Abstract
This experiment examined the effects on memory of interactions of cycloheximide dose and training foot shock intensity. Mice received injections of cycloheximide (120 mg/kg, s.c.) or saline 30 min prior to inhibitory avoidance training with shock intensities of 100, 150, 250 or 300 µA (1 sec duration). Memory was tested 48 hr later. The saline control mice showed increasing memory latencies as a function of shock intensity. The ability of cycloheximide to impair memory increased as the training shock intensity increased. In a second experiment, mice were trained with a 200 µA (1 sec duration) shock and received injections of saline or cycloheximide at one of several doses (30, 60 or 120 mg/kg). Under these training conditions, cycloheximide enhanced memory in an inverted-U dose-response manner. These findings are consistent with prior findings suggesting that protein synthesis inhibitors act on memory by altering modulators of memory formation as a secondary consequence of the inhibition of protein synthesis rather than by interfering with training-initiated synthesis of proteins required for memory formation.
Keywords: cycloheximide, protein synthesis, memory enhancement, long-term memory, consolidation, inhibitory avoidance
1. Introduction
In most contemporary theories of memory formation, protein synthesis initiated by training is viewed as a required mechanism for neural plasticity underlying the establishment of new long-lasting memories [1–12]. These theories are in large part based on evidence showing that treatments that interfere with protein synthesis impair the formation of long-lasting memories.
While there is general agreement that these drugs impair memory, there are many reasons to question whether the amnesia produced by the drugs truly reflects a requirement of training-initiated protein synthesis for memory formation [13–20]. Evidence against the requirement for protein synthesis to make new memories includes a wide range of demonstrations. For example, many pharmacological treatments including lidocaine, strychnine, corticosterone, caffeine, nicotine, and drugs that act at noradrenergic receptors block the amnesia produced by the protein synthesis inhibitors anisomycin and cycloheximide [21–25]. Importantly, these drugs do not reverse the amnesia by modifying the extent of inhibition of protein synthesis produced by anisomycin or cycloheximide.
Inhibitory avoidance tasks have been commonly used to investigate the effects of protein synthesis inhibitors on memory. If training-related protein synthesis is necessary for memory formation, then amnesia produced by protein synthesis inhibitors should be independent of the shock level used during training. However, the evidence does not support this prediction. Avoidance training with increased footshock levels protects against the amnesia in both mice [27,28] and rats [26]. In rats, the failure to observe amnesia after injections of the protein synthesis inhibitor, cycloheximide, is clearly dissociated from possible effects of illness or of an interaction of footshock with the extent of protein synthesis inhibition [26]. The failure of protein synthesis inhibitors, even at inhibition levels >90%, to impair memory formation in mice and rats trained with high footshock levels is inconsistent with the view that protein synthesis is necessary for memory. However, the findings appear to be similar to those seen with modulators of memory, where interactions of modulators with training-related release of hormones and neurotransmitters are common and non-linear [29–33].
In studies of modulation of memory formation, memory is reliably enhanced by treatments like epinephrine and glucose and by drugs that promote noradrenergic and cholinergic functions [32–36]. The effects of these and many memory-enhancing treatments follow inverted-U dose-response functions in which intermediate doses enhance memory but high doses impair memory [19,29,32,33,37]. Of particular relevance here, the peak of the inverted-U function varies with the endogenous training-related arousal, as shown clearly for a diverse set of treatments that include amygdala stimulation, ACTH, epinephrine and glucose [38–42]. The nature of this interaction is that a dose that enhances memory at low arousal levels impairs memory at high arousal levels. For example, a dose of epinephrine or glucose that enhances inhibitory avoidance memory trained with low footshock also increases circulating plasma epinephrine levels or blood glucose levels to those seen with greater arousal. Moreover, the same dose administered after a higher footshock impairs memory and increases circulating epinephrine levels beyond those reached endogenously in response to the higher footshock [43,44]. Similar results are seen when examining brain norepinephrine release in response to training and epinephrine levels [39,40]. Epinephrine injected after low footshock resulted in moderate norepinephrine release and memory enhancement. Epinephrine injected after a high footshock results in both high levels of release of norepinephrine release and memory impairment.
Recent findings show that direct intra-amygdala and intra-hippocampal injections of anisomycin impair memory while also producing extraordinarily large increases in release of neurotransmitters, specifically biogenic amines and acetylcholine, at the site of injection [23–25]. These findings, together with those showing that footshock intensity can interact with the amnestic effects of cycloheximide, suggest that protein synthesis inhibitors may act on memory not by directly affecting molecular mechanisms intrinsic to memory consolidation but by altering the release of modulators of memory, presumably a neural response to the deleterious actions of protein synthesis inhibitors on cell processes.
The present experiment examined cycloheximide effects on memory as an interaction of dose and footshock intensity used during training in order to test contrary predictions for the effects of protein synthesis inhibitors on memory according to views of necessity of new protein synthesis for memory formation vs. modulation of memory by these drugs. According to a protein synthesis-dependent perspective, the inhibitor should be amnestic at all shock levels, from low shock levels to high shock levels; the latter is already shown to result in memory formation that is not sensitive to inhibition of protein synthesis [26]. According to an interpretation that protein synthesis inhibitors result in modulation of memory, the efficacy of the inhibitor in impairing memory should increase across lower footshock levels as seen with a wide range of treatments that do not block protein synthesis. In addition, it may be possible to demonstrate enhancement and impairment of memory with a single dose of a protein synthesis inhibitor depending on the intensity of the footshock used during learning. These were the results described previously for cycloheximide effects on memory [45] and are replicated and extended here.
2. Material and methods
2.1 Animals
Male ICR mice (approximately 2 months old; Jackson Laboratories) were used in this experiment. Mice were housed singly with free access to food and water. The mice were maintained on a 7:00 a.m. ON / 7:00 p.m. OFF light-dark cycle. All procedures were approved by the University of Illinois IACUC; the University of Illinois animal facilities are AAALAC-approved.
2.2 Inhibitory avoidance task
Inhibitory avoidance training was conducted in a trough-shaped alley (61 cm long, 15 cm high, 15 cm ceiling width, and 4 cm floor width) that was divided into start and shock compartments by a sliding door. On the training trial, each mouse was placed in the well-lit start compartment (20.3 cm long). After 15 s, the hurdle was lowered and the mouse was allowed access to the dark shock compartment (40.7 cm long). After the animal entered the dark compartment, the door was closed and a footshock (100 – 300 µA in different conditions, 1 sec duration; Lafayette shocker Model 82400, Lafayette, IN) was delivered through the metal plates that comprised the floor and wails of the trough. Upon termination of the footshock, mice remained in the shock compartment for 30 sec before being removed and returned to their home cages. Retention was assessed 48 h later by placing the animals in the start-box and measuring the latency to cross into the dark compartment. The Ns (6–11) are identified for each group in Figures 1 and 2. The results described in Experiments 1 and 2, shown in Figures 1 and 2, came from two different cohorts of mice with differences in memory scores that precluded comparisons across cohorts. The latency data were therefore evaluated statistically only within cohorts. In Experiment 1, the 150 µA intensity did not produce reliable learning and memory and therefore was lower than optimal for demonstrating enhancement of memory. The 250 µA intensity produced relatively robust learning and therefore might decrease the parametric space within which to demonstrate enhancement. Therefore, to maximize the opportunity to observe memory enhancement in Experiment 2 (Figure 2), we used a shock level of 200 µA, i.e. intermediate to the values in Experiment 1. With the difference in cohort groups, the memory scores obtained with the 200 µA intensity in Experiment 2 were somewhat lower than those seen with the 150 µA intensity in Experiment 1. However, the lower mean memory scores were accompanied by lower variance, resulting in low but statistically reliable levels of learning and memory desired to test possible enhancement of memory with cycloheximide.
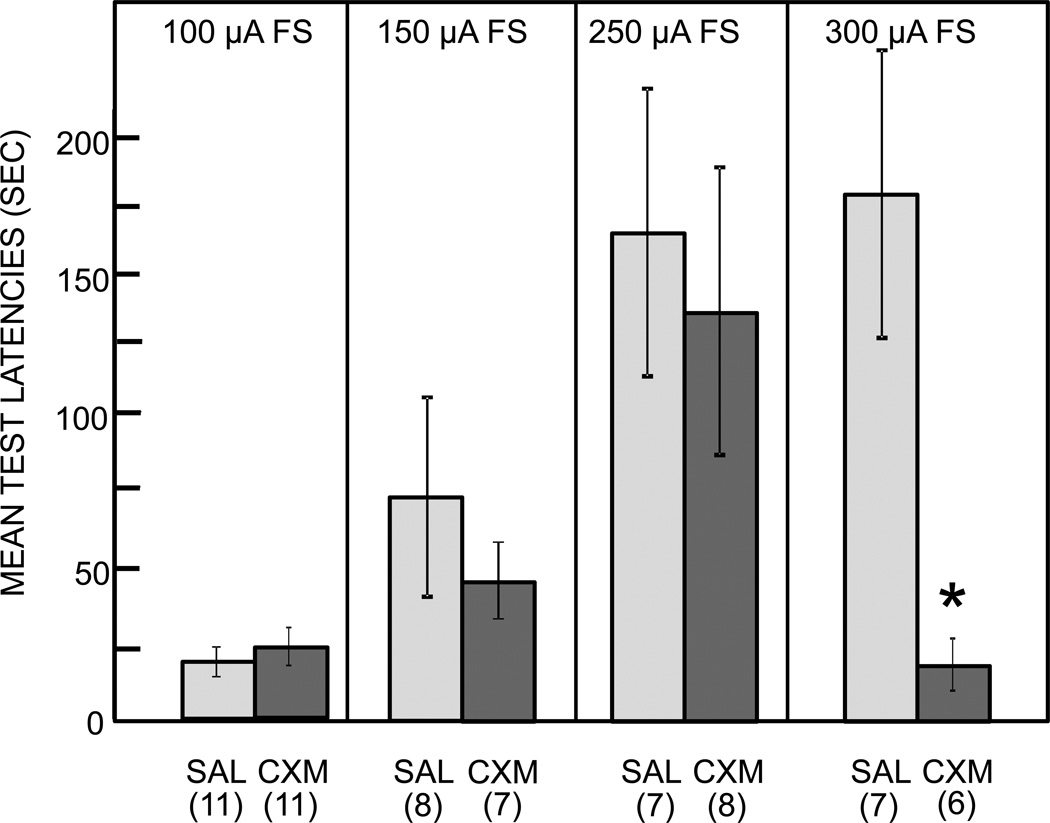
Fig 1.
Emergence of cycloheximide (CXM)-induced memory impairments with increases in footshock (FS) intensity used during inhibitory avoidance training. Mice received an injection of cycloheximide (120 mg/kg) or saline (SAL) prior to training with one of four shock levels. Memory was assessed 48 hr after training. Cycloheximide impaired memory on in those mice trained with the highest shock intensity tested. Ns for each group shown below bars.
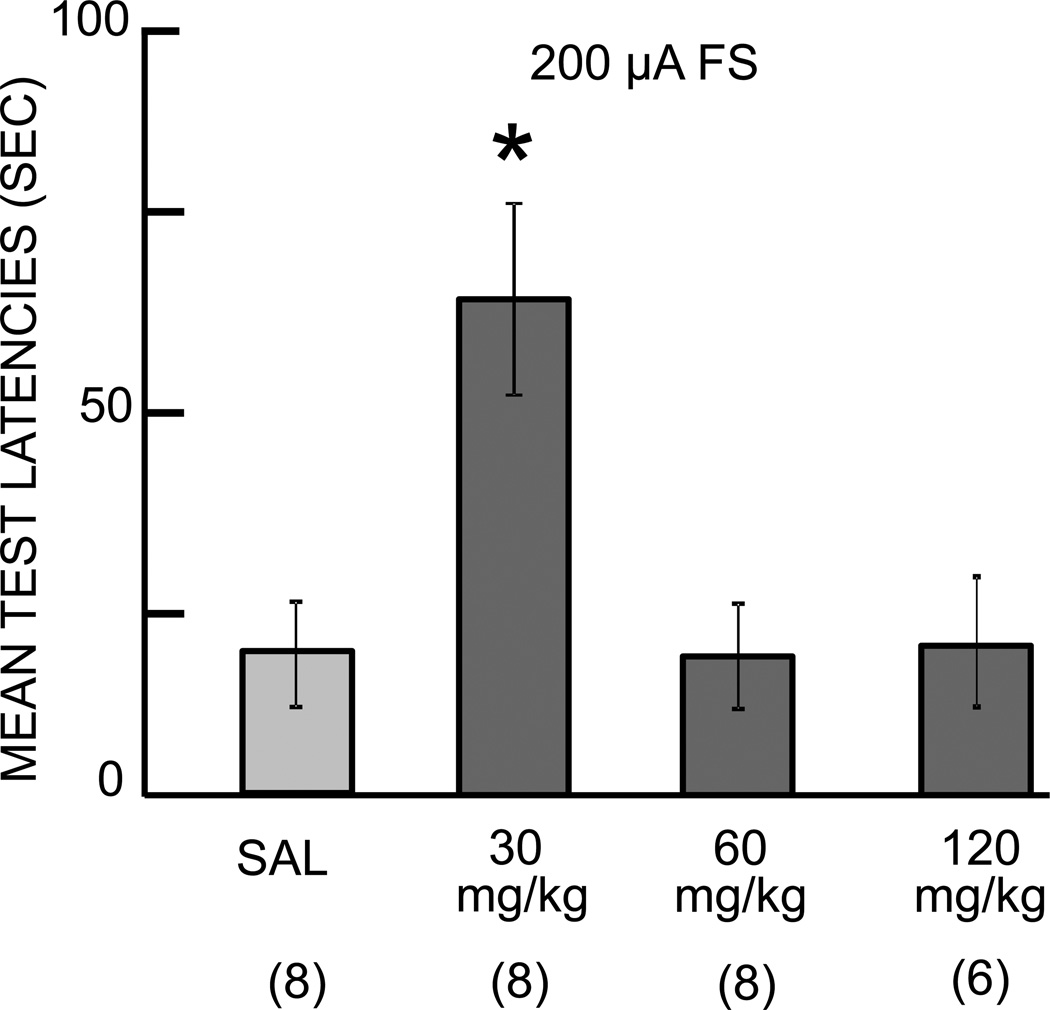
Fig. 2.
Cycloheximide (CXM) enhancement of memory. Using a low footshock (FS) intensity sufficient to produce modest increases in avoidance latencies, cycloheximide enhanced 48-hr memory at the 30 mg/kg dose as compared to the latencies seen in the saline (SAL) control group. The dose-response curve had an inverted-U form, as seen for many modulators of memory formation. Ns for each group are shown below bars. *P<0.05 vs. SAL in 300 µA FS condition.
2.3. Drug injections
Cycloheximide (Sigma Chemical Company) was dissolved in saline and administered IP at concentrations of 0 (saline controls), 30, 60, or 120 mg/kg. Cycloheximide injections were administered 30 min prior to training. The 120 mg/kg dose is commonly used to study amnesia in mice [e.g.: 46–49]. Note that amnestic cycloheximide doses are much lower in rats (1–3 mg/kg: [26,50,51]) than in mice, consistent with a similar difference in LD50s for rats and mice. Cycloheximide doses of 120–150 mg/kg result in approximately 95% inhibition of brain protein synthesis as measured 30–60 min after injection [21,52–54]; the dose of 30 mg/kg produces approximately 80% inhibition of brain protein synthesis [21].
2.5. Data Analysis
Inhibitory avoidance latencies were analyzed using ANOVAs followed by planned post-hoc t-tests.
3. Results
3.1. Cycloheximide impairment of memory was evident only at higher footshock levels
In the first behavioral experiment, mice were trained on an inhibitory avoidance task using one of four relatively low footshock intensities. Significantly increased latencies on the test trial, 48 hr after training, vs. the training trial were taken as evidence of memory. As expected, avoidance latencies on the memory test trials increased as a function of shock intensity (F3,37=7.23, P<0.001). In the saline control groups, latencies on the test trial were significantly higher than those of the training trial in mice trained with either of the highest two shock intensities, 250 and 300 µA (Ps < 0.05 and 0.02, respectively). Latencies did not significantly increase after training with the two low intensities, 100 and 150 µA (Ps < 0.2); observations of mouse behavior during training indicated that the 100 µA shock was below the flinch threshold and the 150 µA elicited clear flinch responses in only about 50% of the mice. Therefore, these two shock levels did not support acquisition of the avoidance response.
The main results of this experiment indicate that the ability of cycloheximide to impair later memory emerged as the training shock intensity increased. All mice in this experiment received a standard amnestic dose, 120 mg/kg, of cycloheximide 30 min prior to training with different footshock intensities. A significant interaction of drug × shock level was evident (F3,57=2.87, P<0.05). As shown in Figure 1, mice that received cycloheximide before training with the highest shock intensity (300 µA) exhibited latencies on the memory test 48 hr after training that were significantly lower than those of the saline controls (P<0.02). In contrast, the same dose of cycloheximide failed to produce significant memory impairments when injected prior to training with any of the three lower footshock intensities tested here, including the 250 µA shock intensity that produced significant memory in controls. Note that cycloheximide had no effect on memory latencies in mice trained with the lowest shock intensities, which did not support learning, indicating that the drug itself did not alter latencies on the memory tests.
3.2. Enhancement of memory with cycloheximide injections
A second behavioral experiment examined a dose-response curve for cycloheximide effects on memory. The basis for this experiment was that other memory-modulating treatments enhance memory after training with low footshock intensities but impair memory after high footshock intensities. The question here was whether a low dose of cycloheximide would enhance memory when administered near the time of training with a low shock level. The mice received cycloheximide injections at 30, 60, or 120 mg/kg prior to training with a 200 µA shock; this shock intensity was chosen to produce significant memory in controls but to produce sufficiently low latencies on 48-hr tests to permit observation of possible enhancement of memory. There was a significant effect of cycloheximide on latencies on the memory test trials (F3,27=6.99, P<0.001). In saline controls, this shock level resulted in latencies on the test trial that were significantly higher than those at training (P<0.05; Figure 2). Injections of the lowest dose of cycloheximide tested, 30 mg/kg, resulted in latencies on the test trial that were significantly higher than those seen in the saline control group. Mice receiving either of the two higher doses of cycloheximide had latencies on the test trial that were comparable to those of the saline group, i.e., the higher doses neither enhanced nor impaired memory under these conditions, resulting in an inverted-U dose-response curve for cycloheximide enhancement of memory. The failure to see impaired memory after the 120 mg/kg dose of cycloheximide here is consistent with the results shown after low footshock training in Figure 1.
4. Discussion
The major findings of these experiments are that the amnestic effectiveness of cycloheximide increases as a function of the shock intensity used during training and that, at low doses combined with low shock intensities, cycloheximide enhances later memory. Note that the cycloheximide doses used here all substantially inhibit brain protein synthesis measured 30–60 min after injection, from ~80% inhibition at the 30 mg/kg dose to ~95% inhibition at doses near 120 mg/kg [21,52–54].
4.1. Cycloheximide impairments emerge with high stress or arousal
The finding that cycloheximide-induced memory impairments are not seen after training with low footshock levels but are seen, at the same dose, after training with higher footshock intensities is analogous to results reported for modulation of memory by ACTH, epinephrine, glucose, norepinephrine, and other treatments [38–44]. Thus, these findings are consistent with those obtained with modulators of memory formation.
Past experiments examining the effects of protein synthesis inhibitors on memory have often examined the effects on memory for tasks that involve relatively high footshock intensities or other high-stress conditions, e.g. habituation to a loud conspecific distress cry, taste aversion training, fear extinction [e.g.: 55–63]. There are also reports of impairment of extinction in appetitive tasks [50,64], and it will be important to assess the nature of neurobiological bases of arousal in these conditions.
It is important to note that the interaction of cycloheximide and footshock level on memory reported here is opposite that reported before [26]. In that report, higher shock intensities overrode the effects of protein synthesis inhibition on memory. Those shock levels were substantially higher (600 µA and higher in rats) than those used in the present experiment and were apparently sufficient to block the ability of modulators of memory to impair memory. Similarly, the amnestic effects of cycloheximide decreased as the training shock intensity increased in mice [27]. Together, the findings suggest that cycloheximide impairment of memory is evident only at moderate shock levels but not at high or low shock levels. These are results expected from an interpretation based on memory modulation as the intervening biological mechanism but are not expected based on interpretations using inhibition of memory-consolidating protein synthesis as the intervening mechanism. The latter view explains neither the absence of amnesia nor the enhancement of memory in the presence of inhibition of protein synthesis inhibitors as a function of training intensity and dose. The findings regarding the interaction of the effects of protein synthesis inhibitors with shock level, presented here and reported earlier [26,27], and the many studies showing that a broad range of drugs rescue amnesia after protein synthesis inhibition suggest a need for alternative interpretations of impairments of memory and neural plasticity by inhibitors of protein synthesis. This is a point made in both early [13] and recent [14–20] reviews.
4.2. Cycloheximide injections enhance memory in an inverted-U manner
As is the case for the interactions of cycloheximide impairments of memory with changing footshock levels, the ability of cycloheximide to enhance memory after training with relatively low shock intensity is also consistent with a cycloheximide effect mediated by modulators of memory. There was an inverted-U dose-response curve for cycloheximide enhancement of memory, analogous to the inverted-U dose-response curves seen for epinephrine, glucose and many other memory-enhancing treatments [30–33,37]. Past evidence shows that direct injections of a different protein synthesis inhibitor, anisomycin, substantially increases release of biogenic amines and acetylcholine [23–25]. Moreover, the memory impairments can be blocked by direct brain injections of drugs that block the non-physiological release of norepinephrine, by drugs that block norepinephrine receptors, and can be mimicked by injections of high doses of norepinephrine itself [23]. These findings, together with the present results, suggest that cycloheximide effects on memory interact with training-related stress and enhance memory in an inverted-U dose-response manner, suggesting that these effects may be mediated by altering neurotransmitter and hormonal modulators of memory.
4.3. Memory-modulating effects of interference with molecular targets associated with memory formation
Generalizing from these findings, it may also be the case that a memory-modulation perspective is appropriate too for treatments that are more selective in their action than gross inhibition of protein synthesis. For example, activation of the transcription factor CREB is often considered to be important for activating training-related protein synthesis important for memory [65–68]. However, other studies indicate that CREB is not necessary for memory or neural plasticity. In mice, conditional-knockout of CREB in mice does not prevent learning, memory, or synaptic plasticity [69,70]. Another experiment shows that CREB antisense treatments interfere with norepinephrine responses to training as well as impairing memory, suggesting that treatments targeting CREB may also act by altering modulators of memory [71]. Analogous to results showing that high footshock blocks cycloheximide-induced memory impairments are recent findings seen in aged rats [72]. Together, the findings appear to be consistent with a description of CREB activation as a modulator of memory [73] rather than as a necessary and sufficient component of the formation of new memories.
Protein Kinase A (PKA) is also proposed as a molecular trigger for memory that engages particular protein synthesis programs [74]. However, PKA inhibitors are also effective or ineffective based on strength of training, in this case manipulated by altering the inter-trial interval [75] and, for long-term potentiation, by manipulating the frequency of tetanizing stimulation at the time of induction [76]. Like other studies that examine interactions of drug effects with training parameters, these findings seem more readily interpreted as effects mediated by modulators of memory rather than effects demonstrating a necessary involvement of PKA and downstream effects on synthesis of proteins necessary for memory consolidation per se.
The present results contribute to a large set of findings supporting the conclusion that impairment of memory with protein synthesis inhibitors is not a function of impairment of training-related proteins needed for memory formation but instead a by-product of actions of protein synthesis inhibitors and perhaps interference with the functions of neuromodulators [cf.: 13,14,16,20,77]. Indeed, it would be surprising if it were not the case that the insult of severe inhibition of systemic and central protein synthesis failed to alter neural function in significant ways, including altering modes of neural communication and neurophysiological activity important for memory formation [78,79]. It is important, however, to constrain this conclusion to the consequences of inhibition of protein synthesis and not to a possible involvement of protein synthesis in memory formation. The main conclusion is that the results obtained with protein synthesis inhibitors, perhaps both general and specific inhibitors, may not provide clear information regarding whether training-related engagement of transcription mechanisms is necessary for memory formation.
Highlights.
Cycloheximide (CXM) impairs memory more as footshock intensity increases.
A low dose of CXM enhances memory in an inverted-U dose-response manner.
CXM enhancement and impairment are both seen during inhibition of protein synthesis.
These results are similar to those obtained with many memory-enhancing treatments.
The results do not support a need for new protein-synthesis in producing long-term memory.
Acknowledgments
The authors thank Dr. Donna L. Korol and Ms. Jamie R. Richards for their excellent technical assistance with these experiments.
Funding for this research was provided by grants from NIDA R21 DA024129, NIA R01 AG07648, and NSF IOS 08-43175 and 10-52464.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflicts of interest regarding the material in this report.
References
- 1.Davis HP, Squire LR. Protein synthesis and memory: A review. Psychol Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- 2.Schafe GE, Nadel NV, Sullivan GM, Harris A, LeDoux JE. Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis, PKA, MAP kinase. Learn Mem. 1999;6:97–110. [PMC free article] [PubMed] [Google Scholar]
- 3.Kandel ER. The molecular biology of memory storage: A dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 4.Kandel ER. The molecular biology of memory storage: A dialog between genes and synapses. Biosci Rep. 2002;21:565–611. doi: 10.1023/a:1014775008533. [DOI] [PubMed] [Google Scholar]
- 5.Dudai Y. Molecular basis of long-term memories: A question of persistence. Curr Opin Neurobiol. 2002;12:211–216. doi: 10.1016/s0959-4388(02)00305-7. [DOI] [PubMed] [Google Scholar]
- 6.Nader K. Memory traces unbound. Trends Neurosci. 2003;26:65–72. doi: 10.1016/S0166-2236(02)00042-5. [DOI] [PubMed] [Google Scholar]
- 7.Kelleher RJ, 3rd, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004;44:59–73. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Alberini CM. The role of protein synthesis during the labile phases of memory: Revisiting the skepticism. Neurobiol Learn Mem. 2008;89:234–246. doi: 10.1016/j.nlm.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costa-Mattioli M, Sonenberg N. Translational control of gene expression: a molecular switch for memory storage. Progr Brain Res. 2008;169:81–95. doi: 10.1016/S0079-6123(07)00005-2. [DOI] [PubMed] [Google Scholar]
- 10.Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang SH, Morris RG. Hippocampal-neocortical interactions in memory formation, consolidation, and reconsolidation. Ann Rev Psychol. 2010;61:49–79. doi: 10.1146/annurev.psych.093008.100523. [DOI] [PubMed] [Google Scholar]
- 12.Redondo RL, Morris RG. Making memories last: the synaptic tagging and capture hypothesis. Nat Rev Neurosci. 2011;12:17–30. doi: 10.1038/nrn2963. [DOI] [PubMed] [Google Scholar]
- 13.Martinez JL, Jr, Jensen RA, McGaugh JL. Attenuation of experimentally-induced amnesia. Progr Neurobiol. 1981;16:155–186. doi: 10.1016/0301-0082(81)90011-3. [DOI] [PubMed] [Google Scholar]
- 14.Radulovic J, Tronson NC. Protein synthesis inhibitors, gene superinduction and memory: too little or too much protein? Neurobiol Learn Mem. 2008;89:212–218. doi: 10.1016/j.nlm.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Routtenberg A. The substrate for long-lasting memory: If not protein synthesis, then what? Neurobiol Learn Mem. 2008;89:225–233. doi: 10.1016/j.nlm.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Routtenberg A, Rekart JL. Post-translational protein modification as the substrate for long-lasting memory. Trends Neurosci. 2005;28:12–19. doi: 10.1016/j.tins.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Rudy JW. Is there a baby in the bathwater? Maybe: Some methodological issues for the de novo protein synthesis hypothesis. Neurobiol Learn Mem. 2008;89:219–224. doi: 10.1016/j.nlm.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Rudy JW, Biedenkapp JC, Moineau J, Bolding K. Anisomycin and the reconsolidation hypothesis. Learn Mem. 2006;13:1–3. doi: 10.1101/lm.157806. [DOI] [PubMed] [Google Scholar]
- 19.Gold PE. The many faces of amnesia. Learn Mem. 2006;13:506–514. doi: 10.1101/lm.277406. [DOI] [PubMed] [Google Scholar]
- 20.Gold PE. Protein synthesis inhibition: Memory formation vs. amnesia. Neurobiol Learn Mem. 2008;89:201–211. doi: 10.1016/j.nlm.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sershen H, Reith MEA, Lajtha A. On the interaction between nicotine and cycloheximide. Brain Res. 1982;251:183–185. doi: 10.1016/0006-8993(82)91290-2. [DOI] [PubMed] [Google Scholar]
- 22.Flood JF, Jarvik ME, Bennett EL, Orme AE, Rosenzweig MR. The effects of stimulants, depressants, and protein synthesis inhibition on retention. Behav Biol. 1977;20:168–183. doi: 10.1016/s0091-6773(77)90734-9. [DOI] [PubMed] [Google Scholar]
- 23.Canal CE, Chang Q, Gold PE. Amnesia produced by release of neurotransmitters after intra-amygdala injections of a protein synthesis inhibitor. Proc Natl Acad Sci USA. 2007;104:12500–12505. doi: 10.1073/pnas.0705195104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi Z, Gold PE. Intrahippocampal infusions of anisomycin produce amnesia: contributions of increased release of norepinephrine, dopamine and acetylcholine. Learn Mem. 2009;16:308–314. doi: 10.1101/lm.1333409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadowski RN, Canal CE, Gold PE. Lidocaine attenuates anisomycin-induced amnesia and release of norepinephrine in the amygdala. Neurobiol Learn Mem. 2011;96:136–142. doi: 10.1016/j.nlm.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Díaz-Trujillo A, Contreras J, Medina AC, Silveyra-Leon GA, Antaramian A, Quírarte GL, Prado-Alcalá RA. Enhanced inhibitory avoidance learning prevents the long-term memory-impairing effects of cycloheximide, a protein synthesis inhibitor. Neurobiol Learn Mem. 2009;91:310–314. doi: 10.1016/j.nlm.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Flood JF, Bennett EL, Rosenzweig MR, Orne AE. Influence of training strength on amnesia induced by pretraining injections of cycloheximide. Physiol Behav. 1972;9:589–600. doi: 10.1016/0031-9384(72)90017-0. [DOI] [PubMed] [Google Scholar]
- 28.Flood JF, Bennett EL, Orme AE, Rosenzweig MR. Effects of protein synthesis inhibition on memory for active avoidance training. Physiol Behav. 1975;14:177–184. doi: 10.1016/0031-9384(75)90163-8. [DOI] [PubMed] [Google Scholar]
- 29.Gold PE, McGaugh JL. A single-trace, two-process view of memory storage processes. In: Deutsch D, Deutsch JA, editors. Short Term Memory. New York: Academic Press; 1975. pp. 355–390. [Google Scholar]
- 30.Conrad CD, Lupien SJ, McEwen BS. Support for a bimodal role for type II adrenal steroid receptors in spatial memory. Neurobiol Learn Mem. 1999;72:39–46. doi: 10.1006/nlme.1998.3898. [DOI] [PubMed] [Google Scholar]
- 31.Diamond DM, Campbell AM, Park CR, Halonen J, Zoladz PR. The temporal dynamics model of emotional memory processing: a synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson law. Neural Plasticity. 2007:60803. doi: 10.1155/2007/60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korol DL, Gold PE. Modulation of learning and memory by adrenal and ovarian hormones. In: Kesner RP, Martinez JL, editors. Neurobiology of Learning and Memory. New York: Elsevier Science; 2007. pp. 243–268. [Google Scholar]
- 33.Gold PE. Memory enhancing drugs. In: Byrne J, editor. Concise Learning and Memory: the Editor’s Selection. Oxford: Elsevier Science; 2009. pp. 605–626. [Google Scholar]
- 34.Power AE, Vazdarjanova A, McGaugh JL. Muscarinic cholinergic influences in memory consolidation. Neurobiol Learn Mem. 2003;80:178–193. doi: 10.1016/s1074-7427(03)00086-8. [DOI] [PubMed] [Google Scholar]
- 35.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 36.McIntyre CK, Power AE, Roozendaal B, McGaugh JL. Role of the basolateral amygdala in memory consolidation. Ann NY Acad Sci. 2003;985:273–293. doi: 10.1111/j.1749-6632.2003.tb07088.x. [DOI] [PubMed] [Google Scholar]
- 37.Koob GF. Arousal, stress, and inverted U-shaped curves: Implications for cognitive functions. In: Lister RG, Weingartner HJ, editors. Perspectives on cognitive neuroscience. New York: Oxford Press; 1991. pp. 300–313. [Google Scholar]
- 38.Gold PE, Hankins L, Edwards RM, Chester J, McGaugh JL. Memory interference and facilitation with posttrial amygdala stimulation: Effect on memory varies with footshock level. Brain Res. 1975;86:509–513. doi: 10.1016/0006-8993(75)90905-1. [DOI] [PubMed] [Google Scholar]
- 39.Gold PE, van Buskirk RB. Enhancement and impairment of memory processes with posttrial injections of adrenocorticotrophic hormone. Behav Biol. 1976;16:387–400. doi: 10.1016/s0091-6773(76)91539-x. [DOI] [PubMed] [Google Scholar]
- 40.Gold PE, van Buskirk RB. Posttraining brain norepinephrine concentrations: correlation with retention performance of avoidance training and with peripheral epinephrine modulation of memory processing. Behav Biol. 1978;23:509–520. doi: 10.1016/s0091-6773(78)91614-0. [DOI] [PubMed] [Google Scholar]
- 41.Gold PE, van Buskirk RB. Effects of α-and β-adrenergic receptor antagonists on post-trial epinephrine modulation of memory: Relationship to post-training brain norepinephrine concentrations. Behav Biol. 1978;24:168–184. doi: 10.1016/s0091-6773(78)93045-6. [DOI] [PubMed] [Google Scholar]
- 42.Gold PE, Vogt J, Hall JL. Posttraining glucose effects on memory: Behavioral and pharmacological characteristics. Behav Neur Biol. 1986;46:145–155. doi: 10.1016/s0163-1047(86)90626-6. [DOI] [PubMed] [Google Scholar]
- 43.McCarty R, Gold PE. Plasma catecholamines: Effects of footshock level and hormonal modulators of memory storage. Horm Behav. 1981;15:168–182. doi: 10.1016/0018-506x(81)90026-x. [DOI] [PubMed] [Google Scholar]
- 44.Hall JL, Gold PE. The effects of training, epinephrine, and glucose injections on plasma glucose levels in rats. Behav Neur Biol. 1986;46:156–176. doi: 10.1016/s0163-1047(86)90640-0. [DOI] [PubMed] [Google Scholar]
- 45.Hall ME. Enhancement of learning by cycloheximide and DDC: A function of response strength. Behav Biol. 1977;21:41–51. doi: 10.1016/s0091-6773(77)92247-7. [DOI] [PubMed] [Google Scholar]
- 46.Tucker AR, Gibbs ME, Stanes MD. Cycloheximide and passive avoidance memory in mice: time-response, dose-response and short-term memory. Pharmacol Biochem Behav. 1976;4:441–446. doi: 10.1016/0091-3057(76)90061-7. [DOI] [PubMed] [Google Scholar]
- 47.Davis HP, Spanis CW, Squire LR. Inhibition of cerebral protein synthesis: performance at different times after passive avoidance training. Pharmacol Biochem Behav. 1976;4:13–16. doi: 10.1016/0091-3057(76)90168-4. [DOI] [PubMed] [Google Scholar]
- 48.Davis HP, Rosenzweig MR, Grove EA, Bennett EL. Investigation of the reported protective effect of cycloheximide on memory. Pharmacol Biochem Behav. 1984;20:405–413. doi: 10.1016/0091-3057(84)90279-x. [DOI] [PubMed] [Google Scholar]
- 49.Nabeshima T, Noda Y, Itoh K, Kameyama T. Role of cholinergic and GABAergic neuronal systems in cycloheximide-induced amnesia in mice. Pharmacol Biochem Behav. 1988;31:405–409. doi: 10.1016/0091-3057(88)90366-8. [DOI] [PubMed] [Google Scholar]
- 50.Cohen J, Gotthard GH. Extinction of appetitive learning is disrupted by cycloheximide and propranolol in the sand maze in rats. Neurobiol Learn Mem. 2011;95:484–490. doi: 10.1016/j.nlm.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 51.McGauran AM, Moore JB, Madsen D, Barry D, O’Dea S, Mahon BP, Commins S. A possible role for protein synthesis, extracellular signal-regulated kinase, and brain-derived neurotrophic factor in long-term spatial memory retention in the water maze. Behav Neurosci. 2008;122:805–815. doi: 10.1037/0735-7044.122.4.805. [DOI] [PubMed] [Google Scholar]
- 52.Geller A, Robustelli F, Barondes SH, Cohen HD, Jarvik ME. Impaired performance by post-trial injections of cycloheximide in a passive avoidance task. Psychopharmacol. 1969;14:371–376. doi: 10.1007/BF00403577. [DOI] [PubMed] [Google Scholar]
- 53.Quinton EE, Kramarcy NR. Memory impairment correlates closely with cycloheximide dose and degree of inhibition of protein synthesis. Brain Res. 1977;131:184–190. doi: 10.1016/0006-8993(77)90041-5. [DOI] [PubMed] [Google Scholar]
- 54.Rainbow TC, Hoffman PL, Flexner LB. Studies of memory: A reevaluation in mice of the effects of inhibitors on the rate of synthesis of cerebral proteins as related to amnesia. Pharmacol Biochem Behav. 1980;12:79–84. doi: 10.1016/0091-3057(80)90419-0. [DOI] [PubMed] [Google Scholar]
- 55.Lamprecht R, Dudai Y. Transient expression of c-Fos in rat amygdala during training is required for encoding conditioned taste aversion memory. Learn Mem. 1996;3:31–41. doi: 10.1101/lm.3.1.31. [DOI] [PubMed] [Google Scholar]
- 56.Peterson GM, Squire LR. Cerebral protein synthesis and long-term habituation. Behav Biol. 1977;21:443–449. doi: 10.1016/s0091-6773(77)90296-6. [DOI] [PubMed] [Google Scholar]
- 57.Houpt TA, Berlin R. Rapid, labile, and protein-synthesis independent short-term memory in conditioned taste aversion. Learn Mem. 1999;6:37–46. [PMC free article] [PubMed] [Google Scholar]
- 58.Vianna MR, Igaz LM, Coitinho AS, Medina JH, Izquierdo I. Memory extinction requires gene expression in rat hippocampus. Neurobiol Learn Memory. 2003;79:199–203. doi: 10.1016/s1074-7427(03)00003-0. (2003) [DOI] [PubMed] [Google Scholar]
- 59.Duvarci S, Nader K. Characterization of fear memory reconsolidation. J Neurosci. 2004;24:9269–9275. doi: 10.1523/JNEUROSCI.2971-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Orsini CA, Maren S. Glutamate receptors in the medial geniculate nucleus are necessary for expression and extinction of conditioned fear in rats. Neurobiol Learn Mem. 2009;92:581–589. doi: 10.1016/j.nlm.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hardt O, Wang SH, Nader K. Storage or retrieval deficit: the yin and yang of amnesia. Learn Mem. 2009;16:224–230. doi: 10.1101/lm.1267409. [DOI] [PubMed] [Google Scholar]
- 62.Inda MC, Muravieva EV, Alberini CM. Memory retrieval and the passage of time: from reconsolidation and strengthening to extinction. J Neurosci. 2011;31:1635–1643. doi: 10.1523/JNEUROSCI.4736-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martinez-Moreno A, Rodriguez-Duran LF, Escobar ML. Late protein synthesisdependent phases in CTA long-term memory: BDNF requirement. Front Behav Neurosci. 2011;5:61. doi: 10.3389/fnbeh.2011.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gotthard GH, Knoppel AB. Cycloheximide produces amnesia for extinction and reconsolidation in an appetitive odor discrimination task in rats. Neurobiol Learn Mem. 2010;93:127–131. doi: 10.1016/j.nlm.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 65.Dash PK, Hochner B, Kandel ER. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature. 1990;345:718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
- 66.Silva AJ, Paylor R, Wehner JM, Tonegawa S. Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- 67.Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schütz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 68.Sekeres MJ, Neve RI, Frankland PW, Josselyn SA. Dorsal hippocampal CREB is both necessary and sufficient for spatial memory. Learn Mem. 2010;17:280–283. doi: 10.1101/lm.1785510. [DOI] [PubMed] [Google Scholar]
- 69.Gass P, Wolfer DP, Balschun D, Rudolph D, Frey U, Lipp HP, Schutz G. Deficits in memory tasks of mice with CREB mutations depend on gene dosage. Learn Mem. 1998;5:274–288. [PMC free article] [PubMed] [Google Scholar]
- 70.Balschun D, Wolfer DP, Gass P, Mantamadiotis T, Welzl H, Schütz G, Frey JU, Lipp HP. Does cAMP response element-binding protein have a pivotal role in hippocampal synaptic plasticity and hippocampus-dependent memory? J Neurosci. 2003;23:6304–6314. doi: 10.1523/JNEUROSCI.23-15-06304.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Canal CE, Chang Q, Gold PE. Intra-amygdala injections of CREB antisense impair inhibitory avoidance memory: Role of norepinephrine and acetylcholine. Learn Mem. 2008;15:677–686. doi: 10.1101/lm.904308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morris KA, Gold PE. Age-related impairments in memory and in CREB and pCREB expression in hippocampus and amygdala following inhibitory avoidance training. 2012 doi: 10.1016/j.mad.2012.03.004. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yin JC, Del Vecchio M, Zhou H, Tully T. CREB as a memory modulator: induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell. 1995;81:107–115. doi: 10.1016/0092-8674(95)90375-5. [DOI] [PubMed] [Google Scholar]
- 74.Abel T, Nguyen PV. Regulation of hippocampus dependent memory by cyclic AMP-dependent protein kinase. Progr Brain Res. 2008;169:97–115. doi: 10.1016/S0079-6123(07)00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sutton MA, Bagnall MW, Sharma SK, Shobe J, Carew TJ. Intermediate-term memory for site-specific sensitization in aplysia is maintained by persistent activation of protein kinase C. J. Neurosci. 2004;24:3600–3609. doi: 10.1523/JNEUROSCI.1134-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim M, Huang T, Abel T, Blackwell KT. Temporal sensitivity of protein kinase A activation in late-phase long term potentiation. PLoS Comp Biol. 2010;6:e1000691. doi: 10.1371/journal.pcbi.1000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prado-Alcalá RA, Salado-Castillo R, Quiroz C, Garín-Aguilar ME, Díaz-Trujillo A, Rivas- Arancibia S, Quírarte GL. Enhanced learning protects the brain against the effects of amnesia treatments. In: Bermudez-Rattoni F, editor. Neural Plasticity and Memory: From Genes to Brain Imaging. London: Taylor and Francis Ltd; 2007. pp. 175–191. [PubMed] [Google Scholar]
- 78.Kleim JA, Bruneau R, Calder K, Pocock D, VandenBerg PM, MacDonald E, Monfils MH, Sutherland RJ, Nader K. Functional organization of adult motor cortex is dependent upon continued protein synthesis. Neuron. 2003;40:167–176. doi: 10.1016/s0896-6273(03)00592-0. [DOI] [PubMed] [Google Scholar]
- 79.Sharma AV, Nargang FE, Dickson CT. Neurosilence: Profound suppression of neural activity following intracerebral administration of the protein synthesis inhibitor anisomycin. J. Neurosci. 2012;32:2377–2387. doi: 10.1523/JNEUROSCI.3543-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]