With its rapid conceptual leaps, cell/molecular biology has converged with the accelerating trajectory of bioengineering, and the outcome of this encounter suggests that replacement of organs is either here or not faraway. Historically, five methods have been used to tackle the problem of the missing organ: transplantation, which suffers from depletion of organ donors; autografting, excising part of a patient's organ to graft and restore organ function in another part of the anatomy where it is needed more; replacement with a permanent prosthesis, which turns out not to be permanent at all, requiring revision surgery after a few to several years of implantation; in vitro synthesis, a popular research direction, in which an organ is first largely synthesized in culture and then is implanted; and in vivo synthesis, which depends on implantation only of the bare minimum required to induce organ regeneration in situ. The last two paradigms are commonly referred to collectively as tissue engineering.
In an article in a recent issue of PNAS, Schechner and colleagues (1) combine in vitro and in vivo approaches. The authors report a significant improvement in methodology that may turn out to be of great value in a frustrating area of clinical research: the treatment of the elderly patient who suffers from pressure (bed) sores. These chronically open wounds, or skin ulcers, typically result from lack of appropriate vascular supply to the skin. If prolonged enough, the lack of blood supply leads to necrosis of the skin and impairment of the mechanism for wound closure. The authors report new methodology for the enrichment of vascular structures in host skin by implanting a skin substitute.
The vascular architecture of skin is quite sophisticated (Fig. 1). Of the two major tissues in skin, the outer epidermis is completely avascular and depends on the underlying dermis for its metabolic support. Blood flow inside the dermis not only plays a nutritional role for the entire organ but also serves as a thermoregulatory device for the organism: e.g., increased blood flow facilitates heat loss in hot conditions. The arteries supplying the skin are located deep below the dermis; from there, two local distribution systems (cutaneous plexus and subpapillary plexus) branch off to supply all parts of the organ. In particular, the subpapillary plexus supplies the epidermis by means of a capillary (papillary) loop inside each of the upward projections of the dermis (dermal papillae). The junction between the epidermis and the dermis is characterized by downward folds of the epidermis (rete ridges), which interdigitate with the dermal papillae (Fig. 1). The “egg carton” topology of the junction maximizes the area of contact between epidermis and dermis, and thus enhances the adhesion of the two tissues to each other, as well as increasing the area for metabolite transfer to the avascular epidermis (2). This interesting architecture of blood vessels must be duplicated after implantation of a skin substitute.
Figure 1.
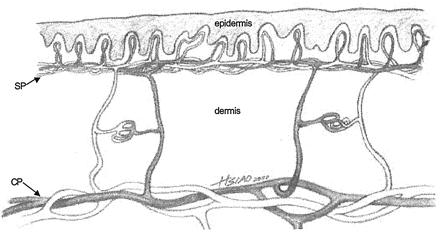
Blood circulation of the skin near the dermal-epidermal junction. Two local distribution systems (cutaneous plexus, CP, and subpapillary plexus, SP) are shown. The subpapillary plexus supplies the epidermis by means of a capillary (papillary) loop inside each of the upward projections of the dermis (dermal papillae). (Adapted from ref. 2.)
Skin has been synthesized in vivo by induced regeneration. This approach, the fifth listed above, consists of synthesizing the appropriate insoluble (nondiffusible) regulator, an analog of extracellular matrix referred to as dermis regeneration template, and seeding it with uncultured autologous keratinocytes (3, 4). The cell-seeded template then is grafted onto a standardized skin-free wound in a rodent model or the swine, prepared by meticulously excising all of the epidermis and the dermis over a substantial area (full-thickness excision). In this protocol, the time spent in culture media in vitro is near zero; almost all of the synthesis takes place in vivo, after implantation to the host. The host's fibroblasts, as well as exogenous keratinocytes, migrate into the molecular scaffold; enzymatic degradation of the scaffold proceeds while new tissue is being synthesized at the skin-free site. Provided that the structure of the nondiffusible regulator, a highly porous macromolecular network, has been maintained within narrowly defined limits (5), a new dermis and an epidermis are simultaneously synthesized at the same rate that the template is being broken down enzymatically. The nondiffusible regulator loses its regenerative activity if its structure does not include the appropriate ligands for binding to the corresponding integrin cell receptors. Activity also is lost if ligand density or if the residence time of the template in vivo have not been adjusted within critical limits (6). Exogenously supplied keratinocytes and endogenous fibroblasts use the template to induce regeneration of the organ at the initially skin-free site. Although the newly synthesized organ has the morphology and function of skin (7, 8) it is less than fully physiological: it lacks appendages, including sweat glands. However, the vascular supply of the new dermis is rich. In particular, the morphology of the dermal papillae is largely restored by the induced regeneration process (Fig. 2).
Figure 2.
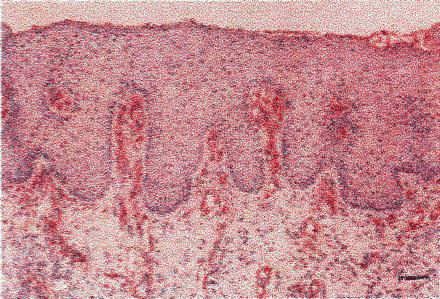
Synthesis of skin in vivo (induced regeneration). Dermis regeneration template, an extracellular matrix analog, was seeded with uncultured autologous keratinocytes and grafted onto a standardized, skin-free (full thickness) wound in the swine. Capillary loops are shown inside the regenerated dermal papillae, detected by immunostaining for factor VIII at day 35 postgrafting. (Scale bar: 75 μm.) [Reproduced with permission from ref. 8. (Copyright 1998, Journal of Investigative Dermatology).]
The in vitro approach relies on first constructing in vitro a “skin equivalent” (9–14). In contrast to the in vivo protocol described above, this methodology consists of extensive in vitro culture of keratinocytes and fibroblasts in a contracted collagen gel, to yield a bilayer consisting of an epidermis and a connective tissue layer, which is grafted on skin-free wounds. A new skin, consisting of an epidermis and a dermis, is synthesized after implantation and subsequent remodeling inside the host (9–14). In addition to lacking appendages, the new skin lacks the downward folds of the epidermis (rete ridges) (9–14); however, these folds harbor the interdigitating dermal papillae that supply the epidermis (Figs. 1 and 2). A possible explanation for the eventual lack of physiological vascularization using this protocol is suggested by Schechner and coworkers (1). When these authors cultured endothelial cells in a collagen-fibronectin gel they observed spontaneous reorganizations into multicellular cords, resembling incipient vessels; however, the cells soon thereafter showed morphologic evidence of apoptosis and died (1).
Schechner and coworkers (1) genetically modified human endothelial cells by retroviral transduction with exogenous Bcl-2 protein. Transduced cells remained viable in culture for days, whereas the controls died early; by day 7, the transduced cells had formed capillary-like structures, a demonstration of the success of this type of transduction in preventing apoptosis in vitro. After grafting under the skin of immunodeficient mice, “vascular beds” consisting of cells transduced by Bcl-2 became differentiated to endothelial-lined vascular structures at much higher density and with much higher branching than cells that had been transduced with a control (green fluorescence protein). In addition, blood vessels formed from cells transduced with Bcl-2 became invested with an outer layer of partly differentiated vascular smooth muscle cells from the murine host whereas control preparations did not. In summary, these investigators (1) succeeded in transforming the protocol for synthesis of the skin equivalent into one that provided for lengthy vascular remodeling in vitro and eventually yielded small arterioles, venules, and capillaries after implantation in vivo.
Can one eventually expect the Bcl-2 transduced endothelial cells to yield dermal papillae? The authors' methodology represents a decided and encouraging improvement; nevertheless, their findings suggest that, in future investigations, an effort could be profitably made to identify the appropriate nondiffusible regulator. Although Schechner and coworkers (1) switched from the type I collagen gel originally used (9–14) to a type I collagen-fibronectin gel, this hardly represents an exhaustive search for the most active nondiffusible regulator. The experience with in vivo synthesis of skin, described above, suggests that such a search would be well worth the effort.
Support for such a future approach further comes from an analysis of the data obtained by a large number of independent investigations conducted either primarily in vitro or in vivo (15). The investigators sought to identify the conditions required for synthesis of two organs, skin and peripheral nerves, as well as their individual tissue components (Fig. 3). The analysis was based on the tissue triad that characterizes the anatomy of most organs: the three tissue layers consisting of the basement membrane at the center, the avascular and extracellular matrix-free, epithelia-like tissue on one side of it and the supporting tissue on the other side, which comprises vacularized connective tissue (2). In skin, the tissues flanking the basement membrane are the epidermis and the dermis; in peripheral nerves, they are the myelinating Schwann cells, long recognized as epithelium-like cells (16), on one side and the endoneurium-perineurium on the other side of the basement membrane (basal lamina); in blood vessels, they are the intima layer comprising endothelial cells on one side and the media-adventitia layer on the other (2).
Figure 3.
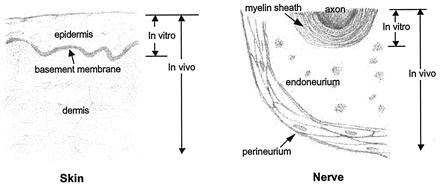
Schematic of empirical conditions required for synthesis of two organs, skin and a peripheral nerve. The sketch shows tissue components that have been synthesized by using the simplest synthetic pathway, i.e., that requiring the smallest number of reactants. Other pathways also have been successfully used (15). In each organ, synthesis of the epithelium-like layer and the basement membrane can be accomplished in vitro; however, synthesis of the supporting (connective) tissue requires an in vivo protocol.
The empirical observations of several independent investigators generally show that in vitro conditions suffice to synthesize the avascular, epithelium-like tissue on one side of the basement membrane but not the vascularized, supporting (connective) tissue on the other side. Furthermore, while syntheses of the epithelium-like tissues and the basement membrane have been observed in the absence of nondiffusible regulators, synthesis of supporting tissues requires their presence (15). For example, synthesis of a near-physiological peripheral nerve connecting two transected nerve stumps across a gap (17) and synthesis of a physiological conjunctiva in a conjunctiva-free bed, which had been prepared by excision to bare sclera (18), are two instances, in addition to the case of skin mentioned above, in which in vivo synthesis of the new organ required the presence of the appropriate nondiffusible regulator. Increased understanding of the conditions required for organ synthesis suggests that rapid advances in the field of organ replacement would follow in future studies from a realization of the requirement for appropriate extracellular matrix-like regulators.
Footnotes
See companion article on page 9191 in issue 16 of volume 97.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.180313497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.180313497
References
- 1.Schechner J S, Nath A K, Zheng L, Kluger M S, Hughes C C W, Sierra-Honigmann M R, Lorber M I, Tellides G, Kashgarian M, Bothwell A L M, Pober J. Proc Natl Acad Sci USA. 2000;97:9191–9196. doi: 10.1073/pnas.150242297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burkitt H G, Young B, Heath J W. Wheater's Functional Histology. 3rd Ed. New York: Livingstone; 1993. [Google Scholar]
- 3.Yannas I V, Burke J F, Warpehoski M, Stasikelis P, Skrabut E M, Orgill D, Giard D J. Trans Am Soc Artif Intern Organs. 1981;27:19–22. [PubMed] [Google Scholar]
- 4.Yannas I V, Burke J F, Orgill D P, Skrabut E M. Science. 1982;215:174–176. doi: 10.1126/science.7031899. [DOI] [PubMed] [Google Scholar]
- 5.Yannas I V, Lee E, Orgill D P, Skrabut E M, Murphy G F. Proc Natl Acad Sci USA. 1989;86:933–937. doi: 10.1073/pnas.86.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yannas I V. Ann NY Acad Sci. 1997;831:280–293. doi: 10.1111/j.1749-6632.1997.tb52203.x. [DOI] [PubMed] [Google Scholar]
- 7.Murphy G F, Orgill D P, Yannas I V. Lab Invest. 1990;62:305–313. [PubMed] [Google Scholar]
- 8.Compton C C, Butler C E, Yannas I V, Warland G, Orgill D P. J Invest Dermatol. 1998;110:908–916. doi: 10.1046/j.1523-1747.1998.00200.x. [DOI] [PubMed] [Google Scholar]
- 9.Bell E, Ehrlich H P, Buttle D J, Nakatsuji T. Science. 1981;211:1052–1054. doi: 10.1126/science.7008197. [DOI] [PubMed] [Google Scholar]
- 10.Bell E, Sher S, Hull B, Merrill C, Rosen S, Chamson A, Asselineau D, Dubertret L, Coulomb B, Lapiere C, et al. J Invest Dermatol. 1983;81:2s–10s. doi: 10.1111/1523-1747.ep12539993. [DOI] [PubMed] [Google Scholar]
- 11.Hull B E, Sher S E, Rosen S, Church D, Bell E. J Invest Dermatol. 1983;81:436–438. doi: 10.1111/1523-1747.ep12522605. [DOI] [PubMed] [Google Scholar]
- 12.English K B, Stayner N, Krueger G G, Tuckett R P. J Invest Dermatol. 1992;99:120–128. doi: 10.1111/1523-1747.ep12616765. [DOI] [PubMed] [Google Scholar]
- 13.Nolte C J M, Oleson M A, Hansbrough J F, Morgan J, Greenleaf G, Wilkins L. J Anat. 1994;185:325–333. [PMC free article] [PubMed] [Google Scholar]
- 14.Hansbrough J F, Morgan J, Greenleaf G, Parikh M, Nolte C, Wilkins L. J Burn Care Rehab. 1994;15:346–353. doi: 10.1097/00004630-199407000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Yannas I V. Tissue and Organ Regeneration in Adults. New York: Springer; 2000. , in press. [Google Scholar]
- 16.Bunge R P, Bunge M B. Trends Neurosci. 1983;6:499–505. [Google Scholar]
- 17.Chamberlain L J, Yannas I V, Hsu H-P, Strichartz G, Spector M. Exp Neurol. 1998;154:315–329. doi: 10.1006/exnr.1998.6955. [DOI] [PubMed] [Google Scholar]
- 18.Hsu, W.-C., Spilker, M. H., Yannas, I. V. & Rubin, P. A. D. (2000) Invest. Ophthalmol. Visual Sci., in press. [PubMed]


