Abstract
Severe keratinocyte dysplasia (SKD) has been reported as a common event in the early post-transplant period of hematopoietic stem cell transplant (HCST) patients.1 The purpose of our study is to determine the possible causes of SKD during the intermediate post-transplant period, and to ascertain its prevalence in skin biopsies. Skin biopsy slides, obtained from HCST recipients who were days 28 to 84 post transplant, were evaluated for SKD. Forty-four examples of SKD were identified in 467 slides, or 9%. Thirty-seven patients were evaluated as cases in a case-control design. SKD was strongly associated with a conditioning regimen containing busulfan with an odds ratio (OR) of 7.25 (p=0.0002). In a multivariate adjusted analysis, SKD was not associated with cyclophosphamide, fludarabine, total body irradiation (TBI), or a nonmyeloablative conditioning regimen. SKD was not associated with clinical acute graft-versus-host disease (GVHD). SKD histology gradually resolved, reaching a normal histology after an average of 241 days. This study finds that severe keratinocyte dysplasia in the period 28 to 84 days post hematopoietic stem cell transplantation is strongly associated with a busulfan conditioning regimen.
Keywords: Skin, Dysplasia, Transplantation, Busulfan
INTRODUCTION
In hematopoietic stem cell transplant recipients we noted the sporadic appearance, in skin biopsies, of severe keratinocyte dysplasia (SKD). The histologic appearance seems distinct from graft-versus-host disease. SKD is characterized by large keratinocytes with large and often irregular nuclei. GVHD is typically characterized by apoptotic keratinocytes and a lymphoid infiltrate.
SKD has been described as a common, albeit poorly documented, effect of chemotherapy in the non-transplant population, and there is a suggestion it is associated with drugs that can disrupt the keratinocyte cell cycle, particularly alkylating agents. 2
There are 2 reports of skin dysplasia in marrow transplant recipients. Castaño et al. found that severe keratinocyte dysplasia is a frequent histologic finding in patients who have had a bone marrow or liver transplant within 60 days post-transplant, being present in 40.9% of this mixed population.1 Although some of the cases occurred in the absence of any chemotherapy, pretreatment with the alkylating agent cyclophosphamide was found to be the only independent chemotherapeutic agent significantly associated with a higher risk of severe dysplasia.
In contrast, Hymes et al. found the incidence of severe keratinocyte dysplasia to be 92% in marrow transplant recipients prepared with the alkylating agent busulfan. SKD was restricted to the interval 15 to 45 days post-transplant.3 They further speculated that mild keratinocyte dysplasia early post transplant, with a median of 13 days, was due to cyclophosphamide.
We observed significant numbers of severe dysplasia in skin biopsies of patients more than three weeks post-hematopoietic stem cell transplant. We set out to determine the incidence of SKD in the post-transplant period from 28 to 84 days and find any association with chemotherapeutic agents, transplantation type, or graft versus host disease.
MATERIALS AND METHODS
Case Identification
We constructed a case-control study using skin biopsy slides from the Fred Hutchinson Cancer Research Center–Seattle Cancer Care Alliance, Seattle, Washington. All skin biopsy slides from 2002 and 2003 that had been obtained from 28 to 84 days after hematopoietic stem cell transplantation were examined, including autografts and non-myeloablative transplants. Most of the skin biopsies were obtained to evaluate the possible presence of cutaneous GVHD. The biopsies were re-examined blindly without knowledge of clinical data or histologic diagnosis.
The stained slides were examined for evidence of keratinocyte dysplasia, which is characterized by disruption of the normal keratinocyte maturation with enlarged cells, enlarged nuclei, irregular nuclear contours, prominent nucleoli, multi-nucleation, as well as frequent normal and abnormal mitotic figures. The process can be focal, multifocal or diffuse, with partial or full epidermal thickness involvement. Only the slides with severe keratinocyte dysplasia (SKD) were included in our study, which is defined as dysplastic keratinocytes that have enlarged nuclei at least twice the diameter of normal cell nuclei, and the extent of dysplasia is at least multifocal, with involvement of the superficial spinous layers.
Case and Control Selection Criteria
A 1:2 case-control design was employed. Only slides from patients following the first hematopoietic stem cell transplant were eligible for the study, and slides from patients post second or third transplants excluded. Cases consisted of all patients with a slide showing severe keratinocyte dysplasia. If a patient had slides showing SKD on more than one date, the slide from the latest date was chosen as the case slide. Two control patients were selected for each case from the set of patients who never showed SKD in a skin biopsy from days 28 to 84. Controls were matched to cases according to days post transplant and age {Minimum of [(absolute value of difference in days post transplant) plus (absolute value of difference in age in years)]}, and each control patient was chosen only once.
GVHD Evaluation
All the case and control slides were examined for histologic evidence of acute GVHD (histologic GVHD) by staff unaware of clinical characteristics or diagnosis. GVHD was graded according to the grading system of Sale et al.,4,5 which requires dyskeratotic keratinocytes (apoptotic cells, “eosinophilic bodies”) in numbers excessive for site, usually but not invariably associated with exocytosis of lymphocytes in the epidermis, the presence of lymphocytes in the dermis, and basal vacuolar changes. Clinical acute GVHD (clinical GVHD) was assessed based on overall severity in all organs by a standard system of evaluation.6 The evaluation of clinical GVHD did not include any histology information. Only histological and clinical grades 2, 3 and 4 (out of 4) were considered positive for acute GVHD for the purposes of our study. The relationships between SKD and donor and patient factors were analyzed using conditional logistic regression, stratified on each case-control set.
Evaluation of Course
To determine the course of SKD, all available subsequent skin biopsies of the cases were examined and evaluated for dysplasia, and less severe changes.
RESULTS
Frequency Determination, Case and Control Selection
A total of 467 skin biopsies were examined. They derived from 352 individuals. Each biopsy was assessed for severe keratinocyte dysplasia (Figure 1). The rate of SKD was 9% of the skin biopsies (44/467 skin biopsies), and 12% of the biopsied individuals (41/352 individuals). Excluding multiple biopsies from the same patient (3) and 2nd and 3rd transplants (4), 37 Cases were identified. Seventy-four age and day post-transplant matched controls were assigned in the 1:2 design. The mean age of cases was 49.6 years and of controls 49.3 years. The mean day of biopsy post-transplant was 61.0 days for cases and 60.9 days for controls.
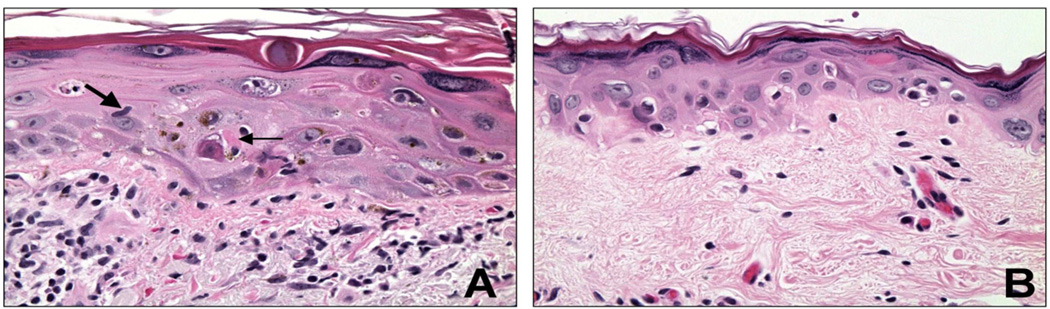
Figure 1.
A) Severe keratinocyte dysplasia (SKD) and GVHD in a patient 35 days post-transplant. The skin exhibits enlarged cells, enlarged nuclei, irregular nuclear contours, and prominent nucleoli. Keratohyaline granules are prominent. A fragmenting nucleus may be seen in the mid epidermis on the left. This skin additionally shows evidence of GVHD, with a lymphocyte (large arrow) and apoptotic cell (small arrow), and abundant lymphocytes in the dermis. B) SKD in a patient 77 days post-transplant. There is epidermal atrophy, along with the features of SKD of enlarged cells, enlarged nuclei, and prominent nucleoli.
Univariate Risk Factors for SKD
The univariate risk factors for severe keratinocyte dysplasia are presented in Table 1. Donor type, whether a matched sibling, an unrelated donor, or an autologous donor was not associated with SKD. The diagnosis of myelodysplastic syndrome (MDS), which also includes non-CML myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms, was significantly associated with SKD in the univariate analysis (p=0.002), with an odds ratio (OR) of 3.16 as compared to acute myeloid leukemia. The conditioning regimen was also significantly associated with SKD, with a busulfan-containing regimen showing an OR of 7.25 (p=0.0002) compared to regimens without busulfan. A cytoclophosphamide containing regimen was also significantly associated with SKD (OR 4.21, p=0.02). A fludarabine containing regimen was significantly associated, with a lower risk of SKD (OR 0.15, p=0.004). Total body irradiation was also associated with a lower risk of SKD (p=0.007), with the application of the low level of radiation, 200 cGy, showing significance with an OR of 0.14. Both fludarabine and 200 cGy of TBI are typically restricted to nonmyeloablative transplants. Any nonmyeloablative conditioning regimen was similarly significantly negatively associated with SKD (OR 0.16, p=0.009).
TABLE 1.
Univariate risk factors for severe keratinocyte dysplasia
| Controls N (%) |
Cases N (%) |
OR (95% CI) | P-value | |
|---|---|---|---|---|
| Gender | ||||
| Female | 31 (42%) | 17 (46%) | 1.0 | 0.67 |
| Male | 43 (58%) | 20 (54%) | 0.84 (0.4–1.9) | |
| Donor type | ||||
| Matched sib | 24 (32%) | 12 (32%) | [1.0] | 0.99 |
| Unrelated | 42 (57%) | 21 (57%) | 1.00 (0.4–2.5) | |
| Autologous | 4 (5%) | 2 (5%) | 1.00 (0.2–6.6) | |
| Other related | 4 (5%) | 2 (5%) | 1.00 (0.2–5.9) | |
| Diagnosis | ||||
| AML | 32 (43%) | 12 (32%) | [1.0] | 0.002 |
| CML | 9 (12%) | 2 (5%) | 0.58 (0.1–3.2) | |
| MDS | 17 (23%) | 21 (57%) | 3.16 (1.2–8.1) | |
| Other | 16 (22%) | 2 (5%) | 0.28 (0.1–1.4) | |
| Conditioning | ||||
| Busulfan in conditioning regimen | ||||
| No | 36 (49%) | 6 (16%) | 1.0 | 0.0002 |
| Yes | 38 (51%) | 31 (84%) | 7.25 (2.1–25) | |
| Cytoxan in conditioning regimen | ||||
| No | 22 (30%) | 5 (14%) | 1.0 | 0.02 |
| Yes | 52 (70%) | 32 (86%) | 4.21 (1.1–16) | |
| Fludarabine in conditioning regimen | ||||
| No | 54 (73%) | 34 (92%) | [1.0] | 0.004 |
| Yes | 20 (27%) | 3 (8%) | 0.15 (0.0–0.7) | |
| TBI in conditioning regimen | ||||
| None | 42 (57%) | 31 (42%) | 1.0 | 0.007 |
| 200 cGy | 16 (22%) | 2 (6%) | 0.14 (0.0–0.7) | |
| >200 cGy | 16 (22%) | 3 (8%) | 0.32 (0.1–1.2) | |
| Nonmyeloablative conditioning | ||||
| No | 58 (78%) | 35 (95%) | 1.0 | 0.009 |
| Yes | 16 (22%) | 2 (5%) | 0.16 (0.0–0.8) | |
| GVHD | ||||
| Clinical acute GHVD (Grade 2–4) | ||||
| No | 22 (30%) | 9 (24%) | 1.0 | 0.53 |
| Yes | 52 (70%) | 28 (76%) | 1.35 (0.5–3.4) | |
| Histological GVHD (Grade 2–4) | ||||
| No | 29 (39%) | 8 (22%) | 1.0 | 0.05 |
| Yes | 45 (61%) | 29 (78%) | 2.61 (1.0–7.0) | |
Multivariate Risk Factors for SKD
To determine what factors were independently associated with SKD, a multivariate model was adopted. After adjustment for the use of busulfan, none of the other conditioning-related factors or the diagnoses was significantly associated with SKD (Table 2). Conversely, the use of busulfan remained significant even after adjustment for these factors. Thus, the apparent associations of SKD with cyclophosphamide, fludarabine, TBI, nonmyeloablative conditioning, and diagnosis are largely explained by the degree to which these factors are associated with the use of busulfan.
TABLE 2.
Multivariate analysis adjusting for the presence of busulfan in the conditioning regimen
| Adjusted* | ||
|---|---|---|
| OR (95% CI) | P | |
| Diagnosis | ||
| AML | [1.0] | 0.24 |
| CML | 0.49 (0.1–2.7) | |
| MDS | 2.00 (0.7–5.7) | |
| Other | 0.46 (0.1–2.7) | |
| Conditioning | ||
| Cytoxan in conditioning regimen | ||
| No | [1.0] | 0.63 |
| Yes | 1.44 (0.3–6.6) | |
| Fludarabine in conditioning regimen | ||
| No | [1.0] | 0.30 |
| Yes | 0.41 (0.1–2.4) | |
| TBI in conditioning regimen | ||
| None | [1.0] | 0.20 |
| 200 cGy | Undefined | |
| >200 cGy | Undefined | |
| Nonmyeloablative conditioning | ||
| No | [1.0] | 0.49 |
| Yes | 0.51 (0.1–3.4) | |
Each factor adjusted for busulfan in conditioning regimen
GVHD and SKD
There was no evidence that clinical GVHD, as assessed without involving pathology data, is associated with SKD (Table 1).
The possible presence of histological GVHD was evaluated in the same skin slide as examined for SKD. The evaluation of GVHD in the SKD cases proved problematic, with difficulty applying the criteria for GVHD diagnosis in skin with a background of large, dysplastic keratinocytes, which might be expected to show more apoptotic cells than normal skin. The univariate analysis showed that SKD was associated with GVHD (OR 2.61, p=0.05) (Table 1).
It is possible that busulfan is associated with both SKD and GVHD-like histologic changes in skin, but is not actually related to clinical GVHD. This would result is the association of busulfan with “histological GVHD”, but not clinical GVHD. To study this possibility, only those patients without clinical GVHD were examined, with the 6 autologous patients excluded. The presence or absence of histological GVHD was stratified for the presence or absence of busulfan treatment (Table 3). Histological GVHD was not significantly associated with busulfan, with 67% of those with busulfan showing GVHD, compared to 50% of those with no busulfan (p=0.55, Fisher exact test). These findings do not support busulfan as an agent related to the diagnosis of histologic GVHD.
TABLE 3.
Histologic GVHD in patients without clinical acute GVHD, stratified according to busulfan treatment*
| Histologic GHVD | ||
|---|---|---|
| GVHD (% of row) | No GVHD | |
| Busulfan | 10 (67%) | 5 |
| No Busulfan | 5 (50%) | 5 |
Autografts are excluded
Evaluation of Course of SKD
To approximate the course of SKD subsequent skin biopsies were examined. The first available biopsy subsequent to the biopsy showing SKD was evaluated. These were often beyond the 84 day post-transplant criterion used for initial inclusion in the study. Twenty-nine cases were available. SKD was found in none on them. The appearance ranged from a lesser degree of keratinocyte dysplasia not reaching the criteria for SKD (7%), to the less damaged moderate keratinocyte atypia exhibiting prominent nucleoli (21%), to slight keratinocyte atypia exhibiting visible nucleoli (52%), to normal (21%). The interval from the time of the SKD biopsy averaged 32, 45, 193, and 241 days, for each appearance type, respectively. These findings suggest that SKD starts resolving within a period of 32 days but reaches normal morphology many months later, averaging 241 days.
Incidence of SKD
To estimate the frequency of busulfan associated SKD in the biopsied patients, we calculated an incidence rate in the individuals prepared with busulfan. Approximately 199 patients were treated with busulfan (extrapolating from the fraction of treated controls and cases), resulting in 31 busulfan-associated SKD cases (Table 1). This suggests, in biopsied patients conditioned with busulfan, 16% developed SKD in the day 28 to 84 post-transplant interval.
DISCUSSION
We investigated severe keratinocyte dysplasia in the day 28 to 84 of the post-transplant period in a case-control study. This design is well suited for ascertaining possible associations with pretransplant factors. In a multivariate model, a preparative regimen containing busulfan was strongly associated with SKD.
The prevalence of SKD was 9% of the biopsies, 12% of the HSCT recipients, and 16% of those prepared with busulfan. Those studied were limited to patients who underwent skin biopsies in the day 28 to 84 time period, and represent about half the patients who were transplanted. Skin biopsies are often taken of clinical lesions, and are routinely taken with or without lesions at departure or about day 80 post transplant. Therefore these estimates only provide approximate frequencies based on a large number of skin biopsies, and do not provide precise population based frequencies. The calculated prevalence of busulfan associated SKD may be subject to further slight inaccuracy due to the sampling variance of controls and the inclusion of censored individuals such as second transplant recipients. Nevertheless, the estimated 16% incidence in busulfan treated patients is the most reliable available for this pathological finding.
Keratinocyte dysplasia following chemotherapy have been described infrequently, but is now well documented to actually occur at high frequencies. It has been variously described as keratinocyte atypia, keratinocyte dysmaturation, or epidermal dysmaturation.2 The cause of the epidermal dysmaturation has been postulated to be a direct cytotoxic action of chemotherapeutic drugs or radiation given before engraftment, affecting keratinocytes.2 In non-transplant patients, these agents have been associated with a specific skin pathology exhibiting an attenuated epidermis, individual keratinocyte necrosis, and cell poor interface dermatitis.7–9 The agents include cyclophosphamide, busulfan, cyclosporine, etoposide, docetaxel, thiotepa, cytarabine, bleomycin, hydroxyurea, methotrexate, and others.
Etoposide and busulfan have been associated with specific pathological abnormalities.3,7,10 The specific keratinocyte pathology associated with busulfan has been described as “‘busulfan’ cells that are abnormally large keratinocytes with extremely large nuclei…[that] demonstrate irregular nuclear contours, bizarre chromatin patterns, and prominent keratohyaline granules.” 7 This description is similar to, but generally more marked than, our independently determined criteria defining severe keratinocyte dysplasia.
The severe form of keratinocyte dysplasia has been described in post-transplant patients as a common lesion, more frequent early post transplant.1,3,4 Castaño et al. reported a 44% incidence of severe keratinocyte dysplasia in the post marrow or liver transplant recipients in the period less than 60 days post transplant.1 They found an independent association with cyclophosphamide and not busulfan.
Hymes et al. found the incidence of severe keratinocyte dysplasia to be 92% in busulfan treated marrow transplant recipients in the interval 15 to 45 days post transplant.3 Since the dose of busulfan was similar to ours (total 16 mg/kg), one possible explanation of the markedly higher incidence is the earlier procurement of the skin biopsies. With weekly biopsies, virtually all of their cases would have been biopsied twice in the 15 to 27 day period, during which they could have found SKD. Our patients were biopsied on day 28 at the earliest. The interval difference is reflected in their median day post-transplant of 35, versus our mean of 61. They further separated out slight dysplasia present early post transplant, median 13 days, and ascribed it to the cyclophosphamide in a TBI-CY regimen, as had Sale et al.4
The most likely explanation is that cyclophosphamide is commonly associated with slight keratinocyte dysplasia early post-transplant and busulfan is commonly associated with severe keratinocyte dysplasia, lasting in some smaller number of cases, later into the post-transplant period.
One of the challenges presented by chemotherapy toxicity occurring after marrow transplantation is its histologic similarity to skin graft-versus-host disease.4,11 However, chemo-radiation toxicity was not associated with findings diagnostic of histologic GVHD after 20 days post transplant.4,12 Others have also found that keratinocyte dysplasia was not associated with the onset of acute GVHD.1,3
Our data suggest that SKD is not associated with clinical GVHD but is associated with histological GVHD (OR 2.61, p=0.05). It is evident from Table 3 that a busulfan preparative regimen fails to be associated with histologic GVHD. This leaves open the possibility that histologic GVHD may tend to be diagnosed in the presence of concurrent SKD. Table 3 also implies that histological GVHD was sometimes diagnosed in the absence of clinical GVHD. This is a widely reported phenomenon, presumably due to the relatively low specificity of the histologic criteria.7,13,14 Further conclusions regarding GVHD are beyond the power of this casecontrol study of SKD.
“What happens to severe keratinocyte dysplasia?” The time course of SKD was studied by evaluating subsequent skin biopsies, when available. A statistical treatment was not attempted. It turned out that SKD resolved rapidly, appearing as slight dysplasia in 32 days on average, and moderate atypia in 45 days. Normal histology was reached only slowly, however, after an average of 241 days following the index biopsy. The conclusion that SKD reverts gradually to normal histology over several months is warranted.
In summary, our study has provided evidence that severe keratinocyte dysplasia is an occasional histologic finding in the day 28 to 84 of the post-transplant period of hematopoietic stem cell transplant patients, with a frequency of 9% of the biopsies, 12% of the biopsied patients, and 16% of biopsied patients prepared with a busulfan-containing conditioning regimen. Preparation for transplant with busulfan was independently and very strongly associated with SKD. Familiarity with this potential association is important for the correct diagnosis when evaluating biopsies from this particular patient population.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institutes of Health CA18029, CA15074, and HL36444.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURE STATEMENT
Authors must disclose any primary financial relationship with a company that has a direct financial interest in the subject matter or products discussed in the submitted manuscript, or with a company that produces a competing product. These relationships (such as honoraria, ownership of stock, patents, or consulting fees) and any direct support of research by a commercial company will be included in the Acknowledgements section of the published article.
N. Li: Nothing to disclose.
K. Guthrie: Nothing to disclose.
B Storer: Nothing to disclose.
P. Martin: Nothing to disclose.
G. Sale: Nothing to disclose.
Z. Argenyi: Nothing to disclose.
D. Myerson: Nothing to disclose.
REFERENCES
- 1.Castaño E, Rodriguez-Peralto JL, Lopez-Rios F, Gomez C, Zimmermann M, Iglesias Diez L. Keratinocyte dysplasia: an usual finding after transplantation or chemotherapy. J Cutan Pathol. 2002;29:579–584. doi: 10.1034/j.1600-0560.2002.291002.x. [DOI] [PubMed] [Google Scholar]
- 2.Henry LB, Horn TD. Chemotherapy and keratinocytes. J Cutan Pathol. 2002;29:575–578. doi: 10.1034/j.1600-0560.2002.291001.x. [DOI] [PubMed] [Google Scholar]
- 3.Hymes SR, Simonton SC, Farmer ER, Beschorner WB, Tutschka PJ, Santos W. Cutaneous busulfan effect in patients receiving bon-marrow transplantation. J Cut Pathol. 1985;12:125–129. doi: 10.1111/j.1600-0560.1985.tb01613.x. [DOI] [PubMed] [Google Scholar]
- 4.Sale GE, Lerner KG, Barker EA, Shulman HM, Thomas ED. The skin biopsy in the diagnosis of acute graft-versus-host disease in man. Am J Pathol. 1977;89:621–635. [PMC free article] [PubMed] [Google Scholar]
- 5.Sale GE. Pathology and recent pathogenetic studies in human graft-versus-host disease. Surv Synth Path Res. 1984;3:235–253. doi: 10.1159/000156929. [DOI] [PubMed] [Google Scholar]
- 6.Martin PJ, Nelson BJ, Appelbaum FR, Anasetti C, Deeg HJ, Hansen JA, et al. Evaluation of a CD5-specific immunotoxin for treatment of acute graft-versus-host disease after allogeneic marrow transplantation. Blood. 1996;88:824–830. [PubMed] [Google Scholar]
- 7.Fitzpatrick TB. The cutaneous histopathology of chemotherapeutic reactions. J Cutan Pathol. 1993;20:1–14. doi: 10.1111/j.1600-0560.1993.tb01242.x. [DOI] [PubMed] [Google Scholar]
- 8.Burgdorf WHC. Cutaneous reactions to chemotherapeutic agents. In: Arndt KA, Leboit PE, Robinson JK, Wintroub BU, editors. Cutaneous Medicine and Surgery. 1st ed. Philadelphia, USA: W.B. Saunders Company; 1996. p. 426. [Google Scholar]
- 9.Horn T. Cutaneous toxicities of drugs. In: Elder D, Elenitsas R, Jaworsky C, Johnson B Jr, editors. Lever’s Histopathology of the Skin. 8th ed. Philadelphia, Pa: Lippincott-Raven; 1997. pp. 289–291. [Google Scholar]
- 10.Yokel BK, Friedman KJ, Farmer ER, Hood AF. Cutaneous pathology following etoposide therapy. J Cutan Pathol. 1987;14:326–330. doi: 10.1111/j.1600-0560.1987.tb01532.x. [DOI] [PubMed] [Google Scholar]
- 11.Horn TD. Acute cutaneous eruption after marrow ablation: roses by other names? J Cutan Pathol. 1994;21:385. doi: 10.1111/j.1600-0560.1994.tb00277.x. [DOI] [PubMed] [Google Scholar]
- 12.LeBoit PE. Subacute radiation dermatitis: a histologic imitator of acute cutaneous graft-versus-host disease. J Am Acad Dermatol. 1989;20:236–241. doi: 10.1016/s0190-9622(89)70028-1. [DOI] [PubMed] [Google Scholar]
- 13.Kohler S, Hendrickson MR, Chao N, Smoller BR. Value of skin biopsies in assessing prognosis and progression of acute graft-versus-host disease. Am J Surg Pathol. 1997;21:988–996. doi: 10.1097/00000478-199709000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Cutler C, Antin JH. Manifestations and treatment of acute graft-versus-host disease. In: Appelbaum F, Forman SJ, Negrin RS, Blume KG, editors. Thomas’ hematopoietic cell transplantation. 4th ed. Chichester, West Sussex: Wiley-Blackwell; 2009. pp. 1287–1303.pp. 1291 [Google Scholar]