Abstract
Bioprinting as a promising but unexplored approach for cartilage tissue engineering has the advantages of high throughput, digital control, and highly accurate placement of cells and biomaterial scaffold to the targeted 3D locations with simultaneous polymerization. This study tested feasibility of using bioprinting for cartilage engineering and examined the influence of cell density, growth and differentiation factors. Human articular chondrocytes were printed at various densities, stimulated transiently with growth factors and subsequently with chondrogenic factors. Samples were cultured for up to 4 weeks to evaluate cell proliferation and viability, mechanical properties, mass swelling ratio, water content, gene expression, ECM production, DNA content, and histology. Bioprinted samples treated with FGF-2/TGF-β1 had the best chondrogenic properties among all groups apparently due to synergistic stimulation of cell proliferation and chondrogenic phenotype. ECM production per chondrocyte in low cell density was much higher than that in high cell seeding density. This finding was also verified by mechanical testing and histology. In conclusion, cell seeding density that is feasible for bioprinting also appears optimal for human neocartilage formation when combined with appropriate growth and differentiation factors.
Keywords: inkjet printing, cartilage tissue engineering, chondrocyte, hydrogel, extracellular matrix, photoplymerization
Introduction
Bioprinting based on thermal inkjet printing technology was introduced in 2003 as an attractive biofabrication approach with the advantages of high throughput, digital control, and highly precise placements of cells, biological factors, and biomaterial scaffolds to the desired two-dimensional (2D) and three-dimensional (3D) locations (Wilson and Boland, 2003). Many successes have been achieved previously using this technology (Boland et al., 2006; Cui and Boland, 2009; Xu et al., 2006). In this study, we developed a 3D bioprinting platform based on a modified Hewlett-Packard (HP) Deskjet 500 thermal inkjet printer for cartilage tissue engineering using synthetic hydrogel scaffold formulated from poly(ethylene glycol) (PEG). PEG hydrogels have demonstrated their capacities to maintain chondrocyte viability and induce chondrogenic extracellular matrix (ECM) deposition (Bryant and Anseth, 2002; Elisseeff et al., 2000). The physical and mechanical properties of PEG hydrogels are tunable to match those of human articular cartilage (Bryant et al., 2004). In addition, PEG is water soluble with low viscosity and can be modified to be photocrosslinkable, which makes it attractive for direct printing to fabricate 3D structures with simultaneous polymerization. In our study, human articular chondrocytes with poly(ethylene) glycol dimethacrylate (PEGDMA; MW, 3400) and photoinitiator were precisely deposited to fabricate cartilage tissue in a layer-by-layer fashion. Bioink was ejected with a volume of 130 pL/drop and delivers digital patterns at 300 dpi (85 μm) resolution (Buskirk et al., 1988; Harmon and Widder, 1988). Even and precise distribution of printed human chondrocytes in 3D hydrogel was achieved with simultaneous polymerization during layer-by-layer assembly, which generated neocartilage with stronger mechanical properties and higher ECM production. By contrast, in manual fabricated constructs or polymerized after printing, the deposited chondrocytes accumulated at the bottom of the 3D constructs instead of their originally deposited positions due to gravity, which led to inhomogeneous neocartilage formation (unpublished observations). Although bioprinting is promising to engineer zonal cartilage based on these observations, previous studies showed significantly higher ECM production with initial cell seeding density at 10–20×106 cells/mL for cell based cartilage tissue engineering with bovine chondrocytes (Sharma et al., 2007) and mesenchymal stem cells (MSCs) (Buxton et al., 2011). However, the maximum cell density for bioprinting is restricted as the optimal printing resolution is only achieved with bioink at or lower than 8×106 cells/mL (Cui et al., 2010). These observations raise concerns that optimal cell densities for cartilage tissue engineering cannot be achieved using bioprinting approaches. However, this has not formally been tested and the effect of growth and differentiation factors on human chondrocytes that are printed at various densities has not yet been examined.
Insulin-transferrin-selenium (ITS+) based medium supplemented with 10ng/mL transforming growth factor-β1 (TGF-β1) is commonly used to maintain chondrocyte chondrogenic phenotype in 3D culture (Grogan et al., 2006). Although TGF-β1 supplemented medium seems sufficient to induce persistent expression of chondrogenic ECM proteins, it is not as efficient for chondrocyte expansion (Yaeger et al., 1997). Fibroblast growth factor-2 (FGF-2) supplemented medium has been shown to be beneficial for chondrocyte monolayer expansion as well as the chondrogenic capacity in terms of ECM synthesis and chondrocyte specific phenotype expression (Jakob et al., 2001; Mandl et al., 2002; Mandl et al., 2004). In this study, we tested the role of these growth factors in chondrocyte proliferation and ECM production for the first time in bioprinted cell-laden 3D hydrogels. Our hypothesis was that proper growth factors stimulation will induce cell proliferation and more chondrogenic ECM deposition in bioprinted cartilage tissue while maintain the high printing resolution. The treated low cell density bioprinted constructs will near or match the functional properties of engineered cartilage with much higher cell densities reported previously for cartilage tissue engineering.
Materials and Methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM) was obtained from Mediatech (Manassas, VA). LIVE/DEAD® Viability/Cytotoxicity kit was purchased from Invitrogen (Carlsbad, CA). Human serum albumin was obtained from Bayer (Elkhart, IN). TGF-β1 and FGF-2 were purchased from PeproTech Inc. (Rocky Hill, NJ). Photoinitiator Irgacure 2959 (I-2959) was purchased from Ciba Specialty Chemicals (Tarrytown, NY). Primers were from Applied Biosystems (Carlsbad, CA). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise noted.
Preparation of PEGDMA
PEGDMA was synthesized as described previously (Lin-Gibson et al., 2004). Briefly, PEG (3 kDa) was dissolved in tetrahydrofuran and reacted with methacryloyl chloride in the presence of triethylamine overnight under nitrogen. Synthesized PEGDMA macromer was purified by precipitation in ethyl ether and lyophilized overnight. Methacrylation of the reaction was over 95% as determined by proton nuclear magnetic resonance (1H NMR).
Human articular chondrocyte isolation
Healthy human articular cartilage was supplied by tissue banks from adult donors (mean±SD age 26.8±6.7) having no history of joint disease. Human tissues were obtained under approval by the Scripps Human Subjects Committee. Articular cartilage and chondrocytes were harvested and isolated from cadavers within 72 h after death as previously described (Blanco et al., 1995; Maier et al., 1993). Briefly, harvested cartilage samples were minced and treated with 0.5 mg/mL trypsin solution at 37°C for 15 min. After removing trypsin, the cartilage tissues were digested with 2 mg/mL type IV clostridial collagenase in DMEM with 5% fetal calf serum for 12 to16 h at 37°C.
The released human articular chondrocytes were rinsed three times with 1X penicillin-streptomycin-glutamine (PSG; Invitrogen) supplemented DMEM and cell viability was determined (> 95%). Isolated chondrocytes were seeded into T175 tissue culture flasks at 5 million cells per flask for monolayer expansion in DMEM supplemented with 10% calf serum and 1X PSG. Cells were incubated at 37°C with humidified air containing 5% CO2. Culture medium was changed every 4 days. Human chondrocytes were used at 80 to 90% confluence (1 to 2 weeks in primary culture). All cells used for this study were from first passage.
Bioink preparation
PEGDMA was prepared in PBS to a final concentration of 10% weight/volume (w/v). Photoinitiator I-2959 was added at 0.05% w/v. Human articular chondrocytes were suspended in filter-sterilized PEGDMA solution at 8×106 cells/mL.
Bioprinting and cell culture
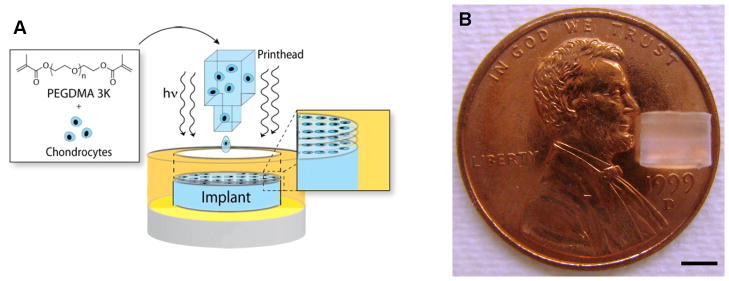
The bioprinting platform was set up as previously described (Cui and Boland, 2009). The printer was UV sterilized for at least 2 h. HP Deskjet pens with 50 firing chambers were rinsed with 70% ethanol for three times followed by sterile water. A long-wave ultraviolet lamp (Model B-100AP, UVP, Upland, CA) was set up above the printing platform with a distance of 25 cm for simultaneous photopolymerization during the printing. UV intensity at the platform was 4 to 8 mW/cm2 according to the manufacturer and it was further verified using a UV light meter (UV513AB, General Tools, New York City, NY). The pen was filled with bioink and covered by aluminum foil to protect from UV exposure. Cylindrical molds with 4 mm internal diameter served as biopaper. The distance between the printhead and biopaper was set at 1 to 2 mm. Digital patterns representing the shape and size of the desired structure were designed in Adobe Photoshop (Adobe Systems, San Jose, CA) and printed layer-by-layer to fabricate a 3D construct (Fig. 1A). ITS+ medium was formulated with 1X insulin-transferrin-selenium (ITS+), 0.1 mM ascorbic acid 2-phosphate, 1.25 mg/mL human serum albumin, 10−7 M dexamethasone, and 1X PSG in DMEM. ITS+ medium supplemented with 10 ng/mL FGF-2 or FGF-2/TGF-β1 combination was used for cell expansion during the first week. 10 ng/mL TGF-β1 supplemented ITS+ medium was used as a standard chondrogenic culture medium for the subsequent weeks 2–4 (Schmitt et al., 2003). Cell-laden hydrogel cultured with standard chondrogenic culture medium from week 1 to 4 was used as control. Cell-laden hydrogel constructs with higher cell density (20×106 cells/mL) cultured with standard chondrogenic culture conditions served as a reference. Medium was changed every 3 days during the culture. Cell viability was measured using Live/Dead Viability/Cytotoxicity assay after printing and during the culture. Hydrogel constructs were imaged using a Zeiss LSM 510 laser scanning microscope (Carl Zeiss, Minneapolis, MN).
Figure 1.
Bioprinted cell-laden constructs for cartilage tissue engineering. A: Schematic of bioprinting with simultaneous photopolymerization process. B: A printed PEG hydrogel construct with 4 mm in diameter and 4 mm in height using layer-by-layer assembly. Scale bar: B = 2 mm.
Mechanical characterization of printed PEGDMA hydrogels
A customized electromechanical testing system with a 50 g load cell (LSB200, Futek, Irvine, CA) and a dual-axis controller (LAC-25, SMAC, Carlsbad, CA) was employed to measure the compressive modulus of hydrogels at room temperature. The hydrogel samples were placed in a parallel plate configuration and the thickness was measured by the axis controller. The hydrogel was compressed in a step-wise manner to a maximum of 20% compressive strain with 4 progressive strain loadings with a test velocity of 0.1 mm/s. Each loading cycle was followed by a relaxation phase to sample equilibrium. The unconfined compressive modulus was determined from the stress-strain profile obtained from the load and displacement data at the end of each relaxation phase (Hoenig et al., 2011). A sample size of three was used for each condition.
Swelling studies of printed PEGDMA hydrogels
Printed PEGDMA hydrogels were allowed to swell to equilibrium in culture medium at 37°C for 48 h before weighing to obtain equilibrium swollen mass. Dry mass was obtained by lyophilizing hydrogels for 48 h. The equilibrium mass swelling ratio (Q) and water content (M) of hydrogel constructs were determined by the following equations.
Here, Ws and Wd represent the weight of hydrogel construct after equilibrium swelling in culture medium, and the dry weight of lyophilized hydrogel construct, respectively.
RNA isolation and gene expression using quantitative real-time PCR (RT-PCR)
Cell-hydrogel constructs were harvested and analyzed for gene expression at week 1, 2, 3, and 4 during the culture (n = 3). Samples were frozen immediately in liquid nitrogen and pulverized with a biopulverization kit. Pulverized powder was carefully collected into a tube containing RNA lysis buffer provided by RNeasy Mini Kit (QIAGEN, Valencia, CA) and incubated at room temperature for 15 min. Lysed solution was transferred into shredder tubes (QIAGEN) and centrifuged at 12,000 g for 2 min. The homogenized solution was then transferred to spin tubes and RNA was purified following the protocol provided with the kit. Total RNA content and purity was quantified using the Nanodrop ND-1000 (Thermo Scientific, Wilmington, DE). Isolated total RNA was reverse transcribed to cDNA using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) following the kit protocol. Quantitative RT-PCR (LightCycler 480II Real-Time PCR System, Roche, Basel, Switzerland) was performed using TaqMan Gene Expression Assay probes (Applied Biosystems) to determine the gene expression of human collagen type I (COL1A1; Hs00164004_m1), collagen type II (COL2A1; Hs01064869_m1), and aggrecan (ACAN; Hs00153936_m1) relative to the expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Hs99999905_m1). GAPDH was used as housekeeping gene for normalization.
Biochemical assays
Samples collected at different time points were lyophilized for at least 48 h before cell lysis. Lyophilized constructs were sliced into small pieces with scalpels and digested with papain or pepsin. Total glycosaminoglycan (GAG) content was extracted by treating each sample with 1 mL papain solution (125 μg/mL papain type III (Worthington Biochemical, Lakewood, NJ), 10 mM l-cysteine, 100 mM phosphate buffer, 10 mM EDTA, pH 6.4) for 16 h at 60°C. Soluble type II collagen was extracted by digesting each sample with 1 mL pepsin solution (100 μg/mL pepsin in 0.05 M acetic acid) for 6 days at 4°C to avoid denaturation.
DNA content in each sample was measured using CyQUANT Cell Proliferation Assay (Invitrogen) following the kit protocol. Results were measured using a TECAN Safire 2 microplate reader (Mannedorf, Switzerland). Total GAG content was determined with dimethymethylene blue dye assay (Farndale et al., 1986). Solubilized type II collagen was measured with an ELISA detection kit (Chondrex, Redmond, WA) following the protocol provided by the manufacturer. GAG and collagen type II contents were normalized to the DNA content and dry weight of each sample to assess biosynthetic activity of embedded human chondrocytes and overall ECM production. DNA content was normalized to the dry weight of each sample to assess the cell growth during the culture. A sample size of three was used.
Histology
Samples were fixed overnight in 10% formaldehyde and transferred to 1X PBS until embedded in paraffin following standard histological protocol. After embedding in paraffin, 6 μm cross sections were cut using a microtome (Microm HM 325, GMI Inc., Ramsey, MN). Representative sections of each construct were stained with Safranin-O/fast green to visualize the cells and proteoglycans in the hydrogels.
Statistical analysis
All the data in this article are reported as mean with standard deviation. Statistical significance was detected with one-way ANOVA at a confidence level of 0.05.
Results
Bioprinted PEGDMA hydrogel with human chondrocytes
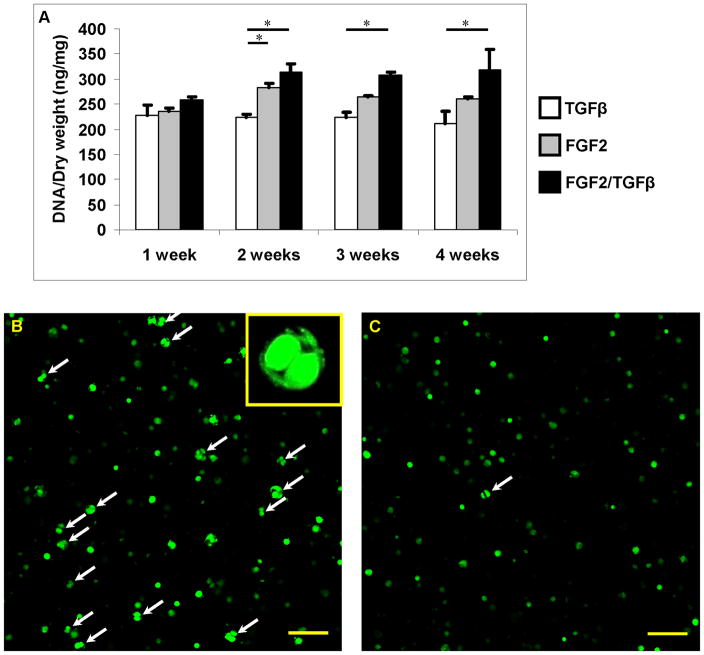
The printing resolution of the bioprinting system was 300 dpi with individual ink drop volume of 130 pL. There are 50 nozzles in each printhead with a firing frequency of 3600 Hz (Buskirk et al., 1988; Harmon and Widder, 1988). Therefore for a representative construct of 4 mm diameter and 4 mm thick, a nominal 0.23 μL of bioink estimated to contain 1823 human chondrocytes (8×106 cells/mL) was printed and photopolymerized for each layer to fabricate the construct in a layer-by-layer fashion. The thickness of each printed layer was about 18 μm. Total firing time of printhead was about 2.2 sec and the printing process was completed between 3 to 4 min to generate the entire construct (Fig. 1B). The viability of human chondrocytes after printing was 84.9±2.2% (n = 3) and there was no significant difference in cell viability during the culture. Total DNA content normalized to dry weight was measured to compare the cell proliferation with different treatments. There was no significant difference in cell proliferation among all groups at week 1 (Fig. 2A). However, constructs cultured with FGF-2 and FGF-2/TGF-β1 supplemented medium showed significantly more cell proliferation compared with TGF-β1 only after two weeks in culture. The combined FGF-2/TGF-β1 treatment demonstrated the highest cell proliferation, which was about 40% more than the TGF-β1 only groups (p = 0.017) (Fig. 2A). There was no significant difference in cell proliferation from week 2 to 4 in each group after switched to TGF-β1 only medium (Fig. 2A). There was visible evidence of more chondrocytes dividing in 3D hydrogel with FGF-2/TGF-β1 (Fig. 2B) compared with TGF-β1 only (Fig. 2C).
Figure 2.
Human chondrocyte proliferation within printed 3D cell-laden PEG hydrogel constructs. A: Cell proliferation within PEG hydrogel treated with TGF-β1 (white bars), FGF-2 (grey bars), and FGF-2/TGF-β1 (black bars) for the first week, then TGF-β1 for all groups. B, C: Confocal images of Calcein AM stained cells treated with FGF-2/TGF-β1 (B) and TGF-β1 only (C) after two weeks in culture. The dividing cells were marked with arrows. Asterisks indicate statistical significance between assigned groups (* p < 0.05) (n = 3). Scale bars: B, C = 100 μm.
Thus, bioprinting yields constructs with high chondrocyte viability. The cells in the constructs respond to FGF-1 and FGF-2/TGF-β1 with a significant increase in cell proliferation.
Mass swelling ratio and mechanical properties of printed PEGDMA scaffolds
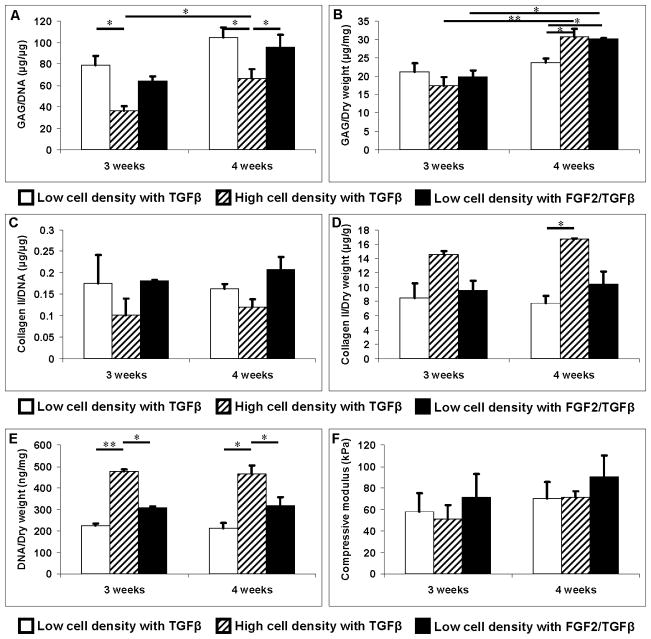
There was no significant difference in mass swelling ratio and equilibrium water content among all groups from week 1 to 4 (Table I). Hydrogel embedded with 8×106 cells/mL and 20×106 cells/mL had compressive modulus of 36.90±3.41 kPa (n = 3) and 29.88±2.33 kPa (n = 3), respectively. Both were lower than the compressive modulus of blank PEG gel without cells (59.99±12.92 kPa, n = 3). There was no significant difference in the stiffness of the cell-laden hydrogels from week 1 to 2 (Table I). At week 3 and 4, however, the compressive modulus of FGF-2/TGF-β1 treated samples was 24% and 28% higher than that of the TGF-β1 only treated samples, respectively. No significant difference in compressive modulus was observed between FGF-2 and TGF-β1 groups. At week 4, all cellular groups had significantly higher compressive modulus than the initial compressive modulus (p < 0.05). Only the FGF-2/TGF-β1 treated samples had significantly higher compressive modulus than the blank gel (p = 0.047). No statically significant difference in compressive modulus was observed in high and low cell density samples at week 3 and 4 although it showed less stiffer in high cell density samples (Fig. 5F). This reduced mechanical property of PEG hydrogel with cells may be due to the interference with photopolymerization process by the embedded cells, as well as the lower stiffness of the encapsulated cells. This lost stiffness can be recovered by the produced ECM during the culture.
Table I.
Properties of printed 10% (w/v) PEGDM with 8×106 cells/mL human chondrocytes cultured at different conditions (n = 3)
| Culture time (weeks) | TGF-β1 | FGF-2 | TGF-β1/FGF-2 | |
|---|---|---|---|---|
| Qa | 1 | 10.87 ± 0.17 | 10.18 ± 0.24 | 11.02 ± 0.31 |
| 2 | 11.07 ± 0.28 | 11.03 ± 0.54 | 10.80 ± 0.25 | |
| 3 | 11.21 ± 0.34 | 10.83 ± 0.07 | 12.00 ± 0.42 | |
| 4 | 12.03 ± 0.39 | 11.53 ± 0.17 | 11.55 ± 0.54 | |
| Mb (%) | 1 | 90.79 ± 0.15 | 90.18 ± 0.23 | 90.92 ± 0.25 |
| 2 | 90.96 ± 0.23 | 90.92 ± 0.45 | 90.74 ± 0.22 | |
| 3 | 91.07 ± 0.27 | 90.77 ± 0.06 | 91.66 ± 0.29 | |
| 4 | 91.68 ± 0.27 | 91.33 ± 0.13 | 91.33 ± 0.43 | |
| Compressive Modulus (kPa) | 1 | 44.07 ± 6.72 | 40.05 ± 2.76 | 46.29 ± 8.38 |
| 2 | 55.74 ± 8.23 | 56.45 ± 11.64 | 55.08 ± 9.34 | |
| 3 | 57.63 ± 17.50 | 58.55 ± 9.68 | 71.55 ± 21.64 | |
| 4 | 70.42 ± 14.95 | 68.10 ± 12.22 | 90.45 ± 19.40 |
Mass swelling ratio
Equilibrium water content
Figure 5.
ECM production normalized to DNA content and dry weight, DNA content normalized to weight, and compressive modulus of human chondrocytes embedded in PEG hydrogel at 8×106 (white bars) and 20×106 cells/mL (upward diagonal bars) cultured at standard chondrogenic condition with TGF-β1 only and 8×106 cells/mL cultured with FGF-2/TGF-β1 for the first week and then TGF-β1 after (black bars). A: GAG production normalized to DNA content (μg/μg). B: GAG production normalized to dry weight (μg/mg). C: Soluble collagen type II production normalized to DNA content (μg/μg). D: Soluble collagen type II production normalized to dry weight (μg/g). E: Total DNA content normalized to dry weight (ng/mg). F: Compressive modulus. Asterisks indicate statistical significance between assigned groups (* p < 0.05; ** p < 0.01) (n = 3).
Gene expression of printed human chondrocytes
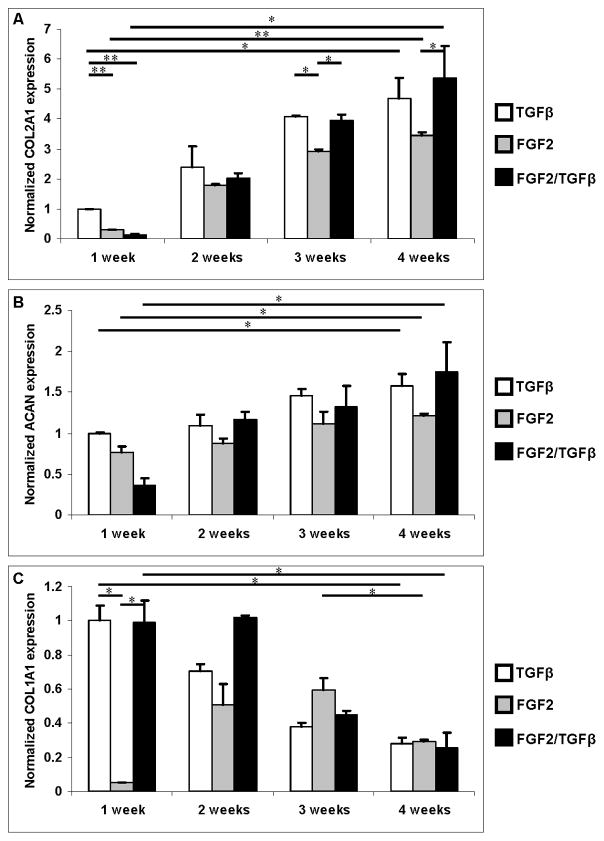
Expression of human collagen type I, collagen type II, and aggrecan genes was measured using quantitative PCR, normalizing to the GAPDH housekeeping gene. To determine the effect of different treatments, relative gene expression of target genes was then normalized to the expression in human chondrocytes cultured at standard chondrogenic condition at week 1. The results of these studies are shown in Figure 3.
Figure 3.
Gene expression of human chondrocytes embedded in PEG hydrogel treated with TGF-β1 (white bars), FGF-2 (grey bars), and FGF-2/TGF-β1 (black bars) for the first week, then TGF-β1 for all groups. Gene expressions were normalized to week 1 with TGF-β1 only. A: Collagen type II expression. B: Aggrecan expression. C: Collagen type I expression. Asterisks indicate statistical significance between assigned groups (* p < 0.05; ** p < 0.01) (n = 3).
Collagen type II and aggrecan expression increased significantly from week 1 to 4 for all groups during the culture (p < 0.05) (Fig. 3A, B). Samples treated with TGF-β1 had significantly higher collagen type II expression than those treated with FGF-2 and FGF-2/TGF-β1 at week 1 (p < 0.01) (Fig. 3A). TGF-β1 group also showed higher aggrecan expression at week 1 but it was not statistically significant (Fig. 3B). FGF-2 treated samples had significantly lower collagen type I expression at week 1 (p < 0.05) (Fig. 3C). This inhibition of collagen type I expression was not observed upon change to TGF-β1 supplemented medium after week 1. Collagen type I expression decreased significantly for other groups from week 1 to 4 during the culture (p < 0.05) and no significant difference in collagen type I expression was observed in the end of the culture in all groups. At week 4, collagen type II expression in FGF-2/TGF-β1 treated group was significantly higher than FGF-2 group (p < 0.05). Aggrecan expression was also higher in the same group comparing with FGF-2 group but this was not significant with p = 0.08. There was no significant difference in collagen type II and aggrecan expression between TGF-β1 and FGF-2/TGF-β1 groups at week 4. TGF-β1 treated groups showed higher chondrogenic gene expression at week 1 comparing to groups treated with FGF-2 and FGF-2/TGF-β1. However, gene expression of all groups showed similar level in the end of culture after the media was switched to standard chondrogenic media after the first week.
Collectively, these results show that the presence of TGF-β1 is essential for the induction or maintenance of the chondrocyte phenotype. Pre-culture or co-culture with FGF-2 to stimulate cell proliferation does not interfere with the chondrogenic effect of TGF-β1.
Biochemical analysis of printed tissue constructs
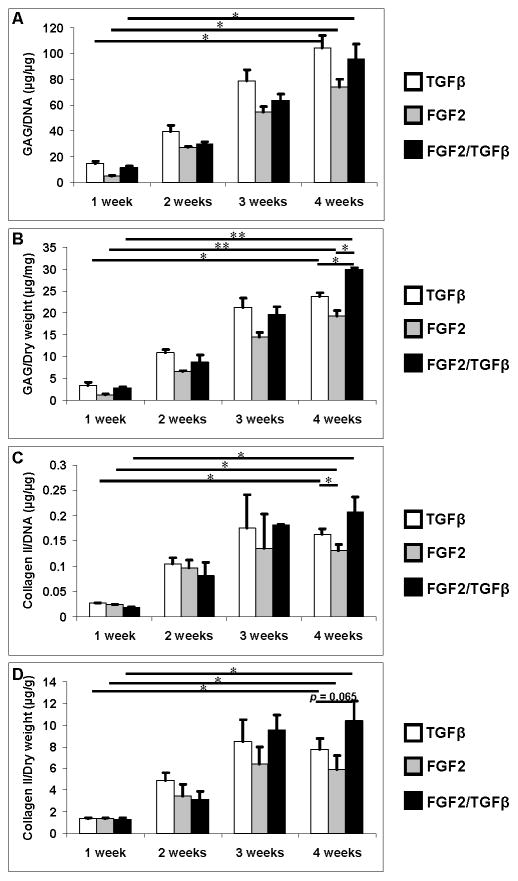
Chondrocyte ECM deposition was evaluated by measuring GAG and collagen type II contents. GAG and collagen II measurements were normalized to the DNA content of each sample to represent the average chondrogenic properties of each cell. Overall ECM production of the neocartilage was normalized to dry weight of each construct, as shown in Figure 4. GAG and collagen type II production increased significantly from week 1 to 4 in all groups. Although the difference in GAG/DNA and collagen type II/DNA is not significant from FGF-2/TGF-β1 group to other two groups, GAG/dry weight of FGF-2/TGF-β1 group was significantly higher at week 4. The collagen type II/dry weight from the same group was also higher than other groups with p = 0.065 at week 4.
Figure 4.
ECM production normalized to DNA content and dry weight of each sample treated with TGF-β1 (white bars), FGF-2 (grey bars), and FGF-2/TGF-β1 (black bars) for the first week, then TGF-β1 for all groups. A: GAG production normalized to DNA content (μg/μg). B: GAG production normalized to dry weight (μg/mg). C: Soluble collagen type II production normalized to DNA content (μg/μg). D: Soluble collagen type II production normalized to dry weight (μg/g). Asterisks indicate statistical significance between assigned groups (* p < 0.05; ** p < 0.01) (n = 3).
PEG hydrogel embedded with 20×106 cells/mL human chondrocytes cultured with standard chondrogenic TGF-β1 supplemented ITS+ medium was also evaluated for ECM production as a reference. GAG/DNA production was significantly lower than that in human chondrocytes at 8×106 cells/mL and treated with same chondrogenic condition or FGF-2/TGF-β1 at week 4 (p < 0.05, Fig. 5A). There was no difference in total GAG production between low and high cell density at week 3. At week 4, although total GAG content at lower cell density cultured in standard chondrogenic condition was significant lower than that in higher cell density, there was no difference in GAG production between higher cell density and lower cell density pre-treated with FGF-2/TGF-β1 (Fig. 5B). Differences in collagen type II/DNA and total collagen type II were not statistically significant (p > 0.05) between higher cell density and lower cell density samples pre-treated with FGF-2/TGF-β1 (Fig. 5C & D). Total DNA content remained the same from week 3 to 4 for low and high cell density packed constructs (Fig. 5E). Total DNA content of high cell density was almost 1.5 times greater than that of low cell density samples pre-treated with FGF-2/TGF-β1.
Histology
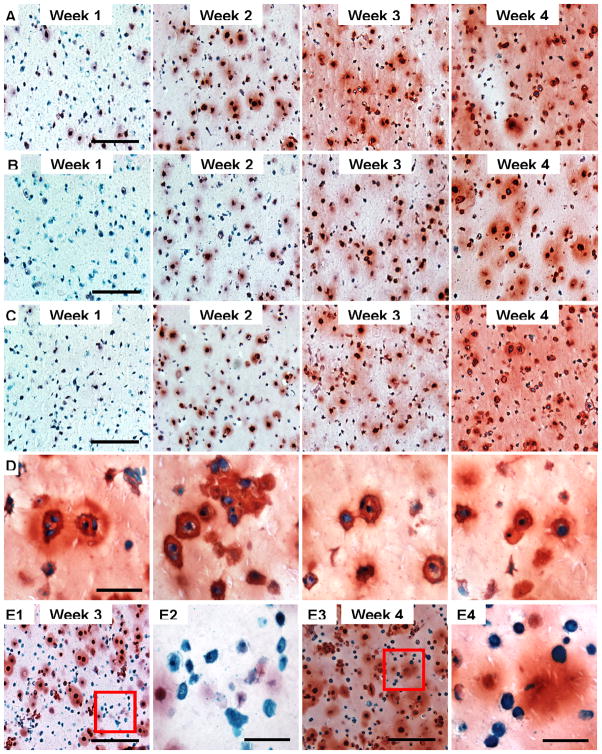
Safranin-O staining showed increased proteoglycan production in PEG encapsulated human chondrocytes from week 1 to 4 in all groups (Fig. 6A, B, & C). At week 1, constructs had more GAG in group cultured at standard chondrogenic condition with TGF-β1 while the group pre-treated with FGF-2 had minimum GAG content. The group treated with FGF-2/TGF-β1 showed overall higher amount of proteoglycan content at week 4, which is consistent with the biochemical data. Many dividing cells were observed from that group (Fig. 6D). Many embedded human chondrocytes in higher cell density seeding constructs had no proteoglycan production at week 3 and 4 (Fig. 6E). Using cell counting by a blinded observor, there were 42.91±7.58% cells without proteoglycan production in high cell density samples and only 10.56±2.84% cells in FGF-2/TGF-β1 group were observed without proteoglycan production at week 4 (p < 0.001).
Figure 6.
Safranin-O staining of cell-laden PEG hydrogel. A: Treated with standard chondrogenic condition with TGF-β1 only from week 1 to 4. B: Treated with FGF-2 for week 1, then TGF-β1 from week 2 to 4. C: Treated with FGF-2/TGF-β1 for week 1, then TGF-β1 from week 2 to 4. D: Proliferating cells in PEG hydrogel with FGF-2/TGF-β1 synergistic treatment at week 4. E: Human chondrocytes in PEG hydrogel photoencapsulated at high cell density (20×106 cells/mL) at week 3 (E1 & E2) and 4 (E3 & E4). E2 and E4 show chondrocytes with no proteoglycan production at week 3 and 4, respectively. Scale bars: A, B, C, E1, E3 = 200 μm; D, E2, E4 = 50 μm.
Discussion
We describe here the first study of synergistic FGF-2 and TGF-β1 stimulations to the bioprinted 3D hydrogel constructs for human chondrocyte proliferation and differentiation, resulting in a functional neocartilage formation. A major goal of this study was to determine feasibility of stimulating proliferation and ECM production of printed cells at levels equivalent to samples seeded at high cell density using conventional methods. For proof of principle we used FGF-2, which is efficient for cell proliferation, and TGF-β1, which is critical for chondrogenic differentiation (Mann et al., 2001). A successful approach would stimulate cell proliferation in 3D hydrogel without delaying neocartilage formation. The bioprinted samples were treated with FGF-2 for the first week and all samples were then cultured with standard chondrogenic medium. The FGF-2/TGF-β1 treated samples demonstrated 40% more cell proliferation compared with TGF-β1 treated group. Therefore the FGF-2/TGF-β1 synergistic treatment, made the initial 8×106 cells/mL seeding density equivalent to a seeding density of over 11×106 cells/mL. This is within the range of cell seeding density for optimal ECM production for cartilage tissue engineering (Buxton et al., 2011; Sharma et al., 2007).
The lack of significant difference in cell proliferation among the different treatments at week 1 reflects the lag time in embedded chondrocyte response to the applied cell proliferation growth factors in PEG hydrogel. By week 2, however, many chondrocytes were actively proliferating in response to FGF-2/TGF-β1, in distinct contrast to the few proliferating cells within standard chondrogenic condition with TGF-β1. There was no significant difference in cell viability among all groups during the culture. This is consistent with previous studies that PEG hydrogel provides a biocompatible environment for chondrocytes (Bryant and Anseth, 2002; Wang et al., 2007).
Increase in chondrogenic phenotype of TGF-β1 treated samples over time was reflected in the increase in collagen type II and aggrecan expression with decrease in collagen type I expression. The lower collagen type II and aggrecan gene expression in FGF-2 treated samples reflected some inhibition of chondrogenesis of embedded human chondrocytes in 3D hydrogel. This is consistent with previous observations that TGF-β1 is more efficient than FGF-2 to promote and maintain chondrogenic phenotype of chondrocytes (Brandl et al., 2010). Although cells proliferated significantly in FGF-2 and FGF-2/TGF-β1 groups compared with TGF-β1 group, there was no significant difference in collagen type II and aggrecan expression compared to TGF-β1 group at week 4. This indicates that the chondrogenic phenotype of human chondrocytes recovered quickly after switching to chondrogenic medium. FGF-2 treated cells showed significantly lower expression in collagen type I expression at week 1. The similar inhibition of collagen type I gene expression was also observed in FGF-2 treated osteoblasts, demonstrating the differences between in vivo and in vitro systems (Boudreaux and Towler, 1996; Hurley et al., 1993). The inhibitory mechanism remains unknown (Chaudhary and Avioli, 2000).
Matrix deposition was consistent with the gene expression. Similar levels of GAG deposition normalized by DNA observed among all groups at week 4 indicate comparable chondrogenesis regardless of initial treatments. However, total GAG production in the FGF-2/TGF-β1 group was significantly higher than other groups, corresponding with the 40% increase of proliferated cells with overall higher ECM production. Similar trends were seen in collagen type II deposition levels as well. The compressive modulus of the FGF-2/TGF-β1 group was also about 28% higher than the others. Collectively, these results indicate that initial supplementation of FGF-2/TGF-β1 increased cell density, gene expression, and matrix deposition by comparable levels.
Having established the beneficial effect of FGF-2 supplementation with TGF-β1, we next compared FGF-2 supplementation of low density (8×106 cells/mL) to a higher initial cell density of 20×106 cells/mL. The initial ratio of high and low cell seeding density reduced from 2.5 to 1.5 by week 4 based on total DNA measurements. This reduced ratio is in part due to the 40% increase in cell population treated with FGF-2/TGF-β1 and in part due to the fact that not all cells at high cell density may have survived or been successfully embedded.
Despite the initially lower cell density, the GAG/DNA and collagen type II/DNA from low cell density group were higher than those from the high cell density group, indicating high density cell seeding does not lead to high ECM production on a per cell basis. Greater ECM production at higher cell density has been reported for bovine articular chondrocytes (Sharma et al., 2007) and MSCs (Buxton et al., 2011) in PEG hydrogels. However, our data suggests optimal cell density for human chondrocytes may be lower. The increase in cell density induced by FGF-2 supplementation coupled with the relative increase in ECM production per cell essentially minimized any differences in overall GAG production per construct between low and high cell density samples by week 3 and 4.
Increased GAG content increases the compressive modulus of cell-laden PEG hydrogel while increased cell density reduces the compressive modulus due to the lower stiffness of cellular cytoplasm as well as possible interference with the photopolymerization process. FGF-2/TGF-β1 treated low cell density samples were stiffer than high cell density samples. Equal amounts of GAG content observed in high and low cell density groups indicate that the higher cell density was responsible for the lower compressive modulus. These observations reveal that from a biomechanical perspective the optimum cell density of human chondrocytes for cartilage tissue engineering may also be lower than 20×106 cells/mL. Lower cell density, when combined with appropriate growth and differentiation factors may in fact be advantageous and result in increased ECM production and superior biomechanical properties.
Safranin-O staining showed all groups had significant increase in GAG production from week 1 to 4. FGF-2 alone or in combination with TGF-β1 suppressed GAG production of chondrocytes in the first week, reflecting the inhibition of GAG production in chondrocytes. However, by week 4, cells treated by FGF-2/TGF-β1 demonstrated the highest amount of GAG production as well as a more homogenous intercellular distribution in the hydrogel, suggesting synergy between the two growth factors, which was also consistent with biochemical analysis data. Furthermore, human chondrocyte doublets were found in FGF-2/TGF-β1 treated samples at week 4, with pericellular matrix staining positive for GAG, indicating that proliferating human chondrocytes also maintained the chondrogenic phenotype. On the other hand, we noted a higher number of encapsulated human chondrocytes without visible pericellular GAG production up to the 4th week in culture in the high cell density samples. This finding was consistent with the lower GAG/DNA ratio for the high density group and strengthening our conclusion that a human chondrocyte density of 20×106 cells/mL may be above the optimal range for cartilage tissue engineering. In striking contrast, with FGF-2/TGF-β1 treatment, near 90% cells at lower cell density exhibited pericellular GAG production at week 4.
UV photocrosslinking can be phototoxic. In pilot studies we assessed cell viability against UV light intensity and exposure time. Conventional casting required 10 minutes or greater exposure at optimal intensity and this reduced cell viability by nearly 40%. Our printing process is much faster than conventional casting because substantially less exposure is needed to crosslink the picoliter volume of each droplet. Therefore 3D printing maintained average cell viability at 90%. Unreacted PEGDMA and photoinitiator I-2959 has been characterized and proven to be biocompatible to the mammalian cells (Bryant et al., 2000). Although these results are provocative, additional studies are necessary to evaluate the performance of this growth factor stimulation in the printed and non-printed (cast) cell-laden hydrogels to fully characterize the results and assess optimal cell density. It is also important to evaluate the stimulation of printed neocartilage formation in vivo.
A major challenge constraining the clinical translation of autologous cell-based cartilage repair is the limited source of chondrocytes available during biopsy. A method of expanding cells after bioprinting to the density required for optimal tissue engineering without compromising the quality of the matrix is extremely valuable. One attractive approach is to directly implant harvested autologous chondrocytes or MSCs for cartilage repair without in vitro expansion. Furthermore, in order to maximize the advantage of bioprinting which delivers cells, growth factors, and biomaterial scaffold precisely to the desired 3D position, limiting initial cell density will greatly optimize the delivery precision. Therefore, the approach developed here will be particularly valuable to supplement our bioprinting to deliver cells with optimal resolution in combination with spatially arranged growth factor gradients to fabricate cartilage tissue which recapitulates the zonal architecture of native tissue.
Acknowledgments
The authors would like to acknowledge Melissa Szeto for harvesting human chondrocytes, Lilo Creighton, William Hui, and Margaret Chadwell for helping with histology; Chien-Chi Lin, Shawn Grogan, Diana Brinson, Sujata Sovani, and Akihiko Hasegawa for constructive suggestions and other technical support. This work was funded by the NIH (AG007996), CIRM (TR1-01216), STSI (UL1 RR025774), and NSF (grant#1011796).
References
- Blanco FJ, Ochs RL, Schwarz H, Lotz M. Chondrocyte Apoptosis Induced by Nitric-Oxide. Am J Pathol. 1995;146:75–85. [PMC free article] [PubMed] [Google Scholar]
- Boland T, Xu T, Damon B, Cui X. Application of inkjet printing to tissue engineering. Biotechnol J. 2006;1:910–917. doi: 10.1002/biot.200600081. [DOI] [PubMed] [Google Scholar]
- Boudreaux JM, Towler DA. Synergistic induction of osteocalcin gene expression: identification of a bipartite element conferring fibroblast growth factor 2 and cyclic AMP responsiveness in the rat osteocalcin promoter. J Biol Chem. 1996;271:7508–7515. doi: 10.1074/jbc.271.13.7508. [DOI] [PubMed] [Google Scholar]
- Brandl A, Angele P, Roll C, Prantl L, Kujat R, Kinner B. Influence of the growth factors PDGF-BB, TGF-beta1 and bFGF on the replicative aging of human articular chondrocytes during in vitro expansion. J Orthop Res. 2010;28:354–360. doi: 10.1002/jor.21007. [DOI] [PubMed] [Google Scholar]
- Bryant SJ, Nuttelman CR, Anseth KS. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J Biomater Sci Polym Ed. 2000;11:439–457. doi: 10.1163/156856200743805. [DOI] [PubMed] [Google Scholar]
- Bryant SJ, Anseth KS. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res. 2002;59:63–72. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- Bryant SJ, Chowdhury TT, Lee DA, Bader DL, Anseth KS. Crosslinking density influences chondrocyte metabolism in dynamically loaded photocrosslinked poly(ethylene glycol) hydrogels. Ann Biomed Eng. 2004;32:407–417. doi: 10.1023/b:abme.0000017535.00602.ca. [DOI] [PubMed] [Google Scholar]
- Buskirk WA, Hackleman DE, Hall ST, Kanarek PH, Low RN, Trueba KE, Vandepoll RR. Development of A High-Resolution Thermal Inkjet Printhead. Hewlett-Packard J. 1988;39:55–61. [Google Scholar]
- Buxton AN, Bahney CS, Yoo JU, Johnstone B. Temporal exposure to chondrogenic factors modulates human mesenchymal stem cell chondrogenesis in hydrogels. Tissue Eng Part A. 2011;17:371–380. doi: 10.1089/ten.tea.2009.0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary LR, Avioli LV. Extracellular-signal regulated kinase signaling pathway mediates downregulation of type I procollagen gene expression by FGF-2, PDGF-BB, and okadaic acid in osteoblastic cells. J Cell Biochem. 2000;76:354–359. [PubMed] [Google Scholar]
- Cui X, Boland T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials. 2009;30:6221–6227. doi: 10.1016/j.biomaterials.2009.07.056. [DOI] [PubMed] [Google Scholar]
- Cui X, Dean D, Ruggeri ZM, Boland T. Cell damage evaluation of thermal inkjet printed Chinese hamster ovary cells. Biotechnol Bioeng. 2010;106:963–969. doi: 10.1002/bit.22762. [DOI] [PubMed] [Google Scholar]
- Elisseeff J, McIntosh W, Anseth K, Riley S, Ragan P, Langer R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J Biomed Mater Res. 2000;51:164–171. doi: 10.1002/(sici)1097-4636(200008)51:2<164::aid-jbm4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Farndale RW, Buttle DJ, Barrett AJ. Improved Quantitation and Discrimination of Sulfated Glycosaminoglycans by Use of Dimethylmethylene Blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- Grogan SP, Barbero A, Winkelmann V, Rieser F, Fitzsimmons JS, O’Driscoll S, Martin I, Mainil-Varlet P. Visual histological grading system for the evaluation of in vitro-generated neocartilage. Tissue Eng. 2006;12:2141–2149. doi: 10.1089/ten.2006.12.2141. [DOI] [PubMed] [Google Scholar]
- Harmon JP, Widder JA. Integrating the Printhead Into the Hp Deskjet Printer. Hewlett-Packard J. 1988;39:62–66. [Google Scholar]
- Hoenig E, Winkler T, Mielke G, Paetzold H, Schuettler D, Goepfert C, Machens HG, Morlock MM, Schilling AF. High amplitude direct compressive strain enhances mechanical properties of scaffold-free tissue-engineered cartilage. Tissue Eng Part A. 2011;17:1401–1411. doi: 10.1089/ten.TEA.2010.0395. [DOI] [PubMed] [Google Scholar]
- Hurley MM, Abreu C, Harrison JR, Lichtler AC, Raisz LG, Kream BE. Basic fibroblast growth factor inhibits type I collagen gene expression in osteoblastic MC3T3-E1 cells. J Biol Chem. 1993;268:5588–5593. [PubMed] [Google Scholar]
- Jakob M, Demarteau O, Schafer D, Hintermann B, Dick W, Heberer M, Martin I. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J Cell Biochem. 2001;81:368–377. doi: 10.1002/1097-4644(20010501)81:2<368::aid-jcb1051>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Lin-Gibson S, Bencherif S, Cooper JA, Wetzel SJ, Antonucci JM, Vogel BM, Horkay F, Washburn NR. Synthesis and characterization of PEG dimethacrylates and their hydrogels. Biomacromolecules. 2004;5:1280–1287. doi: 10.1021/bm0498777. [DOI] [PubMed] [Google Scholar]
- Maier R, Ganu V, Lotz M. Interleukin-11, An Inducible Cytokine in Human Articular Chondrocytes and Synoviocytes, Stimulates the Production of the Tissue Inhibitor of Metalloproteinases. J Biol Chem. 1993;268:21527–21532. [PubMed] [Google Scholar]
- Mandl EW, Jahr H, Koevoet JL, van Leeuwen JP, Weinans H, Verhaar JA, Van Osch GJ. Fibroblast growth factor-2 in serum-free medium is a potent mitogen and reduces dedifferentiation of human ear chondrocytes in monolayer culture. Matrix Biol. 2004;23:231–241. doi: 10.1016/j.matbio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Mandl EW, van d V, Verhaar JA, Van Osch GJ. Serum-free medium supplemented with high-concentration FGF2 for cell expansion culture of human ear chondrocytes promotes redifferentiation capacity. Tissue Eng. 2002;8:573–580. doi: 10.1089/107632702760240490. [DOI] [PubMed] [Google Scholar]
- Mann BK, Schmedlen RH, West JL. Tethered-TGF-beta increases extracellular matrix production of vascular smooth muscle cells. Biomaterials. 2001;22:439–444. doi: 10.1016/s0142-9612(00)00196-4. [DOI] [PubMed] [Google Scholar]
- Schmitt B, Ringe J, Haupl T, Notter M, Manz R, Burmester GR, Sittinger M, Kaps C. BMP2 initiates chondrogenic lineage development of adult human mesenchymal stem cells in high-density culture. Differentiation. 2003;71:567–577. doi: 10.1111/j.1432-0436.2003.07109003.x. [DOI] [PubMed] [Google Scholar]
- Sharma B, Williams CG, Kim TK, Sun DN, Malik A, Khan M, Leong K, Elisseeff JH. Designing zonal organization into tissue-engineered cartilage. Tissue Eng. 2007;13:405–414. doi: 10.1089/ten.2006.0068. [DOI] [PubMed] [Google Scholar]
- Wang DA, Varghese S, Sharma B, Strehin I, Fermanian S, Gorham J, Fairbrother DH, Cascio B, Elisseeff JH. Multifunctional chondroitin sulphate for cartilage tissue-biomaterial integration. Nat Mater. 2007;6:385–392. doi: 10.1038/nmat1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WC, Boland T. Cell and organ printing 1: Protein and cell printers. Anatomical Record Part a-Discoveries in Molecular Cellular and Evolutionary Biology. 2003;272A:491–496. doi: 10.1002/ar.a.10057. [DOI] [PubMed] [Google Scholar]
- Xu T, Gregory CA, Molnar P, Cui X, Jalota S, Bhaduri SB, Boland T. Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials. 2006;27:3580–3588. doi: 10.1016/j.biomaterials.2006.01.048. [DOI] [PubMed] [Google Scholar]
- Yaeger PC, Masi TL, de Ortiz JL, Binette F, Tubo R, McPherson JM. Synergistic action of transforming growth factor-beta and insulin-like growth factor-I induces expression of type II collagen and aggrecan genes in adult human articular chondrocytes. Exp Cell Res. 1997;237:318–325. doi: 10.1006/excr.1997.3781. [DOI] [PubMed] [Google Scholar]