyan P, Yu HH. Effectiveness of Radiotherapy for Elderly Patients with Glioblastoma. Int J Radiat Oncol. 2011;81(1):206–10. doi: 10.1016/j.ijrobp.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 21.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]