Abstract
Approximately 80% of smokers initiate tobacco use during adolescence, suggesting that nicotine initiation and nicotine dependence have a substantial age component. There also is a substantial genetic influence on smoking behaviors such as age of initiation and the development of nicotine dependence. The goal of this study was to examine both genetic background and age dependent effects on oral nicotine self-administration and anxiety-like behaviors in mice. Two inbred mouse strains (C3H/Ibg and C57BL/6J) were assessed for oral nicotine preference during early adolescence (postnatal day 24–35), middle adolescence (postnatal day 36–47), late adolescence (postnatal day 48–59), adulthood (postnatal day 60+) and 2 months following their initial exposure to nicotine. Mice also were assessed for innate anxiety using an elevated zero maze to determine if age and/or genetic background influenced anxiety-like behaviors. Results indicated that initial nicotine preference and nicotine preference two months after an initial exposure are both strain and age dependent. Age also had an effect on some baseline anxiety measures but strain differences for most zero maze measures were present throughout all age groups. In general, early adolescent C3H mice exhibited greater nicotine preference while C57 mice displayed greater preference during middle adolescence and upon a second exposure to nicotine. In contrast, C57 mice exhibited reduced anxiety across all ages tested. These studies indicate that genetic background should be considered when evaluating age-dependent effects of drugs of abuse and baseline anxiety-like behaviors.
Keywords: Mouse, Strain, Nicotine, Adolescent, Anxiety, Nicotine Preference
1. Introduction
In the United States, the use of tobacco products is responsible for about 20% of all premature deaths [1,2] and of the reported 21% of adults in the United States who smoke, 80% initiated the use of tobacco before the age of 18 [3,4]. In fact, not only does smoking almost always start in adolescence, several studies have reported that the earlier in age an individual initiates smoking, the more likely they are to exhibit a history of heavy smoking and reduced success at quitting [5,6,7]
Despite the fact that initiating smoking in adolescence significantly increases the risk for long term smoking only about one third of adolescents who try tobacco go on to become nicotine dependent [8]. Therefore, although adolescence is a critical period for the establishment nicotine dependence, individual variability in liability to nicotine dependence during this stage of development also plays a major role in who does and does not go on to become a regular smoker. The chief cause of individual variability in the progression from experimentation to nicotine dependence in adolescence appears to be genetics. Indeed, reports indicate that between 75% and 85% of individual variability in the development of nicotine dependence can be attributed to genetic factors [9,10]. In addition, studies have suggested that anxiety disorders in adolescents increases the risk for the development of nicotine dependence [11,12,13]. Consequently, whether an individual becomes nicotine dependent is not only determined by his/her age of onset, but also by genetic factors and mental state.
Because age of initiation plays a major role in later smoking behavior, several studies in rodents have explored the influence of age in several measures of nicotine sensitivity (for a recent review see Schramm-Sapyta et al.,[14]). In general, these studies show that adolescent rodents are more sensitive to the reinforcing properties and less sensitive to the aversive properties of nicotine relative to adults. Additionally, a few studies have found that exposure to nicotine during adolescence leads to an increase in the rewarding properties of the drug in adulthood [15,16,17,18].
The studies published to date provide evidence on why adolescence is a period of increased risk for experimentation with drugs of abuse including nicotine. However, they do not explain why two-thirds of human adolescents who experiment with tobacco do not go on to become nicotine dependent. The experiments described in this report used two inbred strains of mice, C3H/Ibg and C57BL6/J, to assess whether genetics and/or, sex differentially impact nicotine preference during defined periods of adolescence and adulthood as well as the effect of nicotine exposure during adolescence on later nicotine preference. Additionally, naïve animals were assessed for innate differences in anxiety-like measures as measured by the elevated zero maze in order to assess whether age, strain and/or sex also differentially affect anxiety.
2. Materials and Methods
2.1 Materials
Free base (−)-nicotine was purchased from Sigma Aldrich (St. Louis, MO), and all nicotine concentrations were calculated as free base.
2.2 Animals
C57BL/6J and C3H/Ibg animals were bred in a specific pathogen free (SPF) facility at the University of Colorado at Boulder. Animals were weaned between postnatal day (pnd) 21 and 25 for all experiments. The animals were maintained on a 12 hour light and 12 hour dark cycle (lights on at 7 a.m.) with food and water ad libitum. The animal colony and testing facilities are maintained at 21 ± 1 degree Celsius with approximately 50% humidity. For these studies, both male and female animals were divided into four different age groups: early adolescent (pnd 24–35), middle adolescent (pnd 36–47), late adolescent (pnd 48–59), and adult (pnd 60+) similar to ages defined by Adriani et al. [19]. All procedures were approved by the University of Colorado’s Institutional Animal Care and Use Committee.
2.3 Oral Nicotine Consumption
A two bottle free choice paradigm was used as previously described [20] with the exception that the nicotine concentrations used in the current study were reduced to 10 μg/ml, 20 μg/ml and 30 μg/ml. Preliminary studies with adolescent animals indicated that animals would not reliably consume measurable amounts of higher nicotine concentrations (25, 50, and 100 μg/ml). Approximately 24–48 hours before the start of the experiment, mice that were either pnd 22–23, pnd 34–35, pnd 46–47, or 60+ days old were weighed, singly housed, and given free access to food and water. At the start of the experiment, mice were given two glass bottles (18 × 150 mm glass culture tubes (Corning, Corning, NY)) fitted with standard stainless steel sipper tubes mounted in rubber stoppers (size 0)). One of the bottles contained fresh tap water and the other contained 10 μg/ml nicotine in fresh tap water. Mice and bottles were weighed daily. The bottles also were rotated each day to account for potential side bias. After four days, the nicotine concentration was increased to 20 μg/ml and animals were given fresh water. Following the four days of 20 μg/ml nicotine solution, the nicotine concentration was increased to 30 μg/ml for four days and animals received fresh water. In addition, cages without mice were fitted with two glass bottles with standard sipper tubes. The bottles were rotated and weighed daily to determine volume lost due to spillage and evaporation during the experiment. Approximately 10–20 animals of each strain, age and sex were tested in the nicotine oral preference paradigm. Data were calculated for all age groups (early, middle, late adolescents and adults) to determine the preference ratio (percent of nicotine consumed relative to total fluid consumed) of nicotine consumption to water consumption at each nicotine concentration.
After initial nicotine preference was measured in each group of animals, animals were returned to their home cage with their littermates for 2 months and then retested using the same oral nicotine preference paradigm. Data was calculated for each group to determine nicotine preference.
2.4 Elevated Zero Maze
Zero maze activity was measured in naive C57BL/6J and C3H/Ibg animals during early adolescence (between pnd 24–35), middle adolescence (between pnd 36–47), late adolescence (48–59), and adulthood (60+ days old). Each age group was an independent set of animals. For testing, mice were transferred to the room with the zero mazes and allowed to acclimate for two hours before the start of the experiment. The zero mazes (AccuScan Instruments, Inc., Columbus, OH) were elevated 109 cm above the floor and composed of a black circular ring, 30 cm in diameter and 5 cm wide. The circular platform was divided into 4 quadrants. Quadrants 1 and 3 were closed sections with 28.5 cm tall clear acrylic plastic pieces surrounding the two sides, while quadrants 2 and 4 were open on both sides but contained a short lip on the edge of each side of the platform. One at a time, animals were placed in closed arm quadrant 1 at the start of the experiment. As soon as the animal was placed in the zero maze, the timer started and data were recorded for 5 minutes. The zero maze was automated and recorded the amount of time spent in open and closed quadrants. However, the total number of entries into open/closed arms was not recorded by the automated system, but this information was scored manually by an experienced experimenter. The zero maze was cleaned with 70% ethanol prior to each animal being tested.
2.5 Statistical Analyses
A threshold of p < 0.05 was set to determine statistical significance, and all data are represented as an average ± SEM. A 3 way (age X strain x sex) repeated measures analysis of variance (ANOVA) was employed for nicotine preference (unless specifically stated). When sphericity was violated, a Greenhouse-Geisser correction was used. A 3 way (age X strain X sex) multivariate analysis of variance (MANOVA) was used to determine statistical significance for zero maze measures. Where appropriate, a Bonferroni post hoc analysis was utilized for age comparisons. If statistical significance was not obtained for any of the factors and there were no significant interactions with the factors, data were collapsed. In some cases, a one-way ANOVA was used to examine potential within strain effects of age. Additionally, a Pearson product-moment correlation coefficient was calculated within each group to examine the relationship between preference during the initial and retest nicotine exposures.
3. Results
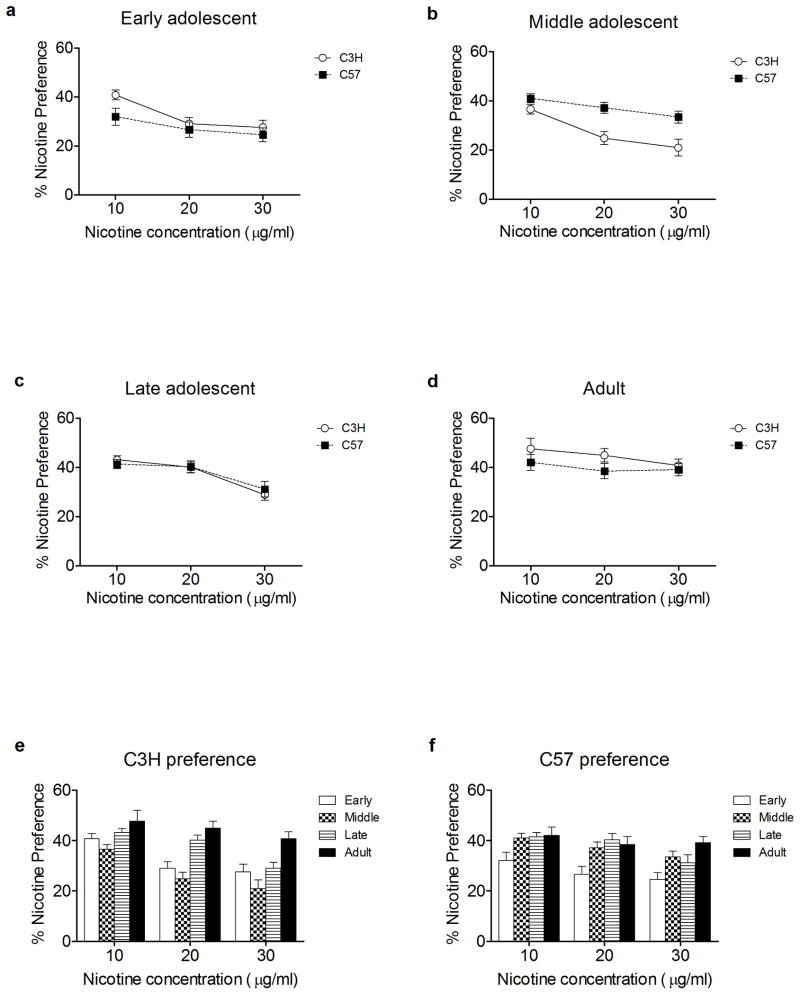
To assess whether there are genetic background, age, and/or sex effects on nicotine intake, male and female C3H and C57 mice were tested for oral nicotine preference (figure 1) during early adolescence (pnd 24–35) (figure 1a), middle adolescence (pnd 36–47) (figure 1b), late adolescence (pnd 48–59) (figure 1c) and adulthood (60+ days) (figure 1d) as described in the methods. A repeated measures ANOVA showed a significant main effect of age on nicotine preference (p = 1.2 × 10−5). Post-Hoc analysis indicated that nicotine preference during early and middle adolescence was decreased relative to late adolescence and adulthood. In addition, there were significant between-subjects interactions for strain x age (p = 0.002) and age × sex (p = 0.031). Early adolescent C3H mice displayed a higher nicotine preference at 10 μg/ml than C57 mice but middle adolescent C57 mice exhibited a higher nicotine preference at the 20 and 30 μg/ml nicotine solutions. Similar results were seen when data was calculated as dose in mg/kg (see supplemental materials). Additionally, females displayed reduced nicotine preference relative to males during middle adolescence and as adults (data not shown). A within-strain repeated measures ANOVA for C3H animals (figure 1e) revealed a significant main effect of age (p = 8.9 × 10−6) with the early (p = 0.007) and middle (p = 1.1 × 10−5) adolescent age groups exhibiting significantly lower preference than the adults. It also was observed that as the nicotine concentrations increased, nicotine preference in early and middle adolescent C3H animals decreased whereas there was little change in nicotine preference for adult C3H animals across nicotine concentrations. A main effect of age on nicotine preference was (figure 1f) also observed in C57 mice (p = 0.007). Furthermore, a post hoc analysis revealed that nicotine preference in early adolescent C57 animals was significantly less than in middle adolescent C57 animals (p = 0.018) and adult C57 animals (p = 0.015) and trended towards being significantly different from late adolescent C57 animals (p = 0.065).
Figure 1.
Age and strain effects on nicotine preference. Female and male early adolescent (pnd 24–35) (a), middle adolescent (pnd 36–47) (b), late adolescent (pnd 48–59) (c), and adult (pnd 60+) (d) C3H/Ibg and C57BL/6J mice were examined for nicotine preference (percentage of total daily fluid consumed from the nicotine bottle) in a 2 bottle choice test. Analysis indicated that there was a main affect of age (p = 1.2 × 10−5) but not strain or sex for nicotine preference. Within strain results showed that early (p = 0.007) and middle adolescent (p = 1.1 × 10−5) C3H/Ibg mice had reduced preference for nicotine relative to adults (e). In contrast, early adolescent C57BL/6J mice exhibited reduced preference for nicotine relative to middle adolescent (p = 0.018) and adult (p = 0.015) animals (f). Data represent mean ± SEM.
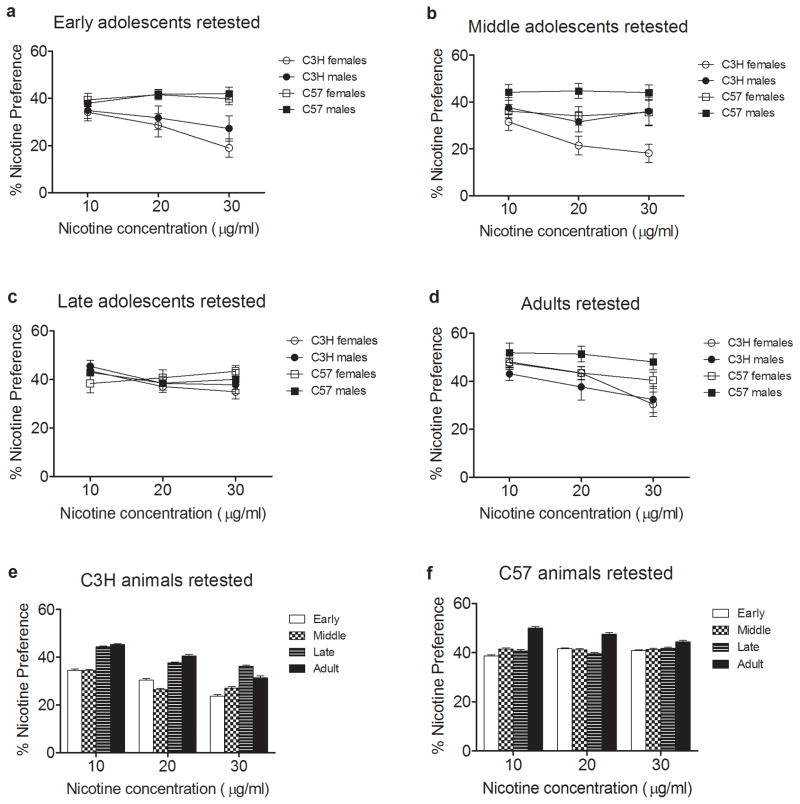
Following their initial two bottle choice trial, mice were returned to their home cages with same sex littermates for 2 months and then were retested for nicotine preference using the same two bottle choice paradigm. A repeated measures ANOVA (figure 2) revealed that age of first nicotine exposure (p = 0.001), strain (p = 1.3 × 10−5), and sex (p = 0.030) each had significant effects on nicotine preference during the second exposure. A post-hoc analysis revealed that animals first exposed to nicotine during early (figure 2a) and middle adolescence (figure 2b) had reduced preference for nicotine relative to animals first exposed during adulthood (p = 0.012 and p = 0.01, respectively) (figure 2d). Additionally, females that were re-exposed to nicotine 2 months following the initial exposure to the drug displayed reduced nicotine preference as compared to males. The sex difference in nicotine preference was most pronounced in animals first exposed to nicotine during middle adolescence or as adults (figures 2b and 2d). Within strain analyses indicated that the effect of age of initial exposure on later nicotine preference was strain dependent (figures 2e and 2f). The reduced preference resulting from initial exposure during early and middle adolescence was observed for C3H mice. However, age of initial exposure did not significantly affect later nicotine preference in C57BL/6 mice. Similar results were obtained when nicotine intake was measured by daily dose (mg/kg) (see supplemental information).
Figure 2.
Influence of age of first exposure, strain and sex on nicotine preference two months following initial free choice intake of nicotine. Two months following initial nicotine exposure during early adolescence (a), middle adolescence (b), late adolescence (c), or adulthood (d) mice were retested for nicotine preference. A repeated measures ANOVA demonstrated that age of first nicotine exposure (p = 0.001), strain (p = 1.3 × 10−5), and sex (p = 0.030) each had a significant effect on nicotine preference in the retested animals. A post hoc analysis further indicated that nicotine preference in re-tested animals first exposed to nicotine during early and middle adolescence was significantly reduced compared to re-tested animals initially exposed to nicotine as adults. Within strain analyses indicated a significant effect of age initially exposed to nicotine on later nicotine preference for C3H/Ibg animals (p = 0.001) (e). A posthoc analysis showed that nicotine preference in re-tested C3H/Ibg mice first exposed to nicotine during late adolescence was significantly more than nicotine preference in animals initially exposed to nicotine during early (p = 0.025) and middle (p = 0.008) adolescence. There was no effect of age initially exposed to nicotine in retested C57BL/6J animals (f). Data represent mean ± SEM.
A Pearson moment correlation coefficient also was computed to assess the relationship between nicotine preference during the initial two bottle choice and later two bottle choice nicotine exposure (table 1). C3H animals first tested during early adolescence displayed no correlation in nicotine preference upon later exposure at any nicotine concentration while C57 animals first tested as early adolescence displayed a negative correlation for nicotine preference at 30 μg/ml when retested (r = −0.445, p = 0.026, n = 25). Middle adolescent C3H mice displayed a positive correlation with later nicotine preference at the 10 μg/ml nicotine (r = 0.502, p = 0.001, n = 38), 20 μg/ml nicotine (r = 0.467, p = 0.003, n = 38) and 30 μg/ml nicotine (r = 0.401, p = 0.012) concentrations. C57 middle adolescents also showered a positive correlation for nicotine preference between initial and retest exposure at 10 μg/ml (r = 0.437, p = 0.005, n = 39) and 20 μg/ml (r = 0.368, p = 0.021, n = 39), but not at 30 μg/ml. C3H mice first tested during late adolescence showed positive correlation for nicotine preference at only the highest nicotine concentration tested (30 μg/ml) (r = 0.336, p = 0.039, n = 38). On the other hand, C57 animals tested for two bottle nicotine preference displayed no correlation with later testing for this age group. When C3H animals were first tested for two bottle nicotine preference as adults displayed a positive correlation for nicotine preference at 30 μg/ml nicotine concentration (r = 0.547, p = 0.015, n = 19). However, no correlation was detected in C57 animals initially tested as adults.
Table 1.
Summary of correlations between retest and original nicotine preference. The Pearson-moment correlation coefficient was calculated for inbred strains originally tested at each age for nicotine preference
| C3H | C57 | ||||||
|---|---|---|---|---|---|---|---|
| 10 μg/ml | 20 μg/ml | 30 μg/ml | 10 μg/ml | 20 μg/ml | 30 μg/ml | ||
| Early Adolescent | Pearson Correlation | .234 | .034 | −.043 | −.115 | −.249 | −.445* |
| Sig. (2-tailed) | .250 | .870 | .836 | .593 | .231 | .026 | |
| N | 26 | 26 | 26 | 24 | 25 | 25 | |
| Middle Adolescent | Pearson Correlation | .502** | .467** | .401* | .437** | .368* | .119 |
| Sig. (2-tailed) | .001 | .003 | .012 | .005 | .021 | .470 | |
| N | 38 | 38 | 38 | 39 | 39 | 39 | |
| Late Adolescent | Pearson Correlation | .217 | .100 | .336* | −.098 | .079 | −.406 |
| Sig. (2-tailed) | .191 | .549 | .039 | .680 | .741 | .076 | |
| N | 38 | 38 | 38 | 20 | 20 | 20 | |
| Adult | Pearson Correlation | .203 | .434 | .547* | .405 | .353 | .329 |
| Sig. (2-tailed) | .404 | .064 | .015 | .096 | .151 | .182 | |
| N | 19 | 19 | 19 | 18 | 18 | 18 | |
Anxiety during adolescence may be a contributing factor to smoking initiation and the development of nicotine dependence [13,11,21]. In addition, there is evidence that the mouse strains used in this study, C3H and C57, differ in anxiety when tested as adults [22,23,24] and that there are age [25,26] and sex [27] dependent effects on anxiety related behaviors in mice. As a result, female and male C3H and C57 animals in each of the four age groups were assessed for innate anxiety using an elevated zero maze in order to thoroughly examine age and sex related differences in this measure of anxiety-related behavior.
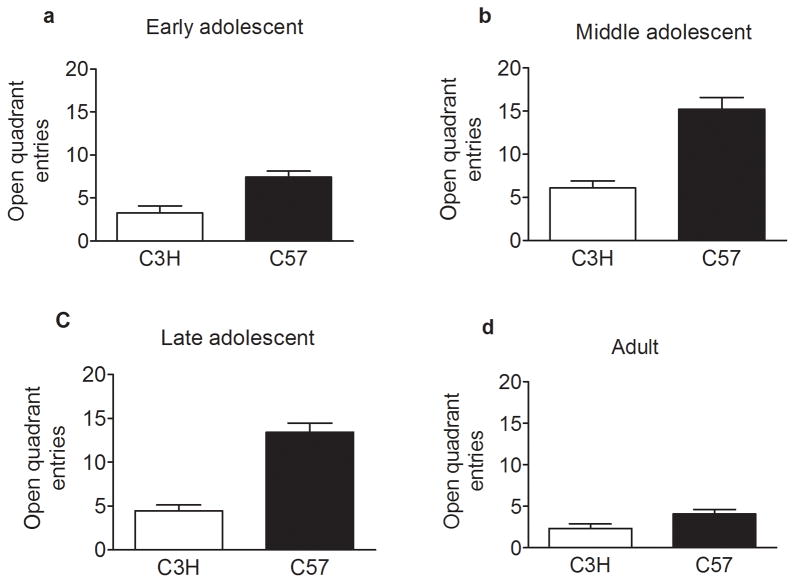
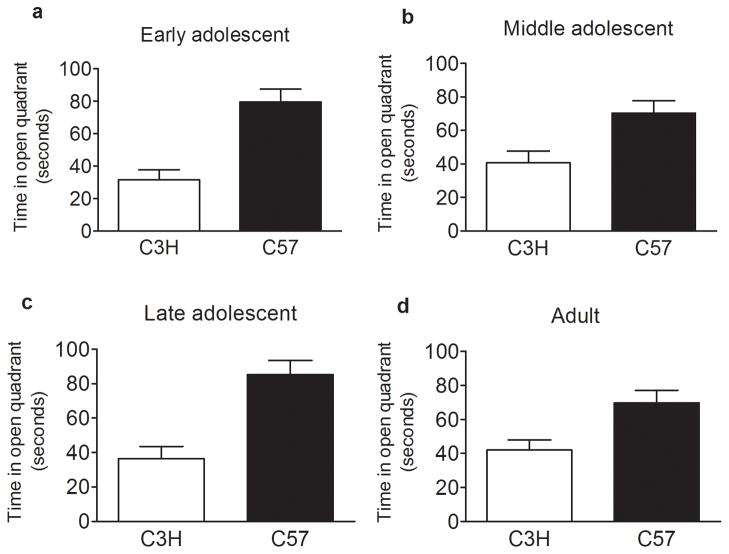
Animals in each age group were scored for open quadrant entries (figure 3) and time spent in the open quadrant (figure 4) of the zero maze. A multivariate analysis of variance (MANOVA) indicated a significant effect of age (p = 9.6 × 10−16), strain (p = 1.3 × 10−17), and an age x strain interaction (p = 2.9 × 10−5) (the data were collapsed on sex due to no effect) for the number of open quadrant entries. Post hoc analyses revealed that early adolescent animals (figure 3a) exhibited fewer open quadrant entries compared to middle adolescents (p = 1.5 × 10−6) (figure 3b) and late adolescents (p = 0.013) (figure 3c). Additionally, adults (figure 3d) displayed decreased open quadrant entries relative to middle adolescents (p = 5.2 × 10−13) (figure 3b) and late adolescents (p = 3.0 × 10−7) (figure 3c). For time spent in the open quadrants (figure 4) there was an effect of strain (p = 9.0 × 10−12) where C57 animals spent more time in the open quadrants compared to C3H animals. There was not an effect of age or a strain by age interaction for time spent in the open quadrant. In summary, C3H animals exhibited lower open quadrant entries and decreased time in the open quadrants across all age groups and therefore, display more anxiety-like behaviors compared to C57 animals regardless of age tested. Age seemed to affect only the number of open quadrant entries whereas strain influenced both open quadrant entries and time spent in open quadrants.
Figure 3.
Age and strain effects on the number of zero maze open quadrant entries. Drug naïve early adolescent (a), middle adolescent (b), late adolescent (c), and adult (d) inbred animals were tested on a zero maze for baseline differences in the number of open quadrant entries. Analysis identified a significant effect of age (p = 9.6 × 10−16), strain (p = 1.3 × 10−17), and an age x strain interaction (p = 2.9 × 10−5) (the data were collapsed on sex due to no effect) with middle and late adolescents exhibiting a greater number of open arm entries than either early adolescent or adult animals. Data represent mean ± SEM.
Figure 4.
Age and strain effects on time spent in open quadrants of an elevated zero maze. Early adolescent (a), middle adolescent (b), late adolescent (c), and adult (d) animals were examined for baseline differences in the total time spent in the open quadrants of the zero maze. A main effect of strain (p = 9.0 × 10−12) but not age was detected for the time spent in the open quadrants. C57 animals spent more time in the open quadrants relative to C3H animals across all age groups. Data represent mean ± SEM.
4. Discussion
In this study, oral nicotine preference in C3H/Ibg and C57BL/6J mice was assessed at 10, 20 and 30 μg/ml nicotine in early (pnd 24), middle (pnd 36), and late adolescent animals (pnd 48) as well as adult animals (60+ days). The inbred mouse strains C3H/Ibg and C57BL/6J were chosen for this study because they have been shown to be near opposite extremes for nearly all measures of nicotine sensitivity when tested as adults [28,29,30,31]. Results from this study indicate that there is a significant effect of age tested, strain x age interaction, and age x sex interaction for nicotine preference. Between strain comparisons indicated that early adolescent mice C3H exhibited greater preference than early adolescent C57BL/6 mice at the 10 μg/ml concentration of nicotine while middle adolescent C57BL/6J mice had greater preference for the 20 and 30 μg/ml nicotine concentrations than did C3H mice. Within strain comparisons indicated that early and middle adolescent mice but not late adolescent mice differed from adults for nicotine preference in C3H mice while only early adolescent animals differed from adults in C57BL/6 mice. In all cases where there was a difference in nicotine preference between adolescents and adults, adolescents consistently had a reduced preference for nicotine.
The nicotine preference data indicate that although age can influence nicotine intake, the influence of age is strain dependent. Moreover, when age-dependent effects on nicotine intake were observed in this study, adolescent animals exhibit reduced preference relative to adults. This finding is in contrast to other intake studies that have shown that adolescent mice, and in particular, early adolescent mice, exhibit higher nicotine preference [32,19,33] or increased sensitivity to nicotine-induced conditioned place preference [34,35] relative to adult animals. It remains to be determined why the data in the current study do not suggest a greater vulnerability to nicotine intake during adolescence. The apparently conflicting results could be due to stain differences and/or procedural differences as the study by Adriani et al. used a different mouse stock (CD-1), a restricted access to fluid and group housing of animals except during their drinking period. It also cannot be ruled out that the adolescent mice in the current study are drinking less nicotine because they are more sensitive to the reinforcing properties of nicotine [36,35,34] and as such, require lower doses to obtain the desired effect. However, the motivation for the observed differences in oral nicotine intake across the age groups is not clear. Several factors such as age and strain differences in sensitivity to the reinforcing or aversive effects of nicotine, tolerance development to nicotine, taste reactivity to nicotine, etc. could each contribute to age and strain effects in nicotine intake. Nonetheless, the data described in this report are consistent with the data reported by Shram et al. [37] who found that adolescent rats have reduced intraveneous nicotine self-administration compared to adults under a progressive ratio design. Thus, it appears that whether adolescent rodents show increased drug intake is paradigm specific and not a universal phenomenon.
Previous studies have shown that exposure to nicotine during adolescence, usually via means of a drug injection, tends to increase nicotine reward in adult animals [16,17,18]. However, this phenomenon was not observed in the current study. Nicotine preference in re-exposed C57BL/6J mice was not affected by the age of initial exposure to nicotine. In contrast, there was an age of initial exposure effect on nicotine preference in C3H mice. However, the effect was for initial exposure during early and middle adolescence to decrease nicotine preference in re-tested mice relative to re-tested animals whose initial exposure to nicotine was as adults. One explanation for these apparently discrepant findings is that in the previously published reports, nicotine exposure during adolescence was forced. Animals were treated with nicotine either via injections or osmotic mini-pumps. In the current study, nicotine dose was controlled by the animals as a result of free choice nicotine intake. These results suggest that when nicotine doses are self-regulated during adolescence, they do not lead to heightened reward for nicotine at later ages and in fact, in some conditions, may lead to reduced reward/drug intake at later ages. This possibility is supported by recent reports by Nesil et al [33,38] who found that free choice oral nicotine consumption in adolescent rats decreased nicotine reinforcement as well as nicotine intake in adults. It is worth noting that significant within-animal correlations were observed between initial nicotine preference and retest preference. Seven of the eight significant correlations were positive indicating that animals with greater nicotine preference during their initial exposure also exhibited greater preference during their second exposure. Most of the positive significant correlations (5 of 7) were for test-retest data from middle adolescence for both C3H and C57BL/6 mice. This finding suggests that innate differences in nicotine preference during middle adolescence are a major determinant of subsequent nicotine intake behavior.
There is evidence that females are more vulnerable to the effects of tobacco. Females generally consume more tobacco products and have more difficulty quitting [39,40,41,42]. However, the influence of sex on oral nicotine intake in rodents is equivocal. For example, in adult rodents, some studies have observed sex differences [20] some have not [43] and others have found that the effect of sex on nicotine intake is measure-dependent [44]. Likewise, sex appears to impact nicotine intake during adolescence in some studies [45] but not others [33]. Consequently, this study sought to further examine whether nicotine preference across various age groups was affected by sex. Results indicated that there was no main effect of sex on initial nicotine preference, although there was a trend toward significance (p = 0.065) with females displaying a lower preference for nicotine than males. However, there was a significant age by sex interaction, indicating that both age of exposure as well as sex influence initial nicotine intake with middle adolescent and adult females displaying lower nicotine preference. Moreover, there was a significant main effect of sex on nicotine preference following the initial nicotine preference with females again displaying decreased nicotine preference. The greatest difference in nicotine preference between females and males was in the groups first exposed to nicotine during middle adolescence and as adults, the two age groups where females initially had reduced preference for nicotine relative to males. This suggests that female mice might be more susceptible to an aversive effect of nicotine at these ages and exposure during this period may lead to long term avoidance of nicotine.
The modest reduction in nicotine preference among middle adolescent and adult females observed in this study is in contrast to the prior studies where sex differences had been observed. In these previous studies, females exhibited greater nicotine intake than males. However, in the previous studies where sex differences were observed, higher nicotine concentrations were used suggesting that increased nicotine preference in females may be nicotine concentration dependent. In support of this possibility, sex differences in nicotine intake were, in fact, concentration dependent in the study reported by Klein et al. [46]. At low concentrations of nicotine (in the range of those used in the current study) no sex difference in nicotine intake was observed while at higher concentrations females consumed more nicotine than males. Nonetheless, it is clear that there is not a consistent effect of sex on nicotine intake in rodents.
Adolescence may not only be a period of altered sensitivity to drugs of abuse, it also is a period of heightened levels of anxiety [47]. Studies in humans suggest that anxiety during adolescence may increase the risk for developing nicotine dependence [21,13,11]. The few studies that have examined age effects on anxiety measures in rodents suggest that age has a modest effect on anxiety measures [48]. The data collected here indicate that age influences anxiety-like behaviors but in a manner that is genetic background and zero maze measure dependent. In general, C57 animals displayed lower anxiety-like behaviors relative to C3H across all age groups for both open quadrant entries and the time spent in the open quadrants. This result is consistent with a study by Tarantino et al. [27] who found that adult C57 animals exhibited more transitions into the open quadrant of the zero maze compared to another substrain of C3H animals. In the current study, age also affected the number of transitions into the open quadrant. Both C3H and C57 middle adolescent animals exhibited more open quadrant entries than any other age group. This finding might suggest an increased exploratory-like behavior that is age but not strain dependent (in the two strains tested).
5. Conclusions
In summary, the results of this study indicate that adolescence does not appear to be universal period of increased nicotine preference. Under conditions of free-choice unrestricted access to nicotine, nicotine preference is either no different or reduced in adolescent animals relative to adult animals and is influenced by both adolescent age and mouse strain. In addition, self-regulated nicotine intake during defined age ranges throughout adolescence does not lead to heightened intake when animals are re-exposed two months following the initial exposure. In fact, free-choice nicotine access during adolescence may actually decrease nicotine preference at a later age depending upon initial age of exposure, mouse strain, and sex. Similarly, the results of this study indicate that anxiety-like measures also are both strain and age dependent as well as dependent on the experimental measure. This study highlights the need for more studies to clarify the relationship between age, sex and strain on nicotine preference and anxiety.
Supplementary Material
Highlights.
adolescence does not appear to be universal period of increased nicotine preference
nicotine preference is effected by age, strain x age, and age x sex interactions
free choice nicotine during adolescence does not increase later nicotine preference
anxiety-like measures are strain and age dependent
Acknowledgments
The experiments in this study were supported by the NIH DA015663, DA017637, DA026918, CA089392, and DA024515.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.CDC. Smoking-Attributable Mortality, Years of Potential Life Lost, and Productivity Losses --- United States, 2000--2004. Morbidity and Mortality Weekly Report. 2008;57:1226–8. [PubMed] [Google Scholar]
- 2.McGinnis JM, Foege WH. Actual causes of death in the United States. JAMA. 1993;270:2207–12. [PubMed] [Google Scholar]
- 3.Chassin L, Presson CC, Sherman SJ, Edwards DA. The natural history of cigarette smoking: predicting young-adult smoking outcomes from adolescent smoking patterns. Health Psychol. 1990;9:701–16. doi: 10.1037//0278-6133.9.6.701. [DOI] [PubMed] [Google Scholar]
- 4.Substance Abuse and Mental Health Services Administration(SAMHSA) OoAS. The NSDUH Report: Trends in Tobacco Use among Adolescents: 2002 to 2008. 2008;17 [Google Scholar]
- 5.Breslau N, Peterson EL. Smoking cessation in young adults: age at initiation of cigarette smoking and other suspected influences. Am J Public Health. 1996;86:214–20. doi: 10.2105/ajph.86.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackesy-Amiti ME, Fendrich M, Goldstein PJ. Sequence of drug use among serious drug users: typical vs atypical progression. Drug Alcohol Depend. 1997;45:185–96. doi: 10.1016/s0376-8716(97)00032-x. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Millar WJ. Age of smoking initiation: implications for quitting. Health Rep. 1998;9:39–46. [PubMed] [Google Scholar]
- 8.Selected cigarette smoking initiation and quitting behaviors among high school students--United States, 1997. MMWR Morb Mortal Wkly Rep. 1998;47:386–9. [PubMed] [Google Scholar]
- 9.Koopmans JR, Slutske WS, Heath AC, Neale MC, Boomsma DI. The genetics of smoking initiation and quantity smoked in Dutch adolescent and young adult twins. Behav Genet. 1999;29:383–93. doi: 10.1023/a:1021618719735. [DOI] [PubMed] [Google Scholar]
- 10.Vink JM, Willemsen G, Boomsma DI. Heritability of smoking initiation and nicotine dependence. Behav Genet. 2005;35:397–406. doi: 10.1007/s10519-004-1327-8. [DOI] [PubMed] [Google Scholar]
- 11.Patton GC, Carlin JB, Coffey C, Wolfe R, Hibbert M, Bowes G. Depression, anxiety, and smoking initiation: a prospective study over 3 years. Am J Public Health. 1998;88:1518–22. doi: 10.2105/ajph.88.10.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonntag H, Wittchen HU, Hofler M, Kessler RC, Stein MB. Are social fears and DSM-IV social anxiety disorder associated with smoking and nicotine dependence in adolescents and young adults? Eur Psychiatry. 2000;15:67–74. doi: 10.1016/s0924-9338(00)00209-1. [DOI] [PubMed] [Google Scholar]
- 13.McKenzie M, Olsson CA, Jorm AF, Romaniuk H, Patton GC. Association of adolescent symptoms of depression and anxiety with daily smoking and nicotine dependence in young adulthood: findings from a 10-year longitudinal study. Addiction. 2010;105:1652–9. doi: 10.1111/j.1360-0443.2010.03002.x. [DOI] [PubMed] [Google Scholar]
- 14.Schramm-Sapyta NL, Walker QD, Caster JM, Levin ED, Kuhn CM. Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacology (Berl) 2009;206:1–21. doi: 10.1007/s00213-009-1585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le MM, Smit AB, Piazza PV. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J Neurosci. 2003;23:4712–6. doi: 10.1523/JNEUROSCI.23-11-04712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adriani W, Deroche-Gamonet V, Le MM, Laviola G, Piazza PV. Preexposure during or following adolescence differently affects nicotine-rewarding properties in adult rats. Psychopharmacology (Berl) 2006;184:382–90. doi: 10.1007/s00213-005-0125-1. [DOI] [PubMed] [Google Scholar]
- 17.Torres OV, Tejeda HA, Natividad LA, O’Dell LE. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacol Biochem Behav. 2008;90:658–63. doi: 10.1016/j.pbb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kota D, Robinson SE, Imad DM. Enhanced nicotine reward in adulthood after exposure to nicotine during early adolescence in mice. Biochem Pharmacol. 2009;78:873–9. doi: 10.1016/j.bcp.2009.06.099. [DOI] [PubMed] [Google Scholar]
- 19.Adriani W, Macri S, Pacifici R, Laviola G. Peculiar vulnerability to nicotine oral self-administration in mice during early adolescence. Neuropsychopharmacology. 2002;27:212–24. doi: 10.1016/S0893-133X(02)00295-6. [DOI] [PubMed] [Google Scholar]
- 20.Li XC, Karadsheh MS, Jenkins PM, Stitzel JA. Genetic correlation between the free-choice oral consumption of nicotine and alcohol in C57BL/6JxC3H/HeJ F2 intercross mice. Behav Brain Res. 2005;157:79–90. doi: 10.1016/j.bbr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Patton GC, Hibbert M, Rosier MJ, Carlin JB, Caust J, Bowes G. Is smoking associated with depression and anxiety in teenagers? Am J Public Health. 1996;86:225–30. doi: 10.2105/ajph.86.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marks MJ, Miner LL, Cole-Harding S, Burch JB, Collins AC. A genetic analysis of nicotine effects on open field activity. Pharmacol Biochem Behav. 1986;24:743–9. doi: 10.1016/0091-3057(86)90584-8. [DOI] [PubMed] [Google Scholar]
- 23.Ennaceur A, Michalikova S, van RR, Chazot PL. Models of anxiety: responses of mice to novelty and open spaces in a 3D maze. Behav Brain Res. 2006;174:9–38. doi: 10.1016/j.bbr.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Milner LC, Crabbe JC. Three murine anxiety models: results from multiple inbred strain comparisons. Genes Brain Behav. 2008;7:496–505. doi: 10.1111/j.1601-183X.2007.00385.x. [DOI] [PubMed] [Google Scholar]
- 25.Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behav Neurosci. 1998;112:1152–66. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- 26.Adriani W, Laviola G. Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behav Pharmacol. 2004;15:341–52. doi: 10.1097/00008877-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Tarantino LM, Gould TJ, Druhan JP, Bucan M. Behavior and mutagenesis screens: the importance of baseline analysis of inbred strains. Mamm Genome. 2000;11:555–64. doi: 10.1007/s003350010107. [DOI] [PubMed] [Google Scholar]
- 28.Marks MJ, Stitzel JA, Collins AC. Genetic influences on nicotine responses. Pharmacol Biochem Behav. 1989;33:667–78. doi: 10.1016/0091-3057(89)90406-1. [DOI] [PubMed] [Google Scholar]
- 29.Miner LL, Collins AC. Strain comparison of nicotine-induced seizure sensitivity and nicotinic receptors. Pharmacol Biochem Behav. 1989;33:469–75. doi: 10.1016/0091-3057(89)90532-7. [DOI] [PubMed] [Google Scholar]
- 30.Marks MJ, Campbell SM, Romm E, Collins AC. Genotype influences the development of tolerance to nicotine in the mouse. J Pharmacol Exp Ther. 1991;259:392–402. [PubMed] [Google Scholar]
- 31.Butt CM, King NM, Hutton SR, Collins AC, Stitzel JA. Modulation of nicotine but not ethanol preference by the mouse Chrna4 A529T polymorphism. Behav Neurosci. 2005;119:26–37. doi: 10.1037/0735-7044.119.1.26. [DOI] [PubMed] [Google Scholar]
- 32.Adriani W, Macri S, Pacifici R, Laviola G. Restricted daily access to water and voluntary nicotine oral consumption in mice: methodological issues and individual differences. Behav Brain Res. 2002;134:21–30. doi: 10.1016/s0166-4328(01)00448-x. [DOI] [PubMed] [Google Scholar]
- 33.Nesil T, Kanit L, Collins AC, Pogun S. Individual differences in oral nicotine intake in rats. Neuropharmacology. 2011;61:189–201. doi: 10.1016/j.neuropharm.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kota D, Martin BR, Damaj MI. Age-dependent differences in nicotine reward and withdrawal in female mice. Psychopharmacology (Berl) 2008;198:201–10. doi: 10.1007/s00213-008-1117-8. [DOI] [PubMed] [Google Scholar]
- 35.Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. J Pharmacol Exp Ther. 2007;322:399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- 36.Shram MJ, Le AD. Adolescent male Wistar rats are more responsive than adult rats to the conditioned rewarding effects of intravenously administered nicotine in the place conditioning procedure. Behav Brain Res. 2010;206:240–4. doi: 10.1016/j.bbr.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 37.Shram MJ, Funk D, Li Z, Le AD. Nicotine self-administration, extinction responding and reinstatement in adolescent and adult male rats: evidence against a biological vulnerability to nicotine addiction during adolescence. Neuropsychopharmacology. 2008;33:739–48. doi: 10.1038/sj.npp.1301454. [DOI] [PubMed] [Google Scholar]
- 38.Nesil T, Yararbas G, Mola G, Kanit L, Pogun S. Previous chronic exposure eliminates the conditioning effect of nicotine in rats. Brain Res Bull. 2011;85:339–45. doi: 10.1016/j.brainresbull.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 39.Pauly JR. Gender differences in tobacco smoking dynamics and the neuropharmacological actions of nicotine. Front Biosci. 2008;13:505–16. doi: 10.2741/2696. [DOI] [PubMed] [Google Scholar]
- 40.Perkins KA. Nicotine discrimination in men and women. Pharmacol Biochem Behav. 1999;64:295–9. doi: 10.1016/s0091-3057(99)00085-4. [DOI] [PubMed] [Google Scholar]
- 41.Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine Tob Res. 2008;10:1245–50. doi: 10.1080/14622200802097506. [DOI] [PubMed] [Google Scholar]
- 42.Schnoll RA, Patterson F, Lerman C. Treating tobacco dependence in women. J Womens Health (Larchmt ) 2007;16:1211–8. doi: 10.1089/jwh.2006.0281. [DOI] [PubMed] [Google Scholar]
- 43.Maehler R, Dadmarz M, Vogel WH. Determinants of the voluntary consumption of nicotine by rats. Neuropsychobiology. 2000;41:200–4. doi: 10.1159/000026660. [DOI] [PubMed] [Google Scholar]
- 44.Li XC, Karadsheh MS, Jenkins PM, Brooks JC, Drapeau JA, Shah MS, Lautner MA, Stitzel JA. Chromosomal loci that influence oral nicotine consumption in C57BL/6J x C3H/HeJ F2 intercross mice. Genes Brain Behav. 2007;6:401–10. doi: 10.1111/j.1601-183X.2006.00266.x. [DOI] [PubMed] [Google Scholar]
- 45.Klein LC, Stine MM, Vandenbergh DJ, Whetzel CA, Kamens HM. Sex differences in voluntary oral nicotine consumption by adolescent mice: a dose-response experiment. Pharmacol Biochem Behav. 2004;78:13–25. doi: 10.1016/j.pbb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Klein LC, Stine MM, Vandenbergh DJ, Whetzel CA, Kamens HM. Sex differences in voluntary oral nicotine consumption by adolescent mice: a dose-response experiment. Pharmacol Biochem Behav. 2004;78:13–25. doi: 10.1016/j.pbb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 47.Abe K, Suzuki T. Prevalence of some symptoms in adolescence and maturity: social phobias, anxiety symptoms, episodic illusions and idea of reference. Psychopathology. 1986;19:200–5. doi: 10.1159/000284448. [DOI] [PubMed] [Google Scholar]
- 48.Lynn DA, Brown GR. The ontogeny of anxiety-like behavior in rats from adolescence to adulthood. Dev Psychobiol. 2010;52:731–9. doi: 10.1002/dev.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cruz AP, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav. 1994;49:171–6. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- 50.Holmes A, Rodgers RJ. Influence of spatial and temporal manipulations on the anxiolytic efficacy of chlordiazepoxide in mice previously exposed to the elevated plus-maze. Neurosci Biobehav Rev. 1999;23:971–80. doi: 10.1016/s0149-7634(99)00030-5. [DOI] [PubMed] [Google Scholar]
- 51.Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology (Berl) 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.