Abstract
Cilia are dynamic organelles that are essential for a vast array of developmental patterning events, including left-right specification, skeletal formation, neural development, and organogenesis. Despite recent advances in understanding cilia form and function, many key ciliogenesis components have yet to be identified. By using a forward genetics approach, we isolated a novel mutant allele (schlei) of the mouse Transmembrane protein 107 (Tmem107) gene, which we show here is critical for cilia formation and embryonic patterning. Tmem107 is required for normal Sonic hedgehog (Shh) signaling in the neural tube and acts in combination with Gli2 and Gli3 to pattern ventral and intermediate neuronal cell types. schlei mutants also form extra digits, and we demonstrate that Tmem107 acts in the Shh pathway to determine digit number, but not identity, by regulating a subset of Shh target genes. Phenotypically, schlei mutants share several features with other cilia mutants; however, spatial restriction of mutant phenotypes and lack of left-right patterning defects in schlei animals suggest differential requirements for Tmem107 in cilia formation in distinct tissues. Also, in contrast to mutants with complete loss of cilia, schlei mutants retain some function of both Gli activator and repressor forms. Together, these studies identify a previously unknown regulator of ciliogenesis and provide insight into how ciliary factors affect Shh signaling and cilia biogenesis in distinct tissues.
Keywords: cilia, Sonic hedgehog (Shh), limb, neural tube, mouse, forward genetics, Tmem107
INTRODUCTION
The molecular nature of several human diseases including Nephronophthisis, Joubert syndrome, Bardet-Biedl Syndrome, Oral-Facial-Digital Syndrome 1, and Meckel Syndrome has been linked to defects in the development, morphology, or function of a small cellular organelle called the cilium (Fliegauf et al., 2007; Sharma et al., 2008; Nigg and Raff, 2009). Cilia are microtubule-based extensions of the cell surrounded by a membrane that is contiguous with, but distinct from, the plasma membrane. They largely fall into two distinct classes: motile cilia, which are thought to function by generating flow in structures such as the embryonic node, lung and the fallopian tubes, and non-motile primary cilia, whose function is less well-defined. What is known is that primary cilia are found on nearly all cells of the vertebrate embryo and in recent years have been shown to play a striking role in a vast array of developmental patterning events, including skeletal formation, the establishment of left-right asymmetry, and organogenesis (Goetz and Anderson, 2010). Long overlooked, cilia have recently emerged as key organelles not only for studying human disease, but also for understanding developmental pathways in many animals. Importantly, primary cilia have recently been implicated in the regulation of several key developmental signaling pathways, the best studied of which is Hedgehog (Hh) signaling (Huangfu et al., 2003; Haycraft et al., 2005; Huangfu and Anderson, 2005; Liu et al., 2005).
Hh acts through a conserved signaling cascade to regulate the development of multiple organs including the eyes, lungs, and limbs. Binding of Hh ligands to their transmembrane receptor Patched (Ptch) relieves Ptch-mediated inhibition of the transmembrane protein Smoothened (Smo), allowing Smo to translocate into the cilium and activate the pathway (Ingham and McMahon, 2001; Rohatgi et al., 2007). Smo acts through an as yet unclear mechanism to modulate the activities of the Gli proteins, which are the transcriptional effectors of Hh signaling. Gli1 is a transcriptional activator, while Gli2 and Gli3 can act as both activators and repressors (Dai et al., 1999; Sasaki et al., 1999). In the absence of the ligand, Gli3 and Gli2 are proteolytically processed into cleaved repressor forms (GliR), but binding of Hh to Ptch and subsequent Smo derepression prevents efficient processing so that the full-length, activator form (GliA) predominates (Wang et al., 2000; Ingham and McMahon, 2001; Pan et al., 2009), resulting in the transcription of Hh target genes.
In vertebrates, primary cilia are required for both positive and negative regulation of the Hh pathway. Both the processing and activation of full-length Gli proteins requires functional cilia (Haycraft et al., 2005; Huangfu and Anderson, 2005; Liu et al., 2005). Sonic hedgehog (Shh), one of three vertebrate Hh homologues, plays a crucial role in the patterning and morphogenesis of a variety of tissues and organs (Ribes and Briscoe, 2009; Traiffort et al., 2010; Harfe, 2011). In the limb, Shh is expressed in a region of the posterior mesenchyme and is required to determine the number and identity of the digits (Riddle et al., 1993; Chiang et al., 2001). In the neural tube, Shh specifies distinct neuronal types in a concentration-dependent manner (Roelink et al., 1994; Tanabe et al., 1995). Mutations that result in near-complete absence of cilia, such as IFT88null or IFT172wim, cause Shh signaling-related defects in the neural tube and limb, including the loss of ventral neuronal cell types and severe polydactyly (Huangfu et al., 2003; Haycraft et al., 2007). Despite an increasing understanding of the relationship between cilia and Shh, many of the components that are required to build cilia and promote Shh signaling during development are unknown.
Through a forward genetics screen, we identified a novel mouse mutant schlei that displays Shh-related defects including preaxial polydactyly, exencephaly, and disrupted ventral neural tube patterning. These phenotypes are also hallmarks of defective cilia, and we show that schlei mutants have decreased numbers of cilia in several developing tissues and organs. By employing new high-throughput sequencing technologies, we demonstrate that the schlei phenotype results from a point mutation in Tmem107, a gene encoding a previously uncharacterized transmembrane protein. We find that Tmem107 acts synergistically with Gli2 as a positive mediator of Shh to specify ventral neuronal cell types, and also acts negatively in combination with Gli3 to constrain the dorsal expansion of intermediate-level neuronal cells. Additionally, Tmem107 functions in the limb to control digit number, but not digit identity, through differential regulation of distinct target genes of the Shh pathway. The effects of Tmem107 on developmental patterning underline the importance of fully functional cilia in Shh signaling. Further, these studies demonstrate the increasing ease and utility of forward genetics screens in the mouse as advances in high-throughput sequencing technologies facilitate the identification of causative mutations.
MATERIAL AND METHODS
Mouse Strains
Mutant lines used included Ptch1tm1Mps (Goodrich et al., 1997), Gli3XT-J (Hui and Joyner, 1993), Smobnb (Caspary et al., 2002), Gli2tm2.1Alj (Bai and Joyner, 2001), and Shhtm1Chg (Chiang et al., 1996). Mutant alleles were genotyped as previously described.
Generation and Mapping of schlei
Initial mapping of the schlei mutation utilized two to five simple sequence length polymorphism markers per chromosome to identify linkage between the polydactyly phenotype and C57BL/6J DNA polymorphisms. schlei was linked to Chromosome 11 and the schlei interval was subsequently narrowed to between markers rs26892691 (68.06 Mb) and D11Mit320 (70.7 Mb). The schlei mutation has been crossed >11 generations onto the C3HeB/FeJ background, which removed more than 99.9% of the original mutagenized C57BL/6J background, supporting the idea that the schlei phenotype is monogenic. Mutant characterization was carried out at various stages of crossing into the C3HeB/FeJ background.
Sequence Capture
A Nimblegen mouse Sequence Capture 385K array was designed to contain oligos complementary to the schlei genomic locus (Chr11:68,058,821-70,766,988 Mb), minus repetitive sequences. Genomic DNA from an e11.5 schlei homozygote was isolated and then sheared by sonication, and adaptors were ligated to the resulting fragments. The adaptor-ligated templates were fractionated by agarose gel electrophoresis and fragments of the desired size were excised. Extracted DNA was amplified by ligation-mediated PCR, purified, and hybridized to the Sequence Capture array. The array was washed, and bound DNA was eluted, purified, and amplified by ligation-mediated PCR (similar to methods employed in (Choi et al., 2009)). The capture and sequencing experiments were performed at the W.M. Keck Foundation for Biotechnology Resources at Yale. This array also contained sequences from Chromosomes 4 & 7, unrelated to the schlei locus. For details about these sequences, please contact the authors.
Sequencing and Mutation Analysis
Captured libraries were sequenced on an Illumina Genome Analyzer II as single-end, 75-bp reads as previously described (Choi et al., 2009). Illumina reads were first trimmed based on their quality scores to remove low-quality regions using the program Btrim (Kong, 2011). A cutoff of 20 for average quality scores within a moving window of size 5-bp was used. Minimum acceptable read length was 25-bp. Other parameters of Btrim were set to defaults. The pre-processed reads were then aligned to the mouse genome reference sequence (mm9) using mapping program BWA (Li and Durbin, 2009). The mapping results were converted into SAMtools pileup format using SAMtools programs (Li et al., 2009). PCR duplicates were removed using the rmdup command from SAMtools. Single nucleotide variations (SNVs) were called using SAMtools' pileup command. Further filtering was performed using in-house scripts to exclude those SNV calls that had less than 3 reads or a SNP score less than 20. Annotation was added based on the UCSC RefSeq gene model ((Pruitt et al., 2009); http://genome.ucsc.edu/).
Obtaining a Second Tmem107 Allele
Generation of the Tmem107tm1Lex allele was previously described (Tang et al., 2010). Cryopreserved sperm from the B6N.129S5-Tmem107tm1Lex/Mmcd strain (identification number 032632-UCD) was obtained from the Mutant Mouse Regional Resource Center, a NCRR-NIH funded strain repository, and was donated to the MMRRC by Genentech, Inc. Tmem107tm1Lex mice were generated via in vitro fertilization by Yale Animal Genomics Services and maintained on a C57Bl/6N background.
Expression Analysis
In situ hybridizations and immunofluorescence analyses were performed using standard methods (Nagy et al., 2003). Monoclonal antibodies that recognize Pax7, Pax6, Nkx6.1, Nkx2.2, and Shh were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the Department of Biological Sciences, The University of Iowa (Iowa City, IA, USA). Additional antibodies used included Olig2 (Millipore, Billerica, MA, USA), acetylated α-tubulin (Sigma-Aldrich, St. Louis, MO, USA), γ-tubulin (Sigma-Aldrich), and Arl13b (gift from Tamara Caspary, Atlanta, GA, USA; (Caspary et al., 2007)).
Skeletal Staining
Skeletons were prepared and stained with Alcian blue and Alizarin red using standard methods (Nagy et al., 2003).
Scanning Electron Microscopy
SEM was performed on open-face preparations of e10.5 neural tubes following standard methods through the Yale Center for Cellular and Molecular Imaging.
Cell Culture
Mouse embryonic fibroblasts (MEFs) were derived using standard methods (Nagy et al., 2003). MEFs were maintained in a 1:1 mix of DMEM:DMEM/F12 + 10% fetal bovine serum (FBS). For cilia staining, cells were plated on coverslips, grown to confluence, and starved for 72 hours in DMEM:DMEM/F12 containing 0.5% FBS.
RESULTS
schlei mutants display cilia defects
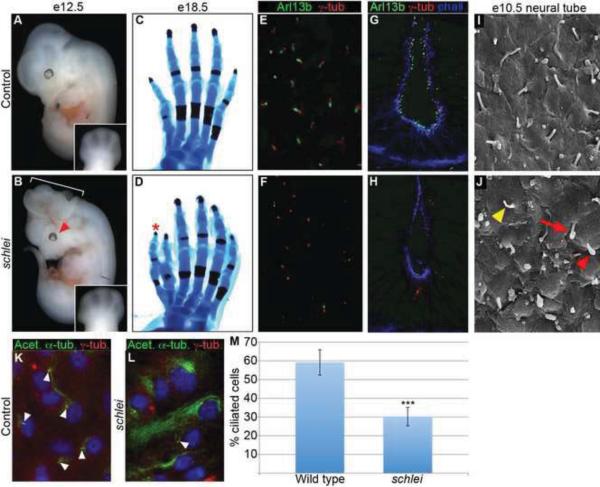
We performed an N-ethyl N-nitrosourea (ENU) screen for recessive mouse mutants at embryonic day (e) 12.5 (similar to (Kasarskis et al., 1998; Weatherbee et al., 2009)) and isolated the schlei mutant based on several characteristics including broadening of the limbs along the anteroposterior axis (Fig. 1A,B). This broadening resolved into preaxial polydactyly at later stages, with most mutant limbs showing at least one ectopic digit (n=23/27 limbs; Fig. 1C,D). Additional features of the schlei mutant phenotype included partially penetrant exencephaly (bracket in Fig. 1B), microphthalmia (arrowhead in Fig. 1B) and skeletal defects (see Fig. S1 in supplementary material), which together phenocopied mutants with abnormal primary cilia (Fliegauf et al., 2007; Goetz and Anderson, 2010). To test whether cilia defects were the proximal cause of the schlei mutant phenotype, we performed immunofluorescence analysis for ciliary markers Arl13b and acetylated α-tubulin. We observed reduced numbers of cilia in schlei mutant limb mesenchyme and lining the lumen of the neural tube based on Arl13b staining (Fig. 1E–H). To confirm the cilia phenotype in the neural tube, we performed scanning electron microscopy (SEM) in the lumen of e10.5 neural tubes. Consistent with Arl13b staining, there was a reduction in the number of cilia lining the schlei neural tube (Fig. 1I,J). In addition, we observed cilia with bulges (red arrowhead in Fig. 1J), long, curled cilia (red arrow in Fig. 1J), and abnormally thin cilia (yellow arrowhead in Fig. 1J) in schlei neural tubes.
Figure 1. schlei mutants display multiple developmental defects and reduced primary cilia formation.
e12.5 schlei mutant embryos display broadening of the limbs (A,B) and a subset of mutants also show exencephaly (60%; n=140; bracket in B) or microphthalmia (66% at e12.5 and older; n=29; arrowhead in B). Early limb broadening resolves into preaxial polydactyly (asterisk) as shown in e18.5 hindlimbs (C,D). Confocal Z-stack projections of immunostaining of transverse cryosections through the limb (E,F) and neural tube (G,H) at e11.5 reveals a reduction in cilia number (as marked by Arl13b in green) in schlei animals (F,H) as compared to wild type (E,G) despite the normal appearance and localization of basal bodies (as marked by γ-tubulin in red). (I,J) Scanning electron microscopy confirms a reduction in number of cilia in the neural tube of schlei animals (J) as compared to wild type (I). Cilia that do form in schlei are malformed, including examples with bulges at the tip (red arrowhead), elongated and curled cilia (red arrow), or thin cilia (yellow arrowhead). Differentiated MEFs from e13.5 wild type (K) and schlei (L) embryos immunostained using acetylated α-tubulin reveal a reduction in cilia number in the mutants (cilia, arrowheads). (M) schlei mutant MEF lines showed fewer cilia than wild-type (n=4 lines of each examined). On average, 59.14 ± 6.75% of wild type cells formed cilia, compared to 30.22 ± 4.90% of mutant cells (p=0.00045 by two-tailed student's t-test with equal variances). In (C,D) hindlimbs are visualized with Alizarin red (bone) and Alcian blue (cartilage) staining. Blue staining in (G,H) = phalloidin. Blue staining in (K,L) = DAPI. Control and schlei mutant images are shown at the same magnification.
We quantified the defect in cilia number using mouse embryonic fibroblasts (MEFs) and found that 59.14% of wild type cells formed cilia (arrowheads in Fig. 1K,L), compared to only 30.22% of mutant cells based on acetylated α-tubulin staining (p=0.00045 by two-tailed student's t-test with equal variances; n=4 cell lines; Fig. 1M). Despite the striking reduction of cilia number in MEFs and the neural tube, not all tissues were equally affected; schlei mutants did not develop left-right patterning defects, and correspondingly, cilia in the embryonic node appeared normal by SEM (data not shown and see Fig. S2 in supplementary material). In addition, schlei mutant kidneys and livers did not display obvious cysts by e18.5 (see Fig. S2 in supplementary material and data not shown). Cilia defects in several, but not all, embryonic tissues suggest that the Schlei gene product is a key component required for ciliogenesis and that it may play a role in the biogenesis or maintenance of specific types of cilia.
Gain and loss of Shh responsiveness in schlei mutant neural tubes
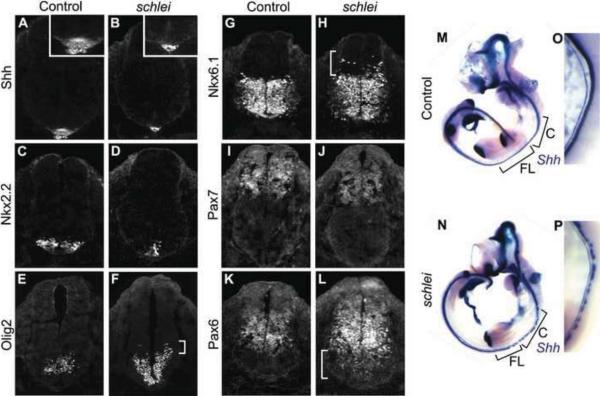
When cilia are lost, neural tube patterning reflects a diminished response to Shh signaling (Huangfu et al., 2003; Huangfu and Anderson, 2005; Liu et al., 2005; May et al., 2005; Houde et al., 2006). To test if the reduced cilia number in schlei neural tubes affected Shh signaling, we examined Shh-specified neuronal subtypes at e10.5. Despite the presence of Shh protein in the notochord, we observed multiple defects in ventral neuronal specification. The number of floorplate cells (Shh+, FoxA2+), which require the highest level of Shh signaling, was dramatically reduced in the cervical and limb regions of schlei mutant neural tubes (Fig. 2A,B; data not shown). V3 interneuron progenitors (Nkx2.2+), which require the next highest level of Shh signaling, were also reduced in number and located more ventrally in schlei neural tubes (Fig. 2C,D). Motor neuron progenitors (Olig2+) were specified in more medial and ventral locations, including the midline (Fig. 2E,F). These data are consistent with a reduced response to Shh signaling.
Figure 2. schlei mutants show disruptions in ventral cell type specification and neuronal subtype mixing in the neural tube.
Sections through e10.5 neural tubes reveal that the floorplate is lost (A,B) and other ventral cell types including V3 interneuron progenitors (Nkx2.2+; C,D) are decreased in number and specified at more ventral locations than in wild type. Cells requiring intermediate levels of Shh signaling, such as motor neuron progenitors (Olig2+; E,F), are both specified more ventrally and expanded dorsally (bracket in F) in schlei. Other intermediate cell types, including a population marked by Nkx6.1 (G,H), are also expanded dorsally in the mutant and are mixed with more dorsal cell types (bracket in H). Some dorsal cell populations, such as the region demarcated by Pax7 expression, appear unchanged in schlei mutants (I,J). In contrast, Pax6 expression, which in wild type embryos (K) is inhibited by high levels of Shh, expands ventrally in schlei (bracket in L). The loss of the floorplate varies based on the axial level as visualized by whole mount in situ hybridization for Shh, with gaps in expression found particularly at the cervical and forelimb levels (M,N). (O,P) Higher magnification views of the cervical regions in e10.5 embryos highlight gaps in floorplate expression of Shh in schlei mutants. C; cervical; FL; forelimb. Control and schlei mutant images are shown at the same magnification.
However, the schlei phenotype departs from most other cilia mutants in that the Olig2+ domain was also expanded dorsally, suggestive of a broader region of intermediate-level Shh signaling (bracket in Fig. 2F). Nkx6.1 marks a broad population of ventral neuronal progenitors and its dorsal expansion in schlei mutants indicates that V2 interneurons (Nkx6.1+; Olig2−) were also specified further from the Shh source (Fig. 2G,H and data not shown). Markers for dorsal cell fates, which are normally repressed by Shh, were either unchanged (Pax7+; Fig. 2I,J) or expanded ventrally (Pax6+; Fig. 2K,L). Overall, these data demonstrate that the schlei mutation is required for the highest-level response to Shh, presumably due to the loss of cilia in the mutant. However, schlei also displays features of other cilia mutants (ex. Arl13bhnn, Mks1krc), in which intermediate Shh targets expand dorsally (Caspary et al., 2007; Weatherbee et al., 2009). Furthermore, the borders of interneuron populations break down and we observed intermingling of different neuronal types (data not shown). Interestingly, Shh expression, which appeared normal in the notochord, was variably lost in gaps from the floorplate, particularly at the cervical and forelimb level, further supporting the hypothesis that there are differential regional requirements for the Schlei gene product (Fig. 2M,N).
The schlei mutation affects Shh signaling downstream of Ptch1 in the neural tube
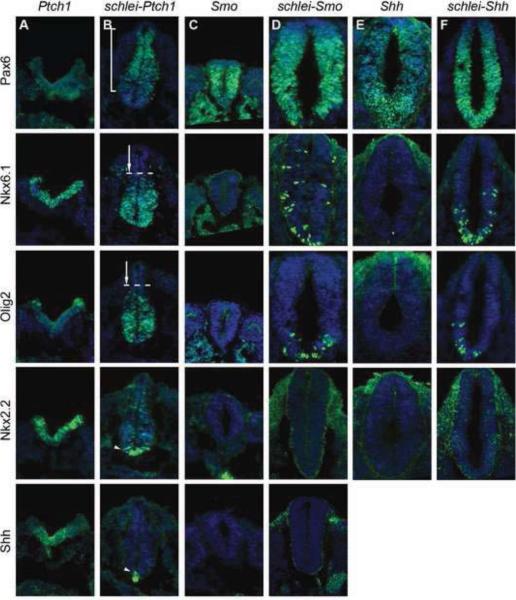
To determine how schlei affects Shh signaling in neuronal specification, we examined e10.5 embryos doubly homozygous for schlei and mutations in Ptch1, Shh or Smo. Ptch1 acts negatively within the pathway and thus Ptch1 mutants have constitutively active Shh signaling, which ventralizes the neural tube (Goodrich et al., 1997). Strikingly, loss of schlei resulted in a significant rescue of the Ptch1 phenotype. Gross morphology in double mutant embryos was partially restored, including head formation, embryo size and neural tube closure (see Fig. S3 in supplementary material). In the neural tube, dorsal cell types (Pax6+) lost in Ptch1 were restored in the double mutant, whereas ventral cell types such as motor neurons, V3 interneurons and floorplate cells were better restricted to a ventral domain than in Ptch1 mutants (Fig. 3A,B). These results indicate that the schlei mutation exerts its effects downstream of Ptch1 to restrict ventral neuronal cell type specification.
Figure 3. The schlei mutation rescues ventral neuronal specification in Shh pathway mutants.
e10.5 neural tubes stained for DAPI (blue) and neuronal markers (green). Expression of the dorsal marker Pax6, which is expanded in Shh (E) and Smo (C) mutants but absent in Ptch1 (A) is rescued and dorsally restricted in schlei-Ptch1 double mutants (B, bracket). In contrast, intermediate-level cells such as the Nkx6.1+ population and Olig2+ motor neuron progenitors, which require positive Shh signaling and are therefore absent in Shh and Smo, are able to form in schlei-Shh (F) and schlei-Smo (D) double mutants. These populations, which are expanded dorsally in Ptch1, are restored to a more ventral location in schlei-Ptch1 double mutants (arrows). High-level Shh targets (Nkx2.2+ V3 interneuron progenitors and Shh+ floorplate cells) are expanded dorsally in Ptch1 animals but are more ventrally restricted in schlei-Ptch1 double mutants (arrowheads). However, schlei is not able to rescue the loss of these cell types in Shh and Smo mutants. All neural tube sections are shown at the same magnification.
The expansion of intermediate Shh targets in schlei mutants suggested that the schlei mutation may also promote their specification. To test this, we analyzed the effect of the schlei mutation in Shh and Smo mutant backgrounds. As Smo is required for all Hh signaling, Smo mutant neural tubes are dorsalized, with a complete absence of Shh-dependent ventral cell types (Wijgerde et al., 2002). The most ventral cell types (floorplate, V3) were absent in both Smo and schlei-Smo mutants (Fig. 3C,D). However, in embryos doubly mutant for schlei and Smo, some Shh-specified cell types, including V2 interneurons and motor neurons, were able to form, but were intermingled with dorsal neurons, indicating that loss of schlei could rescue ventral neuron specification, but not regional restriction in Smo embryos (Fig. 3D). We observed similar results with schlei-Shh double mutants (Fig. 3E,F). Taken together, our analyses of embryos doubly mutant for schlei and components of the Shh pathway show that the Schlei gene product exerts its function downstream of Shh, Ptch1, and Smo. These results suggest that the schlei phenotype is the result not only of a loss of positive Shh function, but that the Schlei gene product can also specify neuronal identities independent of Shh ligand. Previous work has demonstrated that in the absence of shh, reduction of Gli3 levels can result in a rescue of some Shh-dependent cell types (Litingtung and Chiang, 2000), similar to what we observed in the schlei-Shh double mutant. Thus, to determine whether the function of Gli transcription factors in the neural tube was altered in schlei, we examined schlei-Gli2 and schlei-Gli3 embryos.
Schlei acts in combination with Gli2 and Gli3 to pattern the ventral and intermediate neural tube
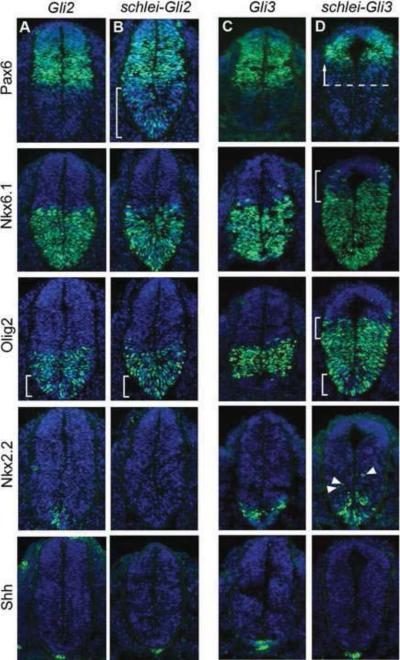
Our results suggested that schlei plays both a positive and negative role in Shh signaling in the neural tube. The Gli transcription factors also have dual roles in Shh-mediated neuronal specification. Gli2 is the predominant transcriptional activator downstream of Shh signaling in the ventral neural tube, and is required to specify ventral cell types such as floorplate and V3 interneurons. Gli2 mutants fail to form a floorplate, while V3 interneurons and motor neuron progenitors are specified more ventrally than in wild type ((Ding et al., 1998; Matise et al., 1998); Fig. 4A). We observed a synergistic effect in schlei-Gli2 double mutants; in addition to the loss of floorplate cells, V3 interneurons were also completely absent (Fig. 4B). This suggests that while both Gli2 and Schlei are required for floorplate specification, the two genes are redundantly required for V3 specification. schlei-Gli2 embryos showed the ventral expansion of Pax6+ cells characteristic of schlei mutants (Fig. 4B). But strikingly, loss of Gli2 blocked the dorsal expansion of V2 interneuron progenitors and motor neuron progenitors (Fig. 4B) normally observed in schlei mutants, indicating that Gli2 is required for the ectopic activation of intermediate-level Shh targets.
Figure 4. schlei interacts genetically with Gli3 and Gli2 to pattern the ventral and intermediate neural tube.
e10.5 neural tubes stained for DAPI (blue) and neuronal markers (green). Expression of Pax6 is similar to wild type in Gli2 (A) and Gli3 (C) mutants, but the ventral border is extended ventrally in schlei-Gli2 (B, bracket), consistent with the schlei single mutant. In schlei-Gli3 embryos, there is a strong dorsal shift in the ventral border of Pax6 (D, arrow). The dorsal expansion of the Nkx6.1+ population observed in schlei is exacerbated in schlei-Gli3 (bracket) but rescued in schlei-Gli2. While intermediate cells such as Olig2+ motor neuron progenitors are comparable to wild type in Gli3 mutants, they are specified more ventrally in Gli2, schlei-Gli2, and schlei-Gli3 (brackets). Additionally, the dorsal boundary of expression of Olig2+ is pushed even further dorsally in schlei-Gli3 (upper bracket) than in schlei, but in schlei-Gli2 is restored to a level similar to wild type. Nkx2.2+ V3 progenitor cells are reduced in number and specified more ventrally in Gli2 mutants, but are completely lost in schlei-Gli2 double mutants. Nkx2.2+ cells are normal in Gli3, but are specified more ventrally and dorsally (arrowheads) in schlei-Gli3 double mutants. The floorplate, as marked by Shh, is normal in Gli3 but is absent in schlei-Gli3, Gli2, and schlei-Gli2 despite normal Shh staining in the notochord, similar to schlei mutants. All neural tube sections are shown at the same magnification.
In the neural tube, Gli3 acts primarily as a repressor and affects the specification of medially located cell populations such as V0 interneurons, but is not required in the most ventral parts of the neural tube ((Persson et al., 2002); Fig. 4C). Intriguingly, loss of Gli3 greatly exacerbated the dorsal expansion of motor neuron progenitors and V2 interneuron progenitors observed in schlei (Fig. 4D). Correspondingly, the Pax6+ domain is restricted to a more dorsal region in the schlei-Gli3 double mutant as compared to controls or the schlei single mutant (Fig. 4D). More ventrally, schlei-Gli3 embryos showed loss of floorplate cells similar to schlei mutants, but also a dorsal expansion of V3 interneuron progenitors (Fig. 4D). Thus, our data indicate that Gli3 acts to constrain widespread dorsal expansion of intermediate cell types in the schlei mutant. Overall, our data are consistent with a model whereby the schlei mutation acts with the Gli genes to specify and define the domains of ventral neuronal cell types of the neural tube.
The Schlei gene is required to restrict Shh targets in the limb
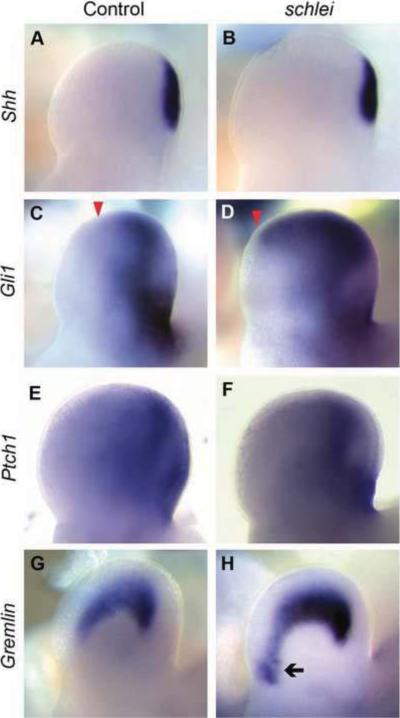
schlei mutants were initially identified based on polydactyly, a common feature in mouse cilia mutants (May et al., 2005; Haycraft et al., 2007; Zeng et al., 2010) as well as human ciliopathies (Ansley et al., 2003; Kyttala et al., 2006; Singla et al., 2010). Shh signaling regulates the number and identity of digits (Riddle et al., 1993; Litingtung et al., 2002; Harfe et al., 2004) by mediating the balance of Gli3 activator and repressor forms (Hui and Joyner, 1993; Litingtung et al., 2002), whereas Gli1 and Gli2 are dispensable for digit patterning (Park et al., 2000; Bai et al., 2002). Since we identified Shh signaling anomalies in schlei neural tubes, we tested whether schlei mutant limbs might have similar patterning changes underlying the polydactyly phenotype. The expression of Shh ligand was normal (Fig. 5A,B); however, we found that its direct targets Gli1 (Fig. 5C,D) and Gremlin (Fig. 5G,H) were anteriorly expanded in schlei limbs, suggesting a broadened response to Shh signaling. Occasionally (n=3/40), schlei mutant limbs displayed a discrete ectopic anterior spot of Gli1 expression in addition to the expanded posterior domain (see Fig. S4 in supplemental material). Expression of Ptch1, an additional direct target of Hh signaling, appeared relatively normal in schlei mutants, suggesting that Shh targets are affected differentially (Fig. 5E,F). Thus, in contrast to mutants with a complete loss of cilia, in which Gli1 and Ptch1 expression are reduced or completely lost (Liu et al., 2005; May et al., 2005; Haycraft et al., 2007; Zeng et al., 2010), there appears to be an upregulation of the Shh pathway in schlei. This indicates that at least some schlei mutant cells can receive and respond to the Shh signal, despite the striking loss of cilia in the mutant limb.
Figure 5. Shh signaling appears expanded in schlei mutant limbs at e11.5.
Despite a normal domain of Shh (A,B), the boundary (arrowhead) of expression of Gli1, a direct target of Shh, is expanded anteriorly in schlei mutants (C,D). Note also the increased width of the mutant limb relative to the control, an early indication of polydactyly (visible in B,D). Expression of Ptch1 appears unchanged in the mutant, suggesting differential sensitivities of various Shh targets (E,F). Gremlin, a downstream target of Shh activation, is expressed ectopically in a distinct region (arrow) in the anterior of the schlei limb (G,H). Control and schlei mutant images are shown at the same magnification.
The schlei mutation affects Gli3 function to regulate digit number, but not identity
In the limb, Shh acts primarily through the Gli3 transcription factor, and the functions of both Gli3A and Gli3R are essential for normal limb development (Wang et al., 2007). Cilia are required for normal processing of full-length Gli3 into Gli3R (Haycraft et al., 2005; Huangfu and Anderson, 2005; Liu et al., 2005), and for the transformation of full-length Gli3 to Gli3A. Complete loss of cilia in limb mesenchyme leads to multiple, unpatterned digits (Haycraft et al., 2007); however, the schlei mutant shows a milder, preaxial polydactyly phenotype (Figs 1, 6A,B). Therefore, we set out to test whether Gli3 processing was altered in schlei mutants. In contrast to other cilia mutants (Haycraft et al., 2005; Huangfu and Anderson, 2005; Liu et al., 2005; May et al., 2005; Zeng et al., 2010), we were unable to detect a difference in Gli3R or Gli3A levels in schlei mutant limbs as measured by Western blot (see Fig. S4 in supplementary material).
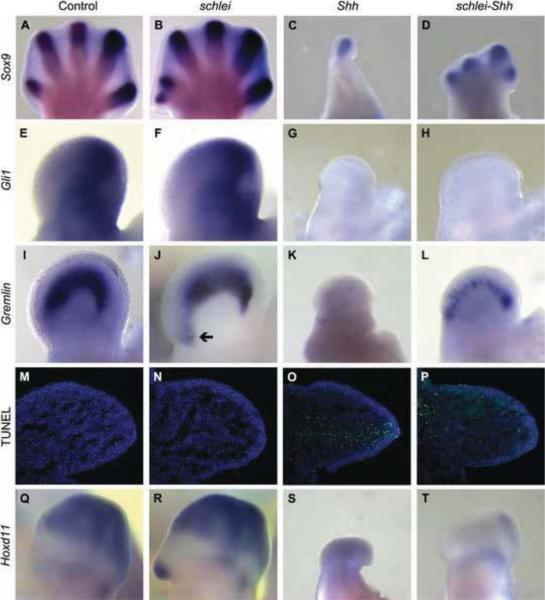
Figure 6. The schlei mutation partially rescues digit number in Shh mutant limbs.
Sox9 marks condensing digits in e13.5 forelimbs (Akiyama et al., 2002), revealing five digits in wild type (A), an ectopic anterior digit in schlei mutants (B), a single digit in Shh mutants (C), and at least four digits in the double mutant (D). The direct positive target of Shh, Gli1, is not activated in Shh (G) or the double mutant (H), compared with the anterior expansion of expression observed in schlei (F) relative to wild type (E). However, expression of Gremlin, which is anteriorly expanded in schlei (arrow in J) as compared to wild type (I), is partially restored in the double mutant (L) despite its complete absence in the Shh mutant (K), suggestive of a loss of Gli3R function. The rescue of digit number in schlei-Shh double mutants may be due in part to a rescue of cell death; TUNEL (green staining) in e10.5 forelimb sections demonstrates that, similar to wild type (M) and schlei (N), double mutant limbs (P) lack the extensive distal cell death observed in Shh mutants (O). Hoxd11 is expressed in the presumptive digits two through five in wild type (Q), and the ectopic digit in schlei variably expresses Hoxd11, indicating posterior identity in the example shown (R). Hoxd11 is absent in both the Shh mutant (S) and the double mutant (T). Blue staining in M–P = DAPI. Control and schlei mutant images are shown at the same magnification.
Although Gli3 processing and levels appeared normal, Western blots cannot reveal protein function. To test if the schlei mutation affects Gli3 function, we examined Shh targets in schlei-Shh double mutants. Our rationale was that in the absence of Shh, only Gli3R should be present and thus we should be able to detect if repressor function was altered in schlei-Shh animals. Strikingly, in contrast to the single digit that develops in Shh mutants ((Chiang et al., 1996); Fig. 6C), in the schlei-Shh double mutant, we observed a rescue of digit number, with four to five digits forming on each double mutant limb, as marked by Sox9 (Fig. 6D). To further investigate this rescue, we examined expression of Gli1 and Gremlin, which are normally lost in Shh mutants (Zuniga et al., 1999; Chiang et al., 2001); Fig. 6E,G,I,K). Gli1 expression, which was expanded in schlei mutants (Fig. 6F), was absent in schlei-Shh limbs (Fig. 6H). In contrast, Gremlin expression, which was also expanded in schlei limbs (Fig. 6J), was partially rescued in double mutants (Fig. 6L), suggesting that the repressive effects of Gli3R are relieved in double mutants.
Since extensive apoptosis occurs in Shh limbs ((Chiang et al., 2001); Fig. 6O), we hypothesized that the rescue of digit number in the schlei-Shh double mutant might be achieved through inhibition of this cell death. Indeed, loss of schlei resulted in a pronounced rescue of limb bud cell death as shown by TUNEL at e10.5 (Fig. 6P). Surprisingly, although loss of schlei function in a Shh mutant background rescued digit number, it did not rescue digit identity based on expression of Hoxd11 (Fig. 6Q–T). Since Hoxd11 marks posterior digits 2–5, the lack of Hoxd11 expression combined with the short digit morphology demonstrated by the Sox9 pattern suggests that the digits that form in schlei-Shh double mutants are all digit 1 (Dolle et al., 1989; Davis and Capecchi, 1994). These data demonstrate that the schlei mutation differentially affects Shh effectors to regulate distinct aspects of limb development like digit number (e.g. through Gremlin) and digit identity (e.g. through Gli1). Together, the morphology and gene expression of schlei-Shh double mutant limbs strongly resembles that of Shh−/−;Gli3+/− mutants (Litingtung et al., 2002). This suggests that loss of schlei may be analogous to reducing but not completely eliminating the function of Gli3R in the limb despite apparent normal Gli3 processing.
Identification of the causative mutation in schlei
Using meiotic recombination mapping we narrowed the genomic interval containing the schlei mutation to 1.88 Megabases on chromosome 11 containing 83 known or predicted transcripts (see Methods). To identify homozygous mutations in this region, we designed and utilized a Nimblegen Sequence Capture array (Hodges et al., 2007; Olson, 2007) followed by massively parallel Solexa sequencing (Bentley et al., 2008; D'Ascenzo et al., 2009). Using this method we identified 22 potential homozygous single nucleotide variants (SNVs; see Table S1 in supplementary material). We were able to eliminate most of these due to low read number, poor consensus score, or location within a repeat element. From the remaining SNVs, we focused on the only exonic mutation, located within the predicted four-pass Transmembrane protein 107 (Tmem107) gene. We identified a single adenine to guanine transition in Tmem107, causing a change from a highly-conserved, negatively charged glutamic acid to a nonpolar glycine (E125G) within the fourth transmembrane domain (see Fig. S5 in supplementary material; Fig. 7A). This missense mutation was confirmed by standard sequencing in multiple independent schlei mutants. Tmem107 is highly conserved within vertebrates, and although obvious orthologs have not been identified in D. melanogaster, they are present in C. elegans, Nematostella and Chlamydomonas, suggesting that Tmem107 may have an ancient role in cilia regulation (see Fig. S6 in supplementary material). Tmem107 is broadly expressed in the embryo ((Tang et al., 2010); see Fig. S5 in supplementary material) and Tmem107 expressed sequence tags have been identified in juvenile and adult mouse tissues (NCBI; (Pontius et al., 2003)) indicating a potential postnatal role for Tmem107.
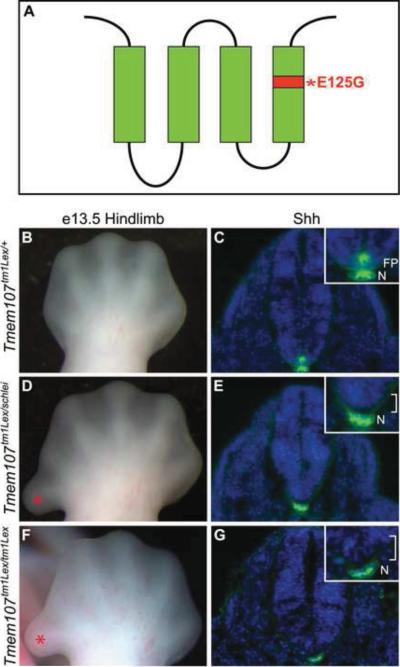
Figure 7. The schlei mutant phenotype results from a point mutation in Tmem107.
(A) Schematic representation of Tmem107 protein, which is predicted to contain four transmembrane domains. Asterisk and red shading, location of the E125G missense mutation in schlei allele. Complementation analysis was carried out by crossing Tmem107tm1Lex males with schlei females. At e10.5, transheterozygous embryos demonstrated that Tmem107tm1Lex fails to complement schlei in anteroposterior limb patterning, as shown by preaxial polydactyly (asterisk) visible at e13.5 (B,D) and floorplate (FP) formation, as shown by staining for Shh (C,E). These phenotypes are also recapitulated in embryos homozygous for the Tmem107tm1Lex mutant allele (F,G). Blue staining in (C,E,G) = DAPI. N = notochord. Brackets in E,G indicate loss of floorplate. Control, transheterozygotes, and homozygous mutant images are shown at the same magnification.
Mice homozygous for a targeted null allele of Tmem107 (Tmem107tm1Lex) have been previously described as embryonic lethal, but their phenotype has not been analyzed (Tang et al., 2010). To confirm that the schlei mutation in Tmem107 underlies the mutant phenotype, we performed a complementation test with the Tmem107tm1Lex allele. While Tmem107tm1Lex heterozygotes appeared grossly normal (Fig. 7B,C), schlei-Tmem107tm1Lex transheterozygotes failed to complement and developed preaxial polydactyly by e13.5 (Fig. 7D) and displayed a loss of the floorplate at e10.5 (Fig. 7E) similar to schlei homozygotes. Furthermore, these defects were also observed in Tmem107tm1Lex/tm1Lex homozygous embryos, and defective neuronal patterning at the hindlimb level of the neural tube in these animals phenocopied that of schlei embryos (Fig. 7F,G; see Fig. S7 in supplemental material). Together these data confirm that the schlei mutant phenotype is caused by the E125G point mutation in Tmem107.
DISCUSSION
Cilia are dynamic organelles with essential functions in signaling, organogenesis, postnatal development and reproduction. Mutations in core cilia-building factors like the Kinesin motor subunits or IFT B complex proteins result in a complete loss of cilia (Marszalek et al., 1999; Rosenbaum and Witman, 2002). Experiments with these mutants have helped define which factors are essential for ciliogenesis and also how complete loss of cilia affects organogenesis and specific signaling pathways. In terms of Hh signaling, we now understand that the formation of GliR and the activation of full-length GliA require cilia (Haycraft et al., 2005; Huangfu and Anderson, 2005; Liu et al., 2005; May et al., 2005), although how cilia fulfill this role remains unresolved. One limitation to studying mutants with complete cilia loss is that the organelle being studied (i.e. the cilium) is absent. Thus, recent studies on mutants that, like schlei, still form some types of cilia (e.g. Tctn1, Mks1;(Reiter and Skarnes, 2006; Weatherbee et al., 2009; Garcia-Gonzalo et al., 2011)) are providing unique insights into the role of cilia in the development of specific tissues and signaling pathways.
Tmem107 is one of only a few transmembrane proteins known to play a role in cilia formation, and it is tempting to speculate that Tmem107 may act within the ciliary membrane itself like Tmem237 (Huang et al., 2011), at the transition zone like Tmem216 (Valente et al., 2010; Garcia-Gonzalo et al., 2011), or both, like Tmem67 (Smith et al., 2006; Garcia-Gonzalo et al., 2011). Although beyond the scope of this study, defining the specific roles of Tmem107 in cilia formation and function should provide key insights into the role of cilia in development and disease.
Tmem107 is essential for ciliogenesis and cilia-mediated signaling in a subset of embryonic tissues
Cilia are required for common signaling pathways in multiple tissues (e.g. Hh) but also play distinct roles in specific organs. However, despite recent advances in understanding cilia function, it remains unclear what genes might contribute to making cilia in different tissues distinct. Using a forward genetics approach, we identified one of these factors, the novel transmembrane protein Tmem107. The schlei mutation in Tmem107 modulates ciliogenesis and Shh signaling in multiple, but not all, tissues of the developing embryo, giving rise to a unique suite of ciliopathy features. schlei animals display polydactyly, microphthalmia, neural patterning defects and skeletal abnormalities common to cilia mutants. However, nodal cilia appear normal in schlei mutants and subsequently, no left-right defects develop (see Fig. S2 in supplementary material). Likewise, another typical feature of ciliopathies, cysts in the kidney and liver, also do not develop in schlei embryos (see Fig. S2 in supplementary material and data not shown). Finally, changes in Shh signaling in the neural tube show regional specificity in schlei mutants. Normally, Shh signaling from the notochord induces Shh in the floorplate throughout the embryo (Roelink et al., 1994; Roelink et al., 1995). However, in schlei mutants, Shh floorplate expression is lost in cervical and forelimb regions (Fig. 2) but still induced in the trunk. Together, these results suggest that Tmem107 is differentially required for cilia formation and/or function in specific tissues within the embryo. This aspect of the schlei phenotype is similar to Tctn1 mutants, which also show tissue-specific defects in ciliary assembly (Reiter and Skarnes, 2006; Garcia-Gonzalo et al., 2011). Thus far, we have demonstrated expression of Tmem107 in all tissues examined, suggesting that the tissue specificity is the result of differential requirement for the gene product in different tissues, as opposed to spatially restricted expression. This raises the intriguing possibility that subsets of primary cilia have divergent composition and/or function, which could be one reason why ciliopathy phenotypes phenocopy Hh defects in some tissues (e.g. limb, neural tube), but have different etiologies in others. If specific populations of primary cilia depend upon distinct genes, this may also explain the spectrum of phenotypes observed across human ciliopathies.
The schlei mutation affects the function of Gli proteins
schlei mutants show distinct differences in Shh signaling compared to animals completely lacking cilia. In the neural tube, schlei embryos display reduced specification of high-level Shh targets (floorplate and V3) but also show an increased dorsal range of intermediate Shh targets (MN and V2). The fact that Shh-dependent cell types are still specified in schlei mutants indicates that Gli activator activity is functional despite the strong reduction in cilia. Specifically, Gli2 must still act as a positive mediator of Shh signaling in schlei mutants as schlei-Gli2 embryos demonstrate a stronger loss of Shh signaling. The exacerbation of the dorsal expansion phenotype in schlei-Gli3 double mutants also demonstrates that some repressive function of Gli3 is retained in schlei mutants. Thus, schlei mutant neural tubes appear to retain both Gli activator and repressor forms but with reduced function (Fig. 8).
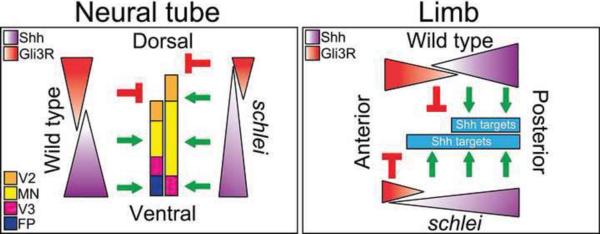
Figure 8. Model of Gli activity in the schlei mutant.
In the neural tube, a ventral gradient of Shh signaling specifies ventral neuronal cell types, while dorsal Gli3R helps restrict the extent of their domain. In the schlei ventral neural tube, the highest level of Shh responsiveness is lost, but there is an increased range of intermediate-level signaling extending more dorsally, mimicking a shallower, but elongated gradient. Gli3 repressor function is also diminished in schlei, and together, this results in the absence of the highest-level ventral target, the floorplate (FP), combined with a broadened domain competent to form motor neurons (MN) and V2 interneurons. In the limb, a posterior gradient of Shh signaling specifies digit identity while anterior Gli3R restricts digit number. Similar to the neural tube, the Shh gradient appears extended in schlei limbs and together with decreased Gli3R function results in an expansion of Shh target gene expression (Gremlin, Gli1) in the anterior portion of the limb.
In the limb, our Western blot analysis showed that anterior and posterior levels of full-length Gli3 and Gli3R are unaffected in schlei mutants. Shh targets are still expressed in schlei limbs, in contrast to complete cilia mutants (Liu et al., 2005; Haycraft et al., 2007), indicating that full-length Gli3 is a functional activator of the Shh pathway. However, the anterior expansion of Shh targets in schlei mutants and the derepression of Gremlin in schlei-Shh double mutants reveal that the function of Gli3R is compromised by the schlei mutation (Fig. 8). The morphology and gene expression patterns in schlei-Shh double mutant limbs strongly resemble those of Shh−/−;Gli3+/− mutants (Litingtung et al., 2002) supporting the idea that the schlei mutation reduces the function of Gli3. Thus, similar to the neural tube, schlei mutant limbs appear to retain some GliR and GliA function despite the reduction in cilia number. This indicates that the few cilia that do form in schlei mutants maintain some ability to regulate Gli protein processing and functionality.
Forward genetics is a key approach to identify genes required for cilia form and function
Mutation discovery in human diseases has been greatly accelerated in recent years by the use of targeted enrichment and next-generation sequencing technologies (Bamshad et al., 2011; Ku et al., 2012). This study, along with a handful of others (Pyrgaki et al., 2011; Sheridan et al., 2011), has begun to apply the same principle to model systems. The result has been a dramatic decrease in the time it takes to progress from phenotype to affected gene, demonstrating that forward genetics screens in mice are a tractable and important avenue for gene discovery. In terms of cilia, bioinformatics and protein sequencing studies have identified hundreds of proteins in the cilium (Ostrowski et al., 2002; Avidor-Reiss et al., 2004; Pazour et al., 2005; Gherman et al., 2006), but determining which of these proteins are essential cilia factors is a daunting endeavor. However, this study and others highlight the immense potential of forward genetics approaches for identifying cilia genes (Huangfu et al., 2003; Garcia-Garcia et al., 2005; May et al., 2005; Liem et al., 2009; Weatherbee et al., 2009; Ko et al., 2010; Cui et al., 2011). Random mutagenesis provides an unbiased approach toward discovery of genes such as Tmem107 that are critical for cilia biogenesis and function, but are not known components of well-studied pathways. Recent and future advances in high-throughput sequencing technology will continue to enhance our ability to identify additional factors in the mouse that are critical regulators of development and disease.
Supplementary Material
The schlei mutation affects cilia number in a subset of developing mouse tissues.
schlei partially disrupts Gli activator and repressor function.
The schlei mutation acts downstream of Shh to control digit number but not identity.
The schlei mutation affects Tmem107, which encodes an ancient transmembrane protein.
Forward genetics plus sequence capture allows rapid discovery of developmental genes.
ACKNOWLEDGEMENTS
We would like to thank Zhaoxia Sun for comments on the manuscript, Emily Mis, Sunjin Lee-Wölfel, Mustafa Khokha, and Martina Brueckner for helpful discussions, and William Horton and Stephanie Andrade for technical assistance. We also thank the Keck microarray resource at Yale for help with sequence capture, and the Yale Center for Cellular and Molecular Imaging for help with scanning electron microscopy.
FUNDING This work was supported by the National Institutes of Health [U01HD43478 to K.V. Anderson and L.A. Niswander; R01NS044385 to K.V. Anderson; R01HD32427 to L.A. Niswander; P30DK090744-01 Pilot & Feasibility funds to S.D. Weatherbee], the National Science Foundation [GRFP Fellowship to K.J. Christopher], and institutional startup funds from the Yale Genetics Department (S.D. Weatherbee).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16(21):2813–28. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM, et al. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature. 2003;425(6958):628–33. doi: 10.1038/nature02030. [DOI] [PubMed] [Google Scholar]
- Avidor-Reiss T, Maer AM, Koundakjian E, Polyanovsky A, Keil T, Subramaniam S, Zuker CS. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell. 2004;117(4):527–39. doi: 10.1016/s0092-8674(04)00412-x. [DOI] [PubMed] [Google Scholar]
- Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL. Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development. 2002;129(20):4753–61. doi: 10.1242/dev.129.20.4753. [DOI] [PubMed] [Google Scholar]
- Bai CB, Joyner AL. Gli1 can rescue the in vivo function of Gli2. Development. 2001;128(24):5161–72. doi: 10.1242/dev.128.24.5161. [DOI] [PubMed] [Google Scholar]
- Bamshad MJ, Ng SB, Bigham AW, Tabor HK, Emond MJ, Nickerson DA, Shendure J. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet. 2011;12(11):745–55. doi: 10.1038/nrg3031. [DOI] [PubMed] [Google Scholar]
- Bentley DR, Balasubramanian S, Swerdlow HP, Smith GP, Milton J, Brown CG, Hall KP, Evers DJ, Barnes CL, Bignell HR, et al. Accurate whole human genome sequencing using reversible terminator chemistry. Nature. 2008;456(7218):53–9. doi: 10.1038/nature07517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary T, Garcia-Garcia MJ, Huangfu D, Eggenschwiler JT, Wyler MR, Rakeman AS, Alcorn HL, Anderson KV. Mouse Dispatched homolog1 is required for long-range, but not juxtacrine, Hh signaling. Curr Biol. 2002;12(18):1628–32. doi: 10.1016/s0960-9822(02)01147-8. [DOI] [PubMed] [Google Scholar]
- Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell. 2007;12(5):767–78. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Harris MP, Simandl BK, Li Y, Beachy PA, Fallon JF. Manifestation of the limb prepattern: limb development in the absence of sonic hedgehog function. Dev Biol. 2001;236(2):421–35. doi: 10.1006/dbio.2001.0346. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383(6599):407–13. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Choi M, Scholl UI, Ji W, Liu T, Tikhonova IR, Zumbo P, Nayir A, Bakkaloglu A, Ozen S, Sanjad S, et al. Genetic diagnosis by whole exome capture and massively parallel DNA sequencing. Proc Natl Acad Sci U S A. 2009;106(45):19096–101. doi: 10.1073/pnas.0910672106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Chatterjee B, Francis D, Yu Q, SanAgustin JT, Francis R, Tansey T, Henry C, Wang B, Lemley B, et al. Disruption of Mks1 localization to the mother centriole causes cilia defects and developmental malformations in Meckel-Gruber syndrome. Dis Model Mech. 2011;4(1):43–56. doi: 10.1242/dmm.006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ascenzo M, Meacham C, Kitzman J, Middle C, Knight J, Winer R, Kukricar M, Richmond T, Albert TJ, Czechanski A, et al. Mutation discovery in the mouse using genetically guided array capture and resequencing. Mamm Genome. 2009;20(7):424–36. doi: 10.1007/s00335-009-9200-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai P, Akimaru H, Tanaka Y, Maekawa T, Nakafuku M, Ishii S. Sonic Hedgehog-induced activation of the Gli1 promoter is mediated by GLI3. J Biol Chem. 1999;274(12):8143–52. doi: 10.1074/jbc.274.12.8143. [DOI] [PubMed] [Google Scholar]
- Davis AP, Capecchi MR. Axial homeosis and appendicular skeleton defects in mice with a targeted disruption of hoxd-11. Development. 1994;120(8):2187–98. doi: 10.1242/dev.120.8.2187. [DOI] [PubMed] [Google Scholar]
- Ding Q, Motoyama J, Gasca S, Mo R, Sasaki H, Rossant J, Hui CC. Diminished Sonic hedgehog signaling and lack of floor plate differentiation in Gli2 mutant mice. Development. 1998;125(14):2533–43. doi: 10.1242/dev.125.14.2533. [DOI] [PubMed] [Google Scholar]
- Dolle P, Izpisua-Belmonte JC, Falkenstein H, Renucci A, Duboule D. Coordinate expression of the murine Hox-5 complex homoeobox-containing genes during limb pattern formation. Nature. 1989;342(6251):767–72. doi: 10.1038/342767a0. [DOI] [PubMed] [Google Scholar]
- Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8(11):880–93. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia MJ, Eggenschwiler JT, Caspary T, Alcorn HL, Wyler MR, Huangfu D, Rakeman AS, Lee JD, Feinberg EH, Timmer JR, et al. Analysis of mouse embryonic patterning and morphogenesis by forward genetics. Proc Natl Acad Sci U S A. 2005;102(17):5913–9. doi: 10.1073/pnas.0501071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gonzalo FR, Corbit KC, Sirerol-Piquer MS, Ramaswami G, Otto EA, Noriega TR, Seol AD, Robinson JF, Bennett CL, Josifova DJ, et al. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat Genet. 2011;43(8):776–84. doi: 10.1038/ng.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherman A, Davis EE, Katsanis N. The ciliary proteome database: an integrated community resource for the genetic and functional dissection of cilia. Nat Genet. 2006;38(9):961–2. doi: 10.1038/ng0906-961. [DOI] [PubMed] [Google Scholar]
- Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11(5):331–44. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277(5329):1109–13. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- Harfe BD. Keeping up with the zone of polarizing activity: New roles for an old signaling center. Dev Dyn. 2011;240(5):915–9. doi: 10.1002/dvdy.22597. [DOI] [PubMed] [Google Scholar]
- Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118(4):517–28. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1(4):e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycraft CJ, Zhang Q, Song B, Jackson WS, Detloff PJ, Serra R, Yoder BK. Intraflagellar transport is essential for endochondral bone formation. Development. 2007;134(2):307–16. doi: 10.1242/dev.02732. [DOI] [PubMed] [Google Scholar]
- Hodges E, Xuan Z, Balija V, Kramer M, Molla MN, Smith SW, Middle CM, Rodesch MJ, Albert TJ, Hannon GJ, et al. Genome-wide in situ exon capture for selective resequencing. Nat Genet. 2007;39(12):1522–7. doi: 10.1038/ng.2007.42. [DOI] [PubMed] [Google Scholar]
- Houde C, Dickinson RJ, Houtzager VM, Cullum R, Montpetit R, Metzler M, Simpson EM, Roy S, Hayden MR, Hoodless PA, et al. Hippi is essential for node cilia assembly and Sonic hedgehog signaling. Dev Biol. 2006;300(2):523–33. doi: 10.1016/j.ydbio.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Szymanska K, Jensen VL, Janecke AR, Innes AM, Davis EE, Frosk P, Li C, Willer JR, Chodirker BN, et al. TMEM237 is mutated in individuals with a Joubert syndrome related disorder and expands the role of the TMEM family at the ciliary transition zone. Am J Hum Genet. 2011;89(6):713–30. doi: 10.1016/j.ajhg.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci U S A. 2005;102(32):11325–30. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426(6962):83–7. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- Hui CC, Joyner AL. A mouse model of greig cephalopolysyndactyly syndrome: the extra-toesJ mutation contains an intragenic deletion of the Gli3 gene. Nat Genet. 1993;3(3):241–6. doi: 10.1038/ng0393-241. [DOI] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15(23):3059–87. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- Kasarskis A, Manova K, Anderson KV. A phenotype-based screen for embryonic lethal mutations in the mouse. Proc Natl Acad Sci U S A. 1998;95(13):7485–90. doi: 10.1073/pnas.95.13.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko HW, Norman RX, Tran J, Fuller KP, Fukuda M, Eggenschwiler JT. Broad-minded links cell cycle-related kinase to cilia assembly and hedgehog signal transduction. Dev Cell. 2010;18(2):237–47. doi: 10.1016/j.devcel.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y. Btrim: A fast, lightweight adapter and quality trimming program for next-generation sequencing technologies. Genomics. 2011;98(2):152–3. doi: 10.1016/j.ygeno.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Ku CS, Cooper DN, Polychronakos C, Naidoo N, Wu M, Soong R. Exome sequencing: Dual role as a discovery and diagnostic tool. Ann Neurol. 2012;71(1):5–14. doi: 10.1002/ana.22647. [DOI] [PubMed] [Google Scholar]
- Kyttala M, Tallila J, Salonen R, Kopra O, Kohlschmidt N, Paavola-Sakki P, Peltonen L, Kestila M. MKS1, encoding a component of the flagellar apparatus basal body proteome, is mutated in Meckel syndrome. Nat Genet. 2006;38(2):155–7. doi: 10.1038/ng1714. [DOI] [PubMed] [Google Scholar]
- Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–60. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25(16):2078–9. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem KF, Jr., He M, Ocbina PJ, Anderson KV. Mouse Kif7/Costal2 is a cilia-associated protein that regulates Sonic hedgehog signaling. Proc Natl Acad Sci U S A. 2009;106(32):13377–82. doi: 10.1073/pnas.0906944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litingtung Y, Chiang C. Specification of ventral neuron types is mediated by an antagonistic interaction between Shh and Gli3. Nat Neurosci. 2000;3(10):979–85. doi: 10.1038/79916. [DOI] [PubMed] [Google Scholar]
- Litingtung Y, Dahn RD, Li Y, Fallon JF, Chiang C. Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature. 2002;418(6901):979–83. doi: 10.1038/nature01033. [DOI] [PubMed] [Google Scholar]
- Liu A, Wang B, Niswander LA. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development. 2005;132(13):3103–11. doi: 10.1242/dev.01894. [DOI] [PubMed] [Google Scholar]
- Marszalek JR, Ruiz-Lozano P, Roberts E, Chien KR, Goldstein LS. Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc Natl Acad Sci U S A. 1999;96(9):5043–8. doi: 10.1073/pnas.96.9.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matise MP, Epstein DJ, Park HL, Platt KA, Joyner AL. Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons in the mouse central nervous system. Development. 1998;125(15):2759–70. doi: 10.1242/dev.125.15.2759. [DOI] [PubMed] [Google Scholar]
- May SR, Ashique AM, Karlen M, Wang B, Shen Y, Zarbalis K, Reiter J, Ericson J, Peterson AS. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev Biol. 2005;287(2):378–89. doi: 10.1016/j.ydbio.2005.08.050. [DOI] [PubMed] [Google Scholar]
- Nagy A, Gertsenstein M, Vintersten K, Behringer R. Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2003. [Google Scholar]
- Nigg EA, Raff JW. Centrioles, centrosomes, and cilia in health and disease. Cell. 2009;139(4):663–78. doi: 10.1016/j.cell.2009.10.036. [DOI] [PubMed] [Google Scholar]
- Olson M. Enrichment of super-sized resequencing targets from the human genome. Nat Methods. 2007;4(11):891–2. doi: 10.1038/nmeth1107-891. [DOI] [PubMed] [Google Scholar]
- Ostrowski LE, Blackburn K, Radde KM, Moyer MB, Schlatzer DM, Moseley A, Boucher RC. A proteomic analysis of human cilia: identification of novel components. Mol Cell Proteomics. 2002;1(6):451–65. doi: 10.1074/mcp.m200037-mcp200. [DOI] [PubMed] [Google Scholar]
- Pan Y, Wang C, Wang B. Phosphorylation of Gli2 by protein kinase A is required for Gli2 processing and degradation and the Sonic Hedgehog-regulated mouse development. Dev Biol. 2009;326(1):177–89. doi: 10.1016/j.ydbio.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HL, Bai C, Platt KA, Matise MP, Beeghly A, Hui CC, Nakashima M, Joyner AL. Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development. 2000;127(8):1593–605. doi: 10.1242/dev.127.8.1593. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Agrin N, Leszyk J, Witman GB. Proteomic analysis of a eukaryotic cilium. J Cell Biol. 2005;170(1):103–13. doi: 10.1083/jcb.200504008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson M, Stamataki D, te Welscher P, Andersson E, Bose J, Ruther U, Ericson J, Briscoe J. Dorsal-ventral patterning of the spinal cord requires Gli3 transcriptional repressor activity. Genes Dev. 2002;16(22):2865–78. doi: 10.1101/gad.243402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontius JU, Wagner L, Schuler GD. UniGene: a unified view of the transcriptome. In: McEntyre J, Ostell J, editors. The NCBI Handbook. National Center for Biotechnology Information; Bethesda, MD: 2003. [Google Scholar]
- Pruitt KD, Harrow J, Harte RA, Wallin C, Diekhans M, Maglott DR, Searle S, Farrell CM, Loveland JE, Ruef BJ, et al. The consensus coding sequence (CCDS) project: Identifying a common protein-coding gene set for the human and mouse genomes. Genome Res. 2009;19(7):1316–23. doi: 10.1101/gr.080531.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrgaki C, Liu A, Niswander L. Grainyhead-like 2 regulates neural tube closure and adhesion molecule expression during neural fold fusion. Dev Biol. 2011;353(1):38–49. doi: 10.1016/j.ydbio.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter JF, Skarnes WC. Tectonic, a novel regulator of the Hedgehog pathway required for both activation and inhibition. Genes Dev. 2006;20(1):22–7. doi: 10.1101/gad.1363606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribes V, Briscoe J. Establishing and interpreting graded Sonic Hedgehog signaling during vertebrate neural tube patterning: the role of negative feedback. Cold Spring Harb Perspect Biol. 2009;1(2):a002014. doi: 10.1101/cshperspect.a002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75(7):1401–16. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- Roelink H, Augsburger A, Heemskerk J, Korzh V, Norlin S, Ruiz i Altaba A, Tanabe Y, Placzek M, Edlund T, Jessell TM, et al. Floor plate and motor neuron induction by vhh-1, a vertebrate homolog of hedgehog expressed by the notochord. Cell. 1994;76(4):761–75. doi: 10.1016/0092-8674(94)90514-2. [DOI] [PubMed] [Google Scholar]
- Roelink H, Porter JA, Chiang C, Tanabe Y, Chang DT, Beachy PA, Jessell TM. Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis. Cell. 1995;81(3):445–55. doi: 10.1016/0092-8674(95)90397-6. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317(5836):372–6. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3(11):813–25. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Nishizaki Y, Hui C, Nakafuku M, Kondoh H. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development. 1999;126(17):3915–24. doi: 10.1242/dev.126.17.3915. [DOI] [PubMed] [Google Scholar]
- Sharma N, Berbari NF, Yoder BK. Ciliary dysfunction in developmental abnormalities and diseases. Curr Top Dev Biol. 2008;85:371–427. doi: 10.1016/S0070-2153(08)00813-2. [DOI] [PubMed] [Google Scholar]
- Sheridan R, Lampe K, Shanmukhappa SK, Putnam P, Keddache M, Divanovic S, Bezerra J, Hoebe K. Lampe1: An ENU-Germline Mutation Causing Spontaneous Hepatosteatosis Identified through Targeted Exon-Enrichment and Next-Generation Sequencing. PLoS One. 2011;6(7):e21979. doi: 10.1371/journal.pone.0021979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla V, Romaguera-Ros M, Garcia-Verdugo JM, Reiter JF. Ofd1, a human disease gene, regulates the length and distal structure of centrioles. Dev Cell. 2010;18(3):410–24. doi: 10.1016/j.devcel.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith UM, Consugar M, Tee LJ, McKee BM, Maina EN, Whelan S, Morgan NV, Goranson E, Gissen P, Lilliquist S, et al. The transmembrane protein meckelin (MKS3) is mutated in Meckel-Gruber syndrome and the wpk rat. Nat Genet. 2006;38(2):191–6. doi: 10.1038/ng1713. [DOI] [PubMed] [Google Scholar]
- Tanabe Y, Roelink H, Jessell TM. Induction of motor neurons by Sonic hedgehog is independent of floor plate differentiation. Curr Biol. 1995;5(6):651–8. doi: 10.1016/s0960-9822(95)00130-8. [DOI] [PubMed] [Google Scholar]
- Tang T, Li L, Tang J, Li Y, Lin WY, Martin F, Grant D, Solloway M, Parker L, Ye W, et al. A mouse knockout library for secreted and transmembrane proteins. Nat Biotechnol. 2010;28(7):749–55. doi: 10.1038/nbt.1644. [DOI] [PubMed] [Google Scholar]
- Traiffort E, Angot E, Ruat M. Sonic Hedgehog signaling in the mammalian brain. J Neurochem. 2010;113(3):576–90. doi: 10.1111/j.1471-4159.2010.06642.x. [DOI] [PubMed] [Google Scholar]
- Valente EM, Logan CV, Mougou-Zerelli S, Lee JH, Silhavy JL, Brancati F, Iannicelli M, Travaglini L, Romani S, Illi B, et al. Mutations in TMEM216 perturb ciliogenesis and cause Joubert, Meckel and related syndromes. Nat Genet. 2010;42(7):619–25. doi: 10.1038/ng.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100(4):423–34. doi: 10.1016/s0092-8674(00)80678-9. [DOI] [PubMed] [Google Scholar]
- Wang C, Ruther U, Wang B. The Shh-independent activator function of the full-length Gli3 protein and its role in vertebrate limb digit patterning. Dev Biol. 2007;305(2):460–9. doi: 10.1016/j.ydbio.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherbee SD, Niswander LA, Anderson KV. A mouse model for Meckel syndrome reveals Mks1 is required for ciliogenesis and Hedgehog signaling. Hum Mol Genet. 2009;18(23):4565–75. doi: 10.1093/hmg/ddp422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijgerde M, McMahon JA, Rule M, McMahon AP. A direct requirement for Hedgehog signaling for normal specification of all ventral progenitor domains in the presumptive mammalian spinal cord. Genes Dev. 2002;16(22):2849–64. doi: 10.1101/gad.1025702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Hoover AN, Liu A. PCP effector gene Inturned is an important regulator of cilia formation and embryonic development in mammals. Dev Biol. 2010;339(2):418–28. doi: 10.1016/j.ydbio.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Zuniga A, Haramis AP, McMahon AP, Zeller R. Signal relay by BMP antagonism controls the SHH/FGF4 feedback loop in vertebrate limb buds. Nature. 1999;401(6753):598–602. doi: 10.1038/44157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.