Abstract
Objective
To characterize the clinical course, therapies, and outcomes of children with fatal and near-fatal asthma admitted to the pediatric intensive care unit (PICU).
Study design
Retrospective chart abstraction across the eight tertiary-care PICUs in the Collaborative Pediatric Critical Care Research Network (CPCCRN). Inclusion criteria: children (1–18 years) admitted 2005 to 2009 (inclusive) for asthma receiving ventilation (near-fatal) or died (fatal). Data collected included medications, ventilator strategies, concomitant therapies, demographics and risk variables.
Results
Of 261 eligible children, 33 (13%) had no previous history of asthma, 218 (84%) survived with no known complications, and 32 (12%) had complications. Eleven (4%) died, 10 having had cardiac arrest before admission. Patients intubated outside the PICU had shorter ventilation (median 25 vs. 84 hours, p<0.001). African-Americans were disproportionately represented by numbers intubated and had shorter durations of intubation. Barotrauma occurred in 15 (6%) children before admission. Pharmacological therapies were highly variable with similar outcomes.
Conclusions
Of children ventilated in CPCCRN PICUs, 96% survived to hospital discharge. Most children who died experienced cardiac arrest prior to admission. Intubation outside the PICU was correlated with shorter ventilation duration. The complications of barotrauma and neuromyopathy were uncommon. Practice patterns varied widely between CPCCRN sites.
Keywords: status asthmaticus, intensive care, death, cause of death, morbidity, intubation, barotraumas, mechanical ventilation, clinical practices, variability, child
The Centers for Disease Control and Prevention notes the prevalence of asthma among U.S. children is high and has increased from 5.8% in 2003 to 9.6% in 2007. Asthma currently affects more than seven million American children (http://www.cdc.gov/nchs/fastats/asthma.htm), with status asthmaticus being the most common medical emergency(1). The National Institutes of Health (NIH) has developed classification and therapy guidelines to address this major public health problem(2). Interestingly, there is a paucity of published data regarding mechanically ventilated children critically ill with status asthmaticus. The NIH guidelines(2) do not address the relatively few children with sufficiently severe asthma to reach the pediatric intensive care unit (PICU). Surveys of asthma management practices in PICUs have demonstrated wide variability, including inconsistent patterns of therapy sequencing and escalation(3–5). Thus, it is difficult to define best practices, develop guidelines, or launch scientific efforts to investigate key questions that might inform clinical management.
Other investigators have recommended the value of descriptive data in asthmatic children with fatal and near-fatal events(6). In this spirit, the Collaborative Pediatric Critical Care Research Network (CPCCRN) investigators conducted an initial, retrospective study of PICU patients with asthma. The purpose was to identify the major areas of variability in drug treatment and ventilatory assistance strategies with the aim of informing later prospective studies, and to ascertain appropriate outcome measures for future trials in critical asthma. Specifically, we sought to characterize the course and therapies used in managing children with near-fatal asthma (i.e., those who received mechanical ventilation and survived), or fatal asthma, admitted to a CPCCRN PICU.
METHODS
A retrospective study was conducted across the eight children’s hospitals of the Collaborative Pediatric Critical Care Research Network (CPCCRN). Patients aged 1 to 18 years of age with a primary admitting diagnosis of an acute exacerbation of asthma or status asthmaticus, who were deemed ill enough to require admission to the PICU of any CPCCRN hospital for ongoing therapy, were defined as having critical asthma. (We acknowledge that our definition of critical asthma is not universally accepted. The term “critical asthma” has been earlier used in clinical practice guidelines in pediatrics(7) where it meant severe asthma in the Emergency Department and did not refer to admission to the PICU.)
All children with critical asthma admitted between January 1, 2005 and December 31, 2009 who received endotracheal intubation and ventilation (near-fatal asthma) or who died (fatal asthma) in the CPCCRN hospital were included in the study. Patients with cystic fibrosis or bronchiolitis were excluded. If a patient was admitted to the hospital more than once during the study period, each hospitalization for critical asthma in which the patient was intubated and ventilated was included. If a patient was admitted to the PICU more than once during the same hospitalization, only the first PICU admission in which the patient was intubated and ventilated was included. Potential cases were identified from PICU logs and hospital databases and then screened by the site Principal Investigator to determine study eligibility. The Institutional Review Board approved the study and granted a waiver of informed consent at each site.
The Data Coordinating Center at the University of Utah trained researchers at each site to review records and collect data. Data were entered into a secure, encrypted Internet site. The Data Coordinating Center staff provided ongoing review of data and contacted researchers at the sites to resolve data queries.
Data collected included (1) demographics such as age, sex, race/ethnicity, height, weight and primary payer type; (2) asthma history such as timing of diagnosis, prior hospital or PICU admissions for asthma, allergies, eczema, psychiatric or behavioral disorders, substance abuse, family history of asthma, chronic asthma medications within 30 days prior to admission, other medical conditions, and non-compliance with asthma therapy; (3) admission data such as hospital and PICU admission and discharge dates, source of admission, mental status, pulse oximetry and vital signs, presence of cardiac arrest or barotrauma prior to admission; (4) ventilation data such as blood gases prior to intubation and extubation, intubation and extubation date/time, initial and final ventilator settings in the PICU; (5) use of inhalational anesthesia, (6) use of extracorporeal membrane oxygenation (ECMO); (7) pharmacological therapies used prior to intubation at referring or CPCCRN hospital, and during and after ventilation; and (8) outcomes including complications (barotrauma, neuromyopathy and CNS deficits), mortality, and mode and cause of death. Mode of death was specified as withdrawal of support, cessation of neurologic function, or failed cardiopulmonary resuscitation. Data on asthma scores was not requested as none of our sites use them.
Statistical Analysis
Descriptive statistics were used to summarize patient characteristics and clinical variables overall and across sites. Categorical data are presented as absolute counts and percentages. Continuous data are presented as means and standard deviations if normally distributed and as medians and inter-quartile ranges if the distributions are skewed. With the exception of pharmacological therapies for which no and not documented were combined, unavailable (missing) values were excluded from calculations of percentages and summary statistics. In survivors only, we evaluated univariable associations between key baseline and early clinical factors with length of mechanical ventilation (LMV), classified as either < 72 or ≥ 72 hours. Statistical testing was based on the Chi-square or Fisher's exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables. Statistical significance was defined as p<0.05 and all analyses were conducted using SAS 9.2 for Windows (Cary, NC).
RESULTS
Cases of near-fatal and fatal asthma (n=261) were identified across the CPCCRN sites during the 5-year study period, of which 260 (99.6%) were intubated and ventilated. One was not intubated and died and 10 (4%) of the intubated patients died. All fatal cases were admitted to the PICU prior to death. Case demographics by study site are shown in Table I (available at www.jpeds.com). When comparing our asthma cohort to overall CPCCRN admissions during the study period, African-American children were over-represented (62% versus 23%; p<0.0001), and Hispanic children were under-represented (10% versus 19%; p=0.0008).
A prior diagnosis of asthma was documented in 226 (87%) cases and 33 (13%) were newly diagnosed during the index hospitalization. Among those previously diagnosed, 132 (63%) had no prior hospital admissions for asthma in the year preceding the index admission, 43 (21%) had one prior admission, 20 (10%) had two and 13 (6%) had three or more. Regarding prior PICU admissions for asthma in the year preceding the index admission, 182 (86%) had none, 21 (10%) had one, 7 (3%) had two and 1 (0.5%) had 3. Among all cases, 76 (29%) had a documented non-food allergy and 51 (20%) had a food allergy. Allergic exposure precipitated the admission in 19/102 (19%) of those with known allergies. A history of eczema was present in 40 (15%) cases, psychiatric or behavioral disorders in 13 (5%), substance abuse in 4 (2%), other medical conditions in 132 (51%) and documented family history of asthma in 151 (58%). Asthma medications used within 30 days prior to admission included short-acting inhaled beta-agonists in 236 (91%), long-acting inhaled beta-agonists in 37 (14%), inhaled corticosteroids in 129 (50%), oral corticosteroids in 55 (21%), leukotriene-receptor antagonists in 64 (25%), inhaled anti-cholinergics in 15 (6%), antihistamines in 9 (4%), methylxanthines in 1 (0.4%) and home supplemental oxygen in 6 (2%). Forty-eight (18%) cases were reported as non-compliant with chronic asthma medications.
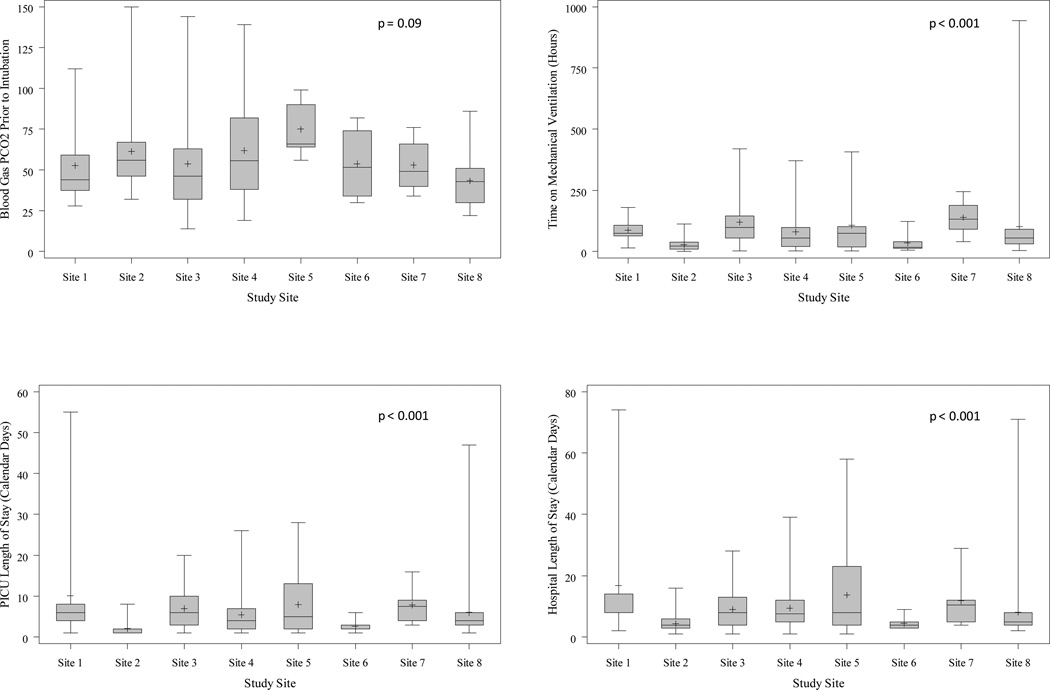
Admission data showed that 141 (54%) cases were transferred to the PICU from an outside emergency department (ED), 86 (33%) from the study site ED, 17 (7%) from a site hospital ward, 13 (5%) were directly admitted to the PICU at the time of hospital admission from a physician’s office or clinic and 4 (2%) from an outside PICU. The mental status of those not intubated prior to PICU admission (n = 81), was reported as alert for 74 (91%) cases, obtunded for 3 (4%), pharmacologically sedated for 3 (4%), and unknown for 1 (1%). Fifteen (6%) cases had pulmonary barotrauma prior to PICU admission including 10 with pneumomediastinum, 6 with subcutaneous emphysema and 4 with pneumothorax. Twenty-five (10%) children had a cardiac arrest prior to PICU admission (16 [64%, p = NS] were African-American), of whom 15 survived, (5 with CNS deficits including one with neuromyopathy and one with residual barotrauma). Ten children died. Figure 1 shows blood gas PCO2 prior to intubation (taken in only 48% of patients) and, for survivors, duration of ventilation, and PICU and hospital lengths of stay by site. The overall distribution of length of ventilation is shown in Figure 2 (available at www.jpeds.com).
Figure 1.
A, Upper left panel: the PCO2 (arterial, capillary or corrected venous (minus 6 torr)) prior to intubation which was not different between sites. Note that only 48% of patients had a blood gas obtained prior to intubation. B, The length of mechanical ventilation which varied significantly between sites. C, The PICU length of stay which varied significantly between sites. D, the Hospital length of stay which varied significantly between sites. The mean (+), median (−), interquartile range (box) and range (whiskers) are plotted in each panel for each site. P values were obtained by Wilcoxon rank-sum test and find overall differences between the CPCCRN PICUs for lengths of PICU and hospital stay and also duration of mechanical ventilation. No multiple comparison tests were done to determine which PICUs were different from the others.
There were 259 patients with documented timing of intubation; 178 (69%) were intubated outside of the CPCCRN PICU. The locations included: referring hospital [125 (70%)], study site ED [36 (20%)], during transport [13 (7%)], operating room [2 (1%)], hospital ward [1 (1%)], and 1 (1%) unknown. Pressure control was the most frequent initial mode of conventional ventilation (63%). High Frequency Oscillatory Ventilation was used in 9 (3%) cases. Mechanical ventilation and blood gas data are shown in Table II. Helium-oxygen gas mixtures were administered prior to intubation for only 5% of those intubated before admission to the PICU vs. 30% of those intubated in the PICU.
Table 2.
Mechanical Ventilation and Blood Gas Data, N=260
| N | n (%) | |
|---|---|---|
| Non-invasive ventilation prior to intubation | 254 | 42 (17) |
| Initial ventilator mode in PICU | 257 | |
| Pressure control | 162 (63) | |
| Volume control | 44 (17) | |
| Pressure-regulated volume control | 36 (14) | |
| Pressure support with PEEP | 15 (6) | |
| Final ventilator mode a | 248 | |
| Pressure control | 84 (34) | |
| Volume control | 50 (20) | |
| Pressure-regulated volume control | 25 (10) | |
| Pressure support with PEEP | 89 (36) | |
| Non-invasive ventilation immediately post-extubation a | 250 | 16 (6) |
| Non-invasive ventilation anytime post-extubation a | 250 | 28 (11) |
| N | median (IQR) | |
| Blood gas values prior to intubation | ||
| pH | 126 | 7.26 (7.17–7.35) |
| PCO2 (torr)b | 126 | 52 (38–68) |
| PaO2 (torr)c | 49 | 109 (65–166) |
| Initial ventilator settings in PICU | ||
| Inspired O2 (%) | 256 | 70 (40–100) |
| Rate (/min)d | 240 | 16 (12–20) |
| Peak inspiratory pressure (cmH2O)e | 161 | 30 (25–36) |
| Tidal volume (ml/kg)f | 77 | 8 (7–9) |
| Inspiratory time (seconds)e | 150 | 0.9 (0.8–1.0) |
| PEEP (cmH2O) | 255 | 5 (5–6) |
| Blood gas values prior to extubation a | ||
| pH | 236 | 7.41 (7.36–7.45) |
| PCO2 (torr)b | 236 | 41 (36–46) |
| PaO2 (torr)c | 156 | 91 (73–110) |
| Final ventilator settings a | ||
| Inspired O2 (%) | 248 | 40 (30–40) |
| PEEP (cmH2O) | 247 | 5 (5–5) |
| Duration of mechanical ventilation, hours a | 216 | 42 (18–84) |
| Duration of non-invasive mechanical ventilation post-extubation, hours | 28 | 27 (17–70) |
Survivors only
Includes arterial, capillary and corrected venous PCO2; correction is venous PCO2 minus 6 torr
Includes arterial PO2 only
Includes cases of pressure control, volume control and pressure-regulated volume control only
Includes cases of pressure control only
Includes cases of volume control and pressure regulated volume control only
Non-invasive positive pressure ventilation was administered to 42 (16%) patients prior to intubation and in 28 (11%) after extubation (Table II). Inhalational anesthesia (isoflurane) was used in 8 (3%) cases (median 74 hours (IQR 52-107). Extracorporeal membrane oxygenation (ECMO veno-arterial) was used in 3 cases (median 139 hours, IQR 116–141). Seventeen (7%) cases were bronchoscoped during ventilation and two (1%) after extubation. Neuromuscular blockade at any time during ventilation (after endotracheal intubation) was used in 170 (65%) patients but its use varied by site (33 to 100%).
The association of demographic and early clinical factors with length of ventilation is shown in Table III. Among 216 patients who survived to hospital discharge where complete ventilation data was available, 148 (69%) were mechanically ventilated for <72 hours and 68 (31%) for ≥72 hours. Children intubated outside the CPCCRN PICUs (80% were African-American) had considerably shorter LMV and ICU stay than those intubated in the PICUs (median LMV: 25 vs. 84 hours, p<0.001; median PICU stay: 56 vs. 137 hours, p<0.001). This observation does not appear to be affected by overall shorter LMV at sites with a preponderance of African-American admissions.
Table 3.
Association of Demographic and Early Clinical Factors with Length of Mechanical Ventilation, N=216a
| Length of Mechanical Ventilation | ||||
|---|---|---|---|---|
| N | < 72 Hours (N=148) | ≥ 72 hours (N=68) | p | |
| median (IQR) | median (IQR) | |||
| Age in years | 216 | 7.5 (2.6, 12.0) | 5.4 (2.1, 9.3) | 0.14 |
| Body mass index | 86 | 17.5 (15.0, 23.5) | 18.1 (15.3, 20.1) | 0.98 |
| n (%) | n (%) | |||
| Sex | 0.88 | |||
| Male | 130 | 90 (69%) | 40 (31%) | |
| Female | 86 | 58 (67%) | 28 (33%) | |
| Race * | < 0.001 | |||
| Black | 123 | 99 (80%) | 24 (20%) | |
| White | 63 | 33 (52%) | 30 (48%) | |
| Asian | 6 | 3 (50%) | 3 (50%) | |
| Other | 2 | 1 (50%) | 1 (50%) | |
| Ethnicity * | 0.03 | |||
| Hispanic or Latino | 15 | 7 (47%) | 8 (53%) | |
| Not Hispanic or Latino | 154 | 115 (75%) | 39 (25%) | |
| Insurance * | 0.17 | |||
| Commercial | 87 | 55 (63%) | 32 (37%) | |
| Medicaid | 119 | 87 (73%) | 32 (27%) | |
| Other | 8 | 4 (50%) | 4 (50%) | |
| Asthma diagnosis * | 0.57 | |||
| New diagnosis | 30 | 22 (73%) | 8 (27%) | |
| Prior diagnosis | 185 | 126 (68%) | 59 (32%) | |
| Known allergic exposure precipitating admission | 0.48 | |||
| Yes | 18 | 11 (61%) | 7 (39%) | |
| No | 198 | 137 (69%) | 61 (31%) | |
| Known cardiac arrest prior to admission | 0.52 | |||
| Yes | 12 | 7 (58%) | 5 (42%) | |
| No | 204 | 141 (69%) | 63 (31%) | |
| Barotrauma evidence prior to admission * | 0.44 | |||
| Yes | 8 | 7 (88%) | 1 (13%) | |
| No | 207 | 140 (68%) | 67 (32%) | |
| Source of admission to PICU | < 0.001 | |||
| Direct admission | 11 | 4 (36%) | 7 (64%) | |
| Transfer from outside ED/ICU | 107 | 85 (79%) | 22 (21%) | |
| Admit through study ED | 83 | 52 (63%) | 31 (37%) | |
| Transfer from floor | 15 | 7 (47%) | 8 (53%) | |
| Intubated Prior to CPCCRN PICU | < 0.001 | |||
| Yes | 136 | 116 (85%) | 20 (15%) | |
| No | 80 | 32 (40%) | 48 (60%) | |
Restricting to patients who survived to hospital discharge and had non-missing value for length of mechanical ventilation.
Data points missing
Pharmacological therapies administered prior to intubation, and during and after mechanical ventilation, were highly variable as shown in Tables IV (available at www.jpeds.com), V, and VI (available at www.jpeds.com), respectively. There was wide site variability for intravenous beta2-agonists with most favoring terbutaline over epinephrine and isoproterenol.
Table 5.
Pharmacologic Therapies Administered During Mechanical Ventilation by Site
| Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 | Site 7 | Site 8 | All | |
|---|---|---|---|---|---|---|---|---|---|
| N=13 | N=68 | N=33 | N=65 | N=15 | N=20 | N=6 | N=40 | N=260 | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Albuterol | |||||||||
| Inhaled, intermittent | 10 (77) | 54 (79) | 30 (91) | 46 (71) | 12 (80) | 14 (70) | 4 (67) | 23 (58) | 193 (74) |
| Inhaled, continuous | 8 (62) | 57 (84) | 8 (24) | 51 (78) | 8 (53) | 7 (35) | (100) | 36 (90) | 181 (70) |
| Corticosteroids, IVa or oral | 13 (100) | 66 (97) | 32 (97) | 63 (97) | 15 (100) | 20 (100) | (100) | 40 (100) | 255 (98) |
| Ipratropium, Inhaled | 4 (31) | 56 (82) | 14 (42) | 9 (14) | 7 (47) | 13 (65) | 5 (83) | 21 (53) | 129 (50) |
| Terbutaline (n, %) | |||||||||
| IV | 4 (31) | 28 (41) | 16 (48) | 32 (49) | 1 (7) | 12 (60) | 3 (50) | 18 (45) | 114 (44) |
| SCb or IMc | 0 | 3 (4) | 0 | 2 (3) | 0 | 0 | 0 | 1 (3) | 6 (2) |
| Inhaled | 0 | 1 (1) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.4) |
| Isoproterenol, IV | 2 (15) | 1 (1) | 0 | 0 | 0 | 0 | 0 | 0 | 3 (1) |
| Epinephrine | |||||||||
| IV | 3 (23) | 7 (10) | 8 (24) | 6 (9) | 3 (20) | 3 (15) | 3 (50) | 8 (20) | 41 (16) |
| SC or IM | 0 | 8 (12) | 3 (9) | 4 (6) | 2 (13) | 2 (10) | 0 | 2 (5) | 21 (8) |
| Inhaled | 3 (23) | 2 (3) | 4 (12) | 5 (8) | 2 (13) | 2 (10) | 0 | 2 (5) | 20 (8) |
| Atropine, IV | 8 (62) | 4 (6) | 3 (9) | 3 (5) | 1 (7) | 0 | 0 | 1 (3) | 20 (8) |
| Aminophylline/Theophylline IV or oral | 6 (46) | 11 (16) | 2 (6) | 2 (3) | 3 (20) | 1 (5) | 2 (33) | 16 (40) | 43 (17) |
| Helium-Oxygen, Inhaled | 0 | 24 (35) | 0 | 13 (20) | 2 (13) | 0 | 1 (17) | 18 (45) | 58 (22) |
| Magnesium sulfate, IV | 6 (46) | 36 (53) | 8 (24) | 22 (34) | 2 (13) | 8 (40) | 5 (83) | 17 (43) | 104 (40) |
| Ketamine, IV | 8 (62) | 12 (18) | 13 (39) | 34 (52) | 7 (47) | 14 (70) | 3 (50) | 30 (75) | 121 (47) |
| Neuromuscular Blockade, IV * | 11 (85) | 47 (69) | 24 (73) | 36 (55) | 5 (33) | 10 (50) | (100) | 31 (78) | 170 (65) |
| Mucolytics, inhaled | 0 | 0 | 0 | 1 (2) | 5 (33) | 0 | 1 (17) | 4 (10) | 11 (4) |
| Nitric oxide, inhaled | 0 | 0 | 6 (18) | 1 (2) | 1 (7) | 0 | 1 (17) | 2 (5) | 11 (4) |
IV is intravenous;
SC is subcutaneous;
IM is intramuscular
Neuromuscular blockade administered during mechanical ventilation, not for intubation
Two hundred eighteen cases (84%) survived PICU admission with no known complications, 32 (12%) with complications and 11 (4%) died. Among survivors with complications, 11 (34%) had central nervous system deficits, 8 (25%) barotrauma and 4 (13%) had neuromyopathy. Five patients (2%) without barotrauma at the time of admission had residual barotrauma at the time of PICU discharge. Six (55%) non-survivors were moribund on arrival to the PICU. Mode of death was cessation of neurologic function in 7 (64%), withdrawal of support in 2 (18%) and failed CPR in 2 (18%). Documented cause of death in the chart was anoxic brain injury in 6 (55%), cardiac arrest in 2 (18%), pneumonia in 1 (9%), multiple organ failure in 1 (9%) and not documented 1 (9%). Four patients had an autopsy.
DISCUSSION
Our data show that most pediatric patients with asthma who are mechanically ventilated and admitted to a CPCCRN PICU survive without short term complications. Ten of the 11 children who died experienced cardiac arrest prior to PICU admission. Geographic location of endotracheal intubation is correlated with LMV. African-American children were significantly over-represented in our sample, and Hispanic children were under-represented. Barotrauma in the course of mechanical ventilation for critical asthma was an uncommon event as was neuromyopathy following steroids and neuromuscular blockade and there is wide variability in the pharmacological treatment of critical asthma over the CPCCRN sites.
In describing contemporary pediatric critical care management for life-threatening childhood asthma the most obvious characteristic is lack of consistency of approach. The lack of standard practice is likely related to conflicting, or lack of, evidence-based data. The lack of national guidelines for critical asthma(2;8–11) reflects this inadequate information.
Patients ultimately admitted to the PICU in this study were more likely to be intubated in the referring or CPCCRN hospital Emergency Department than in the PICU itself. For those 69% of patients intubated prior to PICU admission, the median LMV was shorter (25 hours) compared with 31% eventually intubated within the PICU (84 hours), as reported previously(12). This geographic variability in site of intubations may reflect health care disparities where some children receive less preventive therapy or present later. It could represent over-reaction by clinicians less familiar with children severely ill with asthma, but may also be due to a perceived or real lack of time in the Emergency Department to maximize drug therapy. It is also possible that early intubation and ventilation is beneficial rather than detrimental in status asthmaticus, potentially allowing better drug delivery to the airways, and is safer with modern ventilatory techniques. Many of these children could represent the acute, asphyxial asthmatic (Type 2) with sudden, severe onset but a rapid response to therapy(13). Thus, we could speculate that patients intubated in the PICU and remaining ventilated longer possibly represent the slow onset respiratory failure (Type 1) asthmatics. Furthermore, it is noteworthy that African-American children with near-fatal and fatal asthma were over-represented with respect to their numbers admitted for all diseases to the CPCCRN PICUs (62% vs. 23%). They were also (80%), intubated and ventilated for a shorter time (<72 hours; Table III) whereas LMV for other racial groups were evenly distributed at 50%. These findings were consistent across CPCCRN sites and suggest that African-American children may be particularly prone to acute, asphyxial asthma. Further investigation of this unexpected finding is warranted.
Blood gas values for carbon dioxide tension prior to intubation were available for only 126 (48%) of the 260 intubated patients. Median values were similar across centers (median pH = 7.26 PCO2 = 52 torr) but varied markedly within each center. They are close to those reported by Roberts et al(3) in 2002. Given the variable LMV (median 42; IQR 18–84 hours), and lack of association of initial PCO2 and LMV, carbon dioxide tension may not be a good indicator of severity of the asthma process to the observing clinician, as believed earlier(2). The NIH guidelines(2) do recommend consideration of intubation for children if PaCO2 > 42 torr, without reference.
Pressure control was the most utilized initial mode of ventilation (63%) and this may reflect a gradual acceptance of the proposal by Sarnaik and coworkers that this mode is safe, associated with shorter LMV in pediatric asthma and, theoretically, provides for more uniform ventilation and better inhaled drug distribution than the previously accepted volume control mode(14). Non-invasive ventilation was used overall in 23% of cases both prior to intubation or after extubation for the acute episode. Neither non-invasive nor invasive ventilation in the PICU seemed to cause additional air leaks. Fifteen children had evidence of barotrauma prior to PICU admission and only 5 had a further air leak noted at the time of PICU discharge. ECMO was rarely utilized with only three children (~1%) receiving it, consistent with the ELSO Registry where only 64 children received ECMO for asthma in the 21 years from 1986 to 2007(15).
The use of intravenous beta2 agonists during mechanical ventilation ranged from 27% to 75% across CPCCRN sites. NIH guideline recommendations(2) note a lack of data supporting the intravenous route being superior to inhaled beta2 agonists(16) and specific caution is noted for intravenous isoproterenol which was used at two CPCCRN sites. The NIH guidelines do not address critical asthma so it is difficult to put these recommendations into proper context as other national guidelines advocate intravenous beta2 agonist use if there is no response to continuous inhaled albuterol(8–10). There are at least two studies in severely ill children with asthma supporting intravenous albuterol (salbutamol)(17;18). This suggests a trial of intravenous versus aerosolized beta2 agonists may be feasible and valuable in patients prior to, or without need of, ventilation. We have little information about the incidence of toxicity of beta2-agonists(19;20) by any route in children and further investigation would be of value.
Given our current knowledge of the important role and efficacy of corticosteroids in the management of asthma, and the agreement of many published guidelines(2;8–11), it is rewarding that 98% or 255 of our 260 patients received them during mechanical ventilation. Additionally, 4 of the remaining 5 patients did receive steroids before or after a very short period of ventilation (<3hours). Thus, 99% of children were treated, overall. Notably, 65% of children received neuromuscular blocking agents while ventilated and only 4 cases of subsequent neuromyopathy were reported.
Continued controversy exists about the use of both magnesium and theophylline in acute childhood asthma. A recent meta-analysis for magnesium found it effective in preventing hospitalization and in improving short-term clinical symptom scores and pulmonary function tests(21). Its use is recommended in some published guidelines(2;8;9) but not in others(10). Nonetheless, although magnesium use has not been studied in critical asthma, in this investigation 45% of children received intravenous magnesium prior to intubation, 40% during mechanical ventilation and 6% received this drug after extubation. In contrast, aminophylline was administered to only 4% of children prior to intubation, 17% during mechanical ventilation and 5% after extubation. Aminophylline has been reported to improve several important outcomes in children hospitalized with asthma (though not necessarily critical asthma) in both randomized controlled trials(22–25) and a Cochrane meta-analysis(26). The reasons for this disparity in acceptance are unclear.
Ipratropium bromide is recommended for severe asthma in most published guidelines. Conflicting evidence casts doubt on its efficacy alongside modern selective beta agonists(17;27). Nonetheless, it was used in only 36% of patients in our study prior to intubation and in 50% during mechanical ventilation although there was a wide range of usage (14 – 83%) at individual sites. Overall, its application during ventilation in the CPCCRN PICUs was lower than reported from PHIS data in 2005(4).
Helium-oxygen gas mixtures (heliox) for inhalation have theoretical potential to both decrease work of breathing and improve delivery of inhaled drugs in obstructed airways. BiPAP could potentiate these effects. Nonetheless, although heliox appears useful in models of pediatric ventilation(28) this therapy has had conflicting results in small clinical trials(29;30). In our study, 99 (38%) of children received heliox at some point during their PICU course, but only 22% while ventilated, similar to the 18% noted by Bratton and coworkers for an equivalent group of patients in 2005(4). The role of heliox with or without BiPAP remains to be determined.
There have been several reports of deaths from asthma in children over the past two decades(31–34) but few analyses of PICU management and deaths, all from single centers(35–37). Between 1982 and 1988 in Toronto, 89 children were admitted to the PICU for status asthmaticus(35), one third being mechanically ventilated. Three children died (3.4%) from hypoxic-ischemic encephalopathy following out-of-hospital cardiac arrest(35). In a subsequent three-year review(36), 19 children were ventilated (mean LMV = 42 hours compared with our mean = 73 hours; median = 42.6 hours) with 11 (58%) intubated prior to PICU admission, 14 (74%) extubated within 72 hours and no deaths. Over a six-year period (1982–88) in Melbourne, 27 (10%) required mechanical ventilation and 5 (18.5%) died, 4 having arrested prior to intubation(37). In our study, of 260 ventilated children, 4.3% died, a similar proportion to that of the Toronto report 21 years ago(35).
Recently, Melbourne investigators interviewed 410 of 684 patients admitted to their PICU with asthma over 15 years with a mean follow up of 10.3 years. Eighty-eight per cent continued to have asthma and 5% of those mechanically ventilated during their index admission died within 10 years of discharge(38). These data suggest that children ventilated for asthma are a high risk group and should be studied prospectively, with a view to evaluating longer term outcomes.
Two clinical subsets are described of adults who die from status asthmaticus(39;40). Type 1 (slow onset) patients have poorly controlled, severe asthma with higher mortality. Bronchial mucus plugging is prominent pathologically(41). Type 2 (fast-onset) patients have little or no history of asthma and experience fulminant bronchospasm(40). Pathological examination shows airways without mucus plugging(42). These subsets may not exist in children although Type 2 has been alluded to(13) and clinical experience could support the existence of this subset. The possibility these subsets exist in children as well as adults warrants further investigation. Our autopsy findings were limited to 4 of the 11 patients. All showed characteristic chronic pulmonary changes of asthma with only one having significant bronchial mucus plugging.
Limited conclusions can be derived from this study. It is a retrospective cohort and data from referring Emergency Departments was limited. We could not capture details of drug dosing or toxicity, nor of how drugs were applied with respect to the severity of the asthma. In addition, some elements have missing data and it is possible there is some selection bias with regards to these missing data. We also included children as young as 1 year of age. Each case was adjudicated as having asthma by the principal investigator at each site. Although it is possible that some of these cases had bronchiolitis where longer duration of ventilation would be expected, this proportion of those ventilated <72 hours was the same for those under 2 years of age as for those older (68%).
Development of a critical asthma severity score to guide the intensity of PICU therapeutic interventions and responses would be useful. Although at least 19 “asthma scores” are available(43) none have been derived or validated on children with asthma requiring intensive care unit admission.
In conclusion, this retrospective study of mechanically ventilated patients admitted to CPCCRN PICUs shows that significant variability occurs in anti-asthma therapies and ventilator strategies in critically ill children. Despite marked variability, outcomes are similar and most patients do well. This variability, along with the lack of sensitive tools to grade critical asthma severity and response to therapies, makes it challenging to test hypotheses. All children but one who died experienced a cardiac arrest prior to their PICU admission. The marked over-representation of African-American children in this fatal/near-fatal cohort allows speculation about an alternate pathophysiology (e.g. a predilection to fulminant bronchospasm) or possible health care disparities in this group of children.
Supplementary Material
Acknowledgments
Supported by Eunice Kennedy Shriver National Institute for Child Health and Human Development (NICHD) (cooperative agreements U10-HD050012, U10-HD050096, U10-HD063108, U10-HD049983, U10-HD049981, U10-HD063114, and U10-HD063106), the Obstetric and Pediatric Pharmacology Branch, and the Best Pharmaceuticals for Children Act.
Appendix
CPCCRN members participating in this study included: University of Utah (Data Coordinating Center), Salt Lake City, UT: J. Michael Dean, MD, MBA, Katherine Sward, PhD, RN, Jeri Burr, MS, RN-BC, CCRC, Amy Clark, MS, Richard Holubkov, PhD, Stephanie Bisping, BSN, RN, CCRP, Teresa Liu, MPH, Emily Stock, BS, Rene Enriquez, BS, Anna Davis, MPH, RN, Alecia Peterson, BS; Children’s National Medical Center, Washington DC: John Berger, MD, David Wessel, MD, Jean Reardon, BSN, RN; Children’s Hospital of Pittsburgh, Pittsburgh, PA: Joseph Carcillo, MD, Michael Bell, MD, Alan C. Abraham, BA, CCRC; Children’s Hospital of Michigan, Detroit, MI: Kathleen L. Meert, MD, Sabrina Heidemann, MD, Ann Pawluszka, BSN, RN; Phoenix Children’s Hospital, Phoenix, AZ: Murray Pollack, MD, Heidi Dalton, MD, Christian Lawson, MS, RN; Children’s Hospital of Philadelphia, Philadelphia, PA: Robert Berg, MD, Athena Zuppa, MD, Mary Ann DiLiberto, BS, RN, CCRC; Department of Pediatrics, CS Mott Children’s Hospital at University of Michigan, Ann Arbor, MI: Tom Shanley, MD, Frank Moler, MD, Monica S. Weber, RN, BSN, CCRP; Children’s Hospital Los Angeles, Los Angeles, CA: Christopher J.L. Newth, MD, FRCPC, J. Francisco Fajardo, CLS (ASCP), RN, MD, Jennifer Kwok, BS, Jonathan Serrano, BA; Mattel Children’s Hospital at University of California Los Angeles, Los Angeles, CA: Mary Ann Nyc, Rick Harrison, MD; Washington University, St. Louis, MO: Allan Doctor, MD; National Institute of Child Health and Human Development, Bethesda, MD: Carol Nicholson, MD, Tammara Jenkins, MSN, RN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
Bibliography
- 1.Bohn D, Kissoon N. Acute asthma. Pediatr Crit Care Med. 2001;2:151–163. doi: 10.1097/00130478-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 2.EPR-3.Expert panel report 3, National Institutes of Health, National Heart Lung and Blood Institute, National Asthma Education and Prevention Program. Guidelines for the diagnosis and management of asthma (EPR-3 2007). NIH Publication No. 08-4051. Bethesda, MD: [Accessed January 8, 2012]. http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. [Google Scholar]
- 3.Roberts JS, Bratton SL, Brogan TV. Acute severe asthma: differences in therapies and outcomes among pediatric intensive care units. Crit Care Med. 2002;30:581–585. doi: 10.1097/00003246-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Bratton SL, Odetola FO, McCollegan J, Cabana MD, Levy FH, Keenan HT. Regional variation in ICU care for pediatric patients with asthma. J Pediatr. 2005;147:355–361. doi: 10.1016/j.jpeds.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Bratton SL, Roberts JS. Variation in the use of mechanical ventilation for asthma: how big a gap? Pediatr Crit Care Med. 2007;8:186–187. doi: 10.1097/01.PCC.0000257116.42827.2F. [DOI] [PubMed] [Google Scholar]
- 6.Strunk RC, Nicklas RA, Milgrom H, Ikle D. Fatal and near-fatal asthma questionnaire: prelude to a national registry. J Allergy Clin Immunol. 1999;104:763–768. doi: 10.1016/s0091-6749(99)70285-x. [DOI] [PubMed] [Google Scholar]
- 7.Royal Children's Hospital MA. [Accessed January 8, 2012];Clinical Practice Guidelines Group. Asthma (Acute) 2010 http://www.rch.org.au/clinicalguide/cpg.cfm?doc_id=5251.
- 8.British Thoracic Society and Scottish Intercollegiate Guidelines Network. [Accessed January 8, 2012];British guideline on the management of asthma (revised 2011) http://sign.ac.uk/pdf/sign101.pdf.
- 9.National Asthma Council Australia. [Accessed January 8, 2012];Asthma Management Handbook. 2006 http://www.nationalasthma.org.au/cms/index.php.
- 10.Paediatric Society of New Zealand. [Accessed January 8, 2012];Management of Asthma in children aged 1–15 years. 2005 http://www.paediatrics.org.nz/files/guidelines/Asthmaendorsed.pdf.
- 11.Global Initiative for Asthma (GINA) [Accessed January 8, 2012];Global Strategy for the Diagnosis and Management of Asthma in Children 5 Years and Younger. 2009 http://www.ginasthma.com/.
- 12.Carroll CL, Smith SR, Collins MS, Bhandari A, Schramm CM, Zucker AR. Endotracheal intubation and pediatric status asthmaticus: site of original care affects treatment. Pediatr Crit Care Med. 2007;8:91–95. doi: 10.1097/01.PCC.0000257115.02573.FC. [DOI] [PubMed] [Google Scholar]
- 13.Maffei FA, van der Jagt EW, Powers KS, Standage SW, Connolly HV, Harmon WG, et al. Duration of mechanical ventilation in life-threatening pediatric asthma: description of an acute asphyxial subgroup. Pediatrics. 2004;114:762–767. doi: 10.1542/peds.2004-0294. [DOI] [PubMed] [Google Scholar]
- 14.Sarnaik AP, Daphtary KM, Meert KL, Lieh-Lai MW, Heidemann SM. Pressure-controlled ventilation in children with severe status asthmaticus. Pediatr Crit Care Med. 2004;5:133–138. doi: 10.1097/01.pcc.0000112374.68746.e8. [DOI] [PubMed] [Google Scholar]
- 15.Hebbar KB, Petrillo-Albarano T, Coto-Puckett W, Heard M, Rycus PT, Fortenberry JD. Experience with use of extracorporeal life support for severe refractory status asthmaticus in children. Crit Care. 2009;13:R29. doi: 10.1186/cc7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Travers A, Jones AP, Kelly K, Barker SJ, Camargo CA, Rowe BH. Intravenous beta2-agonists for acute asthma in the emergency department. Cochrane Database Syst Rev. 2001;2:CD002988. doi: 10.1002/14651858.CD002988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Browne GJ, Trieu L, Van Asperen P. Randomized, double-blind, placebo-controlled trial of intravenous salbutamol and nebulized ipratropium bromide in early management of severe acute asthma in children presenting to an emergency department. Crit Care Med. 2002;30:448–453. doi: 10.1097/00003246-200202000-00030. [DOI] [PubMed] [Google Scholar]
- 18.Browne GJ, Penna AS, Phung X, Soo M. Randomised trial of intravenous salbutamol in early management of acute severe asthma in children. Lancet. 1997;349:301–305. doi: 10.1016/S0140-6736(96)06358-1. [DOI] [PubMed] [Google Scholar]
- 19.Meert KL, Clark J, Sarnaik AP. Metabolic acidosis as an underlying mechanism of respiratory distress in children with severe acute asthma. Pediatr Crit Care Med. 2007;8:519–523. doi: 10.1097/01.PCC.0000288673.82916.9D. [DOI] [PubMed] [Google Scholar]
- 20.Chiang VW, Burns JP, Rifai N, Lipshultz SE, Adams MJ, Weiner DL. Cardiac toxicity of intravenous terbutaline for the treatment of severe asthma in children: a prospective assessment. J Pediatr. 2000;137:73–77. doi: 10.1067/mpd.2000.106567. [DOI] [PubMed] [Google Scholar]
- 21.Cheuk DK, Chau TC, Lee SL. A meta-analysis on intravenous magnesium sulphate for treating acute asthma. Arch Dis Child. 2005;90:74–77. doi: 10.1136/adc.2004.050005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wheeler DS, Jacobs BR, Kenreigh CA, Bean JA, Hutson TK, Brilli RJ. Theophylline versus terbutaline in treating critically ill children with status asthmaticus: a prospective, randomized, controlled trial. Pediatr Crit Care Med. 2005;6:142–147. doi: 10.1097/01.PCC.0000154943.24151.58. [DOI] [PubMed] [Google Scholar]
- 23.Yung M, South M. Randomised controlled trial of aminophylline for severe acute asthma. Arch Dis Child. 1998;79:405–410. doi: 10.1136/adc.79.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ream RS, Loftis LL, Albers GM, Becker BA, Lynch RE, Mink RB. Efficacy of iv theophylline in children with severe status asthmaticus. Chest. 2001;119:1480–1488. doi: 10.1378/chest.119.5.1480. [DOI] [PubMed] [Google Scholar]
- 25.Roberts G, Newsom D, Gomez K, Raffles A, Saglani S, Begent J, et al. Intravenous salbutamol bolus compared with an aminophylline infusion in children with severe asthma: a randomised controlled trial. Thorax. 2003;58:306–310. doi: 10.1136/thorax.58.4.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitra AA, Bassler D, Goodman K, Lasserson TJ, Ducharme FM. Intravenous aminophylline for acute severe asthma in children over two years receiving inhaled bronchodilators. Cochrane Database Syst Rev. 2005;2:CD001276. doi: 10.1002/14651858.CD001276.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iramain R, Lopez-Herce J, Coronel J, Spitters C, Guggiari J, Bogado N. Inhaled salbutamol plus ipratropium in moderate and severe asthma crises in children. J Asthma. 2011;48:298–303. doi: 10.3109/02770903.2011.555037. [DOI] [PubMed] [Google Scholar]
- 28.Habib DM, Garner SS, Brandeburg S. Effect of helium-oxygen on delivery of albuterol in a pediatric, volume-cycled, ventilated lung model. Pharmacotherapy. 1999;19:143–149. doi: 10.1592/phco.19.3.143.30920. [DOI] [PubMed] [Google Scholar]
- 29.Kim IK, Phrampus E, Venkataraman S, Pitetti R, Saville A, Corcoran T, et al. Helium/oxygen-driven albuterol nebulization in the treatment of children with moderate to severe asthma exacerbations: a randomized, controlled trial. Pediatrics. 2005;116:1127–1133. doi: 10.1542/peds.2004-2136. [DOI] [PubMed] [Google Scholar]
- 30.Bigham MT, Jacobs BR, Monaco MA, Brilli RJ, Wells D, Conway EM, et al. Helium/oxygen-driven albuterol nebulization in the management of children with status asthmaticus: a randomized, placebo-controlled trial. Pediatr Crit Care Med. 2010;11:356–361. [PubMed] [Google Scholar]
- 31.Matsui T, Baba M. Death from asthma in children. Acta Paediatr Jpn. 1990;32:205–208. doi: 10.1111/j.1442-200x.1990.tb00812.x. [DOI] [PubMed] [Google Scholar]
- 32.Fletcher HJ, Ibrahim SA, Speight N. Survey of asthma deaths in the Northern region, 1970–85. Arch Dis Child. 1990;65:163–167. doi: 10.1136/adc.65.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kravis LP, Kolski GB. Unexpected death in childhood asthma. A review of 13 deaths in ambulatory patients. Am J Dis Child. 1985;139:558–563. doi: 10.1001/archpedi.1985.02140080028026. [DOI] [PubMed] [Google Scholar]
- 34.Robertson CF, Rubinfeld AR, Bowes G. Pediatric asthma deaths in Victoria: the mild are at risk. Pediatr Pulmonol. 1992;13:95–100. doi: 10.1002/ppul.1950130207. [DOI] [PubMed] [Google Scholar]
- 35.Stein R, Canny GJ, Bohn DJ, Reisman JJ, Levison H. Severe acute asthma in a pediatric intensive care unit: six years' experience. Pediatrics. 1989;83:1023–1028. [PubMed] [Google Scholar]
- 36.Cox RG, Barker GA, Bohn DJ. Efficacy, results, and complications of mechanical ventilation in children with status asthmaticus. Pediatric Pulmonology. 1991;11:120–126. doi: 10.1002/ppul.1950110208. [DOI] [PubMed] [Google Scholar]
- 37.Shugg AW, Kerr S, Butt WW. Mechanical ventilation of paediatric patients with asthma: short and long term outcome. J Paediatr Child Health. 1990;26:343–346. doi: 10.1111/j.1440-1754.1990.tb02449.x. [DOI] [PubMed] [Google Scholar]
- 38.Triasih R, Duke T, Robertson CF. Outcomes following admission to intensive care for asthma. Arch Dis Child. 2011;96:729–734. doi: 10.1136/adc.2010.205062. [DOI] [PubMed] [Google Scholar]
- 39.Papiris S, Kotanidou A, Malagari K, Roussos C. Clinical review: severe asthma. Crit Care. 2002;6:30–44. doi: 10.1186/cc1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wasserfallen JB, Schaller MD, Feihl F, Perret CH. Sudden asphyxic asthma: a distinct entity? Am Rev Respir Dis. 1990;142:108–111. doi: 10.1164/ajrccm/142.1.108. [DOI] [PubMed] [Google Scholar]
- 41.McFadden ER., Jr Acute severe asthma. Am J Respir Crit Care Med. 2003;168:740–759. doi: 10.1164/rccm.200208-902SO. [DOI] [PubMed] [Google Scholar]
- 42.Sur S, Crotty TB, Kephart GM, Hyma BA, Colby TV, Reed CE, et al. Sudden-onset fatal asthma. A distinct entity with few eosinophils and relatively more neutrophils in the airway submucosa? Am Rev Respir Dis. 1993;148:713–719. doi: 10.1164/ajrccm/148.3.713. [DOI] [PubMed] [Google Scholar]
- 43.Gorelick MH, Stevens MW, Schultz TR, Scribano PV. Performance of a novel clinical score, the Pediatric Asthma Severity Score (PASS), in the evaluation of acute asthma. Acad Emerg Med. 2004;11:10–18. doi: 10.1197/j.aem.2003.07.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.