Abstract
The Immunodeficiency, Centromeric instability, Facial anomalies (ICF) syndrome is an autosomal recessive disease presenting with immunodeficiency secondary to hypo- or agammaglobulinemia, developmental delay, and facial anomalies. Centromeric instability is the cytogenetic hallmark of the disorder which results from targeted chromosomal rearrangements related to a genomic methylation defect. We describe a patient carrying a homozygous mutation of the ZBTB24 gene, which has been recently shown to be responsible for ICF syndrome type 2. Our patient presented with intellectual disability, multiple café-au-lait spots, and a large cerebral arachnoidal cyst. Although laboratory signs of impaired immune function, such as reduced serum IgM were detected, our patient did not present clinical manifestations of immunodeficiency. Brain malformations have not been reported so far in ICF syndrome and it can be speculated that ZBTB24 mutations may alter cerebral development. Nevertheless, we cannot rule out that the presence of the cerebral cyst in the patient is coincidental.
In summary, our patient illustrates that clinical evidence of immunodeficiency is not a universal feature of ICF2 syndrome type 2 and suggests that brain malformations may be present in other ICF cases.
Keywords: Immunodeficiency, Centromeric instability, Facial anomalies syndrome, ICF syndrome, ZBTB24
INTRODUCTION
Immunodeficiency, centromeric instability, facial anomalies (ICF) syndrome is an autosomal recessive condition presenting with recurrent and, often times severe infections secondary to immunodeficiency with hypo- or agammaglobulinemia and normal number of B and T lymphocytes. Negative selection breakdown and peripheral B cell maturation blockage contribute to agammaglobulinemia in ICF syndrome [Blanco-Betancourt et al., 2004]. The facial anomalies, such as hypertelorism, a broad flat nasal bridge, epicanthal folds, low-set ears, and macroglossia, are rather non-specific. Developmental delay and intellectual disability are often seen in affected patients [Ehrlich et al., 2006]. Hematological diseases and malignancies including macrophage activation syndrome, myelodysplastic syndrome, Hodgkin lymphoma, adrenocortical adenoma, and angiosarcoma have been reported in some affected patients [Andre et al., 2004; Schuetz et al., 2007; Hagleitner et al., 2008; van den Brand et al., 2011]. Life expectancy of ICF patients is significantly reduced because of severe immunodeficiency leading to recurrent, severe and often fatal respiratory and gastrointestinal infections. In these severely affected cases multiple intravenous infusions of immunoglobulins are often needed [De Ravel et al., 2001].
Centromeric instability is the cytogenetic hallmark of the disease and results from DNA hypomethylation, which ultimately leads to the characteristic chromosomal rearrangements and multiradiate structures formed by aggregation of chromosomes 1, 9, and 16 at the position of their centromeres [Tiepolo et al., 1979]. About 60% of ICF syndrome patients carry mutations in the DNA methyltransferase 3B gene (DNMT3B) (ICF1, OMIM #242860) [Hansen et al., 1999; Xu et al., 1999]. In most but not all remaining patients, mutations in the zinc-finger- and BTB-domain-containing 24 gene (ZBTB24) have been more recently discovered (ICF2, OMIM #614069) [de Greef et al., 2011]. Although the number of patients reported is still limited, patients with ICF2 appear to be clinically not distinguishable from ICF1 patients [Chouery et al., 2011; de Greef et al., 2011].
Here, we describe a patient carrying a homozygous missense mutation in the ZBTB24 gene with nonspecific facial dysmorphisms, multiple café-au-lait spots, intellectual disability, a large cerebral arachnoidal cyst, and no clinical manifestations of immunodeficiency.
CLINICAL REPORT
The patient was first evaluated at the age of 8 years because of language delay and learning disability. He was born at term of gestation and multiple cafè-au-lait spots were noted since birth. His gross motor development was delayed as he walked independently at the age of 20 months. He said his first words at 12 months of age but his language was delayed thereafter. He also had learning difficulties at school. He had no history of recurrent and/or severe infections and was never admitted to the hospital. The patient was the fourth child of healthy nonconsanguineous parents originating from the same village in Morocco. Few family members died prematurely for unknown reasons and a first cousin of the index case had developmental delay by report.
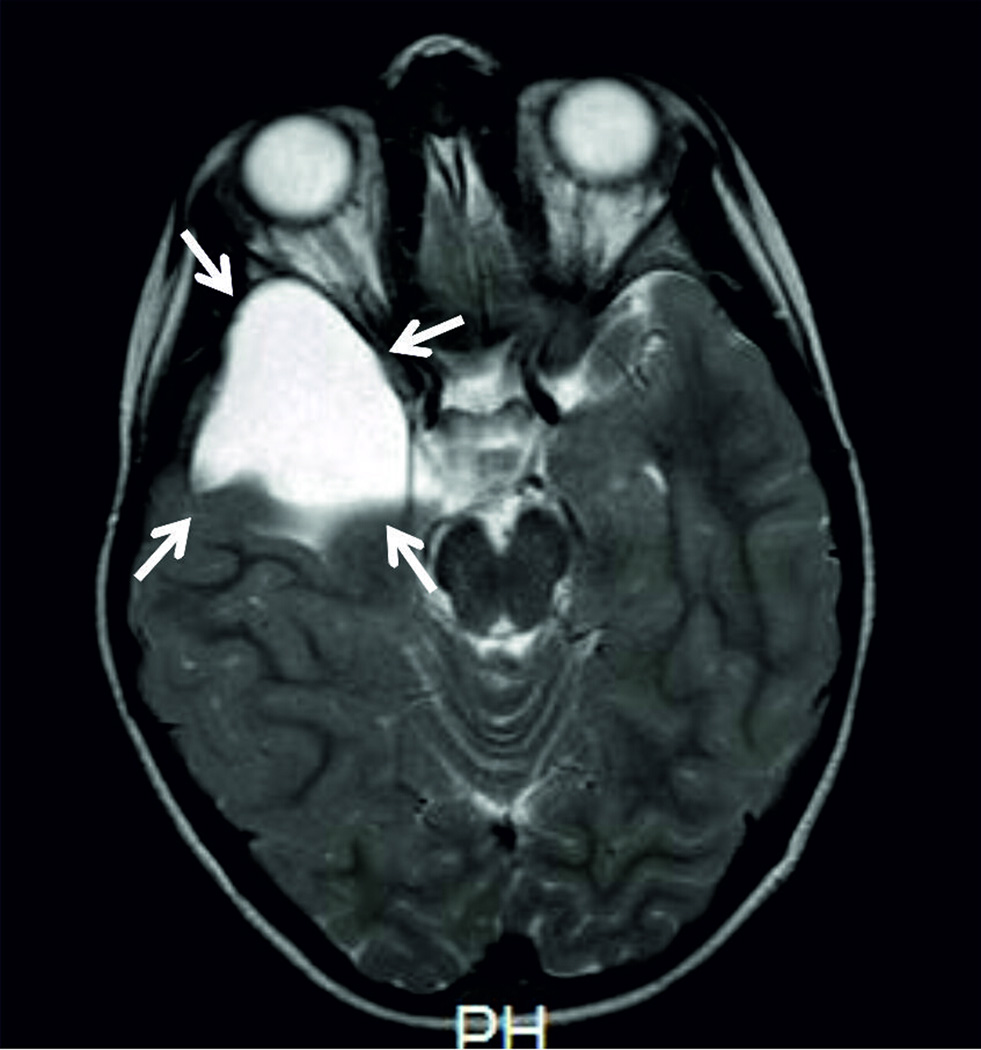
On physical examination at the age of 8 years, his weight was 25.5 Kg (50th–75th centile), his height 130 cm (50th–75th centile), and his head circumference was 53 cm (between mean and +2 SD). He had mild dysmorphic features including broad and depressed nasal bridge and multiple cafè-au-lait spots. Upon Leiter-R, scale measuring nonverbal intelligence [Roid, 1997], he was found to have an Intelligence Quotient (IQ) of 65. Audiometric testing was normal and ophthalmological evaluation did not reveal any abnormality. The brain MRI (1.5 T machine) revealed a large arachnoid cyst, isointense to CSF, in the right temporal region, causing compression of the temporal lobe and lateral ventricle (Fig. 1)
Fig. 1.
Brain MRI (axial TSE, T2w) showing a right large arachnoidal cyst (indicated by the arrows) isointense to CSF, which displaces posteriorly the temporal lobe, causing scalloping of the sphenoid wing.
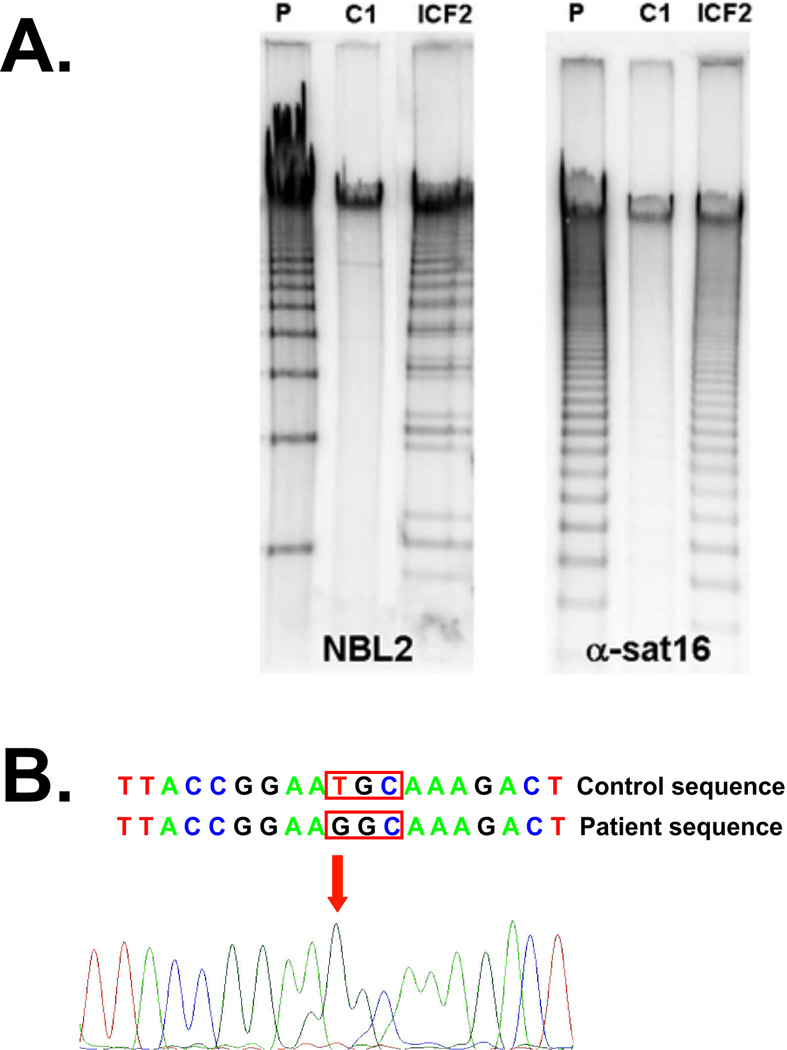
Standard chromosome analysis showed in at least 50% of cells (total of 60 cells examined) the association and duplications of the long/short arms of chromosomes 1 and 16 exhibiting a typical multibranching appearance, that leads to the suspicion of ICF syndrome. Next, we performed a southern blot experiment to analyze the methylation levels of NBL2 (hypomethylated in both ICF1 and ICF2) and α-satellite (hypomethylated in ICF2 and not in ICF1). Both NBL2 and α-satellite repeats were found to be hypomethylated (Fig. 2A), suggesting that the patient does not harbor a defect in DNMT3B gene and is affected by ICF2.
Fig. 2.
A. Southern blot analysis of the NBL2 and α-satellite was performed in the present patient (P), in his father (C1), and in a confirmed ICF2 patient (ICF2) with a known nonsense mutation in ZBTB24 gene (p.R320X). DNA samples (4 µg) were digested overnight with the restriction enzyme EcoR52I (NBL2) or HhaI (α-satellite), separated by linear gel electrophoresis on a 0.8% agarose gel and transferred on a nitrocellulose membrane, which was hybridized with probes targeting the NBL2 or the α-satellite repeat on chromosome 16. B. Sanger sequencing of coding exons of ZBTB24 revealed a homozygous missense mutation (c.1222T>G, p.408C>G).
Therefore, sequencing of the coding regions of ZBTB24 was performed and the patient was found to carry a homozygous missense mutation in the ZBTB24 gene (c.1222T>G), affecting the conserved cysteine residue of the zinc finger domain at the amino acid position 408 (p.408C>G). Both parents were found to be heterozygous for this mutation. Notably, this mutation was previously reported in two other patients with ICF2 [de Greef et al., 2011].
Although there was no evidence from the history and from physical examination of immunodeficiency, given the results of the chromosome analysis and molecular studies, a thorough immunological screening was performed. Serum levels of IgA, and IgG, and IgG subclasses were all within the normal ranges, while IgM levels were found to be reduced (Table I). Peripheral lymphocyte subsets, CD3+, T helper (CD4+), cytotoxic T cells (CD8+), B lymphocytes (CD22+), natural killer (CD16+) were also within the normal ranges (Table I).
TABLE I.
Laboratory findings in the presented patient (age 8 years).
| Patient | Control range | |
|---|---|---|
| IgG (mg/dl) | 10.1 | 6.73–17.34 |
| IgM (mg/dl) | 0.19 | 0.47–3.11 |
| IgA (mg/dl) | 1.47 | 0.41–3.68 |
| IgG1 (mg/dl) | 7.05 | 4.00–10.8 |
| IgG2 (mg/dl) | 1.30 | 0.85–4.10 |
| IgG3 (mg/dl) | 1.77 | 0.13–1.42 |
| IgG4 (mg/dl) | 0.03 | 0.00–1.89 |
| White blood cells (×103/µL) | 6.70 | 4.50–13.50 |
| % lymphocytes | 39.00 | 20.0–51.0 |
| % granulocytes | 51.00 | 40.0–75.0 |
| CD22+ (cells/mm3) | 360.0 | 200.0–1300.0 |
| CD16+ (cells/mm3) | 104.0 | 200.0–400.0 |
| CD3+ (cells/mm3) | 1846.0 | 1000.0–3900.0 |
| CD4+ (cells/mm3) | 854.0 | 560.0–2700.0 |
| CD8+ (cells/mm3) | 728.0 | 330.0–1400.0 |
DISCUSSION
We describe a 8-year-old Moroccan boy affected by ICF2 and presenting with a very mild phenotype including slightly delayed gross motor development, mild cognitive impairment, and non-distinctive dysmorphic facial features. Our patient did not show clinical manifestations of immunodeficiency. Interestingly, the ZBTB24 mutation found in our patient was previously detected in two other patients with classic features of ICF, including immunodeficiency due to hypogammaglobulinemia [de Greef et al., 2011].
In a large survey of 45 ICF syndrome patients, the age at diagnosis ranged from 5 weeks of life to 16 years, and 89% of cases were found to have hypo- or agammaglobulinemia, recognized in early childhood [Hagleitner et al., 2008]. Infections were a prominent clinical feature for the great majority of patients and 40.5% of them died from severe respiratory infections, sepsis, or failure to thrive in an age range comprised between 0.5 to 42 years. In this large series only 1/45 patients did not presented with immunodeficiency [Hagleitner et al., 2008]. Therefore, our patient supports the previous rare observation [Hagleitner et al., 2008] that immunodeficiency is not a universal feature of ICF. Given that immunodeficiency is the clinical feature that often prompts the diagnosis of ICF, we suspect that several patients with ICF syndrome without immunodeficiency might remain undiagnosed.
The current recommendation for investigation of intellectual disability is to perform array CGH analysis [Miller et al., 2010]. It is important to note that the diagnosis in our patient was strongly suggested by standard chromosome analysis and it would have likely been missed by array techniques.
A brain MRI, performed as an investigation of the intellectual disability, revealed a large right temporal arachnoid cyst (Fig. 1). Brain malformations have so far not been reported in ICF syndrome, although most affected patients are usually not investigated by MRI. Whether the association of this abnormality and ICF is fortuitous or is a feature of the underlying syndrome remains to be confirmed by additional reports. ZBTB24 belongs to a large family of transcriptional factors and is ubiquitously expressed, with the highest expression levels in naïve B cells. Interestingly, besides the well-known effect on immune cells, hypomethylation of the related DNMT3B gene results in changes in expression of genes that are critical in neurogenesis [Jin et al., 2008; Martins-Taylor et al., 2012]. Therefore, we speculate that ZBTB24 mutations may result in abnormal cerebral development and although this is the first case of brain malformation in ICF syndrome, it could represent an underestimated complication in children with this disease.
ACKNOWLEDGMENTS
We are grateful to prof. G. Andria for critical review of the manuscript. This research was partly supported by funding from the National Institutes of Health/National Institute of Allergy and Infectious Disease (R21 AI090135).
REFERENCES
- Andre N, Roquelaure B, Caillez M, Chrestian M, Moncla A, Blanco-Betancourt C, Schiff C. Macrophage activation syndrome mimicking life-threatening infection in a patient with variable immunodeficiency, centromeric instability, and facial anomalies. Pediatrics. 2004;114:1127. doi: 10.1542/peds.2004-1271. [DOI] [PubMed] [Google Scholar]
- Blanco-Betancourt CE, Moncla A, Milili M, Jiang YL, Viegas-Pequignot EM, Roquelaure B, Thuret I, Schiff C. Defective B-cell-negative selection and terminal differentiation in the ICF syndrome. Blood. 2004;103:2683–2690. doi: 10.1182/blood-2003-08-2632. [DOI] [PubMed] [Google Scholar]
- Chouery E, Abou-Ghoch J, Corbani S, El Ali N, Korban R, Salem N, Castro C, Klayme S, Azoury-Abou Rjeily M, Khoury-Matar R, Debo G, Germanos-Haddad M, Delague V, Lefranc G, Megarbane A. A novel deletion in ZBTB24 in a Lebanese family with immunodeficiency, centromeric instability, and facial anomalies syndrome type 2. Clin Genet. 2011 doi: 10.1111/j.1399-0004.2011.01783.x. [DOI] [PubMed] [Google Scholar]
- de Greef JC, Wang J, Balog J, den Dunnen JT, Frants RR, Straasheijm KR, Aytekin C, van der Burg M, Duprez L, Ferster A, Gennery AR, Gimelli G, Reisli I, Schuetz C, Schulz A, Smeets DF, Sznajer Y, Wijmenga C, van Eggermond MC, van Ostaijen-Ten Dam MM, Lankester AC, van Tol MJ, van den Elsen PJ, Weemaes CM, van der Maarel SM. Mutations in ZBTB24 are associated with immunodeficiency, centromeric instability, and facial anomalies syndrome type 2. Am J Hum Genet. 2011;88:796–804. doi: 10.1016/j.ajhg.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ravel TJ, Deckers E, Alliet PL, Petit P, Fryns JP. The ICF syndrome: new case and update. Genet Couns. 2001;12:379–385. [PubMed] [Google Scholar]
- Ehrlich M, Jackson K, Weemaes C. Immunodeficiency, centromeric region instability, facial anomalies syndrome (ICF) Orphanet J Rare Dis. 2006;1:2. doi: 10.1186/1750-1172-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagleitner MM, Lankester A, Maraschio P, Hulten M, Fryns JP, Schuetz C, Gimelli G, Davies EG, Gennery A, Belohradsky BH, de Groot R, Gerritsen EJ, Mattina T, Howard PJ, Fasth A, Reisli I, Furthner D, Slatter MA, Cant AJ, Cazzola G, van Dijken PJ, van Deuren M, de Greef JC, van der Maarel SM, Weemaes CM. Clinical spectrum of immunodeficiency, centromeric instability and facial dysmorphism (ICF syndrome) J Med Genet. 2008;45:93–99. doi: 10.1136/jmg.2007.053397. [DOI] [PubMed] [Google Scholar]
- Hansen RS, Wijmenga C, Luo P, Stanek AM, Canfield TK, Weemaes CM, Gartler SM. The DNMT3B DNA methyltransferase gene is mutated in the ICF immunodeficiency syndrome. Proc Natl Acad Sci U S A. 1999;96:14412–14417. doi: 10.1073/pnas.96.25.14412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin B, Tao Q, Peng J, Soo HM, Wu W, Ying J, Fields CR, Delmas AL, Liu X, Qiu J, Robertson KD. DNA methyltransferase 3B (DNMT3B) mutations in ICF syndrome lead to altered epigenetic modifications and aberrant expression of genes regulating development, neurogenesis and immune function. Hum Mol Genet. 2008;17:690–709. doi: 10.1093/hmg/ddm341. [DOI] [PubMed] [Google Scholar]
- Martins-Taylor K, Schroeder DI, Lasalle JM, Lalande M, Xu RH. Role of DNMT3B in the regulation of early neural and neural crest specifiers. Epigenetics. 2012;7 doi: 10.4161/epi.7.1.18750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DT, Adam MP, Aradhya S, Biesecker LG, Brothman AR, Carter NP, Church DM, Crolla JA, Eichler EE, Epstein CJ, Faucett WA, Feuk L, Friedman JM, Hamosh A, Jackson L, Kaminsky EB, Kok K, Krantz ID, Kuhn RM, Lee C, Ostell JM, Rosenberg C, Scherer SW, Spinner NB, Stavropoulos DJ, Tepperberg JH, Thorland EC, Vermeesch JR, Waggoner DJ, Watson MS, Martin CL, Ledbetter DH. Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am J Hum Genet. 2010;86:749–764. doi: 10.1016/j.ajhg.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roid GH, Miller LJ. International Performance Scale-Revised: Examiners Manual. Wood Dale, IL: Stoelting Co.; 1997. [Google Scholar]
- Schuetz C, Barbi G, Barth TF, Hoenig M, Schulz A, Moeller P, Smeets D, de Greef JC, van der Maarel SM, Vogel W, Debatin KM, Friedrich W. ICF syndrome: high variability of the chromosomal phenotype and association with classical Hodgkin lymphoma. Am J Med Genet A. 2007;143A:2052–2057. doi: 10.1002/ajmg.a.31885. [DOI] [PubMed] [Google Scholar]
- Tiepolo L, Maraschio P, Gimelli G, Cuoco C, Gargani GF, Romano C. Multibranched chromosomes 1, 9, and 16 in a patient with combined IgA and IgE deficiency. Hum Genet. 1979;51:127–137. doi: 10.1007/BF00287166. [DOI] [PubMed] [Google Scholar]
- van den Brand M, Flucke UE, Bult P, Weemaes CM, van Deuren M. Angiosarcoma in a patient with immunodeficiency, centromeric region instability, facial anomalies (ICF) syndrome. Am J Med Genet A. 2011;155A:622–625. doi: 10.1002/ajmg.a.33831. [DOI] [PubMed] [Google Scholar]
- Xu GL, Bestor TH, Bourc'his D, Hsieh CL, Tommerup N, Bugge M, Hulten M, Qu X, Russo JJ, Viegas-Pequignot E. Chromosome instability and immunodeficiency syndrome caused by mutations in a DNA methyltransferase gene. Nature. 1999;402:187–191. doi: 10.1038/46052. [DOI] [PubMed] [Google Scholar]