Abstract
Previous studies have shown that housing mice with toys and running wheels increases adult hippocampal neurogenesis and enhances performance on the water maze. However, the relative contribution of running versus enrichment to the neurogenic and pro-cognitive effects is not clear. Recently, it was demonstrated that enrichment devoid of running wheels does not significantly enhance adult hippocampal neurogenesis in female C57BL/6J mice. However, novel toys were not rotated into the cages, and dietary enrichment was not included, so it could be argued that the environment was not enriched enough. In addition, only females were studied, and animals were group-housed, making it impossible to record individual running behavior or to determine the time spent running versus exploring the toys. Therefore, we repeated the study in singly housed male C57BL/6J mice and enhanced enrichment by rotating novel tactile, visual, dietary, auditory, and vestibular stimuli into the cages. Mice were housed for 32 days in one of 4 groups: running-only, enrichment-only, running plus enrichment, and standard cage. The first 10 days BrdU (bromodeoxyuridine) was administered to label dividing cells. The last 5 days mice were tested on the water maze, and then euthanized to measure number of BrdU cells co-labeled with NeuN (neuronal nuclear marker) in the dentate gyrus. Mice in the running-only group ran, on average, greater distances than animals in the running plus enrichment group. The combination of enrichment and running did not significantly increase hippocampal neurogenesis any more than running alone did. Animals in the running-only condition were the only group to show enhanced acquisition on water maze relative to standard cage controls. We confirm and extend the conclusion that environmental enrichment alone does not significantly increase hippocampal neurogenesis or bestow spatial learning benefits in male C57BL/6J mice, even when the modalities of enrichment are very broad.
The idea that experiences during development and as an adult can have long-lasting effects on the chemistry, morphology and physiology of the brain with consequences for behavior and psychology is well established and has led to some of the great discoveries of modern neuroscience. For example, it is now widely believed that cognitive performance in humans is enhanced and protected against decline (associated with a variety of conditions including neurodegenerative disease, inflammation, brain trauma, and normal aging) by behaving in a way that challenges the brain with new learning and exploration experiences and/or by staying aerobically fit (Nilsson et al., 1999; Arendash et al., 2004; Colcombe et al., 2004; Griesbach et al., 2004; van Praag et al., 2005; Cruise et al., 2011; Kohman et al., 2011; Voss et al., 2011). However, the relative contribution of physical exercise versus stimulation from learning and new experiences to the cognitive benefits is not well understood (Kobilo et al., 2011). Knowing how the different components of enrichment (e.g., physical exercise versus sensory exploration) influence the various domains of cognition (e.g., episodic memory, associative learning, spatial learning, executive control, long term memory, short term memory, working memory etc.) could help optimize or tailor treatments for the specific cognitive deficits presented in human subjects.
One common way to model experience-dependent plasticity in animals is to compare rodents housed in an enriched environment as compared to an environment that is deprived of sensory and motor stimuli. A plethora of studies using rats and mice has documented changes in the morphology and physiology of the brain using the environmental enrichment model (e.g., Volkmar and Greenough, 1972; Greenough et al., 1973; Greenough et al., 1978; Greenough et al., 1985; Black et al., 1987; Sirevaag et al., 1988; Black et al., 1991; Kempermann et al., 1997; Johansson and Belichenko, 2002; Kumar et al., 2012). An inevitable limitation in most of these studies is that multiple sensory, social, and physical activity factors are typically combined in the environmental enrichment treatment, making it difficult to determine which of the various enrichment factors or the interaction contributes to the observed effects on biochemistry, neurophysiology and morphology.
Previous studies have shown that housing mice either in an enriched environment that contains toys and running wheels or in an environment with only running wheels increases adult hippocampal neurogenesis and enhances performance on spatial learning tasks (Kempermann et al., 1997; van Praag et al., 1999a; van Praag et al., 1999b; Rhodes et al., 2003; Clark et al., 2008). However, the relative contribution of running versus the sensory stimulation from the toys to the increases in neurogenesis and cognition remains unclear. A study was recently conducted that for the first time attempted to separate the different components of enrichment to evaluate their effects on adult hippocampal neurogenesis (Kobilo et al., 2011). In that study, female C57BL/6J mice were housed in large cages in groups of 10. There were 4 treatment groups: 1) toys only (consisting of a constant set of toys and tunnels), 2) running wheels only (consisting of 10 running wheels), 3) toys and running wheels, and 4) empty cage without toys or running wheels. The mice housed with access to running wheels showed increased neurogenesis as compared to those without running wheel access. Neurogenesis levels of mice housed with both toys and running wheels were comparable to neurogenesis levels of mice housed with only wheels. Finally, mice housed with only toys displayed similar levels of neurogenesis as compared to those housed in an empty cage (Kobilo et al., 2011). Taken together, these data suggest that animals derived no neurogenic benefit from the addition of toys to their environment. However, one possibility is that mice habituated to the presence of the toys. Although the authors rearranged the toys spatially in the cage on a weekly basis, novel toys were never introduced and dietary enrichment was not included. In addition, the mice were group-housed, making it impossible to record individual running behavior or to determine the time spent running versus exploring the toys.
Therefore, we aimed to test the hypothesis that an enriched environment (without running wheels) can increase hippocampal neurogenesis if novel objects are continually introduced and the stimuli activate several different sensory modalities, including tactile, visual, dietary, auditory, and vestibular stimuli. We compared mice housed in a standard environment, an enriched environment (consisting of all the modalities listed above), running wheels only, or both enriched plus running wheels. Mice were singly housed so we could record time spent running and individual distance traveled to use as a covariate in the analysis of neurogenesis. Moreover, in the enriched plus running group, the cage was divided with the enrichment stimuli on one side and the running wheel on the other, with a small partition through which a mouse could cross between the two compartments. This enabled us to record where the animals spent their time by video-tracking. With this approach, we attempted to ascertain the extent to which increases in neurogenesis can be attributed to time spent running on wheels versus engaging with other sensory stimuli (e.g., toys, dietary, vestibular, etc.). We predicted that we would find the highest levels of hippocampal neurogenesis and enhanced cognitive performance in the enriched environment plus running wheel group, followed by the running wheel-only group, the enriched-environment-only group, and, finally, the group housed in the standard cage environment.
2. Experimental procedures
2.1 Animals
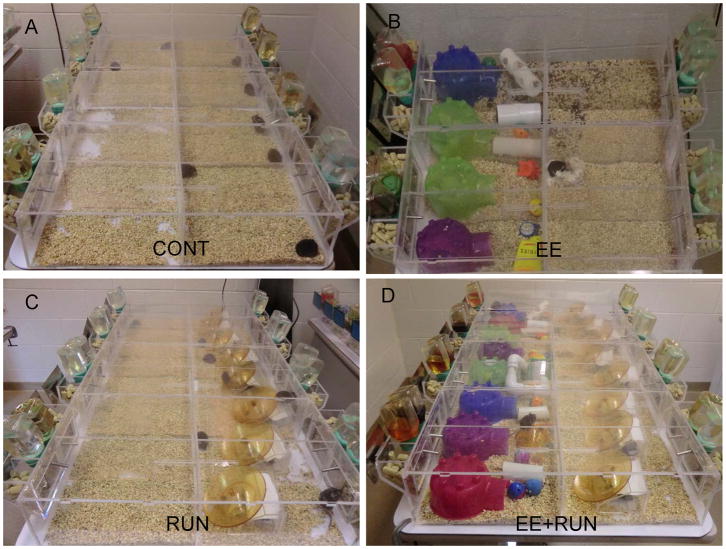
A total of 32 male C57BL/6J mice were used in this study. C57BL/6J was chosen because this strain has been widely used in studies of the effects of environmental enrichment and wheel running on adult hippocampal neurogenesis and water maze performance (van Praag et al., 1999a; van Praag et al., 2005; Clark et al., 2008). The mice were obtained at 5 weeks of age from The Jackson Laboratory (Bar Harbor, ME). Initially, they were housed 4 per cage in standard cages 29 × 19 × 13 cm (L × W × H) for 14 days. On day 15, mice (7 weeks of age) were housed individually in custom-made acrylic home cages with dimensions 67.0 cm × 18.5 cm × 16 cm (L × W × H) and clear plastic lids conducive for video tracking from above (Fig. 1). A clear plastic divider with a small entrance large enough for a mouse to crawl through divided each cage in half so that each side measured 33.5 cm × 18.5 cm × 16 cm (L × W × H). Each mouse could freely move between both sides of its cage, and duration spent on each side was recorded by continuous video tracking (Fig. 1). Animals in adjacent cages could see each other through the clear sides of the cages but could not otherwise interact.
Figure 1.
Treatment groups A) Standard Control (CONT) B) Environmental Enrichment (EE) C) Running (RUN) D) Environmental Enrichment and Running (EE+RUN).
Mice were kept in a climate-controlled environment on a 12 h light/dark cycle (lights off at 10:00 a.m.). Food and water were delivered from both sides so that time spent on one cage side was not artificially inflated by time spent seeking food or water and so the mouse was visible in all areas of the cage when viewed from above (except under EE conditions as described below). Corncob bedding (Harlan 7097) was provided at approximately 2 cm depth. All procedures were approved by the University of Illinois Institutional Animal Care and Use Committee and adhered to NIH guidelines. All measures were taken to minimize the number of mice used as well as the pain and suffering of the animals.
2.2 Treatment Groups (see Fig. 1)
2.2.1 Standard environment (CONT)
Mice were singly housed in the custom-made cages described above. Both sides of the cage were empty except for bedding, regular mouse chow, and plain tap water.
2.2.2 Environmental Enrichment (EE)
Animals were housed in the same cages as above except one side of the cage contained bedding, toys, sweet drinking solutions, and food treats. The other side was empty except for bedding, regular mouse chow, and plain tap water. Certain toys were always present on the enriched side and never rotated. They were 1 plastic igloo, 1 wooden gnaw stick, cotton nesting material, a plastic ball that contained a bell, and a handful of straw. In addition, two of the following toys were rotated into the cage every 4 days in an attempt to engage multiple sensory modalities: auditory (ticking plastic clock, squeeze toy, rattle), visual (mirror, small dome), vestibular (see-saw, smooth winding tunnel), tactile (foam ball, small plastic hedgehog animal toy, towel piece, smooth tunnel, tunnel lined with bubble wrap, tunnel lined with Velcro material, and a tunnel lined with foam) (Fig 1). In addition, every 4 days, the animals received a new drinking solution provided in standard water bottles. They included: apple juice (11.7% sugar), cranberry juice (12.5% sugar), grape juice (16.7%), Kool-Aid (1 of 4 flavors: grape, lemonade, orange, or strawberry; 10.5% sugar), and a syrup consisting of 40% sucrose solution (wt/volume) in plain tap water (Suzuki et al., 2006). Finally, a novel solid food treat was provided every 4 days. Each rotation included one of the following: 5 peanuts, 5 cashews, 1 cracker, 5 Cheerios, 2 mini-carrots, 5 chocolate chips, 1 teaspoon of peanut butter, or 10 unroasted sunflower seeds. The treat was always provided in a plastic weigh-boat placed on top of the igloo.
2.2.3 Running wheels (RUN)
One side of the cage was empty except for bedding, regular mouse chow, and plain tap, whereas the other side, in addition to bedding, food and water, contained a saucer-shaped running disc (Bio-Serv, Frenchtown, NJ, USA) tilted at an angle and attached to a Schwinn pedometer to monitor distance, speed, and cumulative duration of running. Pedometers were recorded and reset daily.
2.2.4 Environmental Enrichment with Running Wheels (EE+RUN)
One side of the cage contained toys, drinking solutions and food treats as described for the EE group, and the other cage side contained the saucer-shaped running disc as described for the RUN group.
2.3 Video Tracking
Eight video cameras mounted on the ceiling of the animal room provided continuous video feed into two separate computers running TopScan video tracking software (Clever Sys Inc, Reston, VA, USA). The video coverage allowed continuous monitoring of all 32 animals at the same time in 32 individual cages (as described above). White lights were placed under the tables holding the cages to produce diffuse light during lights on and were controlled with a timer for 12:12 L:D cycle. Red lights were placed in various positions in the room overhead to illuminate the cages during the dark phase for continuous video tracking. (Mice cannot see red light). The duration spent on each side of the cages was continuously recorded as defined by the location of the center of mass of the animals (1 mm resolution). In the EE condition (as described above), mice were video-tracked only on the empty side because of interference with tunnels and toys that could hide the mouse from view.
2.4 Experimental Design
Mice were housed under the 4 conditions (CONT, EE, RUN, or EE+RUN) for 32 days (n=8 per group). The first 10 days, mice received 50 mg/kg bromodeoxyuridine (BrdU) injections to label dividing cells. The last 5 days, mice were tested for performance on the water maze task. Testing took place during the light phase of the light/dark cycle. Animals were returned to cages immediately after testing. Hence, mice in the EE, RUN, and EE+RUN groups had continuous access to enrichment and/or running throughout the behavioral testing period. The following day after testing, animals were euthanized to measure adult hippocampal neurogenesis.
2.5 Behavioral Performance
2.5.1 Morris water maze
Mice were trained on water maze with 2 two trials per day for 5 days. A trial lasted either 60 s or until the mouse reached the platform and remained on the platform for 10 s. If a mouse did not reach the platform in 60 s, it was gently guided there by hand. Mice were placed back in their cage and allowed to rest for 30 s between trials. One hour after training on day 5, the platform was removed and mice were tested with a probe trial (60 s).
Dimensions and parameters followed Clark et al. (2008). The maze consisted of a circular tub, 100 cm in diameter and 20 cm deep. A square platform made of white plastic mesh 8.5 × 8.5 cm was placed in the middle of one quadrant and submerged 0.5 cm below the surface of the water. Sixty ml of Crayola white tempera paint was added to the water to make the water sufficiently opaque to hide the platform from sight. White was chosen to provide contrast for video tracking from above (black mouse on white background). Water temperature was maintained at 25–26 °C. Topscan (Clever Sys Inc, Reston, VA, USA) video tracking software was used to measure path length, swim speed, and duration spent in the different quadrants of the maze.
2.6 Immunohistochemistry
2.6.1 Tissue preparation
All the mice (n = 32) were anesthetized with 100 mg/kg sodium pentobarbital (i.p.) and then perfused transcardially with 4% paraformaldehyde in phosphate buffer solution (PBS; 0.287% sodium phosphate monobasic anhydrous, 1.102% sodium phosphate dibasic anhydrous, 0.9% sodium chloride in water). Brains were postfixed overnight and then transferred to 30% sucrose in PBS. Brains were sectioned using a cryostat into 40 μm thick coronal sections. Sections were placed into tissue cryoprotectant (30% ethylene glycol, 25% glycerin, 45% PBS) in 24 well plates and stored at −20 °C. Two separate one-in-six series of these sections (i.e. series of sections throughout the rostro-caudal extent of the brain with 240 μm increments separating each section, approximately nine sections) were stained in the following way.
2.6.2 BrdU-DAB
Purpose: To detect BrdU-positive (newly divided) cells in the dentate gyrus. Free floating sections were washed in tissue buffering solution (TBS; 1.3% Trizma hydrochloride, 0.19% Trizma base, 0.9% sodium chloride) and then treated with 0.6% hydrogen peroxide in TBS for 30 min. To denature DNA, sections were treated for 120 min with a solution of 50% de-ionized formamide and 2X SSC buffer, rinsed for 15 min in 2X SCC buffer, then treated with 2 M hydrochloric acid for 30 min at 37 °C, then 0.1 M boric acid in TBS (pH 8.5) for 10 min at room temperature. Sections were then treated (blocked) with a solution of 0.3% Triton-X and 3% goat serum in TBS (TBS-X plus) for 30 min, and then incubated in primary antibody against BrdU made in rat (AbD Serotec, Raleigh, NC, USA, catalog number OBT0030) at a dilution of 1:100 in TBS-X plus for 72 h at 4 °C. Sections were then washed in TBS, blocked with TBS-X plus for 30 min, and then incubated in biotinylated secondary antibody against rat made in goat (Vector, Burlingame, CA, USA, catalog number BA-9400) at 1:250 in TBS-X plus for 100 min at room temperature. Sections were then treated using the ABC system (Vector, Burlingame, CA, USA, catalog number PK-6100) and stained using a diaminobenzidine kit (Sigma, St. Louis, MO, USA, catalog number D4418).
2.6.3 Double-fluorescent label
Purpose: To determine the proportion of BrdU-positive cells in the dentate gyrus that differentiated into neurons. Sections were treated as for BrdU-DAB except a cocktail was used for the primary antibody step. Rat anti-BrdU (1:100; AbD Serotec, Raleigh, NC, USA, catalog number OBT0030) was combined with mouse anti-neuronal nuclear protein (NeuN) (1:50; Millipore, Billerica, MA, USA, catalog number MAB377) for 72 h at 4 °C. Secondary antibodies were conjugated with fluorescent markers (Cy2-green anti-mouse, and Cy3-red anti-rat; Jackson ImmunoResearch, West Grove, PA, USA, catalog numbers 115-225-166 and 112-165-167, respectively) at dilution of 1:250 and also delivered as a cocktail.
2.7 Image Analysis
2.7.1 BrdU-DAB
The entire granule layer (bilateral), represented in the 1-in-6 series, was photographed by systematically advancing the field of view of the Zeiss brightfield light microscope and taking multiple photographs, via camera interfaced to computer, under 10x (total 100x) magnification. Following Clark et al. (2011), to obtain unbiased estimates of BrdU cell numbers, total counts were multiplied by 0.85, under the assumption that 15% of the nuclei counted would intersect with the plane of the section. This was estimated based on the observation that the average size of BrdU nuclei was 6 microns, which is 15% of 40 microns, the thickness of the section. In addition, the volume of the granule layer was measured by tracing the granule layer using ImageJ software (NIH, Bethesda, MD). A comparison of the volumes between groups assumes that there were no group differences in shrinkage of tissue.
2.7.2 Double label
A Leica SP2 laser scanning confocal microscope (using a 40x oil objective, pinhole size 81.35 μm diameter) was used to determine the proportion of dentate gyrus BrdU-positive cells that differentiated into neurons (NeuN+). Dentate gyrus BrdU-positive cells were identified as either co-expressing NeuN or not. Each BrdU-positive cells in the granular layer (represented in the 1-in-6 series) was analyzed by focusing through the tissue in the z-axis to establish co-labeling with NeuN. The total number of new neurons per mouse was calculated as the number of BrdU cells multiplied by average proportion BrdU cell co-expressing NeuN for the designated group. Total number of new neurons per cubic micron was estimated as total numbers divided by volume sampled.
2.8 Statistical analysis
Data were analyzed using SAS (version 9.1) or R (version 2.7.2) statistical software. In all analyses, P < 0.05 was considered statistically significant. Average duration (hr) spent running and average distance run (km/day) were analyzed using 1-way ANOVA with treatment (RUN versus EE+RUN) entered as the factor. Average percent time spent on the EE side was analyzed by 1-way ANOVA with treatment (RUN versus EE+RUN) entered as the factor. Number of BrdU-positive cells, number of new neurons (BrdU+/NeuN+), volume of the dentate gyrus, and number of new neurons per volume, were analyzed using 2-way ANOVA with factors exercise (runner versus sedentary) and enrichment (enriched versus not). Tukey posthoc tests were used to evaluate pair-wise differences between group means. Numbers of new neurons and number of new neurons per volume in the EE+RUN and RUN groups were also analyzed by analysis of covariance, with average distance traveled as the covariate and treatment (EE+RUN versus RUN) as the factor, with the interaction also entered in the model. The proportion of BrdU-labeled cells in the granule cell layer that co-expressed NeuN was analyzed by logistic regression, where proportion (binomial response) was modeled as a linear function of treatment condition (CONT, EE, RUN, EE+RUN).
For water maze acquisition, average path length (m) and latency (sec) to reach the platform per day was analyzed by repeated measures analysis with days as the within-subjects factor and treatment (CONT, EE, RUN, EE+RUN) as the between-subjects factor. For the probe trial, duration (sec) in the target quadrant was analyzed using 1-way ANOVA with treatment (CONT, EE, RUN, EE+RUN) as the factor.
3. Results
3.1 Body mass
Average body mass of the animals at the end of the experiment was 24.5 g (± 0.28 S.E.). No differences between groups or differences from the beginning to the end of the study within individuals were detected.
3.2 Wheel Running
On average, over the 30 day period, mice in the RUN group spent 2.3 hrs/day (± 0.14) running and rotated the disc an equivalent of 6.5 km/day (± 0.49). Mice in the EE+RUN group ran 1.8 hrs/day (± 0.48), and rotated the disc an average of 5.6 km/day (± 1.50). These differences were not statistically significant (Fig. 2).
Figure 2.
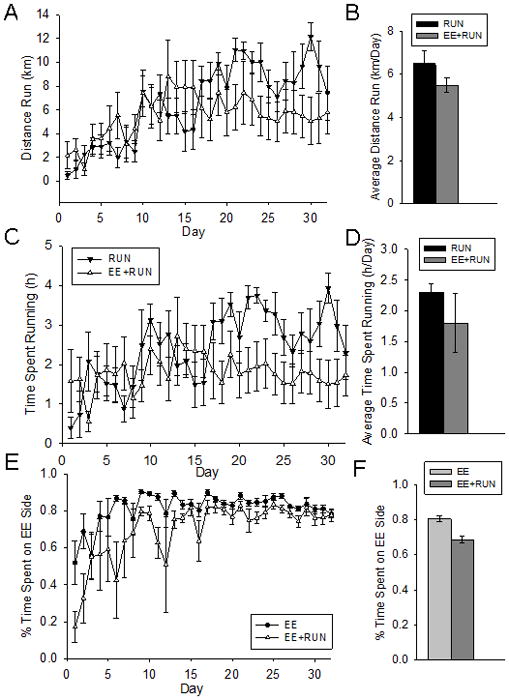
Wheel running and duration spent on the EE side. A) Average distance run on wheels (km) per day in the RUN and EE+RUN groups. B) Average distance run on wheels (km/day) collapsed across the entire 32 day period in the RUN and EE+RUN groups. C) Average time spent running on wheels (h) per day in the RUN and EE+RUN groups. D) Average time spent running on wheels (h/day) collapsed across the entire 32 day period in the RUN and EE+RUN groups. E) Average percent time spent on the EE side per day in the EE and EE+RUN groups. F) Average percent time spent on the EE side collapsed across the entire 32 day period in the EE and EE+RUN groups. Standard error bars shown.
3.3 Environmental Enrichment
Mice in the EE group spent an average of 81% (± 0.02) of their time during the 30-day period on the enriched side, whereas mice in the EE+RUN group spent an average of 68% (± 0.02) on the enriched side (F1,14=24.7, p=0.0002) (see Fig. 2E–2F). Fluid intake was similar in the EE and EE+RUN groups. On average, mice in the EE and the EE+RUN groups drank 10.9 ml (± 2.39) of apple juice, 6.08 ml (± 0.92) of cranberry juice, 8.24 ml (± 1.39) of grape juice, 8.92 ml (± 0.57) of 40% sucrose, and 10.29 ml (± 0.67) of Kool-Aid (collapsed across each flavor) per day. Mice always ate the treats provided within the 4 day rotations. This was accomplished usually within the first day. Many mice were observed moving the weigh boat to the igloo soon after it was placed in the cage.
3.4 Hippocampal Neurogenesis
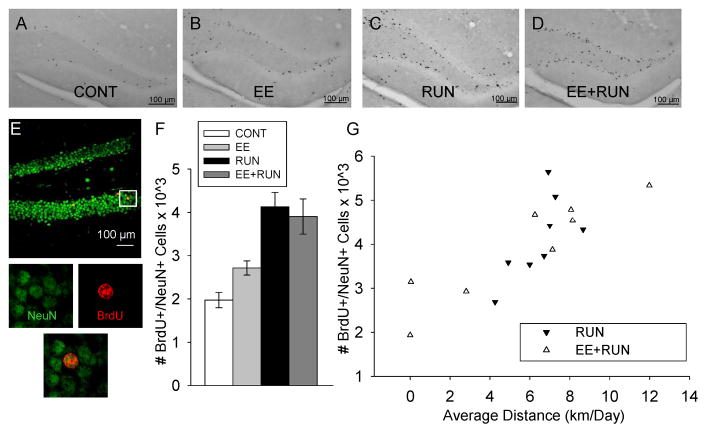
Running produced a 2-fold increase in the total number of BrdU-positive cells and total number of new neurons (BrdU+/NeuN+) in both the RUN and EE+RUN groups relative to their control groups, CONT and EE. Environmental enrichment had no effect. This was indicated by a significant main effect of running on total number of BrdU-positive cells (F1,28=30.3, p<0.0001) and total number of new neurons (F1, 28=33.6, p<0.0001) but no significant effect of enrichment or the interaction between enrichment and running (Fig. 3A–F). The RUN group displayed the highest numbers of new neurons, followed by EE+RUN, EE, and then CONT. All posthoc pairwise differences between groups were significant (p<0.05) except EE versus CONT, and EE+RUN versus RUN.
Figure 3.
Neurogenesis. Representative sections through the dentate gyrus stained for BrdU-DAB from a mouse in the A) CONT B) EE C) RUN and D) EE+RUN groups. E) Photographs of a representative section through the dentate gyrus of a mouse double-stained red for BrdU (bromodeoxyuridine) and green for NeuN (mature neuronal marker). The panels below show the tissue illuminated for each color separately and combined, zoomed in around the BrdU cell, indicating an episode of neurogenesis. F) Average number of new neurons in the dentate gyrus in the 4 groups. Standard error bars shown. G) Number of new neurons in the RUN and EE+RUN groups plotted against average distance run (km/day) across the 32 day study.
The percentage of BrdU-positive cells differentiated as neurons as indicated by co-expression of NeuN (mature neuronal nuclear marker) and BrdU was 80% (± 3.6), 81% (± 3.5), 85% (± 2.4), and 81% (± 2.5) for the CONT, EE, RUN and EE+RUN groups, respectively. These differences were not statistically significant.
Running and environmental enrichment both increased the volume of the granule layer and the effects were additive. This was indicated by a significant main effect of running (F1,28=14.4, p=0.0007) and enrichment (F1, 28=6.0, p=0.02) but no significant interaction. Running increased volume by approximately 20% whereas enrichment increased volume by approximately 12%. The EE+RUN group displayed the largest volume (0.65 μm3 ± 0.041), followed by RUN (0.57 μm3 ± 0.035), EE (0.52 μm3 ± 0.015), and then CONT (0.46 μm3 ± 0.023). Post-hoc comparisons indicated that EE+RUN volume was significantly larger than CONT and EE. No other pair-wise differences were significant.
Running increased the total number of new neurons per volume granule layer (F1, 28 =15.2, p=0.0006). Environmental enrichment had no main effect. However, a significant interaction between running and enrichment was observed (F1, 28=5.8, p=0.02). The interaction was due to enrichment tending to decrease density of new neurons in runners whereas enrichment tended to increase density in sedentary animals. The RUN group displayed the greatest density of new neurons (7.6 ± 0.77 new neurons per cubic mm × 103), followed by EE+RUN (6.0 ± 0.45), EE (5.2 ± 0.34), and then CONT (4.3 ± 0.39). Posthoc comparisons indicated that density of new neurons was significantly greater in RUN than CONT and EE groups. No other pair-wise differences were significant.
Average distance traveled on the disc was significantly correlated with numbers of new neurons in the RUN and EE+RUN groups (F1,12=26.3, p<0.0001) (Fig. 3G). The slope of the relationship between distance run and total number of new neurons was similar between the two groups. After correcting for the influence of distance run by analysis of covariance, total number of new neurons remained similar between the RUN and EE+RUN groups. Average distance traveled on the disc was significantly correlated with numbers of new neurons expressed per cubic mm volume sampled in the RUN and EE+RUN groups (F1,12=19.4, p=0.0009). The slope of the relationship between distance run and numbers of new neurons per volume was steeper for the RUN than the EE+RUN group. This was reflected in a significant main effect of group (F1,12=5.1, p=0.04) and the interaction between group and distance (F1,12=9.4, p=0.01).
3.5 Behavioral Performance: Morris Water Maze
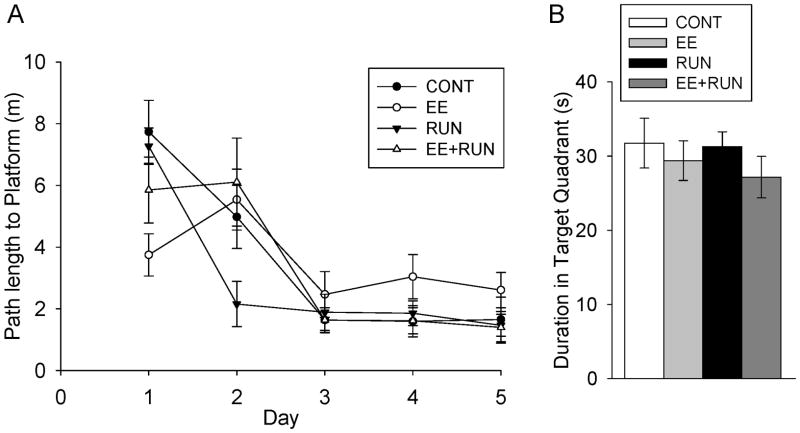
All animals learned the Morris water maze as indicated by decreasing path length (F4,139=28.6, p<0.0001) and latency (F4,139=25.0, p<0.0001) across days. A significant interaction between day and treatment group was detected for both path length (F12,139=3.1, p=0.0007) and latency (F12,139=2.7, p=0.003) to reach the hidden platform. The main effect of treatment was not significant. Animals in the RUN group showed the steepest learning curve as compared with the other groups, as indicated by significant posthoc differences between RUN and all the other groups on day 2 (p<0.05). On the probe test, all groups spent significantly more time in the target quadrant as compared with any other quadrant (p<0.0001 for each group), but no differences between groups were detected.
4. Discussion
Results support and extend results from Kobilo et al. (2011) suggesting that aerobic exercise is the critical variable in environmental enrichment that increases adult hippocampal neurogenesis and performance on the water maze in male C57BL/6J mice. The implication for humans is that physical exercise and not simply mental stimulation may be necessary to enhance total number of new neurons in the hippocampus and to confer associated cognitive gains in the domain of spatial learning.
One of the novel features of our study was the ability to clearly record how much time the animals spent on the enrichment side versus the other side of the cage. We discovered that when enrichment was provided, mice spent nearly all their time on that side (Fig. 2E–F). When both a running disc and enrichment was provided, the mice spent slightly less time on the enrichment side, mostly because they were running on the other side. Many of the animals were observed to make nests in the igloo with the straw and cotton bedding, and therefore, the enrichment side was typically the designated sleeping quarters. Hence, it is not surprising that the majority of time was spent on that side. It was not possible to track individual interactions with the toys due to mice hiding in the toys and tunnels provided.
Mice in the EE+RUN group had access to a running wheel but failed to display the facilitated acquisition of the water maze that mice in the RUN group did (Fig. 4A). This result may seem surprising given that both groups of animals had access to running wheels and ran, on average, at similar levels. Moreover, both groups displayed increased total numbers of new neurons relative to the CONT and EE groups. The finding of a significant post-hoc difference between the RUN and the other groups on day 2 of water maze testing is typical of the modest but significant pro-cognitive effects typically seen as a result of exercise on learning in the water maze and other tasks (Kempermann et al., 1997; van Praag et al., 1999a; van Praag et al., 1999b; Rhodes et al., 2003; Clark et al., 2008). However, given the small effect size, the results should be interpreted with caution. One possible interpretation is that the enhanced performance of the RUN group relative to the EE+RUN group in the water maze could be attributed to the increased density of new neurons. Mice in the EE+RUN group displayed slightly larger volume of the granule layer and fewer new neurons per volume than the RUN group, although these differences were not statistically significant by post-hoc tests. It is not clear what might have contributed to the slightly increased volume in the EE+RUN group relative to the RUN group, because total numbers of new neurons were the same between EE+RUN and RUN groups. It could be larger cells or more space between cells or more processes extending from the cell bodies (Redila and Christie, 2006). The EE+RUN group also displayed a significantly flatter slope than the RUN group for the relationship between distance run and number of new neurons per volume. An alternative possibility is that the new neurons in the RUN group could have displayed greater plasticity because they had not yet integrated into the circuitry as much, compared to the EE+RUN group, in which new neurons could have been already heavily recruited into the processing of the enrichment stimuli (Clark et al., 2012).
Figure 4.
Water maze. A) Average path length (m) to the hidden platform plotted against days with the 4 treatment groups plotted separately. B) Average duration spent in the target quadrant of the probe test in the 4 groups. Standard error bars shown.
Our study also included novel foods as an enrichment manipulation. While all mice received standard rodent chow as their primary diet, mice in the EE and EE+RUN groups also had access to special treats, such as sunflower seeds, peanuts, cashews, peanut butter, carrots, and juice (see Experimental Procedures). While there is evidence that a diet high in sucrose, fat, or calories decreases neurogenesis and hippocampal BDNF levels (Molteni et al., 2002; Lindqvist et al., 2006; Stangl and Thuret, 2009), it is not known how much dietary sucrose, fat, or calories are required to produce these effects. In addition, to the best of our knowledge, no one has documented a reduction of hippocampal neurogenesis due to a more varied diet. In fact, our rotation of treats included various items high in polyunsaturated fatty acids (cashews, peanuts, sunflower seeds), and there is some evidence that a mixed diet that includes polyunsaturated fatty acids may actually enhance hippocampal neurogenesis compared to an isocaloric control diet, at least in serotonin-transporter knock-out rats (Schipper et al., 2011). Therefore, we doubt that the diet component of our environmental enrichment obliterated a significant increase in enrichment-induced neurogenesis that would have been apparent without the dietary manipulation.
It was previously believed that running and enrichment increase neurogenesis via dissociable pathways (Kempermann et al., 1998; van Praag et al., 1999b; Olson et al., 2006). Running, it was thought, mainly increases proliferation of cells, whereas enriched environment increases the survival of cells that had already proliferated (Kempermann et al., 1998; van Praag et al., 1999b; Olson et al., 2006). However, recent studies have demonstrated that the proliferative effect of running is ephemeral and that running mainly increases neurogenesis by increasing the survival of cells, not by increasing proliferation (Kronenberg et al., 2006; Snyder et al., 2009; Clark et al., 2010; Fuss et al., 2010). Therefore, the interpretation of the present results, based on the updated literature, is that aerobic exercise is needed to increase the survival of new neurons in the dentate gyrus. Stimulation from toys and treats has no effect on neurogenesis or cognition, at least in C57BL/6J mice.
In our study, environmental enrichment devoid of running wheels failed to up-regulate hippocampal neurogenesis and improve cognitive performance, even when the modalities of the enrichment were broad. We concluded that aerobic exercise is required to increase neurogenesis and water maze performance in male C57BL/6J mice. However, environmental enrichment can be implemented in many different ways, and it remains a possibility that alternative stimuli or a different environment than that which was included here could produce a different outcome. For example, mice in our study were singly housed, and it is possible that effects of an enriched environment are contingent on interaction with cage mates (i.e. social enrichment) (Schapiro, 2002). However, the RUN group displayed increased hippocampal neurogenesis relative to the CONT despite both groups being singly housed. Furthermore, Kobilo et al. (2011) included large group housing in the enrichment condition and still failed to find an effect of enrichment on neurogenesis in female C57BL/6J mice.
The data in this study demonstrate the relative importance of running over sensory stimulation for increasing adult hippocampal neurogenesis and water maze learning, but that does not imply that enrichment without aerobic exercise has no effect on the brain. For instance, the original studies by Greenough and colleagues used large cages filled with toys without running wheels and found differences in blood vessel density, dendritic spines, and synapses in the visual cortex and cerebellum (Volkmar and Greenough, 1972; Greenough et al., 1973; Greenough et al., 1978; Greenough et al., 1985; Turner and Greenough, 1985; Greenough et al., 1986; Black et al., 1987; Sirevaag and Greenough, 1987; Sirevaag et al., 1988; Black et al., 1991). Therefore, despite the fact that we did not observe a neurogenic effect of environmental enrichment in our study, the possibility remains that environmental enrichment modulates other brain plasticity mechanisms capable of bestowing cognitive benefits. Future studies are needed to discover which brain mechanisms respond to environmental enrichment and whether they are required for gains in cognitive performance akin to those associated with running-induced hippocampal neurogenesis in this study.
Conclusions
In summary, we confirmed and extended the results of Kobilo et al. (2011) by finding that even rotating novel objects that stimulate various sensory modalities into enriched cages in C57BL/6J mice does not produce increases in neurogenesis or performance enhancement on the water maze if the environment does not include the opportunity to run. Hence, the implication is that mere interaction with a stimulating environment does not confer the same neurogenic or cognitive benefits as voluntary aerobic exercise does.
Highlights.
Male C57BL/6J mice were singly housed under 4 conditions for 32 days: standard, EE, run, or both.
Novel toys and treats were rotated into the enrichment conditions every 4 days.
Animals in the run group displayed increased neurogenesis and enhanced water maze performance.
Mere interaction with a stimulating environment does not confer neurogenic or cognitive benefits.
Acknowledgments
This work was supported by NIH grants MH 083807 and DA027487. Shi Chen was supported over the summer 2011 by the Erik Haferkamp Memorial Scholarship. The authors would like to extend their sincere gratitude and appreciation to the Haferkamp family for this incredible undergraduate research opportunity. Thanks to the Beckman Institute Animal Facility staff for routine animal care. Thanks also to the Beckman Institute Imaging Technology Group for their assistance with the microscopic imaging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arendash GW, Garcia MF, Costa DA, Cracchiolo JR, Wefes IM, Potter H. Environmental enrichment improves cognition in aged Alzheimer’s transgenic mice despite stable beta-amyloid deposition. Neuroreport. 2004;15:1751–1754. doi: 10.1097/01.wnr.0000137183.68847.4e. [DOI] [PubMed] [Google Scholar]
- Black JE, Sirevaag AM, Greenough WT. Complex experience promotes capillary formation in young rat visual cortex. Neurosci Lett. 1987;83:351–355. doi: 10.1016/0304-3940(87)90113-3. [DOI] [PubMed] [Google Scholar]
- Black JE, Zelazny AM, Greenough WT. Capillary and mitochondrial support of neural plasticity in adult rat visual cortex. Exp Neurol. 1991;111:204–209. doi: 10.1016/0014-4886(91)90008-z. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Bhattacharya TK, Miller DS, Kohman RA, Deyoung EK, Rhodes JS. New neurons generated from running are broadly recruited into neuronal activation associated with three different hippocampus-involved tasks. Hippocampus. 2012 doi: 10.1002/hipo.22020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155:1048–1058. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Kohman RA, Miller DS, Bhattacharya TK, Brzezinska WJ, Rhodes JS. Genetic influences on exercise-induced adult hippocampal neurogenesis across 12 divergent mouse strains. Genes, brain, and behavior. 2011 doi: 10.1111/j.1601-183X.2010.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PJ, Kohman RA, Miller DS, Bhattacharya TK, Haferkamp EH, Rhodes JS. Adult hippocampal neurogenesis and c-Fos induction during escalation of voluntary wheel running in C57BL/6J mice. Behav Brain Res. 2010;213:246–252. doi: 10.1016/j.bbr.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruise KE, Bucks RS, Loftus AM, Newton RU, Pegoraro R, Thomas MG. Exercise and Parkinson’s: benefits for cognition and quality of life. Acta Neurol Scand. 2011;123:13–19. doi: 10.1111/j.1600-0404.2010.01338.x. [DOI] [PubMed] [Google Scholar]
- Fuss J, Ben Abdallah NM, Vogt MA, Touma C, Pacifici PG, Palme R, Witzemann V, Hellweg R, Gass P. Voluntary exercise induces anxiety-like behavior in adult C57BL/6J mice correlating with hippocampal neurogenesis. Hippocampus. 2010;20:364–376. doi: 10.1002/hipo.20634. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Hwang HM, Gorman C. Evidence for active synapse formation or altered postsynaptic metabolism in visual cortex of rats reared in complex environments. Proc Natl Acad Sci U S A. 1985;82:4549–4552. doi: 10.1073/pnas.82.13.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, McDonald JW, Parnisari RM, Camel JE. Environmental conditions modulate degeneration and new dendrite growth in cerebellum of senescent rats. Brain Res. 1986;380:136–143. doi: 10.1016/0006-8993(86)91437-x. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Volkmar FR, Juraska JM. Effects of rearing complexity on dendritic branching in frontolateral and temporal cortex of the rat. Exp Neurol. 1973;41:371–378. doi: 10.1016/0014-4886(73)90278-1. [DOI] [PubMed] [Google Scholar]
- Greenough WT, West RW, DeVoogd TJ. Subsynaptic plate perforations: changes with age and experience in the rat. Science. 1978;202:1096–1098. doi: 10.1126/science.715459. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Molteni R, Wu A, Gomez-Pinilla F. Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004;125:129–139. doi: 10.1016/j.neuroscience.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Johansson BB, Belichenko PV. Neuronal plasticity and dendritic spines: effect of environmental enrichment on intact and postischemic rat brain. J Cereb Blood Flow Metab. 2002;22:89–96. doi: 10.1097/00004647-200201000-00011. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilo T, Liu QR, Gandhi K, Mughal M, Shaham Y, van Praag H. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Mem. 2011;18:605–609. doi: 10.1101/lm.2283011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, Deyoung EK, Bhattacharya TK, Peterson LN, Rhodes JS. Wheel running attenuates microglia proliferation and increases expression of a proneurogenic phenotype in the hippocampus of aged mice. Brain Behav Immun. 2011 doi: 10.1016/j.bbi.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol Aging. 2006;27:1505–1513. doi: 10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Kumar A, Rani A, Tchigranova O, Lee WH, Foster TC. Influence of late-life exposure to environmental enrichment or exercise on hippocampal function and CA1 senescent physiology. Neurobiol Aging. 2012;33:828 e821–828 e817. doi: 10.1016/j.neurobiolaging.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist A, Mohapel P, Bouter B, Frielingsdorf H, Pizzo D, Brundin P, Erlanson-Albertsson C. High-fat diet impairs hippocampal neurogenesis in male rats. Eur J Neurol. 2006;13:1385–1388. doi: 10.1111/j.1468-1331.2006.01500.x. [DOI] [PubMed] [Google Scholar]
- Molteni R, Barnard RJ, Ying Z, Roberts CK, Gomez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112:803–814. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol. 1999;39:569–578. doi: 10.1002/(sici)1097-4695(19990615)39:4<569::aid-neu10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16:250–260. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- Redila VA, Christie BR. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience. 2006;137:1299–1307. doi: 10.1016/j.neuroscience.2005.10.050. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, van Praag H, Jeffrey S, Girard I, Mitchell GS, Garland T, Jr, Gage FH. Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Behav Neurosci. 2003;117:1006–1016. doi: 10.1037/0735-7044.117.5.1006. [DOI] [PubMed] [Google Scholar]
- Schapiro SJ. Effects of social manipulations and environmental enrichment on behavior and cell-mediated immune responses in rhesus macaques. Pharmacol Biochem Behav. 2002;73:271–278. doi: 10.1016/s0091-3057(02)00779-7. [DOI] [PubMed] [Google Scholar]
- Schipper P, Kiliaan AJ, Homberg JR. A mixed polyunsaturated fatty acid diet normalizes hippocampal neurogenesis and reduces anxiety in serotonin transporter knockout rats. Behav Pharmacol. 2011;22:324–334. doi: 10.1097/FBP.0b013e328347881b. [DOI] [PubMed] [Google Scholar]
- Sirevaag AM, Black JE, Shafron D, Greenough WT. Direct evidence that complex experience increases capillary branching and surface area in visual cortex of young rats. Brain Res. 1988;471:299–304. doi: 10.1016/0165-3806(88)90107-1. [DOI] [PubMed] [Google Scholar]
- Sirevaag AM, Greenough WT. Differential rearing effects on rat visual cortex synapses. III. Neuronal and glial nuclei, boutons, dendrites, and capillaries. Brain Res. 1987;424:320–332. doi: 10.1016/0006-8993(87)91477-6. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Glover LR, Sanzone KM, Kamhi JF, Cameron HA. The effects of exercise and stress on the survival and maturation of adult-generated granule cells. Hippocampus. 2009;19:898–906. doi: 10.1002/hipo.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangl D, Thuret S. Impact of diet on adult hippocampal neurogenesis. Genes Nutr. 2009;4:271–282. doi: 10.1007/s12263-009-0134-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Yamane T, Fushiki T. Inhibition of fatty acid beta-oxidation attenuates the reinforcing effects and palatability to fat. Nutrition. 2006;22:401–407. doi: 10.1016/j.nut.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Turner AM, Greenough WT. Differential rearing effects on rat visual cortex synapses. I. Synaptic and neuronal density and synapses per neuron. Brain Res. 1985;329:195–203. doi: 10.1016/0006-8993(85)90525-6. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999a;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999b;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmar FR, Greenough WT. Rearing complexity affects branching of dendrites in the visual cortex of the rat. Science. 1972;176:1445–1447. doi: 10.1126/science.176.4042.1445. [DOI] [PubMed] [Google Scholar]
- Voss MW, Nagamatsu LS, Liu-Ambrose T, Kramer AF. Exercise, brain, and cognition across the life span. J Appl Physiol. 2011;111:1505–1513. doi: 10.1152/japplphysiol.00210.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Nelson NW, Savik K, Wyman JF, Dysken M, Bronas UG. Affecting Cognition and Quality of Life via Aerobic Exercise in Alzheimer’s Disease. West J Nurs Res. 2011 doi: 10.1177/0193945911420174. [DOI] [PMC free article] [PubMed] [Google Scholar]